Race, Socioeconomic Status, and Cerebellum Cortex Fractional Anisotropy in Pre-Adolescents
Abstract
1. Introduction
Aims
2. Methods
2.1. The ABCD Study Design and Setting
2.2. Ethics
2.3. Samples and Sampling
2.4. Image Acquisition and dMRI
2.5. Variables
2.5.1. Dependent Variable
2.5.2. Independent Variable
2.5.3. Covariates
2.5.4. Moderator
2.6. Data Analysis
3. Results
3.1. Descriptives
3.2. Model Fits
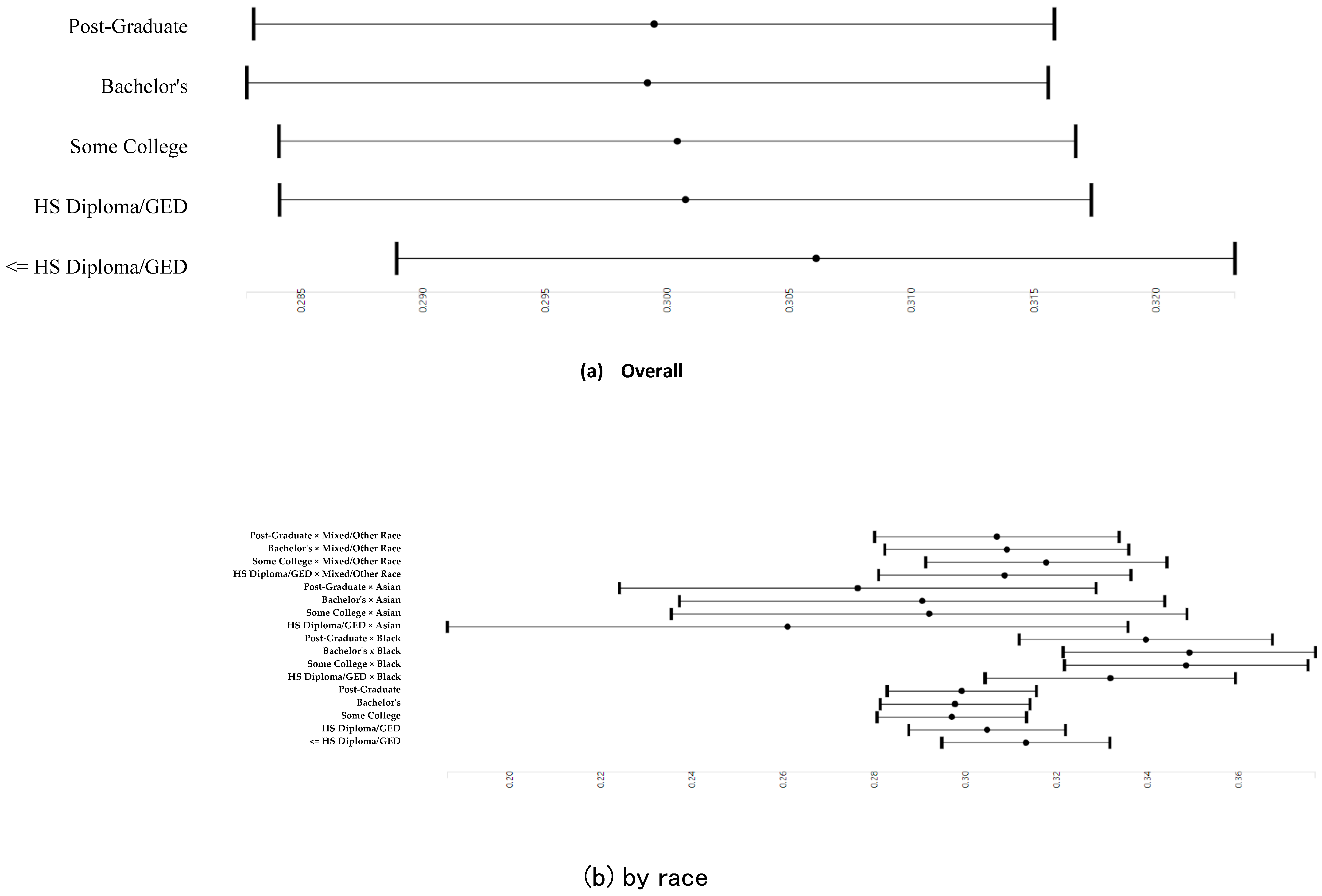
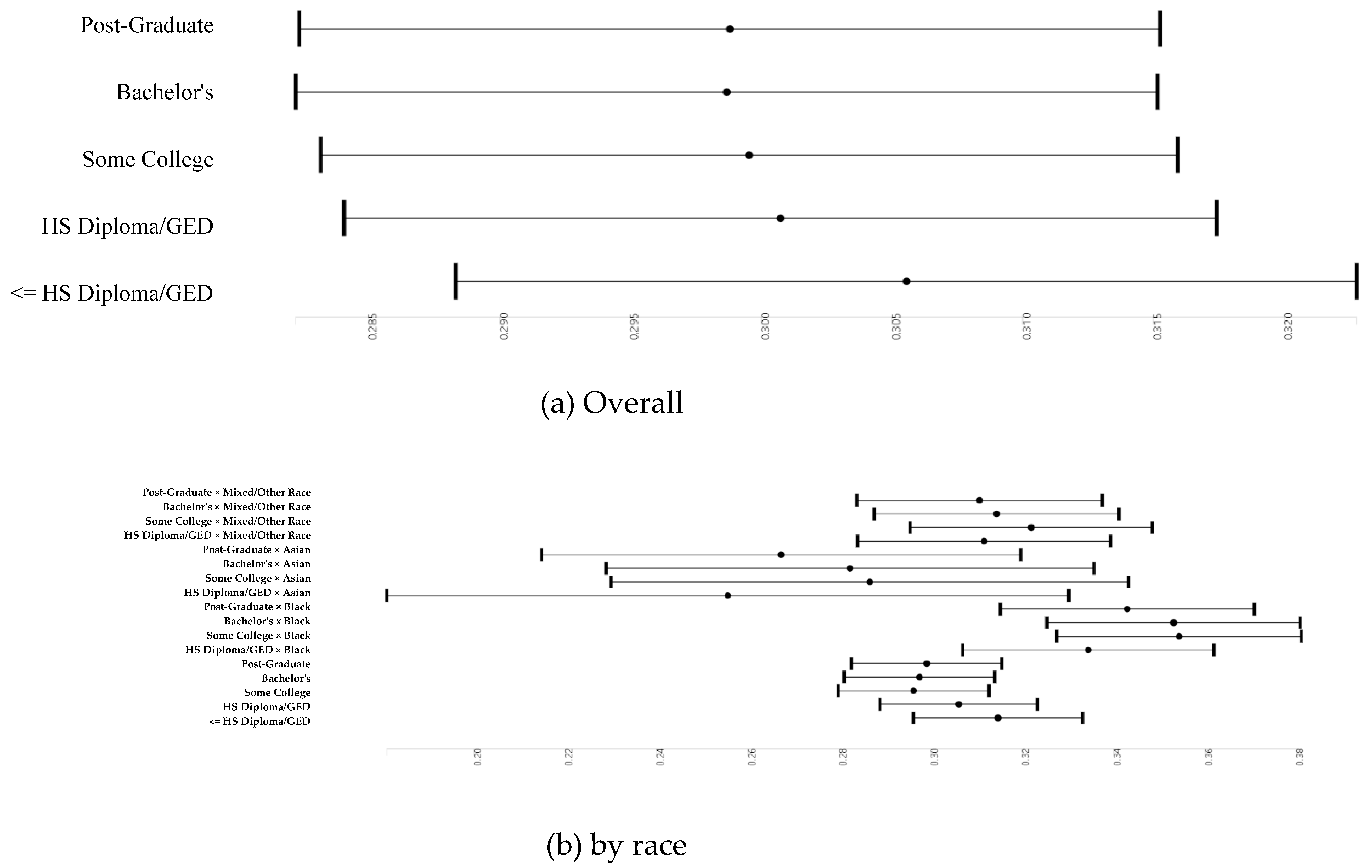
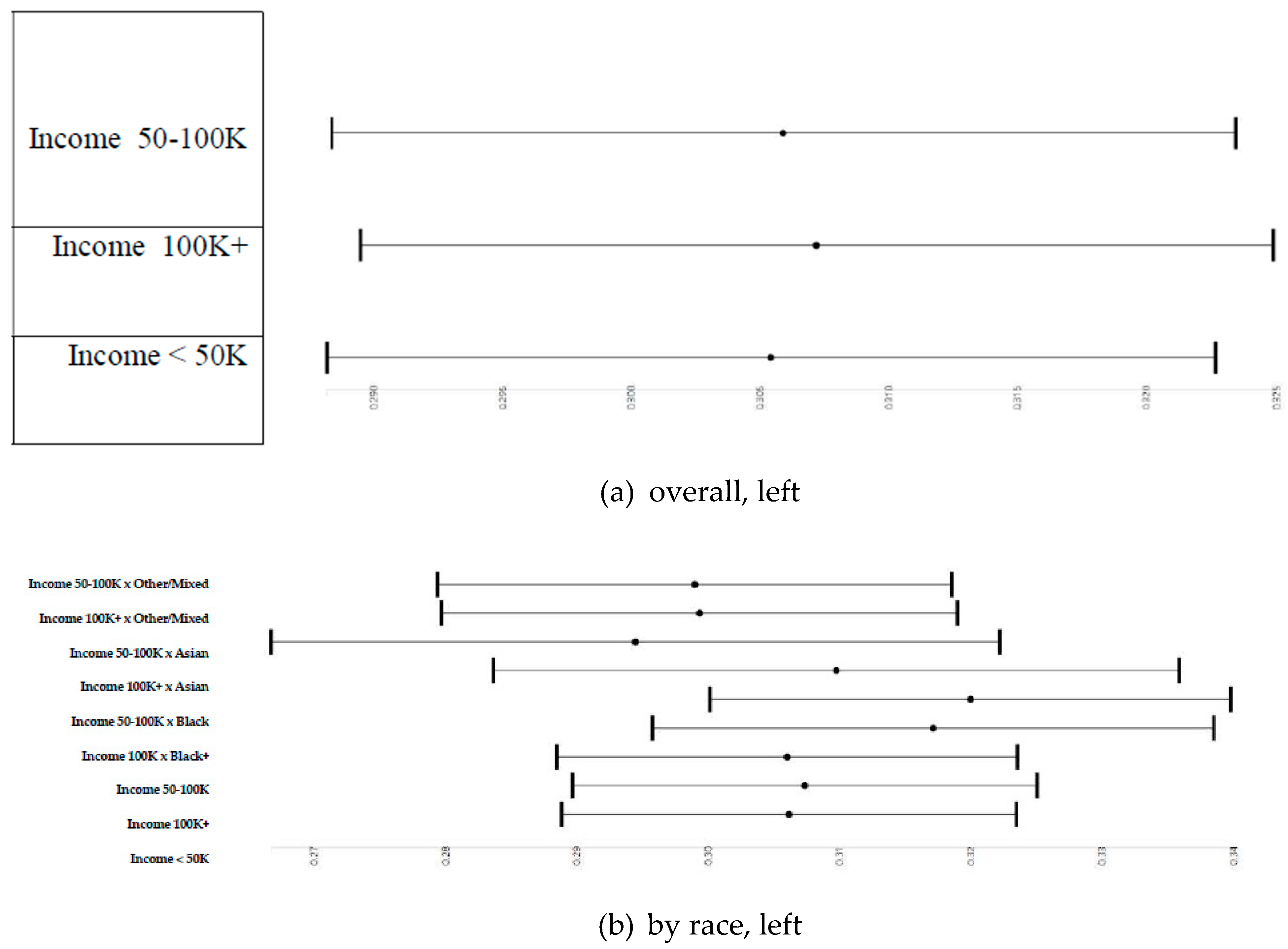
3.3. Parental Education and Right and Left Cerebellum Cortex Fractional Anisotropy
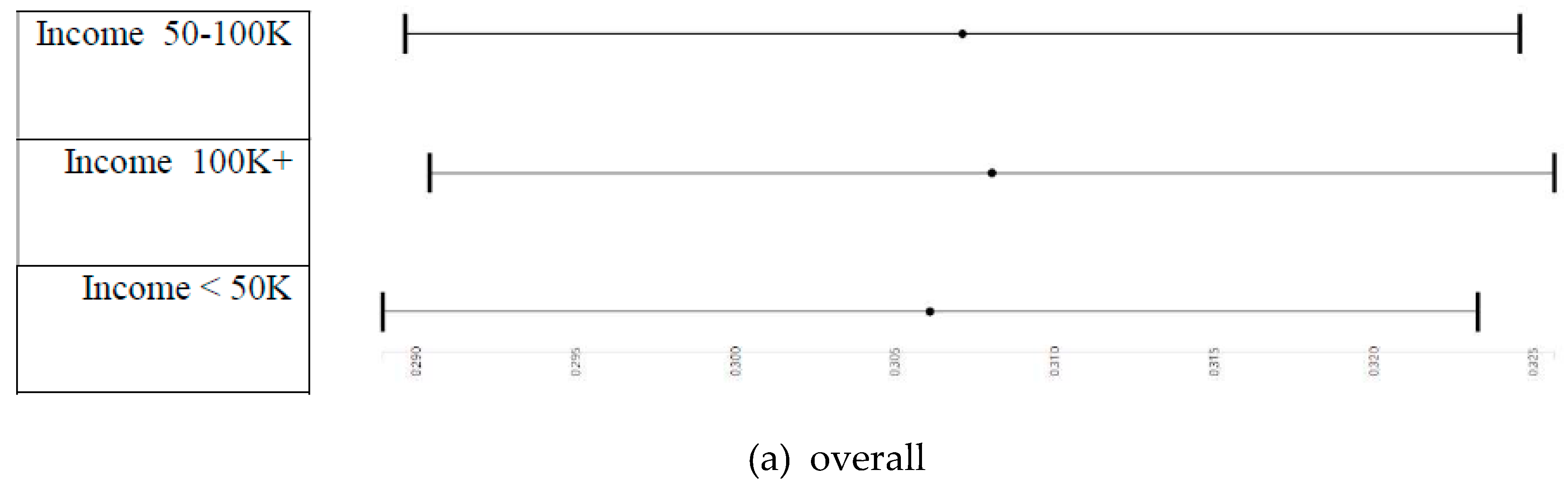
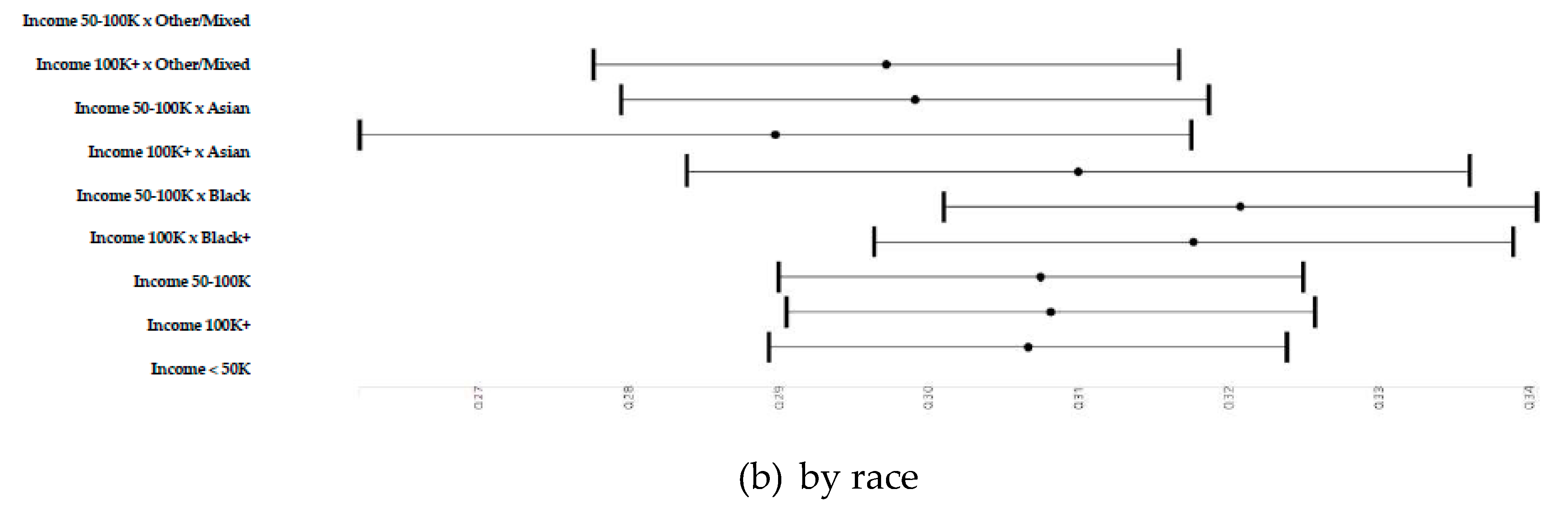
3.4. Household Income and Right and Left Cerebellum Cortex Fractional Anisotropy
3.5. Household Income and Right and Left Cerebellum Cortex Fractional Anisotropy
3.6. Parental Education and Right and Left Cerebellum Cortex Fractional Anisotropy Overall and by Race
3.7. Household Income and Right and Left Cerebellum Cortex Fractional Anisotropy
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Model Formula
| dmri_dti.fa_subcort.aseg_cerebellum.cortex.rh ~ high.educ.bl + household.income.bl + race.4level + married.bl + age + sex + hisp |
| Random: ~(1|rel_family_id) |
| dmri_dti.fa_subcort.aseg_cerebellum.cortex.lh ~ high.educ.bl + household.income.bl + race.4level + married.bl + age + sex + hisp |
| Random: ~(1|rel_family_id) |
| dmri_dti.fa_subcort.aseg_cerebellum.cortex.rh ~ high.educ.bl + household.income.bl + race.4level + married.bl + age + sex + hisp + high.educ.bl * race.4level |
| Random: ~(1|rel_family_id) |
| dmri_dti.fa_subcort.aseg_cerebellum.cortex.lh ~ high.educ.bl + household.income.bl + race.4level + married.bl + age + sex + hisp + high.educ.bl * race.4level |
| Random: ~(1|rel_family_id) |
Appendix A.2. Distribution of Study Variables and Models Assumptions



References
- Eluvathingal, T.J.; Chugani, H.T.; Behen, M.E.; Juhasz, C.; Muzik, O.; Maqbool, M.; Chugani, D.C.; Makki, M. Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics 2006, 117, 2093–2100. [Google Scholar] [CrossRef]
- Deary, I.J.; Bastin, M.E.; Pattie, A.; Clayden, J.D.; Whalley, L.J.; Starr, J.M.; Wardlaw, J.M. White matter integrity and cognition in childhood and old age. Neurology 2006, 66, 505–512. [Google Scholar] [CrossRef]
- Lim, K.O.; Hedehus, M.; Moseley, M.; de Crespigny, A.; Sullivan, E.V.; Pfefferbaum, A. Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch. Gen. Psychiatry 1999, 56, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.O.; Choi, S.J.; Pomara, N.; Wolkin, A.; Rotrosen, J.P. Reduced frontal white matter integrity in cocaine dependence: A controlled diffusion tensor imaging study. Biol. Psychiatry 2002, 51, 890–895. [Google Scholar] [CrossRef]
- Ma, X.; Coles, C.D.; Lynch, M.E.; Laconte, S.M.; Zurkiya, O.; Wang, D.; Hu, X. Evaluation of corpus callosum anisotropy in young adults with fetal alcohol syndrome according to diffusion tensor imaging. Alcohol Clin. Exp. Res. 2005, 29, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Kubicki, M.; Park, H.; Westin, C.F.; Nestor, P.G.; Mulkern, R.V.; Maier, S.E.; Niznikiewicz, M.; Connor, E.E.; Levitt, J.J.; Frumin, M.; et al. DTI and MTR abnormalities in schizophrenia: Analysis of white matter integrity. Neuroimage 2005, 26, 1109–1118. [Google Scholar] [CrossRef]
- Hoeft, F.; Barnea–Goraly, N.; Haas, B.W.; Golarai, G.; Ng, D.; Mills, D.; Korenberg, J.; Bellugi, U.; Galaburda, A.; Reiss, A.L. More is not always better: Increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. J. Neurosci. 2007, 27, 11960–11965. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.E.; Correia, S.; Brennan–Krohn, T.; Malloy, P.F.; Laidlaw, D.H.; Schulz, S.C. Frontal white matter integrity in borderline personality disorder with self–injurious behavior. J. Neuropsychiatry Clin. Neurosci. 2007, 19, 383–390. [Google Scholar] [CrossRef]
- Ashtari, M.; Cottone, J.; Ardekani, B.A.; Cervellione, K.; Szeszko, P.R.; Wu, J.; Chen, S.; Kumra, S. Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Arch. Gen. Psychiatry 2007, 64, 1270–1280. [Google Scholar] [CrossRef]
- Kraus, M.F.; Susmaras, T.; Caughlin, B.P.; Walker, C.J.; Sweeney, J.A.; Little, D.M. White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain 2007, 130, 2508–2519. [Google Scholar] [CrossRef]
- Mueller, H.P.; Unrath, A.; Sperfeld, A.D.; Ludolph, A.C.; Riecker, A.; Kassubek, J. Diffusion tensor imaging and tractwise fractional anisotropy statistics: Quantitative analysis in white matter pathology. Biomed. Eng. Online 2007, 6, 42. [Google Scholar] [CrossRef]
- Keller, T.A.; Kana, R.K.; Just, M.A. A developmental study of the structural integrity of white matter in autism. Neuroreport 2007, 18, 23–27. [Google Scholar] [CrossRef]
- Munoz Maniega, S.; Lymer, G.K.; Bastin, M.E.; Marjoram, D.; Job, D.E.; Moorhead, T.W.; Owens, D.G.; Johnstone, E.C.; McIntosh, A.M.; Lawrie, S.M. A diffusion tensor MRI study of white matter integrity in subjects at high genetic risk of schizophrenia. Schizophr. Res. 2008, 106, 132–139. [Google Scholar] [CrossRef]
- Ouyang, X.; Tao, H.J.; Liu, H.H.; Deng, Q.J.; Sun, Z.H.; Xu, L.; Liu, Z.N.; Xue, Z.M. White matter integrity deficit in treatment–naive adult patients with major depressive disorder. East Asian Arch. Psychiatry 2011, 21, 5–9. [Google Scholar] [PubMed]
- Guo, W.B.; Liu, F.; Chen, J.D.; Xu, X.J.; Wu, R.R.; Ma, C.Q.; Gao, K.; Tan, C.L.; Sun, X.L.; Xiao, C.Q.; et al. Altered white matter integrity of forebrain in treatment–resistant depression: A diffusion tensor imaging study with tract–based spatial statistics. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 38, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Walther, S.; Hugli, S.; Hofle, O.; Federspiel, A.; Horn, H.; Bracht, T.; Wiest, R.; Strik, W.; Muller, T.J. Frontal white matter integrity is related to psychomotor retardation in major depression. Neurobiol. Dis. 2012, 47, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Sahyoun, C.P.; Belliveau, J.W.; Mody, M. White matter integrity and pictorial reasoning in high–functioning children with autism. Brain. Cogn. 2010, 73, 180–188. [Google Scholar] [CrossRef]
- Im, W.Y.; Ha, J.H.; Kim, E.J.; Cheon, K.A.; Cho, J.; Song, D.H. Impaired White Matter Integrity and Social Cognition in High–Function Autism: Diffusion Tensor Imaging Study. Psychiatry Investig. 2018, 15, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Yu, Q.; Herold, F.; Liu, Z.; Wang, J.; Zhu, L.; Xiong, X.; Chen, A.; Muller, P.; Kramer, A.F.; et al. Mini–Basketball Training Program Improves Social Communication and White Matter Integrity in Children with Autism. Brain Sci. 2020, 10, 803. [Google Scholar] [CrossRef]
