Fat-Free Mass Normalization Impacts Cardiorespiratory Fitness in Overweight Adolescents
Abstract
1. Introduction
2. Materials and Methods
2.1. Body Composition and Anthropometric Measurement
2.2. Cardiorespiratory Fitness Test
2.3. Statistical Analysis
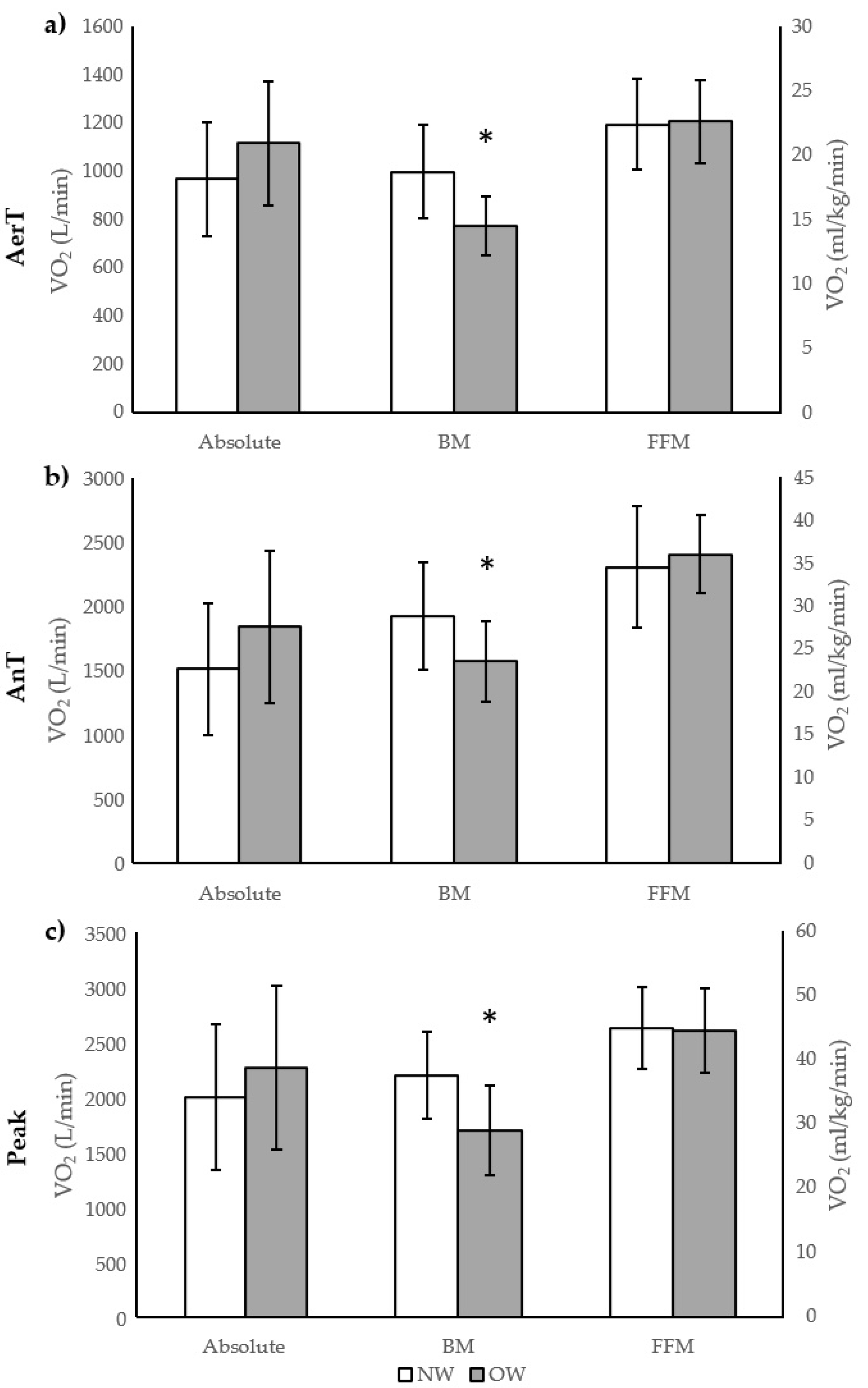
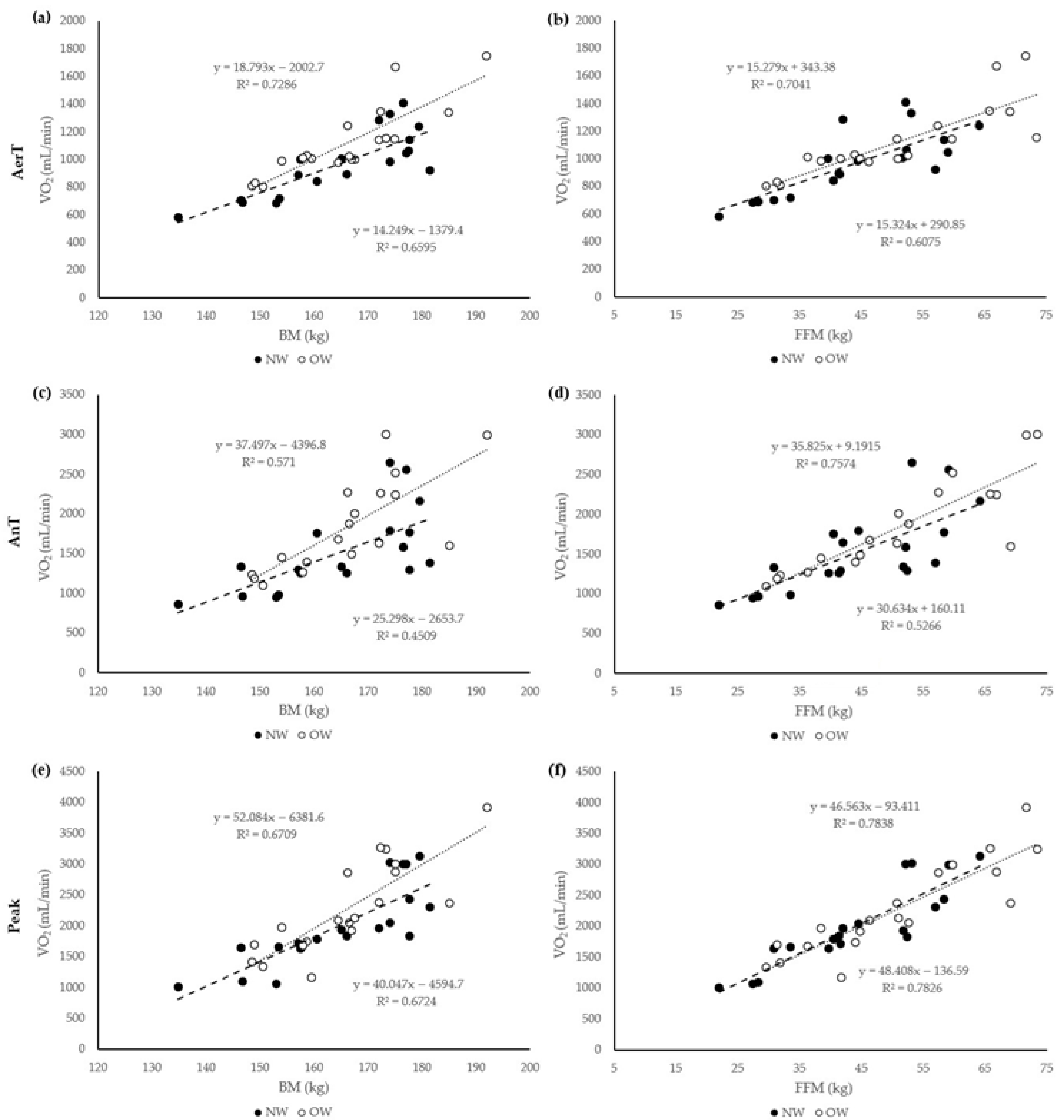
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VO2 | Oxygen Consumption |
| VO2AerT | VO2 at the aerobic threshold |
| VO2AnT | VO2 at the anaerobic threshold |
| VO2peak | VO2 at peak exercise |
| CRF | Cardiorespiratory Fitness |
| BM | Body Mass |
| FM | Fat Mass |
| FFM | Fat-Free Mass |
| BMI | Body Mass Index |
| BF% | Body Fat Percentage |
| HR | Heart Rate |
| NW | Normal Weight |
| OW | Overweight |
| ANCOVA | Analysis of Covariance |
References
- Ogden, C.L.; Carroll, M.D.; Lawman, H.G.; Fryar, C.D.; Kruszon-Moran, D.; Kit, B.K.; Flegal, K.M. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 Through 2013–2014. JAMA 2016, 315, 2292–2299. [Google Scholar] [CrossRef]
- Daniels, S.R.; Arnett, D.K.; Eckel, R.H.; Gidding, S.S.; Hayman, L.L.; Kumanyika, S.; Robinson, T.N.; Scott, B.J.; St Jeor, S.; Williams, C.L. Overweight in Children and Adolescents: Pathophysiology, Consequences, Prevention, and Treatment. Circulation 2005, 111, 1999–2012. [Google Scholar] [CrossRef]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H.; American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and Cardiovascular Disease: Pathophysiology, Evaluation, and Effect of Weight Loss: An Update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar] [CrossRef]
- Lister, N.B.; Baur, L.A.; Felix, J.F.; Hill, A.J.; Marcus, C.; Reinehr, T.; Summerbell, C.; Wabitsch, M. Child and adolescent obesity. Nat. Rev. Dis. Primers 2023, 9, 24. [Google Scholar] [CrossRef]
- Kim, H.; Collier, S.R.; Bonavolontà, V.; Lassiter, A.; Wait, S.; Meucci, M. Cardiorespiratory Fitness Is an Indicator of Arterial Stiffness and Aortic Blood Pressure in Healthy Adolescents. Children 2024, 11, 1078. [Google Scholar] [CrossRef]
