Recent Advances in Catalytic Compounds Developed by Thermal Treatment of (Zr-Based) Metal–Organic Frameworks
Abstract
1. Introduction

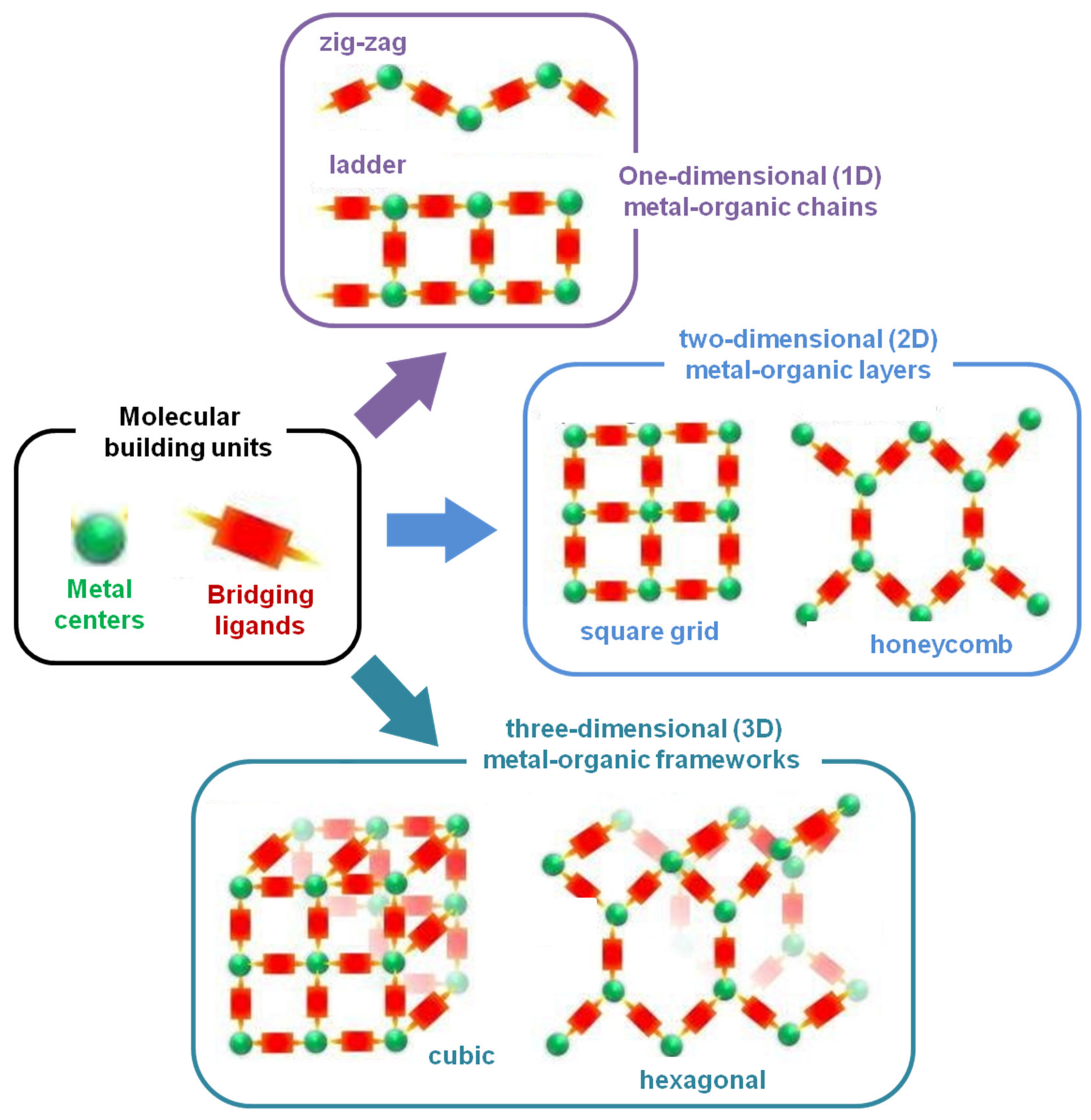
2. Preparation Strategies of MOF Compounds
3. Potential of MOF Applications
4. MOFs with Zirconium Centers

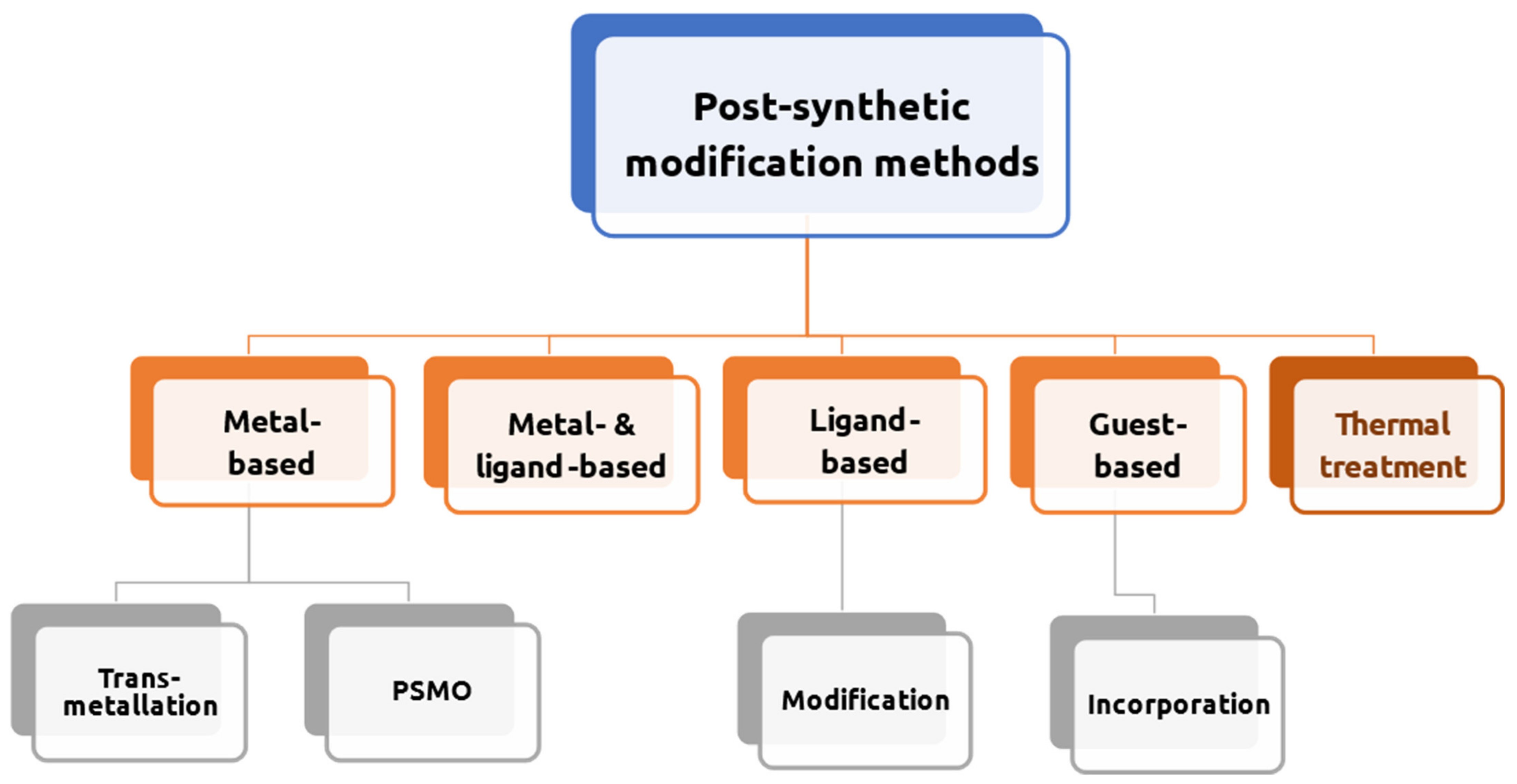
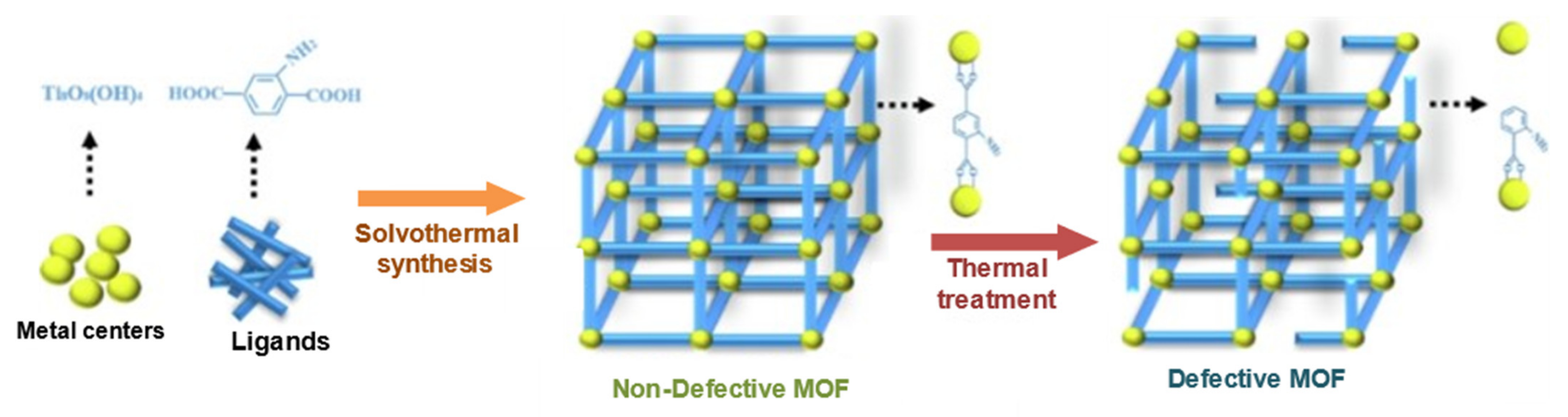
5. Derivatives of MOFs by Heat Treatment
6. MOF-Derived Compounds as Catalysts
6.1. Defective Zr-Based MOFs for Catalysis
6.2. Thermally Modified MOFs for Catalysis
7. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zhai, Q.G.; Bu, X.H.; Zhao, X.; Li, D.S.; Feng, P.Y. Pore Space Partition in Metal-Organic Frameworks. Acc. Chem. Res. 2017, 50, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.W.; Xue, R.X.; Ma, Y.J.; Ge, Y.Z.; Wang, Z.J.; Qiao, X.W.; Zhou, P. Study on the performance of a MOF-808-based photocatalyst prepared by a microwave-assisted method for the degradation of antibiotics. Rsc. Adv. 2021, 11, 32955–32964. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Pang, W.; Huo, Q. Modern Inorganic Synthetic Chemistry; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Ha, J.; Lee, J.H.; Moon, H.R. Alterations to secondary building units of metal–organic frameworks for the development of new functions. Inorg. Chem. Front. 2019, 7, 2–27. [Google Scholar] [CrossRef]
- Morritt, G.H.; Michaels, H.; Freitag, M. Coordination polymers for emerging molecular devices. Chem. Phys. Rev. 2022, 3, 011306. [Google Scholar] [CrossRef]
- Wißmann, G.; Schaate, A.; Lilienthal, S.; Bremer, I.; Schneider, A.M.; Behrens, P. Modulated synthesis of Zr-fumarate MOF. Microporous Mesoporous Mater. 2012, 152, 64–70. [Google Scholar] [CrossRef]
- Raptopoulou, C.P. Metal-Organic Frameworks: Synthetic Methods and Potential Applications. Materials 2021, 14, 310. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Nguyen, H.L.; Yaghi, O.M. Reticular Chemistry and Harvesting Water from Desert Air. ACM 2020, 1, 18–25. [Google Scholar] [CrossRef]
- Chang, Z.; Yang, D.H.; Xu, J.; Hu, T.L.; Bu, X.H. Flexible Metal-Organic Frameworks: Recent Advances and Potential Applications. Adv. Mater. 2015, 27, 5432–5441. [Google Scholar] [CrossRef] [PubMed]
- Darabdhara, J.; Ahmaruzzaman, M. Recent developments in MOF and MOF based composite as potential adsorbents for removal of aqueous environmental contaminants. Chemosphere 2022, 304, 135261. [Google Scholar] [CrossRef]
- Han, Y.; Yang, H.; Gua, X. Synthesis Methods and Crystallization of MOFs. In Synthesis Methods and Crystallization; IntechOpen: London, UK, 2020. [Google Scholar]
- Geyer, F.L.; Rominger, F.; Vogtland, M.; Bunz, U.H.F. Interpenetrated Frameworks with Anisotropic Pore Structures from a Tetrahedral Pyridine Ligand. Cryst. Growth Des. 2015, 15, 3539–3544. [Google Scholar] [CrossRef]