- Hamilton, L.S.; Levitt, J.G.; O’Neill, J.; Alger, J.R.; Luders, E.; Phillips, O.R.; Caplan, R.; Toga, A.W.; McCracken, J.; Narr, K.L. Reduced white matter integrity in attention–deficit hyperactivity disorder. Neuroreport 2008, 19, 1705–1708. [Google Scholar] [CrossRef]
- Davenport, N.D.; Karatekin, C.; White, T.; Lim, K.O. Differential fractional anisotropy abnormalities in adolescents with ADHD or schizophrenia. Psychiatry Res. 2010, 181, 193–198. [Google Scholar] [CrossRef] [PubMed]
- King, J.B.; Yurgelun–Todd, D.; Stoeckel, A.; DiMuzio, J.M.; Lopez–Larson, M.P. Sex differences in white matter integrity in youths with attention–deficit/hyperactivity disorder: A pilot study. Front Neurosci. 2015, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Kerchner, G.A.; Racine, C.A.; Hale, S.; Wilheim, R.; Laluz, V.; Miller, B.L.; Kramer, J.H. Cognitive processing speed in older adults: Relationship with white matter integrity. PLoS ONE 2012, 7, e50425. [Google Scholar] [CrossRef] [PubMed]
- Genova, H.M.; DeLuca, J.; Chiaravalloti, N.; Wylie, G. The relationship between executive functioning, processing speed, and white matter integrity in multiple sclerosis. J. Clin. Exp. Neuropsychol. 2013, 35, 631–641. [Google Scholar] [CrossRef]
- Manoach, D.S.; Ketwaroo, G.A.; Polli, F.E.; Thakkar, K.N.; Barton, J.J.; Goff, D.C.; Fischl, B.; Vangel, M.; Tuch, D.S. Reduced microstructural integrity of the white matter underlying anterior cingulate cortex is associated with increased saccadic latency in schizophrenia. Neuroimage 2007, 37, 599–610. [Google Scholar] [CrossRef]
- Walther, S.; Federspiel, A.; Horn, H.; Razavi, N.; Wiest, R.; Dierks, T.; Strik, W.; Muller, T.J. Alterations of white matter integrity related to motor activity in schizophrenia. Neurobiol. Dis. 2011, 42, 276–283. [Google Scholar] [CrossRef]
- Liu, X.; Lai, Y.; Wang, X.; Hao, C.; Chen, L.; Zhou, Z.; Yu, X.; Hong, N. Reduced white matter integrity and cognitive deficit in never–medicated chronic schizophrenia: A diffusion tensor study using TBSS. Behav. Brain Res. 2013, 252, 157–163. [Google Scholar] [CrossRef]
- Xiao, J.; He, Y.; McWhinnie, C.M.; Yao, S. Altered white matter integrity in individuals with cognitive vulnerability to depression: A tract–based spatial statistics study. Sci. Rep. 2015, 5, 9738. [Google Scholar] [CrossRef]
- Ray, N.R.; O’Connell, M.A.; Nashiro, K.; Smith, E.T.; Qin, S.; Basak, C. Evaluating the relationship between white matter integrity, cognition, and varieties of video game learning. Restor. Neurol. Neurosci. 2017, 35, 437–456. [Google Scholar] [CrossRef]
- Gold, B.T.; Powell, D.K.; Xuan, L.; Jicha, G.A.; Smith, C.D. Age-related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiol. Aging 2010, 31, 512–522. [Google Scholar] [CrossRef]
- Brinkman, T.M.; Reddick, W.E.; Luxton, J.; Glass, J.O.; Sabin, N.D.; Srivastava, D.K.; Robison, L.L.; Hudson, M.M.; Krull, K.R. Cerebral white matter integrity and executive function in adult survivors of childhood medulloblastoma. Neuro Oncol. 2012, 14 (Suppl. 4), iv25–iv36. [Google Scholar] [CrossRef]
- Borghesani, P.R.; Madhyastha, T.M.; Aylward, E.H.; Reiter, M.A.; Swarny, B.R.; Schaie, K.W.; Willis, S.L. The association between higher order abilities, processing speed, and age are variably mediated by white matter integrity during typical aging. Neuropsychologia 2013, 51, 1435–1444. [Google Scholar] [CrossRef]
- Passamonti, L.; Fairchild, G.; Fornito, A.; Goodyer, I.M.; Nimmo–Smith, I.; Hagan, C.C.; Calder, A.J. Abnormal anatomical connectivity between the amygdala and orbitofrontal cortex in conduct disorder. PLoS ONE 2012, 7, e48789. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.B.; Liu, F.; Xue, Z.M.; Gao, K.; Wu, R.R.; Ma, C.Q.; Liu, Z.N.; Xiao, C.Q.; Chen, H.F.; Zhao, J.P. Altered white matter integrity in young adults with first–episode, treatment–naive, and treatment–responsive depression. Neurosci. Lett. 2012, 522, 139–144. [Google Scholar] [CrossRef]
- Choi, S.; Han, K.M.; Won, E.; Yoon, B.J.; Lee, M.S.; Ham, B.J. Association of brain–derived neurotrophic factor DNA methylation and reduced white matter integrity in the anterior corona radiata in major depression. J. Affect. Disord. 2015, 172, 74–80. [Google Scholar] [CrossRef]
- Lee, R.; Arfanakis, K.; Evia, A.M.; Fanning, J.; Keedy, S.; Coccaro, E.F. White Matter Integrity Reductions in Intermittent Explosive Disorder. Neuropsychopharmacology 2016, 41, 2697–2703. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.A.; Cui, J.; Fukunaga, R.; Nickerson, L.D.; Rauch, S.L.; Rosso, I.M. Disruption of white matter structural integrity and connectivity in posttraumatic stress disorder: A TBSS and tractography study. Depress. Anxiety 2017, 34, 437–445. [Google Scholar] [CrossRef]
- Moeller, F.G.; Hasan, K.M.; Steinberg, J.L.; Kramer, L.A.; Dougherty, D.M.; Santos, R.M.; Valdes, I.; Swann, A.C.; Barratt, E.S.; Narayana, P.A. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine–dependent subjects: Diffusion tensor imaging. Neuropsychopharmacology 2005, 30, 610–617. [Google Scholar] [CrossRef]
- Bell, R.P.; Foxe, J.J.; Nierenberg, J.; Hoptman, M.J.; Garavan, H. Assessing white matter integrity as a function of abstinence duration in former cocaine–dependent individuals. Drug Alcohol Depend. 2011, 114, 159–168. [Google Scholar] [CrossRef][Green Version]
- Li, P.; Tsapanou, A.; Qolamreza, R.R.; Gazes, Y. White matter integrity mediates decline in age–related inhibitory control. Behav. Brain Res. 2018, 339, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Bucur, B.; Madden, D.J.; Spaniol, J.; Provenzale, J.M.; Cabeza, R.; White, L.E.; Huettel, S.A. Age–related slowing of memory retrieval: Contributions of perceptual speed and cerebral white matter integrity. Neurobiol. Aging 2008, 29, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Rogalski, E.J.; Murphy, C.M.; deToledo–Morrell, L.; Shah, R.C.; Moseley, M.E.; Bammer, R.; Stebbins, G.T. Changes in parahippocampal white matter integrity in amnestic mild cognitive impairment: A diffusion tensor imaging study. Behav. Neurol. 2009, 21, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.D.; Szeszko, P.R.; Radua, J.; Ikuta, T.; Gruner, P.; DeRosse, P.; Zhang, J.P.; Giorgio, A.; Qiu, D.; Tapert, S.F.; et al. White matter development in adolescence: Diffusion tensor imaging and meta–analytic results. Schizophr Bull 2012, 38, 1308–1317. [Google Scholar] [CrossRef]
