Determination of Volatile Organic Compounds in Some Epipactis, Neottia, and Limodorum Orchids Growing in Basilicata (Southern Italy)
Abstract
1. Introduction
2. Materials and Methods
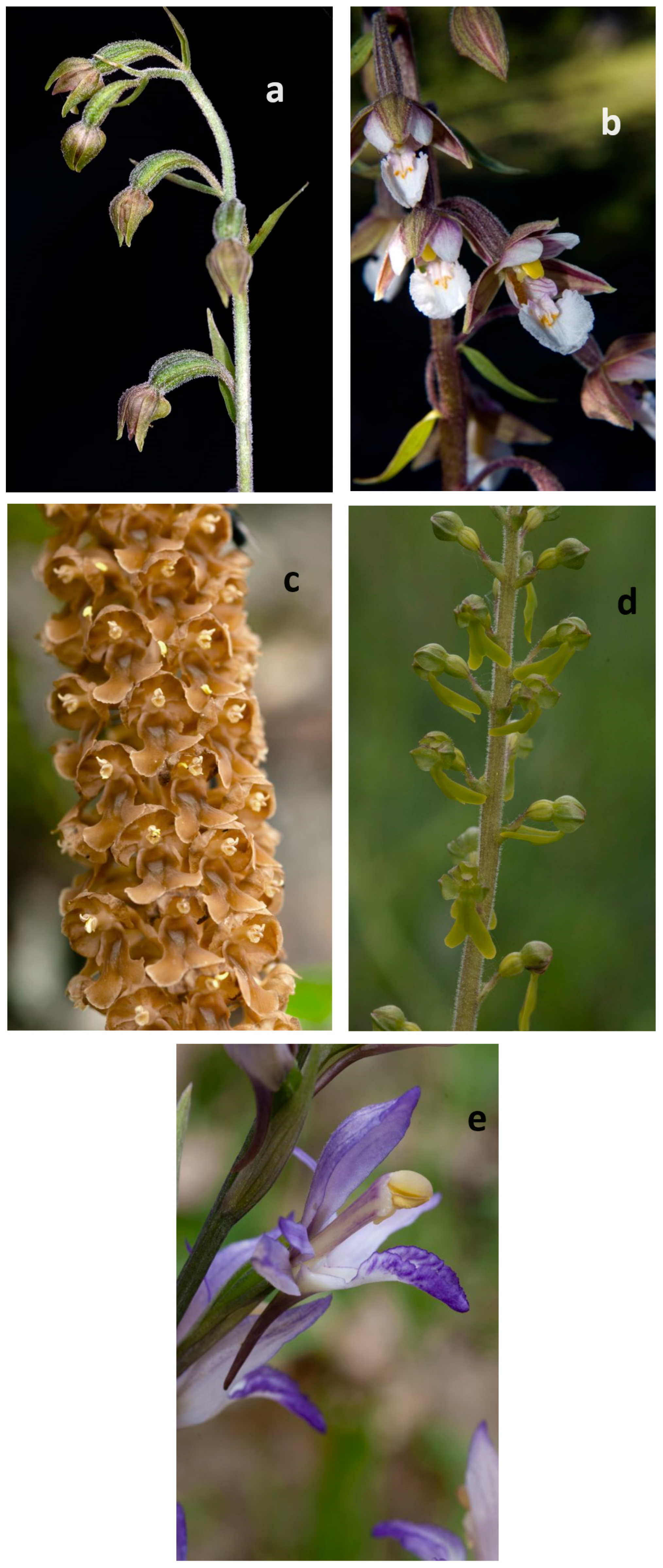
2.1. Plant Material
2.2. Analysis of Volatile Organic Compounds
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chase, M.W.; Cameron, K.M.; Freudenstein, J.V.; Pridgeon, A.M.; Salazar, G.A.; Van Den Berg, C.; Schuiteman, A. An updated classification of Orchidaceae. Bot. J. Linn. Soc. 2015, 177, 151–174. [Google Scholar] [CrossRef]
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Vakhrameeva, M.G.; Tatarenko, I.V. Ecological characteristics of orchids of European Part of Russia. Acta Univ. Wratislav. 2001, 79, 49–54. [Google Scholar]
- Ackerman, J.D. Mechanisms and evolution of food-deceptive pollination systems in orchids. Lindleyana 1986, 1, 108–113. [Google Scholar]
- Tremblay, R.L.; Ackerman, J.D.; Zimmerman, J.K.; Calvo, R.N. Variation in sexual reproduction in orchids and its evolutionary consequences: A spasmodic journey to diversification. Biol. J. Linn. Soc. 2005, 84, 1–54. [Google Scholar] [CrossRef]
- Dressler, R. The Orchids: Natural History and Classification; Harvard University Press: Cambridge, MA, USA, 1981; p. 356. [Google Scholar]
- Tremblay, R.L. Trends in the pollination ecology of the Orchidaceae: Evolution and systematics. Can. J. Bot. 1992, 70, 642–650. [Google Scholar] [CrossRef]
- Johnson, S.D.; Hobbhahn, N. Generalized pollination, floral scent chemistry, and a possible case of hybridization in the African orchid Disa fragrans. S. Afr. J. Bot. 2010, 76, 739–748. [Google Scholar] [CrossRef]
- Nilsson, L.A. The evolution of flowers with deep corolla tubes. Nature 1988, 334, 147–149. [Google Scholar] [CrossRef]
- Jakubska-Busse, A.; Prządo, D.; Steininger, M.; Anioł-Kwiatkowska, J.; Kadej, M. Why do pollinators become ”sluggish”? Nectar chemical constituents from Epipactis helleborine L. (Orchidaceae). Appl. Ecol. Environ. Res. 2005, 2, 29–38. [Google Scholar] [CrossRef]
- Claessens, J.; Kleynen, J. Allogamie- und Autogamie-Tendenzen bei einigen Vertreten der Gattung Epipactis. Ber. Arbeitskr. Heim. Orch. 1996, 12, 4–16. [Google Scholar]
- Claessens, J.; Kleynen, J. The pollination of European Orchids Part 3: Limodorum and Epipactis. J. Hardy Orchid Soc. 2014, 11, 64–71. [Google Scholar]
- Nilsson, L.A. Pollination ecology of Epipactis palustris (Orchidaceae). Bot. Notiser 1978, 131, 355–368. [Google Scholar]
- Procházka, F.; Velisek, V. Orchideje Naši Přirody; Academia Ved: Praha, Czech Republic, 1983; p. 284. [Google Scholar]
- Brantjes, N.B. Ant, bee and fly pollination in Epipactis palustris (L.) Crantz (Orchidaceae). Acta Bot. Neerl. 1981, 30, 59–68. [Google Scholar] [CrossRef]