- Sud, D.; Kaur, G. A comprehensive review on synthetic approaches for metal-organic frameworks: From traditional solvothermal to greener protocols. Polyhedron 2021, 193, 114897. [Google Scholar] [CrossRef]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Udaya Rajesh, R.; Mathew, T.; Kumar, H.; Singhal, A.; Thomas, L. Metal-organic frameworks: Recent advances in synthesis strategies and applications. Inorg. Chem. Commun. 2024, 162, 112223. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, J.; Zhang, P.; Dai, S. Mechanochemical synthesis of metal–organic frameworks. Polyhedron 2019, 162, 59–64. [Google Scholar] [CrossRef]
- Hhan, N.A.; Kang, I.J.; Seok, H.Y.; Jhung, S.H. Facile synthesis of nano-sized metal-organic frameworks, chromium-benzenedicarboxylate, MIL-101. Chem. Eng. J. 2011, 166, 1152–1157. [Google Scholar]
- Dutt, S.; Kumar, A.; Singh, S. Synthesis of Metal Organic Frameworks (MOFs) and Their Derived Materials for Energy Storage Applications. Clean Technol. 2023, 5, 140–166. [Google Scholar] [CrossRef]
- Ameloot, R.; Stappers, L.; Fransaer, J.; Alaerts, L.; Sels, B.F.; De Vos, D.E. Patterned Growth of Metal-Organic Framework Coatings by Electrochemical Synthesis. Chem. Mater. 2009, 21, 2580–2582. [Google Scholar] [CrossRef]
- Zahn, G.; Schulze, H.A.; Lippke, J.; König, S.; Sazama, U.; Fröba, M.; Behrens, P. A water-born Zr-based porous coordination polymer: Modulated synthesis of Zr-fumarate MOF. Microporous Mesoporous Mater. 2015, 203, 186–194. [Google Scholar] [CrossRef]
- Butova, V.V.; Pankin, I.A.; Burachevskaya, O.A.; Vetlitsyna-Novikova, K.S.; Soldatov, A.V. New fast synthesis of MOF-801 for water and hydrogen storage: Modulator effect and recycling options. Inorganica Chim. Acta 2021, 514, 1200250. [Google Scholar] [CrossRef]
- Schaate, A.; Roy, P.; Godt, A.; Lippke, J.; Waltz, F.; Wiebcke, M.; Behrens, P. Modulated Synthesis of Zr-Based Metal–Organic Frameworks: From Nano to Single Crystals. Chem. Eur. J. 2011, 17, 6643–6651. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Dou, Y.B.; Xie, L.H.; Rutledge, W.; Li, J.R.; Zhou, H.C. Zr-based metal-organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, C.; Nagarjun, N.; Roy, S.; Mostakim, S.; Volkmer, D.; Dhakshinamoorthy, A.; Biswas, S. A Zr-Based Metal–Organic Framework with a DUT-52 Structure Containing a Trifluoroacetamido-Functionalized Linker for Aqueous Phase Fluorescence Sensing of the Cyanide Ion and Aerobic Oxidation of Cyclohexane. Inorg. Chem. 2021, 60, 4539–4550. