Abstract
The basic hydrolysis of Malachite Green (MG) in the presence of β-Cyclodextrin (β-CD) has been studied using UV-Vis spectroscopic techniques and at 20 °C. β-CD was found to catalyze the basic hydrolysis. Indeed, this basic hydrolysis is catalyzed by the interaction cyclodextrin hydroxyl group, in its deprotonated form with the carbocation in the host-guest complex. The proposed model has been successfully applied to a reaction catalyzed by CD. It considers two simultaneous pathways in the aqueous medium involving free hydroxyl ions and the substrate-CD complex. The model allows us to obtain the kinetic parameters including the bimolecular rate constant between MG and HO− in bulk water (kw = 1.47 ± 0.01 mol−1s−1), the rate constant between MG and the deprotonated hydroxyl group of β-CD inside the host-guest complex (kCD = 0.25 ± 0.03 s−1) and the binding constant of MG inside the β-CD (KS = 2500 ± 50). This behavior is like the hydrolysis of Cristal Violet (CV) in the same reaction media.
1. Introduction
Cyclic oligosaccharides made up of several glucose units linked together by α-1,4 glycosidic bonds are known as cyclodextrins (CDs) [1,2,3]. Normally this family of compounds is formed by structures of between six and eight glucopyranosides (α-Cd, β-CD, γ-CD, and δ-CD respectively) [4]. Topologically, these compounds have a toroidal shape whose openings are exposed to the primary and secondary hydroxyl groups of the glucopyranose [5]. Due to this peculiar structure, the interior cavity of the cone has a lower hydrophilic character as compared to its exterior, which is hydrophilic in nature, with which it is capable of harboring hydrophobic molecules inside, giving rise to inclusion complexes (the host-guest system) through non-covalent interactions when the size, shape and polarity of these molecules is adequate [5,6]. The stabilization of the guest molecule is given by different factors being Van der Waals and hydrophobic forces or H-bonds, among others [7]. In any case, the study of these inclusion complexes is a key part of what is known as supramolecular chemistry [8,9]. Another interesting aspect of CDs is they can modulate the reactivity related with their capacity to form guest host complexes with small and medium sized molecules [10]. According to Iglesias and Fernández (1998) [10], these changes in reactivity are the result of host-host interactions and vary significantly depending on the nature of the reagents and the reaction. In this way we can observe both increases and decreases in the reaction speed, with which in some cases CD have been used as stabilizers and in others as potential phase transfer catalysts. In addition, in some cases CDs can participate directly in the chemical reaction [10].
Malachite green (MG) is a triphenylmethane cationic dye which is used in the pigment industry [11] to color silk, wool and leather [12]. This compound is also used as a therapeutic agent for fish, since this compound present antifungal activity [13]. The common name of this compound is associated with its intense green color [14], presenting a strong absorption band in the VIS region at λ = 621 nm, with an extinction coefficient of ε = 105 M−1cm−1 (log ε = 5.02) [15]. This band disappears during the hydrolysis process, changing from a colored compound to a colorless compound, which facilitates its spectrophotometric monitoring and is the reason why it is a very popular reaction in chemical kinetics labs in undergraduate studies, as occurs with his analogous compound Crystal violet (CV) [11].
In relation to the uses of MG in commercial aquaculture and ornamental aquariums, a controversial application is its use as an antimicrobial agent for the treatment of the oomycete fungi on fish and fish eggs because its adverse effects on human immune and reproductive systems [16]. Different studies concluded the important fungal effect against oomycete fungus such as Saprolegnia [17], Haliphthoros [18] or Aphanomcyces invades [19]. Due to its effects on health, numerous studies in the literature analyze the physical-chemical properties of this compound [14,20,21,22].
According to Leis et al. (1993) the MG alkaline fading is a reaction with a long chemical tradition [23]. It takes place through a nucleophilic attack of the OH− on the carbocation [24] (Scheme 1). This hydrolysis, together with that of other analogs (such as crystal violet –CV–) was used for the construction of the Ritchie N+ nucleophilicity scale [25].