- Jakubska-Busse, A.; Kadej, M. The pollination of Epipactis Zinn, 1757 (Orchidaceae) species in Central Europe—The significance of chemical attractants, floral morphology and concomitant insects. Acta Soc. Bot. Polon. 2011, 80, 49–57. [Google Scholar] [CrossRef]
- Romano, V.A.; Navazio, G.; Zanpino, A. Nuove stazioni di specie rare di Orchidaceae per la Basilicata. GIROS Not. 2013, 52, 81–88. [Google Scholar]
- Dressler, R.L. The orchids. In Natural History and Classification; Harvard University Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Chase, M.W.; Barrett, R.L.; Cameron, K.N.; Freudenstein, J.V. DNA data and Orchidaceae systematics: A new phylogenetic classification. In Orchid Conservation; Dixon, K.M., Ed.; Natural History Publication: Borneo, Indonesia, 2003; pp. 69–89. [Google Scholar]
- Zhou, T.; Jin, X.-H. Molecular systematics and the evolution of mycoheterotrophy of tribe Neottieae (Orchidaceae, Epidendroideae). PhytoKeys 2018, 94, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Pignatti, S. Flora d’Italia; Edagricole Editore: Bologna, Italy, 1982. [Google Scholar]
- Tison, J.-M.; Jauzein, P.; Michaud, H. Flore de la France Méditerranéenne Continentale; Naturalia Publications: Turriers, France, 2014. [Google Scholar]
- Jersáková, J.; Minasiewicz, J.; Selosse, M.-A. Biological flora of Britain and Ireland: Neottia nidus-avis. J. Ecol. 2022, 110, 2246–2263. [Google Scholar] [CrossRef]
- Summerhayes, V.S. Wild Orchids of Britain; Collins: New York, NY, USA, 1951. [Google Scholar]
- Ziegenspeck, H. Orchidaceae. In Lebensgeschichte Der Blütenpflanzen Mitteleuropas; Band 1, Abteilung 4; Eugen Ulmer: Stuttgart, Germany, 1936. [Google Scholar]
- Claessens, J.; Kleynen, J. The Flower of the European Orchid. Form and Function; Schrijen-Lippertz: Voerendaal, The Netherlands, 2011. [Google Scholar]
- Darwin, C. On the Various Contrivances by Which British and Foreign Orchids Are Fertilised by Insects; John Murray: London, UK, 1862. [Google Scholar]
- Müller, H. The Fertilisation of Flowers; McMillan: New York, NY, USA, 1883. [Google Scholar]
- Burns-Balogh, P.; Szlachetko, D.L.; Dafni, A. Evolution, pollination, and systematics of the tribe Neottieae (Orchidaceae). Plant Syst. Evol. 1987, 156, 91–115. [Google Scholar] [CrossRef]
- Reinhard, H.R.; Golz, P.; Peter, R.; Wildermuth, H. Die Orchideen der Schweiz und Angrenzender Gebiete; Fotorotar Verlag: Zurich, Switzerland, 1991. [Google Scholar]
- Van Der Cingel, N.A. An Atlas of Orchid Pollination: America, Africa, Asia and Australia; Balkena Publishers: Rotterdam, The Netherlands, 2001. [Google Scholar]
- Tamm, C.O. Survival and flowering of some perennial herbs. II. The behaviour of some orchids on permanent plots. Oikos 1972, 23, 23–28. [Google Scholar]