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.C.; Viana, A.M.; de Castro, B.; Cunha-Silva, L.; Balula, S.S. Synergistic combination of the nanoporous system of MOF-808 with a polyoxomolybdate to design an effective catalyst: Simultaneous oxidative desulfurization and denitrogenation processes. Sustain. Energ. Fuels 2021, 5, 4032–4040. [Google Scholar] [CrossRef]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to Metal-Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.J.; Zheng, H.Q.; Chen, J.; Zhuang, W.E.; Lin, Y.X.; Su, J.W.; Huang, Y.B.; Cao, R. Encapsulation of Phosphotungstic Acid into Metal-Organic Frameworks with Tunable Window Sizes: Screening of PTA@MOF Catalysts for Efficient Oxidative Desulfurization. Inorg. Chem. 2018, 57, 13009–13019. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Wang, Y.W.; Jiang, H.-L.; Xu, Q. Metal–Organic Frameworks as Platforms for Catalytic Applications. Adv. Mater. 2017, 30, 1703663. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Cai, X.; Jiang, H.L. Improving MOF stability: Approaches and applications. Chem. Sci. 2019, 10, 10209–10230. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Gu, Y.; Ye, G.; Xu, W.; Zhou, W.; Sun, Y. Creation of Active Sites in MOF-808(Zr) by a Facile Route for Oxidative Desulfurization of Model Diesel Oil. Chem. Sel. 2020, 5, 244–251. [Google Scholar] [CrossRef]
- De Vos, A.; Hendrickx, K.; Van der Voort, P.; Van Speybroeck, V.; Lejaeghere, K. Missing Linkers: An Alternative Pathway to UiO-66 Electronic Structure Engineering. Chem. Mater. 2017, 29, 3006–3019. [Google Scholar] [CrossRef] [PubMed]
- Meilikhov, M.; Yusenko, K.; Esken, D.; Turner, S.; Van Tendeloo, G.; Fischer, R.A. Metals@MOFs—Loading MOFs with Metal Nanoparticles for Hybrid Functions. Eur. J. Inorg. Chem. 2010, 2010, 3701–3714. [Google Scholar] [CrossRef]
- Sholl, D.S.; Lively, R.P. Defects in Metal-Organic Frameworks: Challenge or Opportunity? J. Phys. Chem. Lett. 2015, 6, 3437–3444. [Google Scholar] [CrossRef] [PubMed]
- Dissegna, S.; Hardian, R.; Epp, K.; Kieslich, G.; Coulet, M.V.; Llewellyn, P.; Fischer, R.A. Using water adsorption measurements to access the chemistry of defects in the metal-organic framework UiO-66. Crystengcomm 2017, 19, 4137–4141. [Google Scholar] [CrossRef]
- Hardian, R.; Dissegna, S.; Ullrich, A.; Llewellyn, P.L.; Coulet, M.V.; Fischer, R.A. Tuning the Properties of MOF-808 via Defect Engineering and Metal Nanoparticle Encapsulation. Chem. Eur. J. 2021, 27, 6804–6814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Kauer, M.; Halbherr, O.; Epp, K.; Guo, P.H.; Gonzalez, M.I.; Xiao, D.J.; Wiktor, C.; Xamena, F.X.L.I.; Woll, C.; et al. Ruthenium Metal-Organic Frameworks with Different Defect Types: Influence on Porosity, Sorption, and Catalytic Properties. Chem. Eur. J. 2016, 22, 14297–14307. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Müller, U.; Yaghi, O.M. “Heterogeneity within Order” in Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2015, 54, 3417–3430. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Yan, L.; Cao, L.; Daia, P.; Gu, X.; Zhao, X. Continuous synthesis for zirconium metal-organic frameworks with high quality and productivity via microdroplet flow reaction. Chin. Chem. Lett. 2017, 29, 849–853. [Google Scholar] [CrossRef]