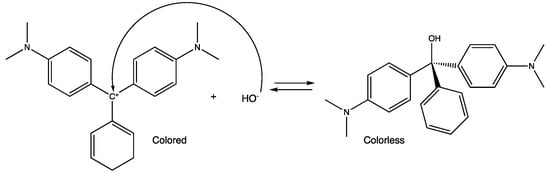
Scheme 1.
Malachite Green basic hydrolysis reaction mechanism.
Our aim is to evaluate the effect exerted by the presence of β-Cyclodextrin on the basic hydrolysis of malachite green.
2. Materials and Methods
β-CD was supplied by Sigma-Aldrich (Darmstadt, Germany) and produced by produced by Wacker Chemie (Burghausen, Germany) in the highest purity available (≥98%) and it was used as received, keeping in mind that commercial β-CD has an H2O content of 8 mol/mol (CAS no. 7585-39-9). It was all deprotonated under the alkaline conditions used ([NaOH] > 0.1 M) (pKa CD = 12.2) [26]. Due to the deprotonation of β-CD, [OH−] was obtained by subtracting the concentration of CD from that of NaOH. NaOH and MG were supplied by Sigma-Aldrich (Darmstadt, Germany). NaOH solutions were titrated with potassium hydrogen phthalate supplied by Sigma-Aldrich (Darmstadt, Germany).
The reaction was followed by UV-Vis spectroscopy, monitoring the disappearance of the MG in the band of λ = 621 nm using a Variant Cari 60 spectrophotometer supplied by Agilent (Santa Clara, CA, USA). All the experiments were carried out at 25.0 ± 0.1 °C using a thermostat-cryostat supplied by Poly-Science (Niles, IL, USA). The kinetic experiments were carried out under pseudo-first order conditions, keeping the concentration of MG (approx. 10−5 M) always much lower than that of NaOH. The obtained absorbance/time data were fitted by first order integrated equations, and the values of the pseudo first order rate constants (ko) were reproducible to within 3%.
3. Results and Discussion
The rate constant of the basic hydrolysis of MG in water was determined to maintain consistency with the results obtained throughout this work. The constant was determined by varying the [NaOH] (0.01–0.15 M) keeping the [MG] constant (10−5 M). Figure 1 shows the dependence of the observed rate constant of pseudo first order (ko) and [NaOH], from which a value for the hydrolysis of bimolecular rate constant, kw = 1.46 ± 0.03 M−1s−1 has been obtained (R2 = 0.9986). This value is compatible with previous one in the literature [23,27].
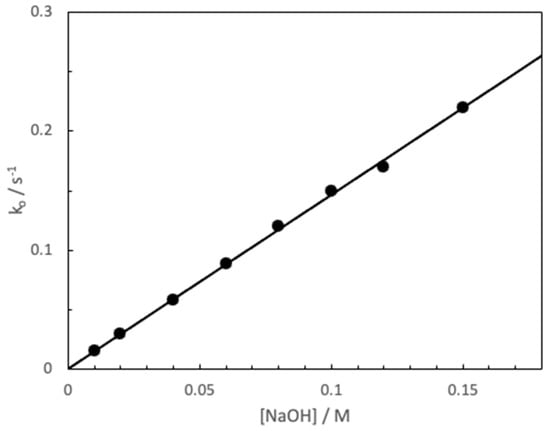
Figure 1.
Influence of [NaOH] upon the pseudo fist order rate constant, ko, of alkaline fading of MG. [MG] = 10−5 M, T = 25 °C.
Since the cyclodextrin cavity has a lower polarity than that of bulk water, the influence of the dielectric constant (ε) on the MG basic hydrolysis reaction has also been analyzed using dioxane-water mixtures. For this, ε value was varied between 24.54 and 74.43, maintaining a constant MG and NaOH concentrations ([MG] = 1.46 × 10−5 M and [NaOH] = 9.98 × 10−4 M). A significant decrease in the rate constant was observed as the dielectric constant increased (Figure 2).
