Scientific and Technical Insights into Hancornia speciosa Gomes for Biotechnological Applications
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Scientific Advancement
3.2. Biological Activity
3.2.1. Anti-Inflammatory Activity
3.2.2. Antihypertensive Activity
3.2.3. Antioxidant Activity
3.2.4. Antidiabetic Activity
3.3. Lupeol and Other Triterpenes
3.4. Technological Prospection
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| H. speciosa Gomes | Hancornia speciosa Gomes |
| INPI | Instituto Nacional de Propriedade Intelectual |
| WIPO | World Intellectual Property Organization |
| ISSR | Inter-Simple Sequence Repeat |
| HPLC-DAD-MS/MS | High performance liquid chromatography coupled with a diode array detector and tandem mass spectrometry techniques |
| UPLC-ESI-MS | ultra-performance liquid chromatography-electrospray ionization-tandem mass spectrometry |
| GC-FID | gas chromatographs with flame ionization detector |
| IL-1 β | interleukin-1 beta |
| IL-6 | interleukin-6 |
| IL-12 | interleukin-12 |
| TNF-α | Tumor necrosis factor alpha |
| FTIR | Fourier Transform Infrared |
| MTT | Methyl-thiazolyl-tetrazolium |
| SBP | systolic blood pressure |
| SAH | systemic arterial hypertension |
| MRM | Multiple Reaction Monitoring |
| DPPH | 2,2-Difenil-1-Picrilidrazil |
| ABTS | 2,2-Azino-Bis(3-Etilbenzotiazolin)-6-sulfônico |
| DCM | Dichloromethane |
| AST | aspartate aminotransferase |
| ALT | alanine aminotransferase |
| PTX | pentoxifylline |
| SEIDES | State Secretariat for Inclusion, Assistance and Social Development |
| ITP—UNIT | Institute of Technology and Research—Tiradentes University |
| UFS | Federal University of Sergipe |
| FAPEMIG | Research Support Foundation of the State of Minas Gerais |
| UFMS | Federal University of Mato Grosso do Sul |
| UFPB | Federal University of Paraíba |
| UFCG | Federal University of Campina Grande |
| IF Baiano | Federal Institute of Education, Science and Technology of Bahia |
| UNICAMP | Campinas State University |
| UFRPE | Rural Federal University of Pernambuco |
| UFRN | Federal University of Rio Grande Do Norte |
| UFC | Federal University of Ceará |
| DIRPA | Patent Directorate of the National Institute of Industrial Property |
References
- Savi, D.C.; Aluizio, R.; Glienke, C. Brazilian Plants: An Unexplored Source of Endophytes as Producers of Active Metabolites. Planta Med. 2019, 85, 619–636. [Google Scholar] [PubMed]
- Sasson, A.; Malpica, C. Bioeconomy in Latin America. New Biotechnol. 2018, 40, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Narain, N.; França, F.R.M.; Neta, M.T.S.L. Mangaba—Hancornia speciosa. In Exotic Fruits Reference Guide; Elsevier: Amsterdam, The Netherlands, 2018; pp. 305–318. ISBN 9780128031384. [Google Scholar]
- Reis, V.H.d.O.T.; Santee, C.M.; Loubet Filho, P.S.; Santos, T.G.; Amianti, C.; Filiú, W.F.d.O.; Rafacho, B.P.M.; Portugal, L.C.; Dos Santos, E.F. The Effects of Supplementing Hancornia speciosa (Mangaba) on Bowel Motility and Inflammatory Profile of Wistar Rats. J. Med. Food 2019, 22, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.C. Mangabeira. 2021. Available online: https://www.embrapa.br/busca-de-imagens/-/midia/5603004/mangabeira (accessed on 10 August 2025).
- Nunes, S.C. Mangabeira. 2021. Available online: https://www.embrapa.br/busca-de-imagens/-/midia/5603002/mangabeira (accessed on 10 August 2025).
- Da Silva Júnior, J.F.; Muniz, A.V.C.D.S.; Lédo, A.D.S.; Maia, M.C.C.; Carvalhaes, M.A.; Silva, S.M.D.C.; Dulloo, E.; Alercia, A. Descriptors for Mangaba (Hancornia speciosa Gomes). In Bioversity International, Rome, Italy and Brazilian Agriculture Research Corporation; Bioversity International and Brazilian Agriculture Research Corporation, Embrapa: Brasília, Brazil, 2018; pp. 1–56. [Google Scholar]
- IBGE PEVS—Produção Da Extração Vegetal e Da Silvicultura. Available online: https://sidra.ibge.gov.br/pesquisa/pevs/quadros/brasil/2021 (accessed on 10 August 2025).
- Ribeiro Neto, J.A.; Pimenta Tarôco, B.R.; Batista dos Santos, H.; Thomé, R.G.; Wolfram, E.; Maciel de A Ribeiro, R.I. Using the Plants of Brazilian Cerrado for Wound Healing: From Traditional Use to Scientific Approach. J. Ethnopharmacol. 2020, 260, 112547. [Google Scholar] [CrossRef]