- Li, J.; Huang, J.-Y.; Meng, Y.-X.; Li, L.; Zhang, L.-L.; Jiang, H.-L. Zr- and Ti-based metal–organic frameworks: Synthesis, structures and catalytic applications. Chem. Commun. 2023, 59, 2541–2559. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, Z.; Xu, Y.; Dai, F.; Zhang, L.; Sun, D. Porous zirconium metal-organic framework constructed from 2D → 3D interpenetration based on a 3,6-connected kgd net. Inorg. Chem. 2014, 53, 7086–7088. [Google Scholar] [CrossRef] [PubMed]
- Dreischarf, A.C.; Lammert, M.; Stock, N.; Reinsch, H. Green Synthesis of Zr-CAU-28: Structure and Properties of the First Zr-MOF Based on 2,5-Furandicarboxylic Acid. Inorg. Chem. 2017, 56, 2270–2277. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.; Lee, S.; Kang, E.; Kim, Y.; Choe, W. Metal-organic frameworks as advanced adsorbents for pharmaceutical and personal care products. Coord. Chem. Rev. 2020, 425, 213526. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Gandara, F.; Zhang, Y.B.; Na, K.; Yaghi, O.M.; Klemperer, W.G. Superacidity in sulfated metal-organic framework-808. J. Am. Chem. Soc. 2014, 136, 12844–12847. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Langmi, H.W.; North, B.C.; Mathe, M.; Bessarabov, D. Modulated synthesis of zirconium-metal organic framework (Zr-MOF) for hydrogen storage applications. Int. J. Hydrogen Energy 2014, 39, 890–895. [Google Scholar] [CrossRef]
- Mandal, S.; Natarajan, S.; Mani, P.; Pankajakshan, A. Post-Synthetic Modification of Metal–Organic Frameworks Toward Applications. Adv. Funct. Mater. 2021, 31, 2006291. [Google Scholar] [CrossRef]
- Howarth, A.J.; Liu, Y.L.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Chemical, thermal and mechanical stabilities of metal–organic frameworks. Nat. Rev. Mater. 2016, 1, 15018. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 455–460. [Google Scholar] [CrossRef]
- Qiu, T.; Liang, Z.; Guo, W.; Tabassum, H.; Gao, S.; Zou, R. Metal–Organic Framework-Based Materials for Energy Conversion and Storage. ACS Energy Lett. 2020, 5, 520–532. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, J.; Peng, Q.; Li, Y. A general strategy for nanocrystal synthesis. Nature 2005, 437, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Datta, K.K.; Kulkarni, C.; Eswaramoorthy, M. Aminoclay: A permselective matrix to stabilize copper nanoparticles. Chem. Commun. 2010, 46, 616–618. [Google Scholar] [CrossRef]
- Fomina, I.G.; Dobrokhotova, Z.V.; Aleksandrov, G.G.; Bogomyakov, A.S.; Novotortsev, V.M.; Eremenko, I.L. Influence of the nature of organic ligands on the character of thermal decomposition products of CoII and NiII pivalate complexes with amino derivatives of pyridine. Russ. Chem. Bull. 2010, 59, 1175–1185. [Google Scholar] [CrossRef]