- Nilsson, L.A. The pollination ecology of Listera ovata (Orchidaceae). Nord. J. Bot. 1981, 1, 461–480. [Google Scholar] [CrossRef]
- Nepi, M. Beyond nectar sweetness: The hidden ecological role of non-protein amino acids in nectar. J. Ecol. 2014, 102, 108–115. [Google Scholar] [CrossRef]
- Brzosko, E.; Bajguz, A.; Chmur, M.; Burzyńska, J.; Jermakowicz, E.; Mirski, P.; Zieliński, P. How are the flower structure and nectar composition of the generalistic orchid Neottia ovata adapted to a wide range of pollinators? Int. J. Mol. Sci. 2021, 22, 2214. [Google Scholar] [CrossRef] [PubMed]
- Stpiczyńska, M.; Nepi, M.; Zych, M. Nectaries and male-biased nectar production in protandrous flowers of a perennial umbellifer Angelica sylvestris L. (Apiaceae). Plant Syst. Evol. 2015, 301, 1099–1113. [Google Scholar] [CrossRef][Green Version]
- Zych, M.; Junker, R.R.; Nepi, M.; Stpiczynska, M.; Stolarska, B.; Roguz, K. Spatiotemporal variation in the pollination systems of a supergeneralist plant: Is Angelica sylvestris (Apiaceae) locally adapted to its most effective pollinators? Ann. Bot. 2019, 123, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Bergström, G.; Groth, I.; Pellmyr, O.; Endress, P.K.; Thien, L.B.; Hübener, A.; Francke, W. Chemical basis of a highly specific mutualism: Chiral esters attract pollinating beetles in Eupomatiaceae. Phytochemistry 1991, 30, 3221–3225. [Google Scholar] [CrossRef]
- Girlanda, M.; Selosse, M.A.; Cafasso, D.; Brilli, F.; Delfine, S.; Fabbian, R.; Ghignone, S.; Pinelli, P.; Segreto, R.; Loreto, F.; et al. Inefficient photosynthesis in the Mediterranean orchid Limodorum abortivum is mirrored by specific association to ectomycorrhizal Russulaceae. Mol. Ecol. 2006, 15, 491–504. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, M.; Lorenz, R.; Racioppi, R.; Romano, V.A. Fragrance components of Platanthera bifolia subsp. osca. Nat. Prod. Res. 2017, 31, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, M.; Lorenz, R.; Mecca, M.; Racioppi, R.; Romano, V.A.; Viggiani, L. Fragrance components of Platanthera bifolia subsp. osca and Platanthera chlorantha collected in several sites in Italy. Nat. Prod. Res. 2020, 34, 2857–2861. [Google Scholar]
- D’Auria, M.; Lorenz, R.; Mecca, M.; Racioppi, R.; Romano, V.A. Aroma components of Cephalanthera orchids. Nat. Prod. Res. 2021, 35, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Mecca, M.; Racioppi, R.; Romano, V.A.; Viggiani, L.; Lorenz, R.; D’Auria, M. Volatile organic compounds from Orchis species found in Basilicata (Southern Italy). Compounds 2021, 1, 83–93. [Google Scholar] [CrossRef]
- D’Auria, M.; Lorenz, R.; Mecca, M.; Racioppi, R.; Romano, V.A. The composition of the aroma of Serapias orchids in Basilicata (Southern Italy). Nat. Prod. Res. 2021, 35, 4068–4072. [Google Scholar]