- Torres-Rêgo, M.; Furtado, A.A.; Bitencourt, M.A.O.; Lima, M.C.J.d.S.; de Andrade, R.C.L.C.; de Azevedo, E.P.; Soares, T.d.C.; Tomaz, J.C.; Lopes, N.P.; da Silva-Júnior, A.A.; et al. Anti-Inflammatory Activity of Aqueous Extract and Bioactive Compounds Identified from the Fruits of Hancornia speciosa Gomes (Apocynaceae). BMC Complement. Altern. Med. 2016, 16, 275. [Google Scholar] [CrossRef]
- Bitencourt, M.A.O.; Torres-Rêgo, M.; de Souza Lima, M.C.J.; Furtado, A.A.; de Azevedo, E.P.; do Egito, E.S.T.; da Silva-Júnior, A.A.; Zucolotto, S.M.; Fernandes-Pedrosa, M.d.F. Protective Effect of Aqueous Extract, Fractions and Phenolic Compounds of Hancornia speciosa Fruits on the Inflammatory Damage in the Lungs of Mice Induced by Tityus serrulatus Envenomation. Toxicon 2019, 164, 1–9. [Google Scholar] [CrossRef]
- Yamashita, F.d.O.; Torres-Rêgo, M.; Gomes, J.A.d.S.; Félix-Silva, J.; Passos, J.G.R.; Ferreira, L.d.S.; da Silva-Júnior, A.A.; Zucolotto, S.M.; Fernandes-Pedrosa, M.d.F. Mangaba (Hancornia speciosa Gomes) Fruit Juice Decreases Acute Pulmonary Edema Induced by Tityus serrulatus Venom: Potential Application for Auxiliary Treatment of Scorpion Stings. Toxicon 2020, 179, 42–52. [Google Scholar] [CrossRef]
- Pegorin, G.S.; Leite, M.N.; Antoniassi, M.; Chagas, A.L.D.; Santana, L.A.; Garms, B.C.; Marcelino, M.Y.; Herculano, R.D.; Cipriani Frade, M.A. Physico-Chemical Characterization and Tissue Healing Changes by Hancornia speciosa Gomes Latex Biomembrane. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 938–948. [Google Scholar] [CrossRef]
- Silva, G.C.; Braga, F.C.; Lemos, V.S.; Cortes, S.F. Potent Antihypertensive Effect of Hancornia speciosa Leaves Extract. Phytomedicine 2016, 23, 214–219. [Google Scholar] [CrossRef]
- Moreira, L.N.; Silva, G.C.; Câmara, D.V.; Pádua, R.M.; Lemos, V.S.; Braga, F.C.; Cortes, S.F. The Cyclitol L-(+)-Bornesitol as an Active Marker for the Cardiovascular Activity of the Brazilian Medicinal Plant Hancornia speciosa. Biol. Pharm. Bull. 2019, 42, 2076–2082. [Google Scholar] [CrossRef]
- Moreira, L.N.; Feltrin, C.; Gonçalves, J.E.; de Castro, W.V.; Simões, C.M.O.; de Pádua, R.M.; Cortes, S.F.; Braga, F.C. Determination of L-(+)-Bornesitol, the Hypotensive Constituent of Hancornia speciosa, in Rat Plasma by LC-MS/MS and Its Application on a Pharmacokinetic Study. Biomed. Pharmacother. 2020, 132, 110900. [Google Scholar] [CrossRef]
- Tang, F.; Yan, H.L.; Wang, L.X.; Xu, J.F.; Peng, C.; Ao, H.; Tan, Y.Z. Review of Natural Resources with Vasodilation: Traditional Medicinal Plants, Natural Products, and Their Mechanism and Clinical Efficacy. Front. Pharmacol. 2021, 12, 627458. [Google Scholar] [CrossRef]
- Pereira, A.B.D.; Gomes, J.H.d.S.; Pereira, A.C.; de Pádua, R.M.; Côrtes, S.F.; Sena, M.M.; Braga, F.C. Definition of Chemical Markers for Hancornia speciosa Gomes by Chemometric Analysis Based on the Chemical Composition of Extracts, Their Vasorelaxant Effect and α-Glucosidase Inhibition. J. Ethnopharmacol. 2022, 299, 115692. [Google Scholar] [CrossRef]
- Pereira, A.C.; Pereira, A.B.D.; Moreira, C.C.L.; Botion, L.M.; Lemos, V.S.; Braga, F.C.; Cortes, S.F. Hancornia speciosa Gomes (Apocynaceae) as a Potential Anti-Diabetic Drug. J. Ethnopharmacol. 2015, 161, 30–35. [Google Scholar] [CrossRef]
- Neto, L.S.; Moraes-Souza, R.Q.; Soares, T.S.; Pinheiro, M.S.; Leal-Silva, T.; Hoffmann, J.C.; Américo, M.F.; Campos, K.E.; Damasceno, D.C.; Volpato, G.T. A Treatment with a Boiled Aqueous Extract of Hancornia speciosa Gomes Leaves Improves the Metabolic Status of Streptozotocin-Induced Diabetic Rats. BMC Complement. Med. Ther. 2020, 20, 114. [Google Scholar] [CrossRef]
- Tomazi, R.; Figueira, Â.C.; Ferreira, A.M.; Ferreira, D.Q.; de Souza, G.C.; Pinheiro, W.B.d.S.; Neto, J.R.P.; da Silva, G.A.; de Lima, H.B.; Hage-Melim, L.I.d.S.; et al. Hypoglycemic Activity of Aqueous Extract of Latex from Hancornia speciosa Gomes: A Study in Zebrafish and in Silico. Pharmaceuticals 2021, 14, 856. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, K.S.; Aloufa, M.A.I. Knowledge, Use, and Management of Mangaba (Hancornia speciosa Gomes) by Extrativist Communities on the Coast of Rio Grande Do Norte, Northeast Brazil. Acta Bot. Bras. 2021, 35, 276–289. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Bieski, I.G.C.; Balogun, S.O.; Martins, D.T.d.O. Ethnobotanical Study of Medicinal Plants Used by Ribeirinhos in the North Araguaia Microregion, Mato Grosso, Brazil. J. Ethnopharmacol. 2017, 205, 69–102. [Google Scholar] [CrossRef]