- Oshri, A.; Hallowell, E.; Liu, S.; MacKillop, J.; Galvan, A.; Kogan, S.M.; Sweet, L.H. Socioeconomic hardship and delayed reward discounting: Associations with working memory and emotional reactivity. Dev. Cogn. Neurosci. 2019, 37, 100642. [Google Scholar] [CrossRef]
- Palma–Coca, O.; Hernandez–Serrato, M.I.; Villalobos–Hernandez, A.; Unikel–Santoncini, C.; Olaiz–Fernandez, G.; Bojorquez–Chapela, I. Association of socioeconomic status, problem behaviors, and disordered eating in Mexican adolescents: Results of the Mexican National Health and Nutrition Survey 2006. J. Adolesc. Health 2011, 49, 400–406. [Google Scholar] [CrossRef]
- Sirin, S.R. Socioeconomic status and academic achievement: A meta–analytic review of research. Rev. Edu. Res. 2005, 75, 417–453. [Google Scholar] [CrossRef]
- Fluss, J.; Ziegler, J.C.; Warszawski, J.; Ducot, B.; Richard, G.; Billard, C. Poor reading in French elementary school: The interplay of cognitive, behavioral, and socioeconomic factors. J. Dev. Behav. Pediatr. 2009, 30, 206–216. [Google Scholar] [CrossRef]
- Machlin, L.; McLaughlin, K.A.; Sheridan, M.A. Brain structure mediates the association between socioeconomic status and attention–deficit/hyperactivity disorder. Dev. Sci. 2019, e12844. [Google Scholar] [CrossRef]
- Assari, S.; Caldwell, C.H. Family Income at Birth and Risk of Attention Deficit Hyperactivity Disorder at Age 15: Racial Differences. Children 2019, 6, 10. [Google Scholar] [CrossRef]
- Jablonska, B.; Kosidou, K.; Ponce de Leon, A.; Wettermark, B.; Magnusson, C.; Dal, H.; Dalman, C. Neighborhood Socioeconomic Characteristics and Utilization of ADHD Medication in Schoolchildren: A Population Multilevel Study in Stockholm County. J. Atten. Disord. 2020, 24, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.P.; Cleary, S.D. Racial and ethnic disparities in parent–reported diagnosis of ADHD: National Survey of Children’s Health (2003, 2007, and 2011). J. Clin. Psychiatry 2016, 77, 52–59. [Google Scholar] [CrossRef]
- Heshmat, R.; Qorbani, M.; Ghoreshi, B.; Djalalinia, S.; Tabatabaie, O.R.; Safiri, S.; Noroozi, M.; Motlagh, M.E.; Ahadi, Z.; Asayesh, H.; et al. Association of socioeconomic status with psychiatric problems and violent behaviours in a nationally representative sample of Iranian children and adolescents: The CASPIAN–IV study. BMJ Open 2016, 6, e011615. [Google Scholar] [CrossRef]
- Feldstein Ewing, S.W.; Hudson, K.A.; Caouette, J.; Mayer, A.R.; Thayer, R.E.; Ryman, S.G.; Bryan, A.D. Sexual risk–taking and subcortical brain volume in adolescence. Ann. Behav. Med. 2018, 52, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Kaleta, D.; Usidame, B.; Dziankowska–Zaborszczyk, E.; Makowiec–Dabrowska, T. Socioeconomic Disparities in Age of Initiation and Ever Tobacco Smoking: Findings from Romania. Cent. Eur. J. Public Health 2015, 23, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Barreto, S.M.; de Figueiredo, R.C.; Giatti, L. Socioeconomic inequalities in youth smoking in Brazil. BMJ Open 2013, 3, e003538. [Google Scholar] [CrossRef]
- Moore, G.F.; Littlecott, H.J. School– and family–level socioeconomic status and health behaviors: Multilevel analysis of a national survey in wales, United Kingdom. J. Sch. Health 2015, 85, 267–275. [Google Scholar] [CrossRef]
- Silveira, C.M.; Siu, E.R.; Anthony, J.C.; Saito, L.P.; de Andrade, A.G.; Kutschenko, A.; Viana, M.C.; Wang, Y.P.; Martins, S.S.; Andrade, L.H. Drinking patterns and alcohol use disorders in Sao Paulo, Brazil: The role of neighborhood social deprivation and socioeconomic status. PLoS ONE 2014, 9, e108355. [Google Scholar] [CrossRef]
- Gerra, G.; Benedetti, E.; Resce, G.; Potente, R.; Cutilli, A.; Molinaro, S. Socioeconomic Status, Parental Education, School Connectedness and Individual Socio–Cultural Resources in Vulnerability for Drug Use among Students. Int. J. Environ. Res. Public Health 2020, 17, 1306. [Google Scholar] [CrossRef]
- Javanbakht, A.; King, A.P.; Evans, G.W.; Swain, J.E.; Angstadt, M.; Phan, K.L.; Liberzon, I. Childhood Poverty Predicts Adult Amygdala and Frontal Activity and Connectivity in Response to Emotional Faces. Front. Behav. Neurosci. 2015, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Masten, C.L.; Telzer, E.H.; Eisenberger, N.I. An FMRI investigation of attributing negative social treatment to racial discrimination. J. Cogn. Neurosci. 2011, 23, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zou, Q.; Hu, J.; Tang, W.; Mao, Y.; Gao, L.; Zhu, J.; Jin, Y.; Wu, X.; Lu, L.; et al. Intrinsic Functional Connectivity Patterns Predict Consciousness Level and Recovery Outcome in Acquired Brain Injury. J. Neurosci. 2015, 35, 12932–12946. [Google Scholar] [CrossRef]
- Noble, K.G.; Houston, S.M.; Brito, N.H.; Bartsch, H.; Kan, E.; Kuperman, J.M.; Akshoomoff, N.; Amaral, D.G.; Bloss, C.S.; Libiger, O. Family income, parental education and brain structure in children and adolescents. Nat. Neurosci. 2015, 18, 773. [Google Scholar] [CrossRef]
- Assari, S.; Boyce, S.; Bazargan, M.; Caldwell, C.H. African Americans’ Diminished Returns of Parental Education on Adolescents’ Depression and Suicide in the Adolescent Brain Cognitive Development (ABCD) Study. Euro. J. Investig. Health Psych. Educ. 2020, 10, 656–668. [Google Scholar]
- Assari, S. Parental Education on Youth Inhibitory Control in the Adolescent Brain Cognitive Development (ABCD) Study: Blacks’ Diminished Returns. Brain Sci. 2020, 10, 312. [Google Scholar] [CrossRef] [PubMed]
- Assari, S.; Boyce, S.; Akhlaghipour, G.; Bazargan, M.; Caldwell, C.H. Reward Responsiveness in the Adolescent Brain Cognitive Development (ABCD) Study: African Americans’ Diminished Returns of Parental Education. Brain Sci. 2020, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, L.M.; Chiang, J.J.; Vause, K.; Hoffer, L.; Alpert, K.; Parrish, T.B.; Wang, L.; Miller, G.E. Subcortical structural variations associated with low socioeconomic status in adolescents. Hum. Brain Mapp. 2020, 41, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Willis, A.W.; Schootman, M.; Kung, N.; Wang, X.Y.; Perlmutter, J.S.; Racette, B.A. Disparities in deep brain stimulation surgery among insured elders with Parkinson disease. Neurology 2014, 82, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Parker, N.; Wong, A.P.; Leonard, G.; Perron, M.; Pike, B.; Richer, L.; Veillette, S.; Pausova, Z.; Paus, T. Income inequality, gene expression, and brain maturation during adolescence. Sci. Rep. 2017, 7, 7397. [Google Scholar] [CrossRef] [PubMed]
- Assari, S. Health Disparities due to Diminished Return among Black Americans: Public Policy Solutions. Soc. Issu. Policy Rev. 2018, 12, 112–145. [Google Scholar] [CrossRef]