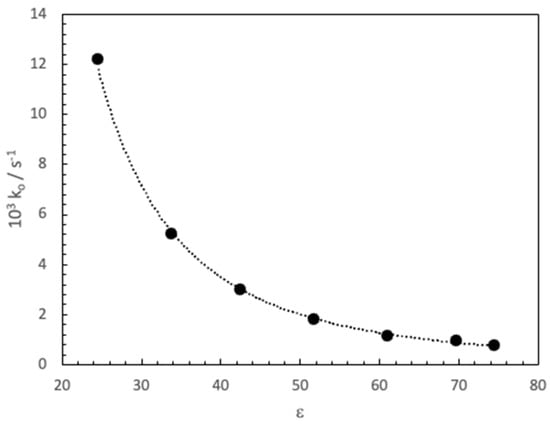
Figure 2.
Variation of the pseudo-first order rate constant, ko, with the dielectric constant (dioxane-water mixtures) for the basic hydrolysis of MG. ([MG] = 1.46 × 10−5 M and [NaOH] = 9.98 × 10−4 M, T = 25 °C).
An interesting piece of information would be the evaluation of the radius of the activated complex for the reaction using the double sphere model [28]. From the fits of the experimental data to Equation (1)—where zA and zB are the ions charge, e is the electron charge, ε the dielectric constant, σ≠ is the active complex radius and k0 is the rate constant in a high dielectric constant medium (e = ∞) (Figure 3) we obtain a value for the radius of the complex σ≠ 5.1 Å (R2 = 0.9803).
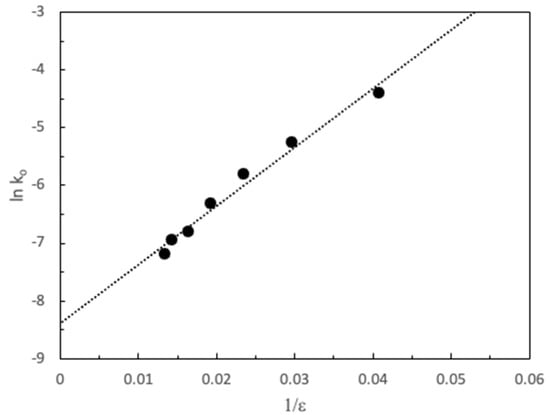
Figure 3.
Double sphere model applied to the influence of the dielectric constant on the pseudo-first order rate constant, ko, for the basic hydrolysis of MG. ([MG] = 1.46 × 10−5 M and [NaOH] = 9.98 × 10−4 M, T = 25 °C).
After determining the influence of the dielectric constant on the alkaline fading of MG, a study of the ionic strength on the rate constant of the hydrolysis process has been carried out. NaClO4 was used as electrolyte to set the ionic strength, which varied between 4.99 × 10−4 and 0.59 M. As would be expected, a decrease in the rate constant is observed as the ionic strength of the medium increases (Figure 4).
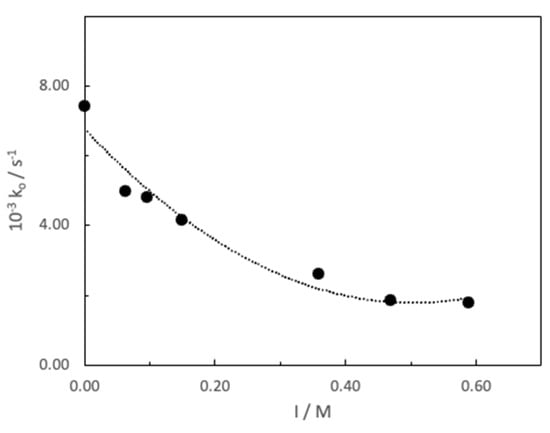
Figure 4.
Influence of ionic strength (fixed with NaClO4−) on the pseudo-first-order constant, ko, for the basic hydrolysis of MG ([MG] = 7.33 × 10−6 M and [NaOH] = 4.99 × 10−3 M, T = 25 °C).
The values obtained fit the Brönsted-Bjerrum equation—Equation (2)—based on a simple Debye model [29].
where zA and zB are the ions charge, I is the ionic strength and k0 is the rate constant at I = 0.