- Almeida, L.M.; Nogueira, C.A.; Borges, P.P.; do Prado, A.D.L.; Gonçalves, P.J. state of the art of scientific literature on Hancornia speciosa: Trends and gaps. Rev. Bras. Frutic. 2016, 38, e-869. [Google Scholar] [CrossRef]
- Morais, G.C.; Resende, R.T.; Chaves, L.J. Improve for Better Use: Exploitation Potential, Advances and Gaps in the Conservation and Breeding of Hancornia speciosa—A Review. TreeDimensional 2022, 9, 1–13. [Google Scholar] [CrossRef]
- Nunes, V.V.; Silva-Mann, R.; Souza, J.L.; Calazans, C.C. Pharmaceutical, Food Potential, and Molecular Data of Hancornia speciosa Gomes: A Systematic Review. Genet. Resour. Crop Evol. 2022, 69, 525–543. [Google Scholar] [CrossRef]
- D’abadia, P.L.; Lemes, S.R.; Demelo-Reis, P.R.; Júnior, R.d.S.L.; Gonçalves, P.J.; Reis, D.D.S.; Caixeta, G.A.B.; Amaral, V.C.S.; Almeida, L.M. Tissue Healing Changes on Wounds in Rats after Treatment with Hancornia speciosa Latex in Cream-Gel Formulation. Acta Cir. Bras. 2022, 37, e371001. [Google Scholar] [CrossRef]
- Martins, K.L.E.; Thomaz, M.M.; Magno, L.N.; Vinaud, M.C.; Almeida, L.M.; Gonçalves, P.J.; Lino Junior, R.d.S. Macroporous Latex Biomembrane from Hancornia speciosa Modulates the Inflammatory Process and Has a Debridement Effect on Wound Healing in Rats. Acta Cir. Bras. 2023, 38, e385323. [Google Scholar] [CrossRef]
- Bitencourt, M.A.O.; Torres-Rêgo, M.; Daniele-Silva, A.; Furtado, A.A.; Lima, M.C.J.d.S.; da Silva-Júnior, A.A.; Zucolotto, S.M.; Araújo, R.M.; Fernandes-Pedrosa, M.d.F. Aqueous Extract and Solvent Fractions of Hancornia speciosa Fruits, Rutin, and Chlorogenic Acid Attenuate the Edema, Inflammation, and Myonecrosis Caused by Bothrops Jararaca Snake Venom in Mice. Rev. Bras. Farmacogn. 2024, 34, 585–594. [Google Scholar] [CrossRef]
- Bastos, K.X.; Dias, C.N.; Nascimento, Y.M.; Da Silva, M.S.; Langassner, S.M.Z.; Wessjohann, L.A.; Tavares, J.F. Identification of Phenolic Compounds from Hancornia speciosa (Apocynaceae) Leaves by UHPLC Orbitrap-HRMS. Molecules 2017, 22, 143. [Google Scholar] [CrossRef]
- de Lima, J.P.; Fante, C.A.; Freitas Pires, C.R.; Nunes, E.E.; Alves, R.R.; de Siqueira Elias, H.H.; Nunes, C.A.; de Barros Vilas Boas, E.V. The Antioxidative Potential and Volatile Constituents of Mangaba Fruit over the Storage Period. Sci. Hortic. 2015, 194, 1–6. [Google Scholar] [CrossRef]
- Lima Neto, G.A.; Kaffashi, S.; Luiz, W.T.; Ferreira, W.R.; Dias Da Silva, Y.S.A.; Pazin, G.V.; Violante, I.M.P. Quantificação de Metabólitos Secundários e Avaliação Da Atividade Antimicrobiana e Antioxidante de Algumas Plantas Selecionadas Do Cerrado de Mato Grosso. Rev. Bras. Plantas Med. 2015, 17, 1069–1077. [Google Scholar] [CrossRef]
- Santos, U.P.; Campos, J.F.; Torquato, H.F.V.; Paredes-Gamero, E.J.; Carollo, C.A.; Estevinho, L.M.; De Picoli Souza, K.; Dos Santos, E.L. Antioxidant, Antimicrobial and Cytotoxic Properties as Well as the Phenolic Content of the Extract from Hancornia speciosa Gomes. PLoS ONE 2016, 11, e0167531. [Google Scholar] [CrossRef] [PubMed]
- Penido, A.B.; De Morais, S.M.; Ribeiro, A.B.; Alves, D.R.; Rodrigues, A.L.M.; Dos Santos, L.H.; De Menezes, J.E.S.A. Medicinal Plants from Northeastern Brazil against Alzheimer’s Disease. Evid.-Based Complement. Altern. Med. 2017, 2017, 1753673. [Google Scholar] [CrossRef]
- Dutra, R.L.T.; Dantas, A.M.; Marques, D.d.A.; Batista, J.D.F.; Meireles, B.R.L.d.A.; de Magalhães Cordeiro, Â.M.T.; Magnani, M.; Borges, G.d.S.C. Bioaccessibility and Antioxidant Activity of Phenolic Compounds in Frozen Pulps of Brazilian Exotic Fruits Exposed to Simulated Gastrointestinal Conditions. Food Res. Int. 2017, 100, 650–657. [Google Scholar] [CrossRef]
- Dos Santos, U.P.; Tolentino, G.S.; Morais, J.S.; De Picoli Souza, K.; Estevinho, L.M.; Dos Santos, E.L. Physicochemical Characterization, Microbiological Quality and Safety, and Pharmacological Potential of Hancornia speciosa Gomes. Oxid. Med. Cell Longev. 2018, 2018, 2976985. [Google Scholar] [CrossRef]
- Maia, J.D.; de Ávila, C.R.; Mezzomo, N.; Lanza, M. Evaluation of Bioactive Extracts of Mangaba (Hancornia speciosa) Using Low and High Pressure Processes. J. Supercrit. Fluids 2018, 135, 198–210. [Google Scholar] [CrossRef]