- Liu, B.; Shioyama, H.; Akita, T.; Xu, Q. Metal-organic framework as a template for porous carbon synthesis. J. Am. Chem. Soc. 2008, 130, 5390–5391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, A.; Zhong, M.; Zhang, Z.; Zhang, X.; Zhou, Z.Z.; Bu, X.-H. Metal–Organic Frameworks (MOFs) and MOF-Derived Materials for Energy Storage and Conversion. Electrochem. Energy Rev. 2018, 2, 29–104. [Google Scholar] [CrossRef]
- He, J.; Xu, Y.; Wang, W.; Hu, B.; Wang, Z.; Yang, X.; Wang, Y.; Yang, L. Ce(III) nanocomposites by partial thermal decomposition of Ce-MOF for effective phosphate adsorption in a wide pH range. Chem. Eng. J. 2020, 379, 122431. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Zou, R.Z.; Xu, Q.X. Metal–organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 2015, 8, 1837–1866. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, X.; Wang, Z.; Lü, M.; Wu, B.; Yan, C.; Yuan, A.; Yang, H. Controlled pyrolysis of MIL-88A to Fe2O3@C nanocomposites with varied morphologies and phases for advanced lithium storage. J. Mater. Chem. A 2017, 5, 25562–25573. [Google Scholar] [CrossRef]
- Shen, K.; Chen, X.; Chen, J.; Li, Y. Development of MOF-Derived Carbon-Based Nanomaterials for Efficient Catalysis. ACS Catal. 2016, 6, 5887–5903. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Vinu, A.; Serre, C.; Jhung, S.H. MOF-derived carbonaceous materials enriched with nitrogen: Preparation and applications in adsorption and catalysis. Mater. Today 2019, 25, 88–111. [Google Scholar] [CrossRef]
- Chinthakindi, S.; Purohit, A.; Singh, V.; Tak, V.; Goud, D.R.; Dubey, D.K.; Pardasani, D. Iron oxide functionalized graphene nano-composite for dispersive solid phase extraction of chemical warfare agents from aqueous samples. J. Chromatogr. A 2015, 1394, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Oar-Arteta, L.; Wezendonk, T.; Sun, X.; Kapteijn, F.; Gascon, J. Metal organic frameworks as precursors for the manufacture of advanced catalytic materials. Mater. Chem. Front. 2017, 1, 1709–1745. [Google Scholar] [CrossRef]
- Matthes, P.R.; Schonfeld, F.; Zottnick, S.H.; Muller-Buschbaum, K. Post-Synthetic Shaping of Porosity and Crystal Structure of Ln-Bipy-MOFs by Thermal Treatment. Molecules 2015, 20, 12125–12153. [Google Scholar] [CrossRef] [PubMed]
- Mo, G.L.; Wang, L.X.; Luo, J.H. Controlled thermal treatment of NH2-MIL-125(Ti) for drastically enhanced photocatalytic reduction of Cr(VI). Sep. Purif. Technol. 2021, 277, 119643. [Google Scholar] [CrossRef]
- Tan, X.; Wu, Y.; Lin, X.; Zeb, A.; Xu, X.; Luo, Y.; Liu, J. Application of MOF-derived transition metal oxides and composites as anodes for lithium-ion batteries. Inorg. Chem. Front. 2020, 7, 4939–4955. [Google Scholar] [CrossRef]