Figure 5 shows the fit of experimental data to Equation (2) (R2 = 0.9203). Obviously, the fulfillment of this equation is fortuitous, because the Debye model is only valid for very low ionic strengths. However, it is relatively common that the data on the variation of the reaction rate with the ionic strength between ionic species (in our case MG+ and HO−) fit in a “formal” way to the Debye model, however, it is not It is possible to rigorously identify the parameters in contracts with which the Debye model assigns. In any case, the inhibition of the reaction by increasing the salinity of the medium is in accordance with all the predictions from simple electrostatic theories [29]. It should be noted that unlike happened with CV, no anomalous behavior is observed between the carbocation and ClO4−. This would indicate that in the case of MG, unlike what occurs with CV, ion pairs are not formed between these two species [30].
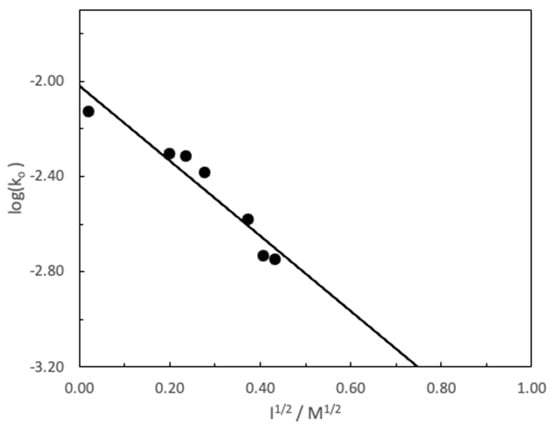
Figure 5.
Influence of ionic strength on the pseudo-first-order constant, ko, for the basic hydrolysis of MG ([MG] = 7.33 × 10−6 M and [NaOH] = 4.99 × 10−3 M, T = 25 °C) ionic strength fixed with NaClO4−.
Once the basic hydrolysis reaction of MG in an aqueous media has been characterized, the alkaline hydrolysis of MG in the presence of β-CD has been analyzed. The hydrolysis reaction is assessed by varying [CD] between 0 and 0.015 M. As can be seen in Figure 6, a catalytic effect of β-CD is observed in this reaction. Indeed, the observed rate constant increases as the concentration of CD present in the medium increases until reaching a leveling off.
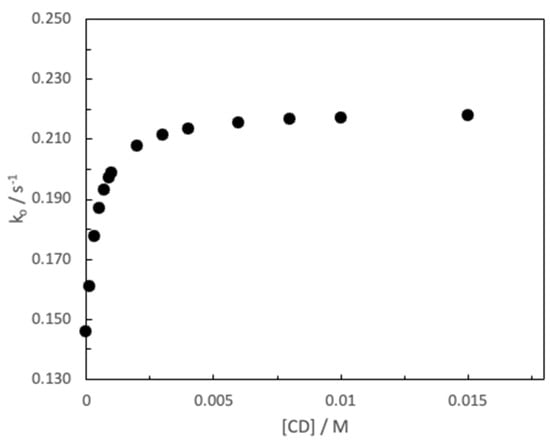
Figure 6.
Influence of β-CD concentration on the pseudo-first-order constant, ko, for the basic hydrolysis of MG. ([MG] = 1.46 × 10−5 M and [NaOH] = 0.1 M, T = 25 °C).
This observed catalysis is consistent with the possibility of a nucleophilic attack by an ionized CD hydroxyl group on the MG+ associated with the CD [24]. This behavior is like that reported in the literature (i.e., cleavage of aryl esters in the presence of CD [31,32] or that observed for CV hydrolysis [33,34]. A direct attack of OH− on the MG that is associated with the MG-CD complex should be ruled out, since given the important effect of the dielectric constant on the reaction rate (vide supra), it would imply a greater catalytic effect of the presence of cyclodextrins in the medium. Applying the model presented in Scheme 2, assuming a substrate that undergoes an uncatalyzed reaction in each medium and a catalyzed reaction through a 1:1 substrate/CD complex. In the scheme, kw corresponds with the rate constant in the bulk water, kCD is the catalytic rate constant in the presence of CD and Ks is the binding constant of MG to the CD cavity.