- Mecca, M.; Racioppi, R.; Romano, V.A.; Viggiani, L.; Lorenz, R.; D’Auria, M. The scent of Himantoglossum species found in Basilicata (Southern Italy). Compounds 2021, 1, 164–173. [Google Scholar] [CrossRef]
- Romano, V.A.; Rosati, L.; Fascetti, S.; Cittadini, A.M.R.; Racioppi, R.; Lorenz, R.; D’Auria, M. Spatial and temporal Variability of the floral scent emitted by Barlia robertiana (Loisel.) Greuter, a Mediterranean food-deceptive orchid. Compounds 2022, 2, 37–53. [Google Scholar] [CrossRef]
- Mecca, M.; Racioppi, R.; Romano, V.A.; Viggiani, L.; Lorenz, R.; D’Auria, M. Volatile organic compounds in Dactylorhiza species. Compounds 2022, 2, 121–130. [Google Scholar] [CrossRef]
- D’Auria, M.; Lorenz, R.; Mecca, M.; Racioppi, R.; Romano, V.A.; Viggiani, L. Fragrance components of Gymnadenia conopsea and Gymnadenia odoratissima collected at several sites in Italy and Germany. Nat. Prod. Res. 2022, 36, 3435–3439. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, M.; Lorenz, R.; Mecca, M.; Racioppi, R.; Romano, V.A.; Viggiani, L. The scent of Neotinea orchids from Basilicata (Southern Italy). Nat. Prod. Res. 2022, 36, 3741–3743. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, M.; Lorenz, R.; Mecca, M.; Racioppi, R.; Romano, V.A. Composition of the scent in some Ophrys orchids growing in Basilicata (Southern Italy): A solid-phase microextraction study coupled with gas chromatography and mass spectrometry. Compounds 2023, 3, 573–583. [Google Scholar] [CrossRef]
- D’Auria, M.; Emanuele, L.; Lorenz, R.; Mecca, M.; Racioppi, R.; Romano, V.A.; Viggiani, L. HS-SPME-GC–MS determination of the scent of Anacamptis taxa (fam. Orchidaceae) from Basilicata (Southern Italy). Nat. Prod. Res. 2023, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Perkins, J.; Hayashi, T.; Peakall, R.; Flematti, G.R.; Bohman, B. The volatile chemistry of orchid pollination. Nat. Prod. Rep. 2023, 40, 819–839. [Google Scholar] [CrossRef] [PubMed]
| Compound | r.t. a [min.] | KI a | Area% ± 0.03 | ||||
|---|---|---|---|---|---|---|---|
| E. microphylla | E. palustris | N. nidus avis | N. ovata | L. abortivum | |||
| Mesityl oxide | 4.97 | 804 | 4.95 | ||||
| 2,2,4,6,6-Pentamethylhept-3-ene | 9.31 | 1030 | 0.41 | 1.24 | 0.62 | 0.45 | |
| Limonene | 10.60 | 1039 | 40.05 | 5.22 | 1.14 | 1.08 | 0.22 |
| Linalool | 10.65 | 1101 | 1.78 | ||||
| β-Terpineol | 10.72 | 1130 | 0.62 | ||||
| Dodecane | 13.09 | 1200 | 1.13 | ||||
| Decanal | 13.23 | 1204 | 3.18 | 0.77 | |||
| 2-Dodecene | 13.36 | 1222 | 0.80 | ||||
| Bornyl acetate | 14.79 | 1285 | 1.70 | ||||
| Tridecane | 14.97 | 1300 | 3.31 | 3.02 | |||
| α-Terpinyl acetate | 15.81 | 1355 | 2.69 | ||||
| 2,4,4,6,6,8,8-Heptamethyl-2-nonene | 16.12 | 1365 | 1.47 | 1.69 | |||
| Farnesane | 16.26 | 1378 | 1.54 | ||||
| 2,4,4,6,6,8,8-Heptamethyl-1-nonene | 16.45 | 1385 | 3.74 | 8.90 | 0.77 | 6.07 | |