- Yang, S.J.; Im, J.H.; Kim, T.; Lee, K.; Park, C.R. MOF-derived ZnO and ZnO@C composites with high photocatalytic activity and adsorption capacity. J. Hazard. Mater. 2011, 186, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Garcia, H.; Xamena, F.X.L.I. Engineering Metal Organic Frameworks for Heterogeneous Catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Pascanu, V.; González Miera, G.; Inge, A.K.; Martín-Matute, B. Metal–Organic Frameworks as Catalysts for Organic Synthesis: A Critical Perspective. J. Am. Chem. Soc. 2019, 141, 7223–7234. [Google Scholar] [CrossRef] [PubMed]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal–Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, S.; Tariq, S.; Luque, R.; Verpoort, F. Self-sacrifice MOFs for heterogeneous catalysis: Synthesis mechanisms and future perspectives. Mater. Today 2022, 55, 137–169. [Google Scholar] [CrossRef]
- Jiao, L.; Jiang, H.-L. Metal-organic frameworks for catalysis: Fundamentals and future prospects. Chin. J. Catal. 2023, 45, 1–5. [Google Scholar] [CrossRef]
- Dandan, L.; Hai-Qun, X.; Long, J.; Hai-Long, J. Metal-organic frameworks for catalysis: State of the art, challenges, and opportunities. Energy Chem. 2019, 1, 100005. [Google Scholar]
- Vermoortele, F.; Bueken, B.; Le Bars, G.; Van de Voorde, B.; Vandichel, M.; Houthoofd, K.; Vimont, A.; Daturi, M.; Waroquier, M.; Van Speybroeck, V.; et al. Synthesis Modulation as a Tool To Increase the Catalytic Activity of Metal–Organic Frameworks: The Unique Case of UiO-66(Zr). J. Am. Chem. Soc. 2013, 135, 11465–11468. [Google Scholar] [CrossRef] [PubMed]
- Mautschke, H.H.; Drache, F.; Senkovska, I.; Kaskel, S.; Llabrés i Xamena, F.X. Catalytic properties of pristine and defect-engineered Zr-MOF-808 metal organic frameworks. Cat. Sci. Technol. 2018, 8, 3610–3616. [Google Scholar] [CrossRef]
- Marx, S.; Kleist, W.; Baiker, A. Synthesis, structural properties, and catalytic behavior of Cu-BTC and mixed-linker Cu-BTC-PyDC in the oxidation of benzene derivatives. J. Catal. 2011, 281, 76–87. [Google Scholar] [CrossRef]
- Kozachuk, O.; Luz, I.; Llabres i Xamena, F.X.; Noei, H.; Kauer, M.; Albada, H.B.; Bloch, E.D.; Marler, B.; Wang, Y.; Muhler, M.; et al. Multifunctional, defect-engineered metal-organic frameworks with ruthenium centers: Sorption and catalytic properties. Angew. Chem. Int. Ed. Engl. 2014, 53, 7058–7062. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Liu, D.; Niu, W.; Zhang, S.; Chen, R.; Wang, Y.; Zhao, P.; Jiang, H.; Zhao, Y.; Yang, L.; et al. Defect-engineered MOF-808 with highly exposed Zr sites as highly efficient catalysts for catalytic transfer hydrogenation of furfural. Fuel 2022, 327, 125085. [Google Scholar] [CrossRef]
- Otake, K.-i.; Ye, J.; Mandal, M.; Islamoglu, T.; Buru, C.T.; Hupp, J.T.; Delferro, M.; Truhlar, D.G.; Cramer, C.J.; Farha, O.K. Enhanced Activity of Heterogeneous Pd(II) Catalysts on Acid-Functionalized Metal–Organic Frameworks. ACS Catal. 2019, 9, 5383–5390. [Google Scholar] [CrossRef]