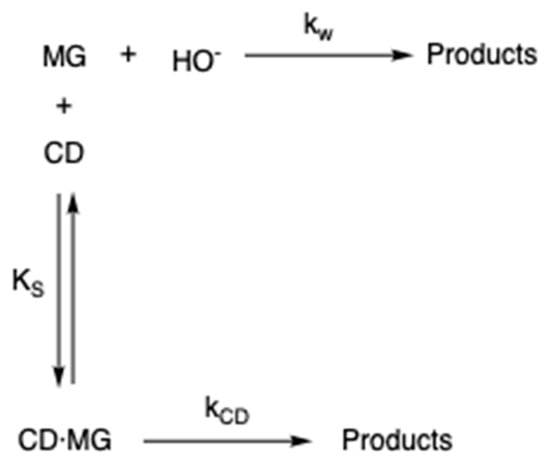
Scheme 2.
Mechanism of basic hydrolysis of MG in the presence of CD, where kw is the rate constant in water, kCD is the rate constant in CD and Ks is the binding constant of MG to the CD.
From this mechanism using the rate equations and the formation equilibrium of the inclusion complex, Equation (3) can be easily obtained [34].
The fit of Equation (2) to the experiment results yields a value of the pseudo-first orther rate kw = 1.47 ± 0.01 mol−1s−1, which is compatible with the value obtained in water kw = 1.46 ± 0.03 M−1s−1 (vide supra) [23]. The value of catalytic constant in the presence of CD was estimated as kCD = 0.25 ± 0.03 s−1. In this sense the ratio kCD/kw was 0.17, which is so close to the equivalent ratio for basic hydrolysis of CV (kCD/kw = 0.15) obtained in previous experiments [30]. The binding constant of MG to CD was evaluated in KS = 2500 ± 50. Figure 7 shows the experimental results compared to those obtained from adjusting them to Equation (1). As can be, this was satisfactory. Indeed, the solid line represents the adjustment of ko and ko,t values to the slope 1 line, for which a value of R2 = 0.9989 has been obtained. This R2 value demonstrates the good fit of the theoretical model to the experimental data.
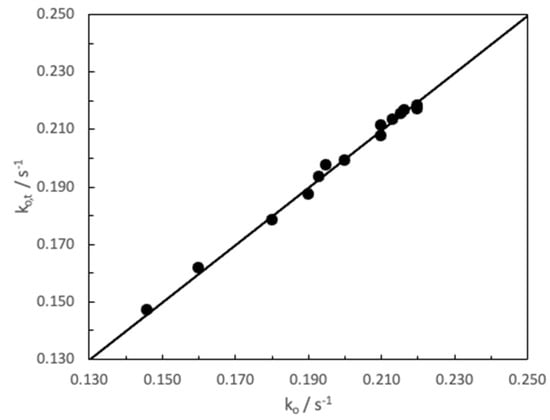
Figure 7.
Experimental results (ko) vs. theoretical results (ko,t)precited by Equation (3) obtained from Scheme 2 ([MG] = 1.46 × 10−5 M and [NaOH] = 0.1 M, T = 25 °C).
Another aspect to underline, which confirms the validity of the model, is the value obtained for the MG binding constant to cyclodextrin cavity. As quote above, a value of Ks = 2500 has been obtained, which is like the CV value obtained in the literature (Ks = 2750) [24]. The ratio between MG and CV binding constant is 0.91 which is too close to the log(P) ratio between MG and CV equal to 0.89, log(P)MG = 6.65 and log(P)CV = 7.48. In fact, if we compare the values of the association constants of different substrates taken from the literature, an acceptable correlation can be observed between the values of the formation constants of the host-guest complexes and the log(P) values of the substrates [31,32,34]. This linear relationship is shown in Figure 8 (R2 = 0.9514). The compounds included in this figure were chosen due to the similarity of the procedure for obtaining the association constants. In this way, the values of the MG and CV constants were obtained from kinetic data of the basic hydrolysis processes of respective carbocations in the presence of CD. Regarding the values of the association constants of N-methyl-N-nitroso-p-toluene sulfonamide (MNTS), they were obtained by the same procedure from the kinetic data of the basic hydrolysis of this compound in the presence of CDs. Regarding p-nitrophenyl acetate (pNPA), they were obtained from kinetic data of the hydrolysis of this compound in the presence of CDs and mixtures of CDs and micellar aggregates. Regarding acetonitrile, its association constant was obtained using the hydrolysis process of MNTS in the presence of CDs as a chemical probe. The pH conditions used in all cases were similar so all constants can be compared. It is well known that the Log P value of a substance is a suitable way to quantify its hydrolicity/hydrophobicity, this linear relationshipt between the formation constant of the host-guest complex and the substrates would demonstrate that the main driving force of the formation of said complexes is associated with their hydrophobicity [35].