- de Araújo, R.L.; Savazzi, S.; Fujimori, M.; Deluque, A.; Honório-França, E.L.; Konda, P.B.P.; Honório-França, A.C. Effects of Mangaba (Hancornia speciosa) Fruit Extract Adsorbed onto PEG Microspheres in MCF-7 Breast Cancer Cells Co-Cultured with Blood Cells. Asian Pac. J. Cancer Prev. 2019, 20, 1995–2001. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.M.; Santos, K.S.; Borges, G.R.; Muniz, A.V.C.S.; Mendonça, F.M.R.; Pinheiro, M.S.; Franceschi, E.; Dariva, C.; Padilha, F.F. Separation of Antibacterial Biocompounds from Hancornia speciosa Leaves by a Sequential Process of Pressurized Liquid Extraction. Sep. Purif. Technol. 2019, 222, 390–395. [Google Scholar] [CrossRef]
- Santos, P.S.; Freitas, L.D.S.; Muniz, E.N.; Santana, J.G.S.; Da Silva, A.V.C. Phytochemical and Antioxidant Composition in Accessions of the Mangaba Active Germplasm Bank. Rev. Caatinga 2021, 34, 228–235. [Google Scholar] [CrossRef]
- Almeida, F.L.C.; de Oliveira, E.N.A.; Almeida, E.C.; da Silva, L.N.; Polari, I.d.L.B.; de Magalhães Cordeiro, A.M.T.; de Albuquerque Meireles, B.R.L.; de Sousa Guedes, J.P.; de Souza, W.F.C. Mangaba (Hancornia speciosa Gomes) Beverage as an Alternative Wine. J. Food Process Preserv. 2021, 45, e15779. [Google Scholar] [CrossRef]
- Santos, R.S.; Chaves-Filho, A.B.; Silva, L.A.S.; Garcia, C.A.B.; Silva, A.R.S.T.; Dolabella, S.S.; da Costa, S.S.L.; Miyamoto, S.; Matos, H.R. Bioactive Compounds and Hepatoprotective Effect of Hancornia speciosa Gomes Fruit Juice on Acetaminophen-Induced Hepatotoxicity in Vivo. Nat. Prod. Res. 2022, 36, 2565–2569. [Google Scholar] [CrossRef] [PubMed]
- Panontin, J.F.; Rambo, M.K.D.; Isaac, V.; Seibert, C.S.; Scapin, E. New Antioxidant Lauryl-Free Herbal Shampoo Formulation with a Brazilian Plant Extract. Braz. J. Biol. 2022, 82, e264677. [Google Scholar] [CrossRef]
- Jácome, M.C.d.M.B.; Padilha, C.E.d.A.; Arrais, M.R.d.N.; Leitão, A.L.O.d.S.; de Sousa Júnior, F.C.; Dos Santos, E.S. Valorization of Mangaba Residue (Hancornia speciosa Gomes) for Polygalacturonase Production from Aspergillus Niger IOC 4003 and Fabrication of Active Chitosan Films. Biomass Convers. Biorefin. 2020, 12, 4069–4080. [Google Scholar] [CrossRef]
- D’abadia, P.L.; Costa, A.F.; Firmino, M.T.; Caramori, S.S.; Lino-Junior, R.d.S.; Zanoelo, F.F.; Gonçalves, P.J.; Almeida, L. the role of enzymes in the angiogenic activity of Hancornia speciosa latex. Biosci. J. 2022, 38, 1981–3163. [Google Scholar] [CrossRef]
- Leite, S.P.; Adami, T.B.; Bjerk, T.R.; Souza, M.R.d.R.; Cardoso, A.L.C.; Krause, L.C.; Caramão, E.B. Ultrasonic Assisted Extraction of Bioactive Compounds from Different Parts of Hancornia speciosa Gomes. J. Med. Plants Res. 2020, 14, 300–308. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Jalali, A.; Dabaghian, F.; Akbrialiabad, H.; Foroughinia, F.; Zarshenas, M.M. A Pharmacology-Based Comprehensive Review on Medicinal Plants and Phytoactive Constituents Possibly Effective in the Management of COVID-19. Phytother. Res. 2021, 35, 1925–1938. [Google Scholar] [CrossRef] [PubMed]
- Siddique, H.R.; Saleem, M. Beneficial Health Effects of Lupeol Triterpene: A Review of Preclinical Studies. Life Sci. 2011, 88, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.S.; Lin, L.W.; Wu, C.R. Lupeol and Its Role in Chronic Diseases. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2016; Volume 929, pp. 145–175. [Google Scholar]
- Beserra, F.P.; Xue, M.; De Azevedo Maia, G.L.; Rozza, A.L.; Pellizzon, C.H.; Jackson, C.J. Lupeol, a Pentacyclic Triterpene, Promotes Migration, Wound Closure, and Contractile Effect in Vitro: Possible Involvement of PI3K/Akt and P38/ERK/MAPK Pathways. Molecules 2018, 23, 2819. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, X.; Xie, L.; Deng, M.; Chen, H.; Song, J.; Long, J.; Li, X.; Luo, J. Lupeol and Its Derivatives as Anticancer and Anti-Inflammatory Agents: Molecular Mechanisms and Therapeutic Efficacy. Pharmacol. Res. 2021, 164, 105373. [Google Scholar] [CrossRef]
- Lucetti, D.L.; Lucetti, E.C.P.; Bandeira, M.A.M.; Veras, H.N.H.; Silva, A.H.; Leal, L.K.A.M.; Lopes, A.A.; Alves, V.C.C.; Silva, G.S.; Brito, G.A.; et al. Anti-Inflammatory Effects and Possible Mechanism of Action of Lupeol Acetate Isolated from Himatanthus drasticus (Mart.) Plumel. J. Inflamm. 2010, 7, 60. [Google Scholar] [CrossRef]