- Hao, L.; Stoian, S.A.; Weddle, L.R.; Zhang, Q. Zr-Based MOFs for oxidative desulfurization: What matters? Green Chem. 2020, 22, 6351–6356. [Google Scholar] [CrossRef]
- Ye, G.; Wan, L.; Zhang, Q.; Liu, H.; Zhou, J.; Wu, L.; Zeng, X.; Wang, H.; Chen, X.; Wang, J. Boosting Catalytic Performance of MOF-808(Zr) by Direct Generation of Rich Defective Zr Nodes via a Solvent-Free Approach. Inorg. Chem. 2023, 62, 4248–4259. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Zhang, Y.; Chen, Y.; Liu, C.-J.; Tu, X. Synthesis, characterization and application of defective metal–organic frameworks: Current status and perspectives. J. Mater. Chem. A 2020, 8, 21526–21546. [Google Scholar] [CrossRef]
- Ma, X.; Wang, L.; Zhang, Q.; Jiang, H.-L. Switching on the Photocatalysis of Metal–Organic Frameworks by Engineering Structural Defects. Angew. Chem. Int. Ed. 2019, 58, 12175–12179. [Google Scholar] [CrossRef] [PubMed]
- Vandichel, M.; Hajek, J.; Vermoortele, F.; Waroquier, M.; De Vos, D.E.; Van Speybroeck, V. Active site engineering in UiO-66 type metal–organic frameworks by intentional creation of defects: A theoretical rationalization. Crystengcomm 2015, 17, 395–406. [Google Scholar] [CrossRef]
- Jrad, A.; Al Sabeh, G.; Hannouche, K.; Al Natour, R.; Haidar, O.; Sammoury, H.; Ahmad, M.N.; Hmadeh, M. Critical Role of Defects in UiO-66 Nanocrystals for Catalysis and Water Remediation. ACS Appl. Nano Mater. 2023, 6, 18698–18720. [Google Scholar] [CrossRef]
- Gadipelli, S.; Guo, Z. Postsynthesis Annealing of MOF-5 Remarkably Enhances the Framework Structural Stability and CO2 Uptake. Chem. Mater. 2014, 26, 6333–6338. [Google Scholar] [CrossRef]
- Shen, Y.; Pan, T.; Wang, L.; Ren, Z.; Zhang, W.N.; Huo, F.W. Programmable Logic in Metal-Organic Frameworks for Catalysis. Adv. Mater. 2021, 33, 2007442. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Mei, J.; Xu, H.; Liu, W.; Qu, Z.; Cui, Y.; Yan, N. Research of mercury removal from sintering flue gas of iron and steel by the open metal site of Mil-101(Cr). J. Hazard Mater. 2018, 351, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, J. Metal Organic Framework with Coordinatively Unsaturated Sites as Efficient Fenton-like Catalyst for Enhanced Degradation of Sulfamethazine. Environ. Sci. Technol. 2018, 52, 5367–5377. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, C.; Wang, H.; Zhang, M. Synthesis of highly efficient MnOx catalyst for low-temperature NH3-SCR prepared from Mn-MOF-74 template. Mater. Lett. 2016, 168, 17–19. [Google Scholar] [CrossRef]
- Bo, P.; Chao, F.; Shanshan, L.; Zhang, R. Synthesis of CuO catalyst derived from HKUST-1 temple for the lowtemperature NH3-SCR process. Cat. Today 2018, 314, 122–128. [Google Scholar]