- Mondal, H. Effect of BMI, Body Fat Percentage and Fat Free Mass on Maximal Oxygen Consumption in Healthy Young Adults. J. Clin. Diagn. Res. 2017, 11, CC17–CC20. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and Cardiovascular Disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef] [PubMed]
- Gaesser, G.A.; Angadi, S.S. Obesity treatment: Weight loss versus increasing fitness and physical activity for reducing health risks. iScience 2021, 24, 102995. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, J.; Blais, S.; Chetaille, P.; Bisson, M.; Counil, F.P.; Huard-Girard, T.; Berbari, J.; Boulay, P.; Dallaire, F. New Reference Values for Cardiopulmonary Exercise Testing in Children. Med. Sci. Sports Exerc. 2018, 50, 1125–1133. [Google Scholar] [CrossRef]
- Bhammar, D.M.; Adams-Huet, B.; Babb, T.G. Quantification of Cardiorespiratory Fitness in Children with Obesity. Med. Sci. Sports Exerc. 2019, 51, 2243–2250. [Google Scholar] [CrossRef]
- Imboden, M.T.; Kaminsky, L.A.; Peterman, J.E.; Hutzler, H.L.; Whaley, M.H.; Fleenor, B.S.; Harber, M.P. Cardiorespiratory Fitness Normalized to Fat-Free Mass and Mortality Risk. Med. Sci. Sports Exerc. 2020, 52, 1532–1537. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, B.M.; Zierath, J.R. The Limits of Exercise Physiology: From Performance to Health. Cell Metab. 2017, 25, 1000–1011. [Google Scholar] [CrossRef]
- Bongers, B.C.; Hulzebos, E.H.; Helbing, W.A.; Harkel, A.D.; Van Brussel, M.; Takken, T. Response profiles of oxygen uptake efficiency during exercise in healthy children. Eur. J. Prev. Cardiol. 2016, 23, 865–873. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine; Riebe, D.; Ehrman, J.K.; Liguori, G.; Magal, M. (Eds.) ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2018; 472p. [Google Scholar]
- Meyer, T.; Lucía, A.; Earnest, C.P.; Kindermann, W. A Conceptual Framework for Performance Diagnosis and Training Prescription from Submaximal Gas Exchange Parameters—Theory and Application. Int. J. Sports Med. 2005, 26, S38–S48. [Google Scholar] [CrossRef] [PubMed]