- Chen, Y.F.; Ching, C.; Wu, T.S.; Wu, C.R.; Hsieh, W.T.; Tsai, H.Y. Balanophora spicata and Lupeol Acetate Possess Antinociceptive and Anti-Inflammatory Activities in Vivo and in Vitro. Evid.-Based Complement. Altern. Med. 2012, 2012, 371273. [Google Scholar] [CrossRef]
- Saleem, M. Lupeol, a Novel Anti-Inflammatory and Anti-Cancer Dietary Triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef]
- Somwong, P.; Theanphong, O. Quantitative Analysis of Triterpene Lupeol and Anti-Inflammatory Potential of the Extracts of Traditional Pain-Relieving Medicinal Plants Derris scandens, Albizia procera, and Diospyros rhodocalyx. J. Adv. Pharm. Technol. Res. 2021, 12, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Holanda Pinto, S.A.; Pinto, L.M.S.; Cunha, G.M.A.; Chaves, M.H.; Santos, F.A.; Rao, V.S. Anti-Inflammatory Effect of α, β-Amyrin, a Pentacyclic Triterpene from Protium Heptaphyllum in Rat Model of Acute Periodontitis. Inflammopharmacology 2008, 16, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, L.H.; Palazon, J.; Navarro-Ocaña, A. The Pentacyclic Triterpenes α, β-Amyrins: A Review of Sources and Biological Activities. In Phytochemicals; Rao, V., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 487–502. [Google Scholar]
- Nogueira, A.O.; Oliveira, Y.I.S.; Adjafre, B.L.; de Moraes, M.E.A.; Aragão, G.F. Pharmacological Effects of the Isomeric Mixture of Alpha and Beta Amyrin from Protium Heptaphyllum: A Literature Review. Fundam. Clin. Pharmacol. 2019, 33, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Santana, L.C.L.d.A.; Leite dos Santos, E.A.; Martins de Souza, F. Method for the Production of Aspergillus Niger Lipase Using Waste from Mangaba Pulp Pro-Cessing as Substrate. Patent BR102014013453A2, 26 May 2014. [Google Scholar]
- Porto, A.L.F.; Lima, G.V.M.; Soares, M.T.C.V.; Nascimento, T.P.; do Nascimento, A.K.C.; de Andrade, G.R. Process of Obtaining a New Bactericide from Mangabeira Latex (Hancornia speciosa Gomes). Patent BR102013018181A2, 17 July 2013. [Google Scholar]
- Mann, R.S.; Nunes, V.V.; Rabbani, A.R.C.; de Freitas, B.A.L.; Candido, I.d.J.; Souza, J.L. Solution and Process for Preserving Recalcitran Seeds. Patent BR 102021009165-7, 11 May 2021. [Google Scholar]
- Leite, N.S.; da Silva, R.R.; Marques, J.J.; Tambourgi, E.B.; da Silva, G.F.; Freitas, L.d.S.; Santos, R.M. Use of a Bioadsorvent Produced from Man-Gaba Seeds to Remove Contaminants from Water and Liquid Effluents. Patent BR102017001445A2, 24 January 2017. [Google Scholar]
- Novaes, P.D.; Neves, J.d.S.; Omar, N.F. Composition of Mangabeira Latex and Its Use in Bone Regeneration. Patent BR 102012025418-2, 5 October 2012. [Google Scholar]
- Araújo, A.A.d.S.; Ribeiro, D.S.; Padilha, F.F.; Cardoso, J.C.; Serafini, M.R.; Pinheiro, M.S.; Melo, M.S.; Albuquerque Júnior, R.L.C.; Pereira Filho, R.N. Composition and Process for Obtaining Healing Film and the Film Obtained Thus. Patent PI 1106145-6, 4 November 2011. [Google Scholar]
- Capim, S.L.; Pereira, M.d.A.; Lima, J.P.d.O.; Conceição, Í.M.S.; Castro, J.O.; dos Santos, J.L.; Cavalli, I.S.J.; Lopes, D.d.S.; Gomes, T.C.; Costa, V.S.C.; et al. Pharmaceutical Composition Containing Natural Latex and Use Thereof for the Treatment of Diseases Caused by Intracellular Protozoa. Patent BR 102020014384-5, 14 July 2020. [Google Scholar]
- Braga, F.C.; Côrtes, S.d.F.; da Silva, G.C.; Endringer, D.C. Extract of Hancornia speciosa and Pharmaceutical Composition Thereof. Patent EP2335711A2, 19 May 2009. [Google Scholar]
- Endriger, D.C.; Braga, F.C.; da Silva, G.C.; Côrtes, S.d.F. Standardized Extract and Fraction of Leaves of Hancornia speciosa and Its Pharmaceutical Composition. Patent PI 0802004-3, 19 May 2008. [Google Scholar]
- Pedrosa, M.d.F.F.; Langassner, S.M.Z.; Lima, M.C.J.d.S.; Bitencourt, M.A.O. Extracts, Fractions, Isolated Compounds and Pharmaceutical Composition of Aspidosperma Pyrifolium, Hancornia speciosa, Ipomoea asarifolia and Mimosa tenuiflora Applied in the Treatment of Poisoning Processes by Venomy Animals. Patent BR 102012026958-9, 24 September 2012. [Google Scholar]