- Assari, S. Unequal Gain of Equal Resources across Racial Groups. Int. J. Health Policy Manag. 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Assari, S.; Caldwell, C.H.; Bazargan, M. Association Between Parental Educational Attainment and Youth Outcomes and Role of Race/Ethnicity. JAMA Netw. Open 2019, 2, e1916018. [Google Scholar] [CrossRef]
- Assari, S. Blacks’ Diminished Return of Education Attainment on Subjective Health; Mediating Effect of Income. Brain Sci. 2018, 8, 176. [Google Scholar] [CrossRef]
- Assari, S. Understanding America: Unequal Economic Returns of Years of Schooling in Whites and Blacks. World J. Edu. Res. 2020, 7, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Assari, S.; Preiser, B.; Kelly, M. Education and Income Predict Future Emotional Well-Being of Whites but Not Blacks: A Ten-Year Cohort. Brain Sci. 2018, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Assari, S. College Graduation and Wealth Accumulation: Blacks’ Diminished Returns. World J. Edu. Res. 2020, 7, 1–18. [Google Scholar] [CrossRef]
- Kim, D.J.; Davis, E.P.; Sandman, C.A.; Glynn, L.; Sporns, O.; O’Donnell, B.F.; Hetrick, W.P. Childhood poverty and the organization of structural brain connectome. Neuroimage 2019, 184, 409–416. [Google Scholar] [CrossRef]
- Staff, R.T.; Murray, A.D.; Ahearn, T.S.; Mustafa, N.; Fox, H.C.; Whalley, L.J. Childhood socioeconomic status and adult brain size: Childhood socioeconomic status influences adult hippocampal size. Ann. Neurol. 2012, 71, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Lawson, G.M.; Camins, J.S.; Wisse, L.; Wu, J.; Duda, J.T.; Cook, P.A.; Gee, J.C.; Farah, M.J. Childhood socioeconomic status and childhood maltreatment: Distinct associations with brain structure. PLoS ONE 2017, 12, e0175690. [Google Scholar] [CrossRef]
- Noble, K.G.; Houston, S.M.; Kan, E.; Sowell, E.R. Neural correlates of socioeconomic status in the developing human brain. Dev. Sci. 2012, 15, 516–527. [Google Scholar] [CrossRef]
- Baxendale, S.; Heaney, D. Socioeconomic status, cognition, and hippocampal sclerosis. Epilepsy Behav. 2011, 20, 64–67. [Google Scholar] [CrossRef]
- McDermott, C.L.; Seidlitz, J.; Nadig, A.; Liu, S.; Clasen, L.S.; Blumenthal, J.D.; Reardon, P.K.; Lalonde, F.; Greenstein, D.; Patel, R.; et al. Longitudinally mapping childhood socioeconomic status associations with cortical and subcortical morphology. J. Neurosci. 2019, 39, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.L.; Bathula, D.; Costa Dias, T.G.; Iyer, S.P.; Fenesy, M.C.; Musser, E.D.; Stevens, C.A.; Thurlow, B.L.; Carpenter, S.D.; Nagel, B.J. Altered cortico–striatal–thalamic connectivity in relation to spatial working memory capacity in children with ADHD. Front. Psychiatry 2012, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Rosch, K.S.; Crocetti, D.; Hirabayashi, K.; Denckla, M.B.; Mostofsky, S.H.; Mahone, E.M. Reduced subcortical volumes among preschool–age girls and boys with ADHD. Psychiatry Res. Neuroimag. 2018, 271, 67–74. [Google Scholar] [CrossRef]
- Tsatsanis, K.D.; Rourke, B.P.; Klin, A.; Volkmar, F.R.; Cicchetti, D.; Schultz, R.T. Reduced thalamic volume in high–functioning individuals with autism. Biolog. Psychiatry 2003, 53, 121–129. [Google Scholar] [CrossRef]
- Huang, X.; Pu, W.; Li, X.; Greenshaw, A.J.; Dursun, S.M.; Xue, Z.; Liu, H.; Liu, Z. Decreased left putamen and thalamus volume correlates with delusions in first–episode schizophrenia patients. Front. Psychiatry 2017, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Young, K.A.; Holcomb, L.A.; Yazdani, U.; Hicks, P.B.; German, D.C. Elevated neuron number in the limbic thalamus in major depression. Amer. J. Psychiatry 2004, 161, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Nugent, A.C.; Davis, R.M.; Zarate, C.A., Jr.; Drevets, W.C. Reduced thalamic volumes in major depressive disorder. Psychiatry Res. Neuroimag. 2013, 213, 179–185. [Google Scholar] [CrossRef]
- Young, K.A.; Bonkale, W.L.; Holcomb, L.A.; Hicks, P.B.; German, D.C. Major depression, 5HTTLPR genotype, suicide and antidepressant influences on thalamic volume. Brit. J. Psychiatry 2008, 192, 285–289. [Google Scholar] [CrossRef]
- Adriano, F.; Spoletini, I.; Caltagirone, C.; Spalletta, G. Updated meta–analyses reveal thalamus volume reduction in patients with first–episode and chronic schizophrenia. Schizophrenia Res. 2010, 123, 1–14. [Google Scholar] [CrossRef]
- Hair, N.L.; Hanson, J.L.; Wolfe, B.L.; Pollak, S.D. Association of Child Poverty, Brain Development, and Academic Achievement. JAMA Pediatr. 2015, 169, 822–829. [Google Scholar] [CrossRef]
- Hanson, J.L.; Nacewicz, B.M.; Sutterer, M.J.; Cayo, A.A.; Schaefer, S.M.; Rudolph, K.D.; Shirtcliff, E.A.; Pollak, S.D.; Davidson, R.J. Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biol. Psychiatry 2015, 77, 314–323. [Google Scholar] [CrossRef]
- McEwen, B.S.; Gianaros, P.J. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann. N. Y. Acad. Sci. 2010, 1186, 190–222. [Google Scholar] [CrossRef]
- Suchy–Dicey, A.; Shibata, D.; Cholerton, B.; Nelson, L.; Calhoun, D.; Ali, T.; Montine, T.J.; Longstreth, W.T.; Buchwald, D.; Verney, S.P. Cognitive Correlates of MRI–defined Cerebral Vascular Injury and Atrophy in Elderly American Indians: The Strong Heart Study. J. Int. Neuropsychol. Soc. 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- McLean, J.; Krishnadas, R.; Batty, G.D.; Burns, H.; Deans, K.A.; Ford, I.; McConnachie, A.; McGinty, A.; McLean, J.S.; Millar, K.; et al. Early life socioeconomic status, chronic physiological stress and hippocampal N–acetyl aspartate concentrations. Behav. Brain Res. 2012, 235, 225–230. [Google Scholar] [CrossRef]
- Brito, N.; Noble, K.G. Socioeconomic status and structural brain development. Front. Neurosci. 2014, 8. [Google Scholar] [CrossRef]
- Johnson, S.B.; Riis, J.L.; Noble, K.G. State of the Art Review: Poverty and the Developing Brain. Pediatrics 2016, 137. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.L.; Chandra, A.; Wolfe, B.L.; Pollak, S.D. Association between income and the hippocampus. PLoS ONE 2011, 6, e18712. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Kong, X.; Hong, Y.; Cheon, B.; Liu, J. Pathway to neural resilience: Self–esteem buffers against deleterious effects of poverty on the hippocampus. Hum. Brain Mapp. 2016, 37, 3757–3766. [Google Scholar] [CrossRef]
- Howell, B.R.; Godfrey, J.; Gutman, D.A.; Michopoulos, V.; Zhang, X.; Nair, G.; Hu, X.; Wilson, M.E.; Sanchez, M.M. Social subordination stress and serotonin transporter polymorphisms: Associations with brain white matter tract integrity and behavior in juvenile female macaques. Cereb. Cortex 2014, 24, 3334–3349. [Google Scholar] [CrossRef]