- Hampl, S.E.; Hassink, S.G.; Skinner, A.C.; Armstrong, S.C.; Barlow, S.E.; Bolling, C.F.; Avila Edwards, K.C.; Eneli, I.; Hamre, R.; Joseph, M.M. Clinical Practice Guideline for the Evaluation and Treatment of Children and Adolescents With Obesity. Pediatrics 2023, 151, e2022060640. [Google Scholar] [CrossRef]
- Fields, D.A.; Goran, M.I.; McCrory, M.A. Body-composition assessment via air-displacement plethysmography in adults and children: A review. Am. J. Clin. Nutr. 2002, 75, 453–467. [Google Scholar] [CrossRef]
- Tseh, W.; Caputo, J.L.; Keefer, D.J. Validity and Reliability of the BOD POD® S/T Tracking System. Int. J. Sports Med. 2010, 31, 704–708. [Google Scholar] [CrossRef]
- Guidetti, L.; Meucci, M.; Bolletta, F.; Emerenziani, G.P.; Gallotta, M.C.; Baldari, C. Validity, reliability and minimum detectable change of COSMED K5 portable gas exchange system in breath-by-breath mode. PLoS ONE 2018, 13, e0209925. [Google Scholar] [CrossRef]
- Sharma, V.K.; Subramanian, S.K.; Arunachalam, V. Evaluation of body composition and its association with cardio respiratory fitness in south Indian adolescents. Indian J. Physiol. Pharmacol. 2013, 57, 399–405. [Google Scholar]
- Cooper, D.M.; Leu, S.-Y.; Taylor-Lucas, C.; Lu, K.; Galassetti, P.; Radom-Aizik, S. Cardiopulmonary Exercise Testing in Children and Adolescents with High Body Mass Index. Pediatr. Exerc. Sci. 2016, 28, 98–108. [Google Scholar] [CrossRef]
- Ekelund, U.; Franks, P.W.; Wareham, N.J.; Åman, J. Oxygen Uptakes Adjusted for Body Composition in Normal-Weight and Obese Adolescents. Obes. Res. 2004, 12, 513–520. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Więcek, M.; Szymura, J.; Szyguła, Z.; Wiecha, S.; Cempla, J. The Influence of Increased Body Fat or Lean Body Mass on Aerobic Performance. PLoS ONE 2014, 9, e95797. [Google Scholar] [CrossRef]
- Inostroza-Mondaca, M.; Valdes, O.; Ramírez-Campillo, R.; Granacher, U. Muscle Strength, Muscle Morphology, and Oxidative Capacity in Normal Weight Versus Overweight and Obese Youth: A Systematic Review with Meta-Analysis. Research Square. 4 June 2025. Available online: https://www.researchsquare.com/article/rs-6497659/v1 (accessed on 15 May 2025).
