Abstract
Background/Objectives: Cannabis is the most widely used illicit substance worldwide, particularly among young adults, with growing acceptance following medical and recreational legalization. Although generally perceived as a drug with low acute toxicity, an expanding body of evidence indicates that cannabinoids can exert relevant cardiovascular effects, including arrhythmias, myocardial ischemia, and sudden cardiac death (SCD). These mechanisms are mediated through complex, dose-dependent interactions among CB1 and CB2 receptors, autonomic imbalance, and endothelial dysfunction. Nevertheless, cannabis-related fatalities remain underestimated in both clinical and forensic settings. Case presentation: Three cases of sudden unexpected death in previously healthy men aged 28, 37, and 37 years are described. All were found deceased at home under non-suspicious circumstances. Forensic autopsies ruled out trauma, coronary atherosclerosis, congenital malformations, or cardiomyopathy. Histological analyses consistently revealed polymorphic myocardial alterations, including interstitial edema, fiber disruption, and focal myocytolysis, without inflammatory infiltrates or necrosis. Toxicological examinations demonstrated the presence of Δ9-tetrahydrocannabinol (THC) and metabolites in peripheral blood and urine, while alcohol and other illicit drugs tested negative. In each case, the cause of death was attributed to arrhythmic sudden cardiac death in temporal association with cannabis use. Conclusions: This case series, integrated with a narrative review of current literature, supports the hypothesis that cannabis consumption can contribute to fatal arrhythmias even in young adults without conventional cardiovascular risk factors. The convergence of autopsy, histopathological, and toxicological findings suggests a potential causal link between THC exposure and sudden unexpected death. These results highlight the importance of systematic postmortem investigations in suspected drug-related fatalities and underscore the need for greater awareness among clinicians, forensic pathologists, and policymakers regarding the underestimated cardiovascular toxicity of cannabis.
1. Introduction
Cannabis is the most widely used illicit substance globally, especially among adolescents and young adults, with a growing trend linked to progressive legalization for both recreational and therapeutic use [1,2]. While cannabidiol (CBD) is known for its anti-inflammatory and anticonvulsant properties, the psychoactive effects of cannabis are mainly attributed to delta-9-tetrahydrocannabinol (THC) [3]. In clinical settings, synthetic cannabinoids such as dronabinol and nabilone have been employed for the treatment of chemotherapy-induced nausea, with off-label application in pain management [4,5]. Nonetheless, thanks to increased medical applications, cannabis continues to be perceived by the general public as a drug with low acute toxicity and minimal cardiovascular risk [2].
This perception contrasts with a growing body of evidence suggesting a possible association between cannabis consumption and adverse cardiovascular events, including arrhythmias, acute coronary syndromes, and sudden cardiac death (SCD) [6,7]. The mechanisms through which cannabinoids exert their cardiovascular effects are complex and not fully understood but appear to involve activation of both CB1 and CB2 receptors located in the central nervous system, myocardium, and vascular endothelium [8,9,10,11,12]. The cardiovascular effects of cannabis are mediated mainly through cannabinoid receptors CB1 and CB2. CB1 receptors, expressed in the myocardium, endothelial cells, and the cardiac conduction system, exert negative chronotropic and inotropic effects, as well as endothelial dysfunction, paradoxical coronary vasodilation, and proarrhythmic activity. In the acute phase, cannabis smoking may also lead to tachycardia via increased sympathetic tone and reduced parasympathetic activity. Conversely, CB2 receptors, predominantly found in immune cells and vascular endothelium, mediate anti-inflammatory and anti-atherosclerotic effects, but their role is less prominent in cardiovascular regulation [8,13].
In chronic cannabis users and smokers, CB1-mediated mechanisms prevail, contributing to myocardial ischemia through vasospasm and reduced coronary reserve, as well as promoting arrhythmogenesis and pro-thrombotic states due to endothelial dysfunction. This imbalance explains the higher cardiovascular vulnerability observed in regular cannabis consumers [14].