- Cassiers, L.L.M.; Sabbe, B.G.C.; Schmaal, L.; Veltman, D.J.; Penninx, B.; Van Den Eede, F. Structural and Functional Brain Abnormalities Associated With Exposure to Different Childhood Trauma Subtypes: A Systematic Review of Neuroimaging Findings. Front. Psychiatry 2018, 9, 329. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.W.; Swain, J.E.; King, A.P.; Wang, X.; Javanbakht, A.; Ho, S.S.; Angstadt, M.; Phan, K.L.; Xie, H.; Liberzon, I. Childhood Cumulative Risk Exposure and Adult Amygdala Volume and Function. J. Neurosci. Res. 2016, 94, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, A.; Kim, P.; Swain, J.E.; Evans, G.W.; Phan, K.L.; Liberzon, I. Sex–Specific Effects of Childhood Poverty on Neurocircuitry of Processing of Emotional Cues: A Neuroimaging Study. Behav. Sci. 2016, 6, 28. [Google Scholar] [CrossRef]
- Assari, S.; Boyce, S.; Bazargan, M. Subjective Socioeconomic Status and Children’s Amygdala Volume: Minorities’ Diminish Returns. NeuroSci 2020, 1, 59–74. [Google Scholar] [CrossRef]
- Dotson, V.M.; Kitner–Triolo, M.H.; Evans, M.K.; Zonderman, A.B. Effects of race and socioeconomic status on the relative influence of education and literacy on cognitive functioning. J. Int. Neuropsychol. Soc. 2009, 15, 580. [Google Scholar] [CrossRef]
- Assari, S. Parental education and spanking of American children: Blacks’ diminished returns. World J. Edu. Res. 2020, 7, 19–44. [Google Scholar] [CrossRef] [PubMed]
- Assari, S.; Boyce, S.; Bazargan, M. Subjective Family Socioeconomic Status and Adolescents’ Attention: Blacks’ Diminished Returns. Children 2020, 7, 80. [Google Scholar] [CrossRef]
- Assari, S.; Caldwell, C.H.; Mincy, R. Family Socioeconomic Status at Birth and Youth Impulsivity at Age 15; Blacks’ Diminished Return. Children 2018, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Assari, S.; Islam, S. Diminished Protective Effects of Household Income on Internalizing Symptoms among African American than European American Pre-Adolescents. J. Economy. Trade Mark. Manag. 2020, 2. [Google Scholar] [CrossRef]
- Assari, S.; Caldwell, C.H. High Risk of Depression in High–Income African American Boys. J. Racial Ethn. Health Disparities 2018, 5, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Assari, S.; Caldwell, C.H.; Zimmerman, M.A. Family Structure and Subsequent Anxiety Symptoms; Minorities’ Diminished Return. Brain Sci. 2018, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Assari, S.; Boyce, S.; Caldwell, C.H.; Bazargan, M. Minorities’ Diminished Returns of Parental Educational Attainment on Adolescents’ Social, Emotional, and Behavioral Problems. Children 2020, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.; Bazargan, M.; Caldwell, C.H.; Zimmerman, M.A.; Assari, S. Parental Educational Attainment and Social Environmental of Urban Public Schools in the U.S.: Blacks’ Diminished Returns. Children 2020, 7, 44. [Google Scholar] [CrossRef]
- Alcohol Research: Current Reviews Editorial Staff. NIH’s Adolescent Brain Cognitive Development (ABCD) Study. Alcohol Res. 2018, 39, 97. [Google Scholar]
- Volkow, N.D.; Koob, G.F.; Croyle, R.T.; Bianchi, D.W.; Gordon, J.A.; Koroshetz, W.J.; Perez-Stable, E.J.; Riley, W.T.; Bloch, M.H.; Conway, K.; et al. The conception of the ABCD study: From substance use to a broad NIH collaboration. Dev. Cogn. Neurosci. 2018, 32, 4–7. [Google Scholar] [CrossRef]
- Karcher, N.R.; O’Brien, K.J.; Kandala, S.; Barch, D.M. Resting–State Functional Connectivity and Psychotic–like Experiences in Childhood: Results From the Adolescent Brain Cognitive Development Study. Biol. Psychiatry 2019, 86, 7–15. [Google Scholar] [CrossRef]
- Lisdahl, K.M.; Sher, K.J.; Conway, K.P.; Gonzalez, R.; Feldstein Ewing, S.W.; Nixon, S.J.; Tapert, S.; Bartsch, H.; Goldstein, R.Z.; Heitzeg, M. Adolescent brain cognitive development (ABCD) study: Overview of substance use assessment methods. Dev. Cogn. Neurosci. 2018, 32, 80–96. [Google Scholar] [CrossRef]
- Luciana, M.; Bjork, J.M.; Nagel, B.J.; Barch, D.M.; Gonzalez, R.; Nixon, S.J.; Banich, M.T. Adolescent neurocognitive development and impacts of substance use: Overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Dev. Cogn. Neurosci. 2018, 32, 67–79. [Google Scholar] [CrossRef]
- Auchter, A.M.; Hernandez Mejia, M.; Heyser, C.J.; Shilling, P.D.; Jernigan, T.L.; Brown, S.A.; Tapert, S.F.; Dowling, G.J. A description of the ABCD organizational structure and communication framework. Dev. Cogn. Neurosci. 2018, 32, 8–15. [Google Scholar] [CrossRef]
- Garavan, H.; Bartsch, H.; Conway, K.; Decastro, A.; Goldstein, R.Z.; Heeringa, S.; Jernigan, T.; Potter, A.; Thompson, W.; Zahs, D. Recruiting the ABCD sample: Design considerations and procedures. Dev. Cogn. Neurosci. 2018, 32, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Casey, B.; Cannonier, T.; Conley, M.I.; Cohen, A.O.; Barch, D.M.; Heitzeg, M.M.; Soules, M.E.; Teslovich, T.; Dellarco, D.V.; Garavan, H. The adolescent brain cognitive development (ABCD) study: Imaging acquisition across 21 sites. Dev. Cognit. Neurosci. 2018, 32, 43–54. [Google Scholar] [CrossRef]
- Dosenbach, N.U.; Koller, J.M.; Earl, E.A.; Miranda–Dominguez, O.; Klein, R.L.; Van, A.N.; Snyder, A.Z.; Nagel, B.J.; Nigg, J.T.; Nguyen, A.L. Real–time motion analytics during brain MRI improve data quality and reduce costs. Neuroimage 2017, 161, 80–93. [Google Scholar] [CrossRef]
- Epstein, J.N.; Casey, B.; Tonev, S.T.; Davidson, M.; Reiss, A.L.; Garrett, A.; Hinshaw, S.P.; Greenhill, L.L.; Vitolo, A.; Kotler, L.A. Assessment and prevention of head motion during imaging of patients with attention deficit hyperactivity disorder. Psychiatry Res. Neuroimag. 2007, 155, 75–82. [Google Scholar] [CrossRef]
- Holland, D.; Kuperman, J.M.; Dale, A.M. Efficient correction of inhomogeneous static magnetic field–induced distortion in Echo Planar Imaging. Neuroimage 2010, 50, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Treiber, J.M.; White, N.S.; Steed, T.C.; Bartsch, H.; Holland, D.; Farid, N.; McDonald, C.R.; Carter, B.S.; Dale, A.M.; Chen, C.C. Characterization and correction of geometric distortions in 814 diffusion weighted images. PLoS ONE 2016, 11, e0152472. [Google Scholar] [CrossRef] [PubMed]
- Hagler, D.J., Jr.; Hatton, S.; Cornejo, M.D.; Makowski, C.; Fair, D.A.; Dick, A.S.; Sutherland, M.T.; Casey, B.; Barch, D.M.; Harms, M.P. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage 2019, 202, 116091. [Google Scholar] [CrossRef] [PubMed]