| Tetradecane | 16.55 | 1400 | 2.38 | 5.34 | 8.03 | 4.22 | 3.87 |
| Methyleugenol | 16.60 | 1408 | 1.31 | ||||
| Dodecanal | 16.74 | 1415 | 1.36 | ||||
| 1-Methoxydodecane | 17.00 | 1424 | 1.02 | ||||
| Geranylacetone | 17.46 | 1451 | 0.61 | ||||
| 2,6-di-t-butylbenzoquinone | 17.79 | 1472 | 1.17 | 2.78 | 1.74 | 2.06 | 2.32 |
| Pentadecane | 18.14 | 1500 | 5.51 | 12.03 | 7.79 | 15.22 | 6.86 |
| β-Cadinene | 18.74 | 1520 | 3.62 | ||||
| Elemicin | 19.07 | 1554 | 1.24 | 7.94 | |||
| Tetradecanal | 19.86 | 1581 | 1.36 | 3.63 | |||
| Hexadecane | 20.36 | 1600 | 7.70 | 8.38 | 6.10 | ||
| Heptadecane | 21.06 | 1700 | 3.25 | 7.31 | 7.38 | 10.30 | 6.28 |
| Pristane | 21.14 | 1708 | 1.29 | 4.21 | 3.89 | 3.38 | |
| 2-(Dodecyloxy)-ethanol | 21.82 | 1731 | 6.32 | ||||
| 2-Benzylideneoctanal | 21.86 | 1746 | 2.94 | 1.59 | 2.90 | ||
| Benzyl benzoate | 22.15 | 1762 | 1.02 | ||||
| 3,5-di-t-butyl-4-hydroxybenzaldehyde | 22.22 | 1772 | 1.36 | 2.09 | |||
| Octadecane | 22.41 | 1800 | 1.58 | 3.89 | 4.84 | 5.62 | 4.55 |
| Hexadecanal | 22.67 | 1819 | 1.75 | ||||
| 5,9,13-trimethyl-4,8,12-tetradecenal | 23.01 | 1880 | 1.14 | 1.70 | 7.11 | 5.23 | 4.59 |
| Nonadecane | 23.70 | 1900 | 1.17 | 2.16 | 3.56 | 3.00 | 3.62 |
| 7,9-Di-t-butyl-1-oxaspiro [4,5]deca-6,9-diene-2,8-dione | 24.19 | 1919 | 1.66 | ||||
| Palmitic acid | 24.48 | 1950 | 1.09 | ||||
| Eicosane | 24.93 | 2000 | 1.21 | 2.44 | 2.55 | 2.30 | 2.99 |
| Kaur-16-ene | 25.86 | 2031 | 19.67 | ||||
| Heinecosane | 26.18 | 2100 | 10.60 | 4.99 | 5.26 | ||
| Docosane | 27.22 | 2200 | 0.65 | 1.20 | 1.61 | 2.23 | 2.45 |
| 9-Tricosene | 28.12 | 2271 | 4.46 | ||||
| Tricosane | 28.40 | 2300 | 1.21 | 4.85 | 1.48 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Auria, M.; Lorenz, R.; Racioppi, R.; Romano, V.A. Determination of Volatile Organic Compounds in Some Epipactis, Neottia, and Limodorum Orchids Growing in Basilicata (Southern Italy). Compounds 2024, 4, 366-375. https://doi.org/10.3390/compounds4020022
D’Auria M, Lorenz R, Racioppi R, Romano VA. Determination of Volatile Organic Compounds in Some Epipactis, Neottia, and Limodorum Orchids Growing in Basilicata (Southern Italy). Compounds. 2024; 4(2):366-375. https://doi.org/10.3390/compounds4020022
Chicago/Turabian StyleD’Auria, Maurizio, Richard Lorenz, Rocco Racioppi, and Vito Antonio Romano. 2024. "Determination of Volatile Organic Compounds in Some Epipactis, Neottia, and Limodorum Orchids Growing in Basilicata (Southern Italy)" Compounds 4, no. 2: 366-375. https://doi.org/10.3390/compounds4020022
APA StyleD’Auria, M., Lorenz, R., Racioppi, R., & Romano, V. A. (2024). Determination of Volatile Organic Compounds in Some Epipactis, Neottia, and Limodorum Orchids Growing in Basilicata (Southern Italy). Compounds, 4(2), 366-375. https://doi.org/10.3390/compounds4020022