- Barbosa, A.M.; Dariva, C.; Santos, K.S.; Franceschi, E.; Borges, G.R.; Borges, J.F.d.C.; da Costa, J.C.F.; Pinheiro, M.S.; Polloni, A.E.; Padilha, F.F. Polymeric Films Containing Mangaba Extract against Multi-Resistant Bacteria. Patent BR102020024121A2, 26 November 2020. [Google Scholar]
- Capim, S.L.; Pereira, M.d.A.; Lima, J.P.d.O.; Conceição, Í.M.S.; Castro, J.O.; dos Santos, J.L.; Cavalli, I.S.J.; Lopes, D.d.S.; Gomes, T.C.; Costa, V.S.C.; et al. Pharmaceutical Formulation Containing Natural Latex and Its Use for the Treatment of Cutaneous Wounds. Patent BR 102021005471-9, 23 March 2021. [Google Scholar]
- Barbosa, A.M.; Dariva, C.; Padilha, F.F.; Santos, K.S.; Franceschi, E.; Borges, G.R.; Muniz, A.V.C.d.S.; Pinheiro, M.S. Process for Obtaining a Product Containing Bioactive Compounds with Antibacterial Action against Multi-Resistant Bacteria. Patent BR 102018076511-6, 19 December 2018. [Google Scholar]
- Rafacho, B.P.M.; Rodrigues, B.M.; Santee, C.M.; dos Santos, E.F.; Corrêa, A.D.; de Souza, H.S.; Reis, V.H.d.O.T. Mangaba Juice Laxative. Patent BR102019019437A2, 18 September 2019. [Google Scholar]
- Droppa-Almeida, D.; Gaspar, L.M.A.C.; Bastos, B.F.; Dória, A.C.S.; da Silva, G.A.; Alves, L.L.; Mendonça, M.; Franceschi, E.; Padilha, F.F. Use of the Extract Obtained from Hancornia speciosa Gomes—Apocynaceae or Any of Its Derivatives as an Antimicrobial Agent. Patent BR102017025846A2, 30 November 2017. [Google Scholar]
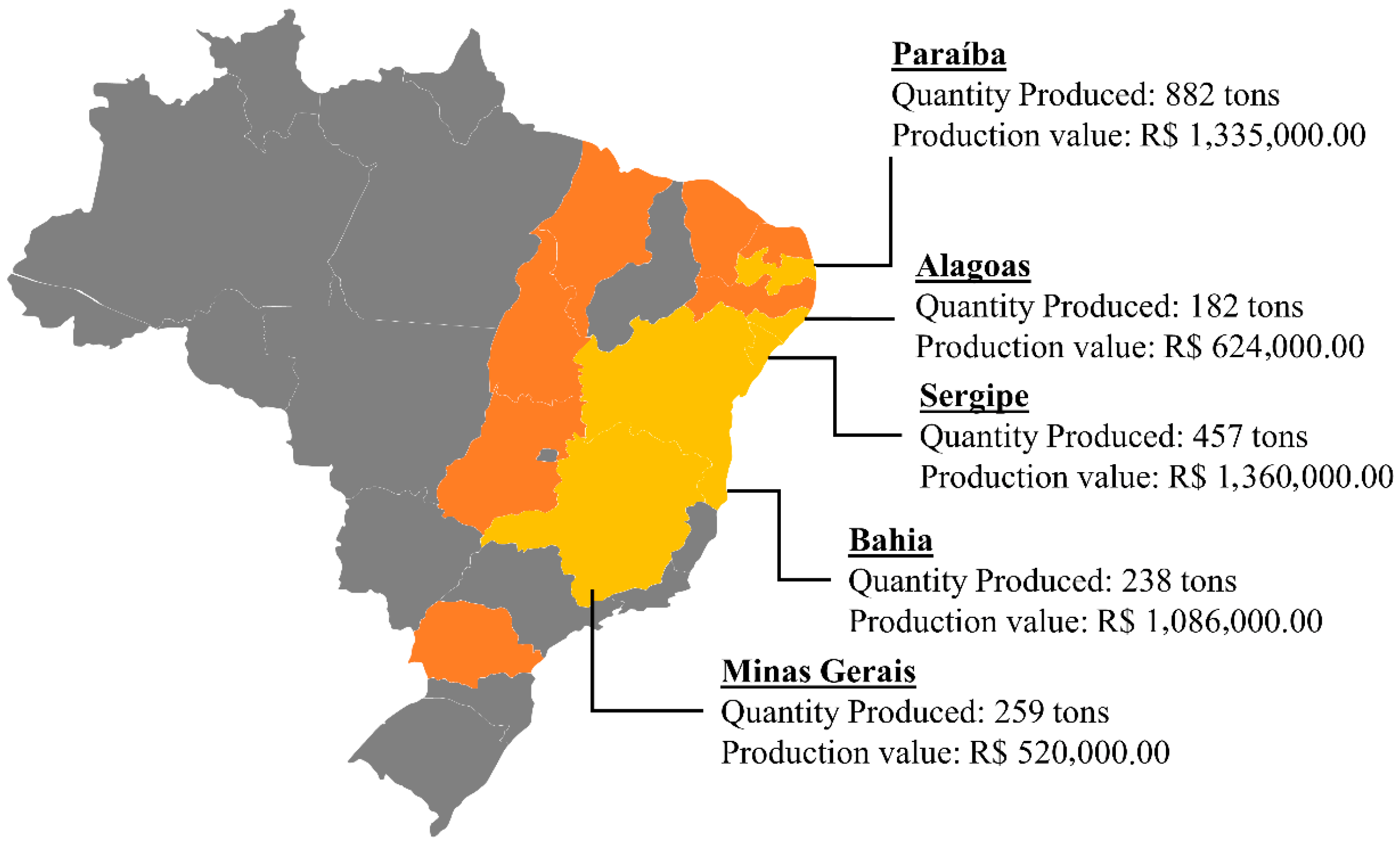



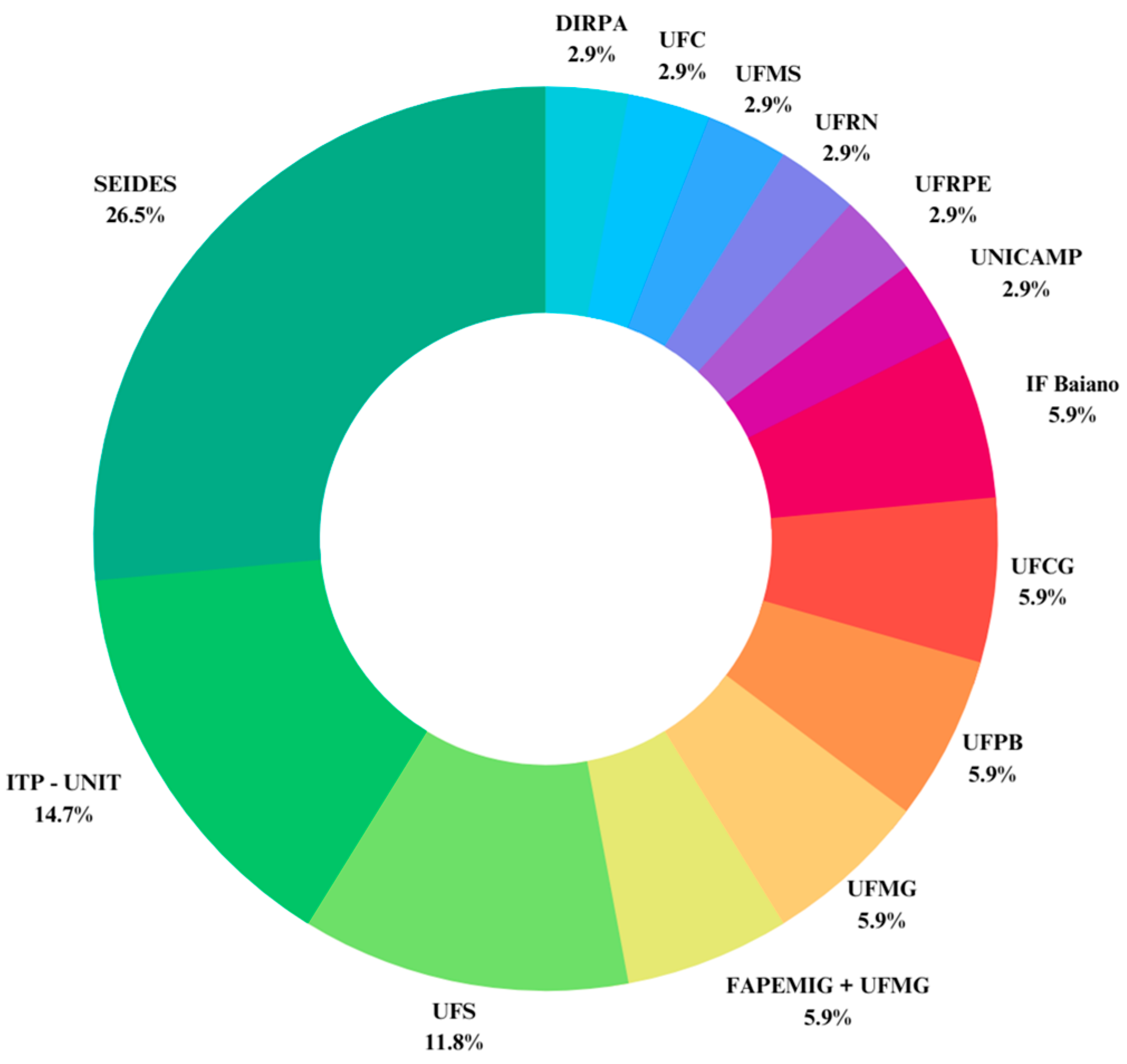
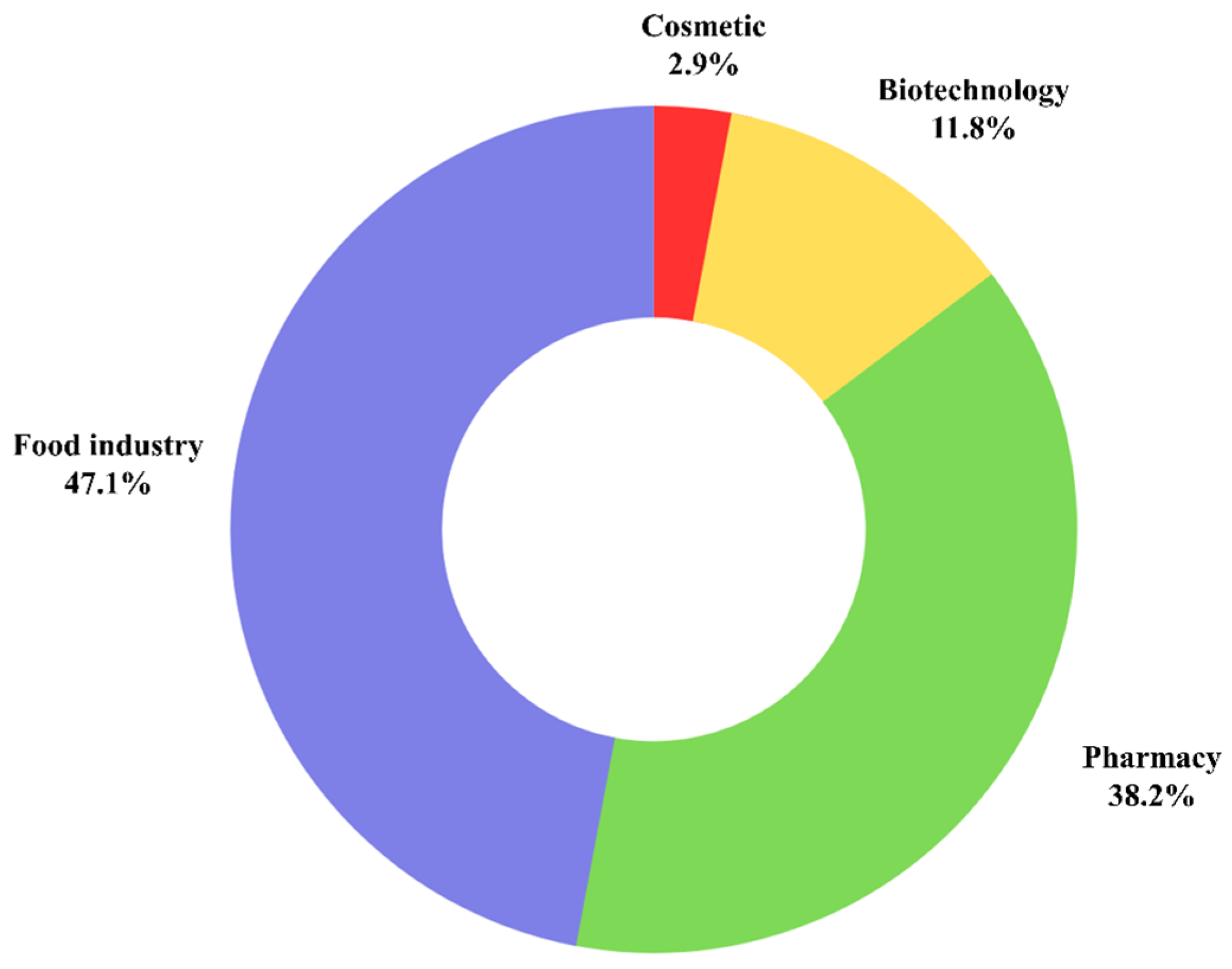
| Application | Reference | Plant Parts | Extraction Method | Solvents | Analytical Method | Main Compounds Identifies |
|---|---|---|---|---|---|---|
| Anti-inflammatory | Torres-Rêgo et al. [10] | Fruits | Decoction | Hot water, 100 °C 1:10, plant/solvent | HPLC-DAD; LC-MS | Chlorogenic acid; Rutin |
| Bitencourt et al. [11] | Fruits | Decoction | Hot water, 100 °C 1:10, plant/solvent | Not identified | Not identified | |
| Reis et al. [4] | Fruits | Did not perform extraction | Did not use | Not used | Not identified | |
| Yamashita et al. [12] | Fruits (juice) | Turboextraction | Purified water, 1:1, plant/solvent | HPLC-DAD-MS/MS | 13 phenolic derivatives | |
| Pegorin et al. [13] | Latex | Latex was centrifuged at 8000× g | Sterilized with ethylene oxide | Not used | Not identified | |
| D’abadia et al. [27] | Latex | Centrifuged at 4 °C for 1 h at 22,000× g | Did not use | Not used | Not identified | |
| Martins et al. [28] | Latex | Latex centrifugado a 3000× g | Água/látex (1:1) | Not used | Not identified | |
| Bitencourt et al. [29] | Fruits | Aqueous extract | Aqueous and solvent extractions with dichloromethane, ethyl acetate and n-butanol | Not used | Rutin, chlorogenic acid | |
| Antihypertensive | Silva et al. [14] | Leaves | Percolation | 96% EtOH | Not used | Not identified |
| Bastos et al. [30] | Leaves | Maceration | Ethanol/water (70:30, v/v) | UHPLC Orbitrap-HRMS | Phenolic derivatives | |
| Moreira et al. [15] | Leaves | Percolation | 96% EtOH | UPLC-ESI-MS | Bornesitol | |
| Moreira et al. [16] | Leaves | Did not perform extraction | Did not use | UPLC-MS/MS | Bornesitol | |
| Pereira et al. [18] | Leaves | Maceration e percolation | 96% EtOH; 70% Ethanol; 50% Ethanol; ethyl acetate/methanol | HPLC-PDA; UPLC-DAD-ESI-MS/MS | Rutin, chlorogenic acid, Bornesitol, 3-O-β-(3′-R-hydroxy)-hexadecanoil-lupeol | |
| Antioxidant | De Lima et al. [31] | Fruits | Centrifuge tubes and extracted sequentially | 40 mL of methanol/water (50:50, v/v); 40 mL of acetone/water (70: 30, v/v) | GCMS–QP | Alcohols (25.00%), aldehydes (25.00%), terpenes (19.44%), other compounds (19.44%), esters (8.34%); ketones (2.78%) |
| Lima Neto et al. [32] | bark, leaves | Maceration | Ethyl alcohol (90%) | Did not use | Not identified | |
| Santos et al. [33] | Leaves | Maceration | Ethanol 96% (1:10) | HPLC-DAD-MS/MS | Quinic acid, Chlorogenic acid, Catechin, Rutin, Isoquercetin | |
| Penido et al. [34] | Bark | Maceration | Ethanol 70% | Did not use | Phenols and flavonoids | |
| Dutra et al. [35] | Fruits | Ultrasonic bath | Methanol/water (50:50, v/v) | HPLC | Phenolic derivatives | |
| Dos Santos et al. [36] | Leaves | Maceration | Ethanol 96% (1:10) | GC-FID | Catechin, Rutin, Isoquercetin | |