- Tisdall, M.D.; Hess, A.T.; Reuter, M.; Meintjes, E.M.; Fischl, B.; van der Kouwe, A.J. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn. Reson. Med. 2012, 68, 389–399. [Google Scholar] [CrossRef]
- White, N.; Roddey, C.; Shankaranarayanan, A.; Han, E.; Rettmann, D.; Santos, J.; Kuperman, J.; Dale, A. PROMO: Real-time prospective motion correction in MRI using image-based tracking. Magn. Magn. Reson. Med. 2010, 63, 91–105. [Google Scholar] [CrossRef]
- Moeller, S.; Yacoub, E.; Olman, C.A.; Auerbach, E.; Strupp, J.; Harel, N.; Uğurbil, K. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn. Reson. Med. 2010, 63, 1144–1153. [Google Scholar] [CrossRef]
- Setsompop, K.; Gagoski, B.A.; Polimeni, J.R.; Witzel, T.; Wedeen, V.J.; Wald, L.L. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn. Reson. Med. 2012, 67, 1210–1224. [Google Scholar] [CrossRef]
- Alexander, A.L.; Lee, J.E.; Lazar, M.; Field, A.S. Diffusion tensor imaging of the brain. Neurotherapeutics 2007, 4, 316–329. [Google Scholar] [CrossRef]
- Hagler Jr, D.J.; Ahmadi, M.E.; Kuperman, J.; Holland, D.; McDonald, C.R.; Halgren, E.; Dale, A.M. Automated white-matter tractography using a probabilistic diffusion tensor atlas: Application to temporal lobe epilepsy. Hum. Brain Mapp. 2009, 30, 1535–1547. [Google Scholar] [CrossRef] [PubMed]
- Barch, D.; Pagliaccio, D.; Belden, A.; Harms, M.P.; Gaffrey, M.; Sylvester, C.M.; Tillman, R.; Luby, J. Effect of Hippocampal and Amygdala Connectivity on the Relationship Between Preschool Poverty and School–Age Depression. Am. J. Psychiatry 2016, 173, 625–634. [Google Scholar] [CrossRef]
- Santelli, J.S.; Lowry, R.; Brener, N.D.; Robin, L. The association of sexual behaviors with socioeconomic status, family structure, and race/ethnicity among US adolescents. Am. J. Public Health 2000, 90, 1582–1588. [Google Scholar] [CrossRef]
- Cohen, S.; Schwartz, J.E.; Epel, E.; Kirschbaum, C.; Sidney, S.; Seeman, T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom. Med. 2006, 68, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Hagger–Johnson, G.E.; Shickle, D.A.; Deary, I.J.; Roberts, B.A. Direct and indirect pathways connecting cognitive ability with cardiovascular disease risk: Socioeconomic status and multiple health behaviors. Psychosom. Med. 2010, 72, 777–785. [Google Scholar] [CrossRef]
- Schulz, A.J.; Mentz, G.; Lachance, L.; Johnson, J.; Gaines, C.; Israel, B.A. Associations between socioeconomic status and allostatic load: Effects of neighborhood poverty and tests of mediating pathways. Am. J. Public Health 2012, 102, 1706–1714. [Google Scholar] [CrossRef]
- Perkins, S.C.; Finegood, E.D.; Swain, J.E. Poverty and language development: Roles of parenting and stress. Innov. Clin. Neurosci 2013, 10, 10–19. [Google Scholar] [PubMed]
- Woods–Jaeger, B.A.; Cho, B.; Sexton, C.C.; Slagel, L.; Goggin, K. Promoting Resilience: Breaking the Intergenerational Cycle of Adverse Childhood Experiences. Health Edu. Behav. 2018, 45, 772–780. [Google Scholar] [CrossRef]
- Emmen, R.A.; Malda, M.; Mesman, J.; van Ijzendoorn, M.H.; Prevoo, M.J.; Yeniad, N. Socioeconomic status and parenting in ethnic minority families: Testing a minority family stress model. J. Fam. Psychol. 2013, 27, 896–904. [Google Scholar] [CrossRef]
- Anton, M.T.; Jones, D.J.; Youngstrom, E.A. Socioeconomic status, parenting, and externalizing problems in African American single–mother homes: A person–oriented approach. J. Fam. Psychol. 2015, 29, 405–415. [Google Scholar] [CrossRef]
- Kiang, L.; Andrews, K.; Stein, G.L.; Supple, A.J.; Gonzalez, L.M. Socioeconomic stress and academic adjustment among Asian American adolescents: The protective role of family obligation. J. Youth Adolesc. 2013, 42, 837–847. [Google Scholar] [CrossRef]
- Danese, A.; Moffitt, T.E.; Harrington, H.; Milne, B.J.; Polanczyk, G.; Pariante, C.M.; Poulton, R.; Caspi, A. Adverse childhood experiences and adult risk factors for age–related disease: Depression, inflammation, and clustering of metabolic risk markers. Arch. Pediatr. Adolesc. Med. 2009, 163, 1135–1143. [Google Scholar] [CrossRef]
- Spann, S.J.; Gillespie, C.F.; Davis, J.S.; Brown, A.; Schwartz, A.; Wingo, A.; Habib, L.; Ressler, K.J. The association between childhood trauma and lipid levels in an adult low–income, minority population. Gen. Hosp. Psychiatry 2014, 36, 150–155. [Google Scholar] [CrossRef]
- Subic–Wrana, C.; Tschan, R.; Michal, M.; Zwerenz, R.; Beutel, M.; Wiltink, J. Childhood trauma and its relation to diagnoses and psychic complaints in patients of an psychosomatic university ambulance. Psychother Psychosom. Med. Psychol. 2011, 61, 54–61. [Google Scholar] [CrossRef]
- Ladebauche, P. Childhood trauma—When to suspect abuse. RN 1997, 60, 38–42, quiz 43. [Google Scholar]
- Parkes, A.; Sweeting, H.; Wight, D. Parenting stress and parent support among mothers with high and low education. J. Fam. Psychol. 2015, 29, 907. [Google Scholar] [CrossRef] [PubMed]
- Assari, S.; Bazargan, M. Unequal Associations between Educational Attainment and Occupational Stress across Racial and Ethnic Groups. Int. J. Envir. Res. Public Health 2019, 16, 3539. [Google Scholar] [CrossRef]
- Chassin, L.; Presson, C.C.; Sherman, S.J.; Edwards, D.A. Parent educational attainment and adolescent cigarette smoking. J. Subst. Abuse 1992, 4, 219–234. [Google Scholar] [CrossRef]
- Kocaoglu, B.; Moschonis, G.; Dimitriou, M.; Kolotourou, M.; Keskin, Y.; Sur, H.; Hayran, O.; Manios, Y. Parental educational level and cardiovascular disease risk factors in schoolchildren in large urban areas of Turkey: Directions for public health policy. BMC Public Health 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Padilla–Moledo, C.; Ruiz, J.R.; Castro-Pinero, J. Parental educational level and psychological positive health and health complaints in Spanish children and adolescents. Child. Care Health Dev. 2016, 42, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Wang, D.; Jackson, A.P. Adverse experiences in early childhood and their longitudinal impact on later behavioral problems of children living in poverty. Child. Abuse Negl. 2019, 98, 104181. [Google Scholar] [CrossRef]
- Barbarin, O.; Bryant, D.; McCandies, T.; Burchinal, M.; Early, D.; Clifford, R.; Pianta, R.; Howes, C. Children enrolled in public pre–K: The relation of family life, neighborhood quality, and socioeconomic resources to early competence. Am. J. Orthopsychiatry 2006, 76, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Assari, S.B.S.; Bazargan, M.; Caldwell, C.H. Diminished Returns of Parental Education in Terms of Youth School Performance: Ruling Out Regression Toward the Mean. Children 2020, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, E.; Assari, M.J.; Farhadian, M.; Chavoshi, E.; Ehsani, H.R. Occupational exposure to mercury vapor in a compact fluorescent lamp factory: Evaluation of personal, ambient air, and biological monitoring. Toxicol. Ind. Health 2019, 35, 304–313. [Google Scholar] [CrossRef]