- Damer, A.; El Meniawy, S.; McPherson, R.; Wells, G.; Harper, M.E.; Dent, R. Association of muscle fiber type with measures of obesity: A systematic review. Obes. Rev. 2022, 23, e13444. [Google Scholar] [CrossRef] [PubMed]
- Marques-Vidal, P.; Marcelino, G.; Ravasco, P.; Oliveira, J.M.; Paccaud, F. Increased body fat is independently and negatively related with cardiorespiratory fitness levels in children and adolescents with normal weight. Eur. J. Cardiovasc. Prev. Rehabil. 2010, 17, 649–654. [Google Scholar] [CrossRef]
- Norman, A.C.; Drinkard, B.; McDuffie, J.R.; Ghorbani, S.; Yanoff, L.B.; Yanovski, J.A. Influence of Excess Adiposity on Exercise Fitness and Performance in Overweight Children and Adolescents. Pediatrics 2005, 115, e690–e696. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Sui, X.; Artero, E.G.; Lee, I.M.; Church, T.S.; McAuley, P.A.; Stanford, F.C.; Kohl, H.W., 3rd; Blair, S.N. Long-Term Effects of Changes in Cardiorespiratory Fitness and Body Mass Index on All-Cause and Cardiovascular Disease Mortality in Men: The Aerobics Center Longitudinal Study. Circulation 2011, 124, 2483–2490. [Google Scholar] [CrossRef] [PubMed]
- Barry, V.W.; Baruth, M.; Beets, M.W.; Durstine, J.L.; Liu, J.; Blair, S.N. Fitness vs. Fatness on All-Cause Mortality: A Meta-Analysis. Prog. Cardiovasc. Dis. 2014, 56, 382–390. [Google Scholar] [CrossRef]
- Blair, S.N.; Kampert, J.B.; Kohl, H.W.; Barlow, C.E.; Macera, C.A.; Paffenbarger, R.S.; Gibbons, L.W. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA 1996, 276, 205–210. [Google Scholar] [CrossRef]
- Lavie, C.J.; De Schutter, A.; Milani, R.V. Healthy obese versus unhealthy lean: The obesity paradox. Nat. Rev. Endocrinol. 2015, 11, 55–62. [Google Scholar] [CrossRef]
- Lubans, D.; Richards, J.; Hillman, C.; Faulkner, G.; Beauchamp, M.; Nilsson, M.; Kelly, P.; Smith, J.; Raine, L.; Biddle, S. Physical Activity for Cognitive and Mental Health in Youth: A Systematic Review of Mechanisms. Pediatrics 2016, 138, e20161642. [Google Scholar] [CrossRef]
- Biddle, S.J.H.; Asare, M. Physical activity and mental health in children and adolescents: A review of reviews. Br. J. Sports Med. 2011, 45, 886–895. [Google Scholar] [CrossRef]