| Maia et al. [37] | Fruits | Low pressure extractions (Cold maceration, ultrasound-assisted and Soxhlet); supercritical fluid extraction | Water, ethanol and n-hexane | CG/FID | Tetradecanal, a-amyrin, trans-oleic acids, palmitic and stearic acids, p-xylene, hap-22(29)-en-beta-ol | |
| De Araújo et al. [38] | Fruits | Maceration | Ethyl alcohol (1:5, m.v−1) | Did not use | Did not identify | |
| Barbosa et al. [39] | Leaves | Pressurized liquid extraction | Hexane, ethyl acetate and, ethanol/water | HPLC | Rutin, L-(+)-bornesitol, quinic acid, chlorogenic acid, and kaempferol | |
| Santos et al. [40] | Fruits | Ultrasound | 5 g of pulp with 100 mL of methanol/water solvent (9:1, v/v) | HPLC-DAD | Rutin, ferulic acid and chlorogenic acid | |
| Almeida et al. [41] | Pulp | Dilution | Water (1/1,5) | HPLC | 2.5 dihydroxybenzoic, gallic, 3.4 dihydroxybenzoic, salicylic, caffeic, and vanillic, as well as flavonoids, such as catechin and rutin | |
| Santos et al. [42] | Fruits | Did not perform extraction | Did not use | Did not use | Vitamin C, rutin and chlorogenic acid (majority) | |
| Panontin et al. [43] | Leaves | Soxhlet and ultrasound-assisted extraction | 70% ethanol | HPLC | Catechin, quercetin, p-coumaric acid, isorhamnetin and morin, rosmarinic acid | |
| Jácome et al. [44] | Mangaba residue | Solid-liquid extraction | 500 mL of ethanol solution (1:1 v v−1) | Did not use | Did not identify | |
| Antidiabetic | Pereira et al. [19] | Leaves | Percolation | 96% ethanol, with fraction of different solvents | ESI–LC–MS | Quinic acid, rutin |
| Neto et al. [20] | Leaves | Decoction | Water (1 L) w/60 g of sample | Did not use | Did not identify | |
| Tomazi et al. [21] | Latex | Ultrasound-assisted extraction | Methanol | HPTLC | Cornoside, dihydrocornoide, and bornesitol |
| Compound | Activity | Part of the Plant | Author |
|---|---|---|---|
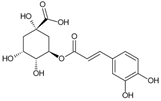 Chlorogenic acid | Antimutagenic; anticarcinogens; Anti-inflammatory, antioxidant; antidiabetic | Fruits, leaves | Torres-Rêgo et al. [10]; Yamashita et al. [12]; Bastos et al. [30]; De Lima et al. [31]; Santos et al. [40]; Jalali et al. [49] |
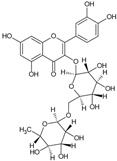 Rutin | Anti-inflammatory, antioxidant, Antihypertensive | Fruit pulp; leaves | Reis et al. [4]; Rêgo et al. [10]; Yamashita et al. [12]; Torres-Bastos et al. [30]; De Lima et al. [31]; Santos et al. [40] |
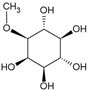 Bornesitol | Anti-inflammatory, antioxidant, Antihypertensive | Fruits; leaves | Yamashita et al. [12]; Silva et al. [14]; Moreira et al. [15]; Moreira et al. [16] |
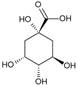 Quinic acid | Anti-inflammatory, antioxidant | Fruits | Yamashita et al. [12] |
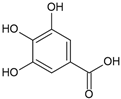 Gallic acid | Antioxidant | Fruit pulp | De Lima et al. [31] |
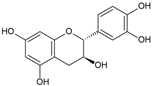 Catechin | Antioxidant; Antimutagenic | Fruit pulp | Reis et al. [4]; De Lima et al. [31]; Panontin et al. [43] |
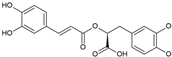 Rosmarinic acid | Anti-inflammatory, antioxidant | Fruit pulp | De Lima et al. [31] |
 Ferulic acid | anti-carcinogenic, antihypertensive, antidiabetic | Fruits | Santos et al. [40] |
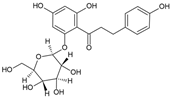 Phlorizin | Antidiabetic | Leaves | Bastos et al. [30] |
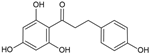 Phloretin | Antidiabetic | Leaves | Bastos et al. [30] |
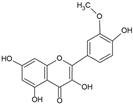 Isorharmnetin | Anti-inflammatory, antioxidant | Leaves | Panontin et al. [43] |
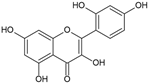 Morin | Anti-inflammatory; Antiallergic activity | Leaves | Panontin et al. [43] |
| Identification | Date | Title | Applicants | Application Area |
|---|---|---|---|---|
| BR 10 2014 013453 0; BR102014013453 | 2014; 2016 | Method for the production of Aspergillus niger lipase using waste from mangaba pulp processing as substrate [61] | Federal University of Sergipe-UFS | Biotechnological |
| BR 10 2013 018181 1; BR102013018181 | 2013; 2016 | Process of obtaining a new bactericide from mangabeira latex (Hancornia speciosa Gomes) [62] | Rural Federal University of Pernambuco-UFRPE | Biotechnological |
| BR 10 2021 009165 7; BR102021009165 | 2021; 2022 | Solution and process for preserving recalcitrant seeds [63] | Federal University of Sergipe-UFS | Biotechnological |