- Chen, X.; Chen, X.; Yu, E.Q.; Cai, S.C.; Jia, H.P.; Chen, J.; Liang, P. In situ pyrolysis of Ce-MOF to prepare CeO2 catalyst with obviously improved catalytic performance for toluene combustion. Chem. Eng. J. 2018, 344, 469–479. [Google Scholar] [CrossRef]
- Lei, L.; Qilei, Y.C.; Jinlong, Y.; Yue, P.; Junhua, L. Hollow-Structural Ag/Co3O4 Nanocatalyst for CO Oxidation: Interfacial Synergistic Effect. ACS Appl. Nano Mater. 2019, 2, 3480–3489. [Google Scholar] [CrossRef]
- Karam, L.; Reboul, J.; Casale, S.; Massiani, P.; El Hassan, N. Porous Nickel-Alumina Derived from Metal-Organic Framework (MIL-53): A New Approach to Achieve Active and Stable Catalysts in Methane Dry Reforming. ChemCatChem 2020, 12, 373–385. [Google Scholar] [CrossRef]
- Chin, K.C.; Leong, L.K.; Lu, S.-Y.; Tsai, D.-H.; Sethupathi, S. Preparation of Metal Organic Framework (MOF) Derived Bimetallic Catalyst for Dry Reforming of Methane. Int. J. Technol. 2019, 10, 1437–1445. [Google Scholar] [CrossRef]
- Khan, I.S.; Ramirez, A.; Shterk, G.; Garzón-Tovar, L.; Gascon, J. Bimetallic Metal-Organic Framework Mediated Synthesis of Ni-Co Catalysts for the Dry Reforming of Methane. Catalysts 2020, 10, 592. [Google Scholar] [CrossRef]
- Toyao, T.; Fujiwaki, M.; Miyahara, K.; Kim, T.-H.; Horiuchi, Y.; Matsuoka, M. Design of Zeolitic Imidazolate Framework Derived Nitrogen-Doped Nanoporous Carbons Containing Metal Species for Carbon Dioxide Fixation Reactions. ChemSusChem 2015, 8, 3905–3912. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Y.; Xu, M.; Villasante, A.G.D.; Asset, T.A.; Liu, Y.L.; Ly, A.; Pan, X.; Atanassov, P.; Zenyuk, I.V. Pyrolysis of Metal Organic Frameworks (MOF): Transformations Leading to Formation of Transition Metal-Nitrogen-Carbon Catalysts. ECS Meet. Abstr. 2021, MA2021-01, 476. [Google Scholar]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, C.E.S.; Balula, S.S.; Cunha-Silva, L. Recent Advances in Catalytic Compounds Developed by Thermal Treatment of (Zr-Based) Metal–Organic Frameworks. Compounds 2024, 4, 315-337. https://doi.org/10.3390/compounds4020017
Ferreira CES, Balula SS, Cunha-Silva L. Recent Advances in Catalytic Compounds Developed by Thermal Treatment of (Zr-Based) Metal–Organic Frameworks. Compounds. 2024; 4(2):315-337. https://doi.org/10.3390/compounds4020017
Chicago/Turabian StyleFerreira, Catarina E. S., Salete S. Balula, and Luís Cunha-Silva. 2024. "Recent Advances in Catalytic Compounds Developed by Thermal Treatment of (Zr-Based) Metal–Organic Frameworks" Compounds 4, no. 2: 315-337. https://doi.org/10.3390/compounds4020017
APA StyleFerreira, C. E. S., Balula, S. S., & Cunha-Silva, L. (2024). Recent Advances in Catalytic Compounds Developed by Thermal Treatment of (Zr-Based) Metal–Organic Frameworks. Compounds, 4(2), 315-337. https://doi.org/10.3390/compounds4020017