- Assari, S.; Caldwell, C.H. Parental Educational Attainment Differentially Boosts School Performance of American Adolescents: Minorities’ Diminished Returns. J. Fam. Reprod. Health 2019, 13, 7–13. [Google Scholar] [CrossRef]
- Assari, S.; Boyce, S. Family’s Subjective Economic Status and Children’s Matrix Reasoning: Blacks’ Diminished Returns. Res. Health Sci. 2020, 6, 1–23. [Google Scholar] [CrossRef]
- Assari, S. Social Determinants of Depression: The Intersections of Race, Gender, and Socioeconomic Status. Brain Sci. 2017, 7, 156. [Google Scholar] [CrossRef]
- Assari, S.; Gibbons, F.X.; Simons, R. Depression among Black Youth; Interaction of Class and Place. Brain Sci. 2018, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Assari, S.; Gibbons, F.X.; Simons, R.L. Perceived Discrimination among Black Youth: An 18–Year Longitudinal Study. Behav. Sci. 2018, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Assari, S. Youth Social, Emotional, and Behavioral Problems in the ABCD Study: Minorities’ Diminished Returns of Family Income. J. Econ. Public Financ. 2020, 6, 1–19. [Google Scholar] [CrossRef]
- Moadab, G.; Bliss–Moreau, E.; Bauman, M.D.; Amaral, D.G. Early amygdala or hippocampus damage influences adolescent female social behavior during group formation. Behav. Neurosci. 2017, 131, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Thames, A.D.; Kuhn, T.P.; Mahmood, Z.; Bilder, R.M.; Williamson, T.J.; Singer, E.J.; Arentoft, A. Effects of social adversity and HIV on subcortical shape and neurocognitive function. Brain Imag. Behav. 2018, 12, 96–108. [Google Scholar] [CrossRef]
- Tottenham, N.; Sheridan, M.A. A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Front. Hum. Neurosci. 2009, 3, 68. [Google Scholar] [CrossRef]
- Clark, U.S.; Miller, E.R.; Hegde, R.R. Experiences of Discrimination Are Associated With Greater Resting Amygdala Activity and Functional Connectivity. Biol. Psychiatry Cogn. Neurosci. Neuroimag. 2018, 3, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Gershoff, E.T.; Ansari, A.; Purtell, K.M.; Sexton, H.R. Changes in parents’ spanking and reading as mechanisms for Head Start impacts on children. J. Fam. Psychol. 2016, 30, 480. [Google Scholar] [CrossRef] [PubMed]
- Neville, H.J.; Stevens, C.; Pakulak, E.; Bell, T.A.; Fanning, J.; Klein, S.; Isbell, E. Family–based training program improves brain function, cognition, and behavior in lower socioeconomic status preschoolers. Proc. Natl. Acad. Sci. USA 2013, 110, 12138–12143. [Google Scholar] [CrossRef] [PubMed]
- Garces, E.; Thomas, D.; Currie, J. Longer–term effects of Head Start. Amer. Econo. Rev. 2002, 92, 999–1012. [Google Scholar] [CrossRef]
- Zigler, E.; Valentine, J. Project Head Start: A Legacy of the War on Poverty. Available online: https://eric.ed.gov/?id=ED183266 (accessed on 22 December 2020).
- Herrnstein, R.J.; Murray, C. The Bell Curve: Intelligence and Class Structure in American Life; Simon and Schuster: New York, NY, USA, 2010. [Google Scholar]
| Level | All | White | Black | Asian | Other/Mixed | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weighted | Weighted | Weighted | Weighted | Weighted | |||||||||
| n | 9565 | 6436 | 1343 | 203 | 1583 | ||||||||
| Age (Month) | 119.07 (7.47) | 119.34(7.49) | 119.12 (7.49) | 119.38(7.49) | 119.10 (7.22) | 119.39(7.24) | 119.83 (7.80) | 120.16(7.80) | 118.70 (7.54) | 118.86(7.62) | 0.097 | 0.102 | |
| Right mean cerebellum cortex fractional anisotropy | 0.26 (0.05) | 0.27(0.05) | 0.26 (0.05) | 0.26(0.05) | 0.26 (0.05) | 0.26(0.05) | 0.28 (0.05) | 0.27(0.05) | 0.28 (0.06) | 0.28(0.06) | <0.001 | <0.001 | |
| Left mean cerebellum cortex fractional anisotropy | 0.27 (0.05) | 0.27(0.05) | 0.26 (0.05) | 0.26(0.05) | 0.26 (0.06) | 0.26(0.06) | 0.28 (0.05) | 0.27(0.05) | 0.28 (0.06) | 0.28(0.06) | <0.001 | <0.001 | |
| Parental education | <HS Diploma | 339 (3.5) | (4.4) | 130 (2.0) | (2.8) | 104 (7.7) | (9.0) | 4 (2.0) | (1.5) | 101 (6.4) | (9.4) | <0.001 | <0.001 |
| HS Diploma/GED | 760 (7.9) | (9.6) | 288 (4.5) | (6.1) | 301 (22.4) | (25.0) | 3 (1.5) | (1.7) | 168 (10.6) | (15.2) | |||
| Some College | 2429 (25.4) | (29.8) | 1348 (20.9) | (26.6) | 533 (39.7) | (41.4) | 16 (7.9) | (8.7) | 532 (33.6) | (40.6) | |||
| Bachelor | 2546 (26.6) | (25.1) | 1926 (29.9) | (28.5) | 201 (15.0) | (13.4) | 52 (25.6) | (26.5) | 367 (23.2) | (18.4) | |||
| Post Graduate Degree | 3491 (36.5) | (31.1) | 2744 (42.6) | (36.1) | 204 (15.2) | (11.3) | 128 (63.1) | (61.6) | 415 (26.2) | (16.5) | |||
| Household income | <50,000 | 2680 (28.0) | (37.7) | 1151 (17.9) | (28.4) | 882 (65.7) | (74.7) | 31 (15.3) | (18.5) | 616 (38.9) | (54.9) | <0.001 | <0.001 |
| >=100,000 | 4147 (43.4) | (31.2) | 3338 (51.9) | (37.4) | 162 (12.1) | (6.2) | 124 (61.1) | (51.4) | 523 (33.0) | (17.6) | |||
| >=50,000 and <100,000 | 2738 (28.6) | (31.2) | 1947 (30.3) | (34.2) | 299 (22.3) | (19.0) | 48 (23.6) | (30.1) | 444 (28.0) | (27.5) | |||
| Married family | No | 2848 (29.8) | (36.6) | 1288 (20.0) | (28.2) | 944 (70.3) | (77.1) | 31 (15.3) | (15.6) | 585 (37.0) | (46.3) | <0.001 | <0.001 |
| Yes | 6717 (70.2) | (63.4) | 5148 (80.0) | (71.8) | 399 (29.7) | (22.9) | 172 (84.7) | (84.4) | 998 (63.0) | (53.7) | |||
| Sex | Female | 4608 (48.2) | (49.2) | 3040 (47.2) | (48.2) | 680 (50.6) | (51.8) | 103 (50.7) | (50.4) | 785 (49.6) | (51.0) | 0.062 | 0.142 |
| Male | 4957 (51.8) | (50.8) | 3396 (52.8) | (51.8) | 663 (49.4) | (48.2) | 100 (49.3) | (49.6) | 798 (50.4) | (49.0) | |||
| Hispanic | No | 7762 (81.2) | (77.7) | 5355 (83.2) | (80.3) | 1276 (95.0) | (92.5) | 184 (90.6) | (94.6) | 947 (59.8) | (45.6) | <0.001 | <0.001 |
| Yes | 1803 (18.8) | (22.3) | 1081 (16.8) | (19.7) | 67 (5.0) | (7.5) | 19 (9.4) | (5.4) | 636 (40.2) | (54.4) |
| Education | Income | |||||||
|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | |||||
| All Main Effects | All Interaction Effects | All Main Effects | All Interaction Effects | All Main Effects | All Interaction Effects | All Main Effects | All Interaction Effects | |
| n | 9565 | 9565 | 9565 | 9565 | 9565 | 9565 | 9565 | 9565 |
| R-squared | 0.01158 | 0.01122 | 0.01634 | 0.01691 | 0.01158 | 0.01122 | 0.01474 | 0.0137 |