| BR 10 2017 001445 2; BR102017001445 | 2017; 2018 | Use of a bioadsorvent produced from mangaba seeds to remove contaminants from water and liquid effluents [64] | Federal University of Sergipe-UFS | Biotechnological |
| BR 10 2012 025418 2 | 2012 | Composition of mangabeira latex and its use in bone regeneration [65] | Campinas State University-UNICAMP | Pharmaceutical |
| PI 1106145-6; BRPI1106145 | 2011; 2014 | Composition and process for obtaining healing film and the film obtained thus [66] | Institute of Technology and Research; Tiradentes University-UNIT | Pharmaceutical |
| BR 10 2020 014387 5; BR102020014387 | 2020; 2021 | Pharmaceutical composition containing natural latex and use thereof for the treatment of diseases caused by intracellular protozoa [67] | Federal Institute of Education, Science and Technology Baiano-IF Baiano | Pharmaceutical |
| WO2009140749; EP2335711 | 2009; 2011 | Extract of Hancornia speciosa and pharmaceutical composition thereof * [68] | Research Support Foundation of the State of Minas Gerais (FAPEMIG); Federal University of Minas Gerais-UFMG | Pharmaceutical |
| PI 0802004-3; BRPI0802004 | 2008; 2009; 2010 | Standardized extract and fraction of leaves of Hancornia speciosa and its pharmaceutical composition * [69] | Research Support Foundation of the State of Minas Gerais (FAPEMIG); Federal University of Minas Gerais-UFMG | Pharmaceutical |
| BR 10 2012 026958 9; BR102012026958 | 2012; 2014 | Extracts, fractions, isolated compounds and pharmaceutical composition of Aspidosperma pyrifolium, Hancornia speciosa, Ipomoea asarifolia and Mimosa tenuiflora applied in the treatment of poisoning processes by venomy animals [70] | Federal University of Rio Grande do Norte-UFRN | Pharmaceutical |
| BR 10 2020 024121 4; BR102020024121 | 2020; 2022 | Polymeric films containing mangaba extract against multi-resistant bacteria [71] | Institute of Technology and Research; Tiradentes University-UNIT | Pharmaceutical |
| BR 10 2021 005471 9; BR102021005471 | 2021; 2022 | Pharmaceutical formulation containing natural latex and its use for the treatment of cutaneous wounds [72] | Federal Institute of Education, Science and Technology Baiano-IF Baiano | Pharmaceutical |
| BR 10 2018 076511 6; BR102018076511 | 2018; 2020 | Process for obtaining a product containing bioactive compounds with antibacterial action against multi-resistant bacteria [73] | Institute of Technology and Research; Tiradentes University-UNIT | Pharmaceutical |
| CA2759877; CA2724971; IN8284/CHENP/2010; CN102202675; US20110183929; JP2015013896; JP2017052792 | 2009; 2011; 2015; 2017 | Standardized extract and fraction from Hancornia speciosa leaves and pharmaceutical composition thereof * [69] | Federal University of Minas Gerais-UFMG | Pharmaceutical |
| BR 10 2019 019437 5; BR102019019437 | 2019; 2021 | Mangaba juice laxative [74] | Federal University of Minas Gerais-UFMG | Pharmaceutical |
| BR 10 2017 025846 7; BR102017025846 | 2017; 2019 | Use of the extract obtained from Hancornia speciosa Gomes—Apocynaceae or any of its derivatives as an antimicrobial agent [75] | Institute of Technology and Research; Tiradentes University-UNIT | Pharmaceutical |
| JP2011520922 | 2011 | Standardized extracts and fractions from Hancornia speciosa leaves and pharmaceutical compositions thereof * [69] | Federal University of Minas Gerais-UFMG | Pharmaceutical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite, S.P.; Krause, L.C.; Jain, S.; Bjerk, T.R. Scientific and Technical Insights into Hancornia speciosa Gomes for Biotechnological Applications. Compounds 2025, 5, 38. https://doi.org/10.3390/compounds5040038
Leite SP, Krause LC, Jain S, Bjerk TR. Scientific and Technical Insights into Hancornia speciosa Gomes for Biotechnological Applications. Compounds. 2025; 5(4):38. https://doi.org/10.3390/compounds5040038
Chicago/Turabian StyleLeite, Sérgio P., Laiza C. Krause, Sona Jain, and Thiago R. Bjerk. 2025. "Scientific and Technical Insights into Hancornia speciosa Gomes for Biotechnological Applications" Compounds 5, no. 4: 38. https://doi.org/10.3390/compounds5040038
APA StyleLeite, S. P., Krause, L. C., Jain, S., & Bjerk, T. R. (2025). Scientific and Technical Insights into Hancornia speciosa Gomes for Biotechnological Applications. Compounds, 5(4), 38. https://doi.org/10.3390/compounds5040038