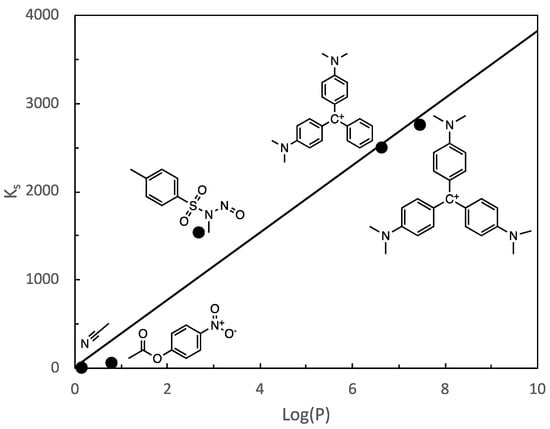
Figure 8.
Relationship between the host-guest complexes formation constant and the log (P) values of substrates.
4. Conclusions
A kinetic model has been applied to the study of the basic hydrolysis of MG in the presence of cyclodextrins. From the reaction mechanism, the kinetic constants of the hydrolysis processes have been obtained (in water and through the formation of the MG-CD host-gues complex) as well as the association constant of MG to CD. A comparison of the rate constants observed in the presence and absence of CD reveals that a catalysis of the hydrolysis process associated with the reaction between MG and deprotonated cyclodextrin occurs. Likewise, from the experimental data, no kinetic evidence is observed that an attack by HO− on the MG-CD occurs. In this way, a kinetic model has been proposed that considers two simultaneous routes in the aqueous medium that involve free hydroxyl ions and the hydroxyl belonging to the deprotonated CD, respectively. Likewise, from the observed results it is suggested that the stoichiometry of the host-guest complex between the CD and the MG is a 1:1 complex. The values obtained for both the kinetic constants and the equilibrium constant are in line with values of similar substances in the literature [24,34], which constitutes another proof of the validity of the proposed kinetic model.
Author Contributions
Conceptualization, J.C.M.; methodology, J.C.M. and A.S.-L.; software, R.R.-F.; validation, A.S.-L. and R.R.-F.; formal analysis, A.S.-L. and R.R.-F.; investigation, A.S.-L. and R.R.-F.; data curation, A.S.-L. and R.R.-F.; writing—original draft preparation, A.S.-L. and R.R.-F.; writing—review and editing, J.C.M.; visualization, A.S.-L.; supervision, J.C.M.; project administration, J.C.M.; funding acquisition, J.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data can be available upon request from the authors.