| ΔR-squared | 0.00044 (0.04%) | 0.00046 (0.05%) | 0.01216 (1.22%) | 0.01272 (1.27%) | 1 × 10−4 (0.01%) | 0.00011 (0.01%) | 0.0103 (1.03%) | 0.00921 (0.92%) |
| Right | Left | |||||||
|---|---|---|---|---|---|---|---|---|
| b | SE | p | sig | b | SE | p | Sig | |
| Parental education (HS Diploma/GED) | −0.0054 | 0.0035 | 0.124 | −0.0048 | 0.0035 | 0.167 | ||
| Parental education (Some College) | −0.0057 | 0.0032 | 0.073 | # | −0.0060 | 0.0032 | 0.057 | # |
| Parental education (Bachelor) | −0.0069 | 0.0034 | 0.041 | * | −0.0069 | 0.0034 | 0.041 | * |
| Parental education (Post Graduate Degree) | −0.0066 | 0.0034 | 0.053 | # | −0.0068 | 0.0034 | 0.048 | * |
| Household income (>=100 K) | 0.0019 | 0.0020 | 0.320 | 0.0018 | 0.0020 | 0.366 | ||
| Household income (>=50 K and <100 K) | 0.0010 | 0.0017 | 0.554 | 0.0005 | 0.0017 | 0.782 | ||
| Race (Black) | −0.0051 | 0.0019 | 0.008 | ** | −0.0040 | 0.0019 | 0.038 | * |
| Race (Asian) | 0.0120 | 0.0035 | 0.001 | ** | 0.0114 | 0.0035 | 0.001 | ** |
| Race (Other/Mixed) | 0.0110 | 0.0017 | < 0.001 | *** | 0.0112 | 0.0017 | < 0.001 | *** |
| Married Family | −0.0042 | 0.0015 | 0.004 | ** | −0.0040 | 0.0015 | 0.008 | ** |
| Age (Months) | −0.0003 | 0.0001 | < 0.001 | *** | −0.0003 | 0.0001 | < 0.001 | *** |
| Sex (Male) | 0.0012 | 0.0011 | 0.260 | 0.0011 | 0.540 | |||
| Right | Left | |||||||
|---|---|---|---|---|---|---|---|---|
| b | SE | p | sig | b | SE | p | sig | |
| Parental education (HS Diploma/GED) | −0.0085 | 0.0054 | 0.117 | −0.0086 | 0.0054 | 0.112 | ||
| Parental education (Some College) | −0.0163 | 0.0048 | 0.001 | *** | −0.0185 | 0.0048 | 0.000 | *** |
| Parental education (Bachelor) | −0.0155 | 0.0049 | 0.002 | ** | −0.0172 | 0.0049 | 0.000 | *** |
| Parental education (Post Graduate Degree) | −0.01405 | 0.0049 | 0.004 | ** | −0.0156 | 0.0049 | 0.001 | ** |
| Household income (>=100 K) | 0.0026 | 0.0020 | 0.188 | 0.0025 | 0.0020 | 0.204 | ||
| Household income (>=50 K and <100 K) | 0.0015 | 0.0017 | 0.371 | 0.0011 | 0.0017 | 0.531 | ||
| Race (Black) | −0.0331 | 0.0071 | < 0.001 | *** | −0.03484 | 0.0071 | < 0.001 | *** |
| Race (Asian) | 0.0428 | 0.0238 | 0.072 | # | 0.0519 | 0.0238 | 0.029 | * |
| Race (Other/Mixed) | 0.0122 | 0.0068 | 0.072 | # | 0.0099 | 0.0068 | 0.145 | |
| Married Family | −0.0043 | 0.0015 | 0.004 | ** | −0.0040 | 0.0015 | 0.006 | ** |
| Age (Months) | −0.0003 | 0.0001 | < 0.001 | *** | −0.0003 | 0.0001 | < 0.001 | *** |
| Sex (Male) | 0.0013 | 0.0011 | 0.218 | 0.0008 | 0.0011 | 0.468 | ||
| Parental education (HS Diploma/GED) × Race (Black) | 0.0185 | 0.0082 | 0.024 | * | 0.0198 | 0.0082 | 0.016 | * |
| Parental education (Some College) × Race (Black) | 0.0352 | 0.0075 | < 0.001 | *** | 0.0396 | 0.0075 | < 0.001 | *** |
| Parental education (Bachelor) × Race (Black) | 0.0359 | 0.0082 | < 0.001 | *** | 0.0384 | 0.0082 | < 0.001 | *** |
| Parental education (Post Graduate Degree) × Race (Black) | 0.0263 | 0.0083 | 0.002 | ** | 0.0283 | 0.0083 | 0.001 | *** |
| Parental education (HS Diploma/GED) × Race (Asian) | −0.0523 | 0.0365 | 0.152 | −0.0591 | 0.0364 | 0.105 | ||
| Parental education (Some College) × Race (Asian) | −0.0212 | 0.0266 | 0.425 | −0.0281 | 0.0266 | 0.292 | ||
| Parental education (Bachelor) × Race (Asian) | −0.0228 | 0.0247 | 0.357 | −0.0324 | 0.0248 | 0.191 | ||
| Parental education (Post Graduate Degree) × Race (Asian) | −0.0369 | 0.0242 | 0.128 | −0.0475 | 0.0243 | 0.050 | # | |
| Parental education (HS Diploma/GED) × Race (Other/Mixed) | −0.0046 | 0.0085 | 0.584 | −0.0031 | 0.0085 | 0.716 | ||
| Parental education (Some College) × Race (Other/Mixed) | 0.0045 | 0.0073 | 0.540 | 0.0073 | 0.0073 | 0.321 | ||
| Parental education (Bachelor) × Race (Other/Mixed) | −0.0042 | 0.0076 | 0.584 | −0.0003 | 0.0076 | 0.970 | ||
| Parental education (Post Graduate Degree) × Race (Other/Mixed) | −0.0064 | 0.0076 | 0.402 | −0.0041 | 0.0076 | 0.591 | ||
| Right | Left | |||||||
|---|---|---|---|---|---|---|---|---|
| b | SE | p | sig | b | SE | p | sig | |
| Household income (>=100 K) | 0.0015 | 0.0022 | 0.490 | 0.0012 | 0.0022 | 0.590 | ||
| Household income (>=50 K and <100 K) | 0.0009 | 0.0021 | 0.684 | −0.0002 | 0.0021 | 0.941 | ||
| Parental education (HS Diploma/GED) | −0.0047 | 0.0035 | 0.174 | −0.0042 | 0.0035 | 0.223 | ||
| Parental education (Some College) | −0.0056 | 0.0032 | 0.077 | # | −0.0060 | 0.0032 | 0.059 | # |
| Parental education (Bachelor) | −0.0069 | 0.0034 | 0.042 | * | −0.0069 | 0.0034 | 0.042 | * |
| Parental education (Post Graduate Degree) | −0.0066 | 0.0034 | 0.055 | # | −0.0067 | 0.0034 | 0.050 | * |
| Race (Black) | −0.0094 | 0.0025 | 0.000 | *** | −0.0083 | 0.0025 | 0.001 | *** |
| Race (Asian) | 0.0146 | 0.0086 | 0.090 | # | 0.0125 | 0.0086 | 0.146 | |
| Race (Other/Mixed) | 0.0154 | 0.0026 | < 0.001 | *** | 0.0148 | 0.0026 | < 0.001 | *** |
| Married Family | −0.0043 | 0.0015 | 0.004 | ** | −0.0040 | 0.0015 | 0.006 | ** |
| Age (Months) | −0.0003 | 0.0001 | < 0.001 | *** | −0.0003 | 0.0001 | < 0.001 | *** |
| Sex (Male) | 0.0012 | 0.0011 | 0.263 | 0.0007 | 0.0011 | 0.544 | ||
| Household income (>=100 K) × Race (Black) | 0.0110 | 0.0059 | 0.059 | # | 0.0110 | 0.0059 | 0.062 | # |
| Household income (>=50 K and <100 K) × Race (Black) | 0.0142 | 0.0043 | 0.001 | ** | 0.0138 | 0.0043 | 0.001 | ** |
| Household income (>=100 K) × Race (Asian) | 0.0033 | 0.0098 | 0.732 | 0.0036 | 0.0098 | 0.712 | ||
| Household income (>=50 K and <100 K) × Race (Asian) | −0.0169 | 0.0109 | 0.124 | −0.0117 | 0.0109 | 0.284 | ||
| Household income (>=100 K) × Race (Other/Mixed) | −0.0075 | 0.0041 | 0.068 | # | −0.0068 | 0.0041 | 0.099 | # |
| Household income (>=50 K and <100 K) × Race (Other/Mixed) | −0.0094 | 0.0040 | 0.019 | * | −0.0072 | 0.0040 | 0.074 | # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assari, S.; Boyce, S. Race, Socioeconomic Status, and Cerebellum Cortex Fractional Anisotropy in Pre-Adolescents. Adolescents 2021, 1, 70-94. https://doi.org/10.3390/adolescents1020007
Assari S, Boyce S. Race, Socioeconomic Status, and Cerebellum Cortex Fractional Anisotropy in Pre-Adolescents. Adolescents. 2021; 1(2):70-94. https://doi.org/10.3390/adolescents1020007
Chicago/Turabian StyleAssari, Shervin, and Shanika Boyce. 2021. "Race, Socioeconomic Status, and Cerebellum Cortex Fractional Anisotropy in Pre-Adolescents" Adolescents 1, no. 2: 70-94. https://doi.org/10.3390/adolescents1020007
APA StyleAssari, S., & Boyce, S. (2021). Race, Socioeconomic Status, and Cerebellum Cortex Fractional Anisotropy in Pre-Adolescents. Adolescents, 1(2), 70-94. https://doi.org/10.3390/adolescents1020007