| Variables | NW | OW |
|---|---|---|
| Age (years) | 14.5 ± 2.3 | 14.0 ± 2.3 |
| Height (cm) | 164.8 ± 13.5 | 166.0 ± 11.7 |
| Weight (kg) | 52.8 ± 13.1 | 79.2 ± 24.1 ** |
| BMI | 19.1 ± 2.9 | 28.2 ± 5.7 ** |
| FM (kg) | 8.6 ± 4.8 | 28.6 ± 14.2 ** |
| FFM (kg) | 44.2 ± 12.1 | 50.7 ± 14.2 |
| FM% | 16.4 ± 7.9 | 35.3 ± 10.3 ** |
| FFM% | 83.6 ± 7.9 | 64.7 ± 10.3 ** |
| RERpeak | 1.20 ± 0.12 | 1.16 ± 0.09 |
| PWRpeak (Watt) | 164 ± 51 | 178 ± 49 |
| Variables | NW | OW | Cohen’s d |
|---|---|---|---|
| VO2AerT (mL/min) | 968 ± 238 | 1117 ± 258 | −0.60 |
| VO2AerTFFM (mL/kg/min) | 22.4 ± 3.5 | 22.6 ± 3.2 | −0.07 |
| VO2AerTBM (mL/kg/min) | 18.7 ± 3.6 | 14.5 ± 2.3 ** | 1.39 |
| VO2AnT (mL/min) | 1514 ± 510 | 1842.4 ± 593 | −0.59 |
| VO2AnTFFM (mL/kg/min) | 34.6 ± 7.1 | 36.1 ± 4.6 | −0.25 |
| VO2AnTBM (mL/kg/min) | 28.8 ± 6.3 | 23.6 ± 4.7 ** | 0.94 |
| VO2peak (mL/min) | 2003 ± 661 | 2266 ± 746 | −0.37 |
| VO2peakFFM (mL/kg/min) | 45.1 ± 6.4 | 44.7 ± 6.6 | 0.06 |
| VO2peakBM (mL/kg/min) | 37.7 ± 6.7 | 29.1 ± 7.0 ** | 1.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oduru, S.; Nagaraj, K.; Charvu, A.; Ravindran, G.; Meucci, M.; Bonavolontà, V. Fat-Free Mass Normalization Impacts Cardiorespiratory Fitness in Overweight Adolescents. Adolescents 2025, 5, 48. https://doi.org/10.3390/adolescents5030048
Oduru S, Nagaraj K, Charvu A, Ravindran G, Meucci M, Bonavolontà V. Fat-Free Mass Normalization Impacts Cardiorespiratory Fitness in Overweight Adolescents. Adolescents. 2025; 5(3):48. https://doi.org/10.3390/adolescents5030048
Chicago/Turabian StyleOduru, Srijan, Kartik Nagaraj, Anvi Charvu, Gautham Ravindran, Marco Meucci, and Valerio Bonavolontà. 2025. "Fat-Free Mass Normalization Impacts Cardiorespiratory Fitness in Overweight Adolescents" Adolescents 5, no. 3: 48. https://doi.org/10.3390/adolescents5030048
APA StyleOduru, S., Nagaraj, K., Charvu, A., Ravindran, G., Meucci, M., & Bonavolontà, V. (2025). Fat-Free Mass Normalization Impacts Cardiorespiratory Fitness in Overweight Adolescents. Adolescents, 5(3), 48. https://doi.org/10.3390/adolescents5030048