Acknowledgments
A. Soria-Lopez thanks the FPU research grant from the Ministry of Science and Innovation of Spain (MCINN) for training (FPU2020/06140).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mejuto, J.C.; Simal-Gandara, J. Host-Guest Complexes. Int. J. Mol. Sci. 2022, 23, 15730. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Mellet, C.O.; Fernández, J.M.G.; Benito, J.M. Cyclodextrin-Based Gene Delivery Systems. Chem. Soc. Rev. 2011, 40, 1586–1608. [Google Scholar] [CrossRef]
- Centeno-Leija, S.; Espinosa-Barrera, L.; Velazquez-Cruz, B.; Cárdenas-Conejo, Y.; Virgen-Ortíz, R.; Valencia-Cruz, G.; Saenz, R.A.; Marín-Tovar, Y.; Gómez-Manzo, S.; Hernández-Ochoa, B.; et al. Mining for Novel Cyclomaltodextrin Glucanotransferases Unravels the Carbohydrate Metabolism Pathway via Cyclodextrins in Thermoanaerobacterales. Sci. Rep. 2022, 12, 730. [Google Scholar] [CrossRef]
- Kinart, Z. Conductometric Studies of Formation the Inclusion Complexes of Phenolic Acids with β-Cyclodextrin and 2-HP-β-Cyclodextrin in Aqueous Solutions. Molecules 2023, 28, 292. [Google Scholar] [CrossRef]
- Connors, K.A. The Stability of Cyclodextrin Complexes in Solution. Chem. Rev. 1997, 97, 1325–1358. [Google Scholar] [CrossRef] [PubMed]
- Bortolus, P.; Grabner, G.; Köhler, G.; Monti, S. Photochemistry of Cyclodextrin Host-Guest Complexes. Coord. Chem. Rev. 1993, 125, 261–268. [Google Scholar] [CrossRef]
- Lehn, J.M. Supramolecular Chemistry. Science 1993, 260, 1762–1763. [Google Scholar] [CrossRef] [PubMed]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry, 2nd ed.; Wiley: Hoboken, NJ, USA, 2013; ISBN 978-0-470-51233-3. [Google Scholar]
- Iglesias, E.; Fernández, A. Cyclodextrin Catalysis in the Basic Hydrolysis of Alkyl Nitrites. J. Chem. Soc. Perkin Trans. 2 1998, 1691–1700. [Google Scholar] [CrossRef]
- Thakur, N.; Thakur, N.; Kumar, K.; Arya, V.; Kumar, A. Encapsulation of Tinospora Cordifolia Plant in Ni Doped TiO2 Nanoparticles for the Degradation of Malachite Green Dye. Nanofabrication 2023, 8. [Google Scholar] [CrossRef]
- Yan, J.; Niu, J.; Chen, D.; Chen, Y.; Irbis, C. Screening of Trametes Strains for Efficient Decolorization of Malachite Green at High Temperatures and Ionic Concentrations. Int. Biodeterior. Biodegrad. 2014, 87, 109–115. [Google Scholar] [CrossRef]
- Lunestad, B.T.; Samuelsen, O. 4—Veterinary Drug Use in Aquaculture. In Improving Farmed Fish Quality and Safety; Lie, Ø., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2008; pp. 97–127. ISBN 978-1-84569-299-5. [Google Scholar]
- Dasmandal, S.; Mandal, H.K.; Kundu, A.; Mahapatra, A. Kinetic Investigations on Alkaline Fading of Malachite Green in the Presence of Micelles and Reverse Micelles. J. Mol. Liq. 2014, 193, 123–131. [Google Scholar] [CrossRef]
- Gessner, T.; Mayer, U. Triarylmethane and Diarylmethane Dyes. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2000; ISBN 9783527306732. [Google Scholar]
- Srivastava, S.; Sinha, R.; Roy, D. Toxicological Effects of Malachite Green. Aquat. Toxicol. 2004, 66, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, L.G.; Roberts, R.J. Towards Strategic Use of Fungicides against Saprolegnia Parasitica in Salmonid Fish Hatcheries. J. Fish Dis. 1992, 15, 1–13. [Google Scholar] [CrossRef]
- Diggles, B.K. A Mycosis of Juvenile Spiny Rock Lobster, Jasus edwardsii (Hutton, 1875) Caused by Haliphthoros Sp., and Possible Methods of Chemical Control. J. Fish Dis. 2001, 24, 99–110. [Google Scholar] [CrossRef]
- Lilley, J.H.; Inglis, V. Comparative Effects of Various Antibiotics, Fungicides and Disinfectants on Aphanomyces Invaderis and Other Saprolegniaceous Fungi. Aquac. Res. 1997, 28, 461–469. [Google Scholar] [CrossRef]
- Deokar, R.; Sabale, A. Biosorption of Methylene Blue and Malachite Green From Binary Solution onto Ulva Lactuca. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 295–304. [Google Scholar]
- Hema, M.; Arivoli, S. Adsorption Kinetics and Thermodynamics of Malachite Green Dye unto Acid Activated Low Cost Carbon. J. Appl. Sci. Environ. Manag. 2008, 12, 43–51. [Google Scholar] [CrossRef]
- Samiey, B.; Toosi, A.R. Kinetics Study of Malachite Green Fading in the Presence of TX-100, DTAB and SDS. Bull. Korean Chem. Soc. 2009, 30, 2051–2056. [Google Scholar] [CrossRef][Green Version]
- Leis, J.R.; Mejuto, J.C.; Pena, M.E. Comparison between the Kinetics of the Alkaline Fading of Carbocation Dyes in Water/Sodium Bis (2-Ethylhexyl) Sulfosuccinate/Isooctane Microemulsions and in Homogeneous Media. Langmuir 1993, 9, 889–893. [Google Scholar] [CrossRef]
- Dasmandal, S.; Mandal, H.K.; Rudra, S.; Kundu, A.; Majumdar, T.; Mahapatra, A. Kinetic Exploration Supplemented by Spectroscopic and Molecular Docking Analysis in Search of the Optimal Conditions for Effective Degradation of Malachite Green. RSC Adv. 2015, 5, 38503–38512. [Google Scholar] [CrossRef]
- Ritchie, C.D. Nucleophilic Reactivities toward Cations. Acc. Chem. Res. 1972, 5, 348–354. [Google Scholar] [CrossRef]
- Saenger, W. Cyclodextrin Inclusion Compounds in Research and Industry. Angew. Chem. Int. Ed. Engl. 1980, 19, 344–362. [Google Scholar] [CrossRef]
- Felix, L.D.; Adesoji, A. Kinetics and Thermodynamic Study of Alkaline Fading of Malachite Green in Aqueous Solution. J. Appl. Fundam. Sci. 2017, 3, 52–57. [Google Scholar]
- Dalal, M. Ionic Reactions: Single and Double Sphere Models. In A Textbook of Physical Chemistry. Volume I; Dalal Institute: Rohtak, India, 2018; pp. 147–152. ISBN 978-81-938720-1-7. [Google Scholar]
- Laider, K.J. Chemical Kinetics, 3rd ed.; Pentice Hall: New York, NY, USA, 1987; ISBN 978-0060438623. [Google Scholar]
- García-Río, L.; Hervés, P.; Leis, J.R.; Mejuto, J.C. Kinetic and Spectroscopic Evidence for the Formation of Ion-Pairs between Crystal Violet and Perchlorate Ion. J. Chem. Res. 1997, 9, 326–327. [Google Scholar] [CrossRef]
- García-Río, L.; Leis, J.R.; Mejuto, J.C.; Pérez-Juste, J. Basic Hydrolysis of M-Nitrophenyl Acetate in Micellar Media Containing β-Cyclodextrins. J. Phys. Chem. B 1998, 102, 4581–4587. [Google Scholar] [CrossRef]
- Alvarez, A.R.; García-Río, L.; Hervés, P.; Leis, J.R.; Mejuto, J.C.; Pérez-Juste, J. Basic Hydrolysis of Substituted Nitrophenyl Acetates in β-Cyclodextrin/Surfactant Mixed Systems. Evidence of Free Cyclodextrin in Equilibrium with Micellized Surfactant. Langmuir 1999, 15, 8368–8375. [Google Scholar] [CrossRef]
- Astray, G.; Cid, A.; Manso, J.A.; Mejuto, J.C.; Moldes, O.A.; Morales, J. Alkaline Fading of Triarylmethyl Carbocations in Self-Assembly Microheterogeneous Media. Prog. React. Kinet. Mech. 2011, 36, 139–165. [Google Scholar] [CrossRef]
- García-Río, L.; Leis, J.R.; Mejuto, J.C.; Navarro-Vázquez, A.; Pérez-Juste, J.; Rodriguez-Dafonte, P. Basic Hydrolysis of Crystal Violet in β-Cyclodextrin/Surfactant Mixed Systems. Langmuir 2004, 20, 606–613. [Google Scholar] [CrossRef]
- Rezanka, M. Synthesis of Cyclodextrin Derivatives. In Cyclodextrin Fundamentals, Reactivity and Analysis; Springer: Cham, Switzerland, 2018; pp. 57–103. ISBN 978-3-319-76158-9. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).