This paper presents a case series of three young adult males with no significant past medical history, found dead in non-suspicious circumstances. In all three cases, they were migrants who had arrived in Italy from North African countries. No medical records were available regarding the medical conditions of their ancestors or descendants. No relevant information about their lifestyle was known to those who knew them. Forensic autopsy excluded traumatic or structural causes of death: no mechanical cardiovascular causes of death (such as intrapericardial hemorrhages, embolism, congenital alterations), non-traumatic non-cardiac causes of sudden death (cerebral, respiratory hemorrhagic or septic shock). Heart examination was performed according to standard gross examination and standard histological examination from guidelines for autopsy investigation of sudden cardiac death [15,16]. Further investigations to exclude channelopathies and plakoglobin deficiency included immunohistochemical techniques. Histopathological examination of the heart revealed nonspecific but suggestive findings of myocardial fiber disruption and edema, compatible with sudden arrhythmic death. These cases contribute to the growing concern regarding the potentially fatal cardiovascular effects of cannabis and highlight the need for increased awareness among clinicians and forensic pathologists. Beyond the description of these cases, this work also includes a narrative review of the available literature, with the aim of contextualizing the forensic and clinical relevance of cannabis-related sudden deaths (no noteworthy pathological changes were found: such as no fatty infiltration of the right ventricular wall, no hypertophic signs, no disarray, no inflammatory foci). Toxicological analyses were positive for THC.
2. Case Series
2.1. Case A
The first case concerned a 28-year-old man who died suddenly in April 2022. The circumstances of death and the absence of traumatic injuries excluded the involvement of third parties. His past medical history was not remarkable. At autopsy, no traumatic lesions were observed and the cardiovascular system did not reveal obstructive coronary atherosclerosis, thrombotic events, or structural cardiomyopathy. The heart was of regular shape, with sparse subepicardial fat, measuring 10 × 11 × 3.5 cm and weighing 400 g. The brain was also grossly normal. Histological examination Figure 1a revealed pericardial layers of regular thickness and the presence of multiple foci of hypercontracted myocardial bundles with myofibrillar rhexis and the formation of transverse hypereosinophilic bands, in the absence of inflammatory infiltrates. Toxicological tests ruled out alcohol and other illicit substances, while Δ9-tetrahydrocannabinol (THC) detected in both blood and urine samples (respectively 64.57 ng/mL and 53.36 ng/mL). Based on these findings, the death was attributed to a sudden arrhythmic cardiac event associated with cannabis use.
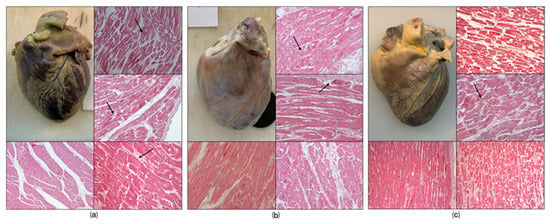
Figure 1.
Histological findings. Macroscopic examination (a,c): revealed no significant alterations (cases a and c); (b) presents a slight pericardial opaque layer. Microscopic examination (a–c): The areas indicated by the arrows in H&E staining are foci of contraction band necrosis. (a) In the H&E pic bottom left represents an almost normal myocardial tissue; (b) the H&E photo at the bottom left shows a slight thickening of the fibrous support network, while to the right of the perivascular hemorrhages. (c) The image at the top right and the one at the bottom right show the segmentation of the myofibers at H&E staining.
2.2. Case B
The second case refers to another 37-year-old man, discovered dead in his residence in May 2020. Testimonial evidence and scene investigation excluded violent circumstances or suspicious external causes, and no cardiovascular history was reported. At autopsy, no traumatic injuries were found. Examination of the main coronary arteries revealed a regular course, orifice, and lumen, with walls of normal thickness. Gross examination of the myocardium did not reveal hypertrophy or scarring, even if appeared enlarged and opaque whitish in color due to the presence of a fibrous layer. Microscopic examination Figure 1b revealed increased thickness of the pericardial layers. In some perivascular and interstitial areas, areas of connective tissue thickening were observed, while in other areas, myocytes with contraction band necrosis were present. Toxicological analysis demonstrated the presence of THC in urine and peripheral blood (respectively 15.36 ng/mL and 67.33 ng/mL), while alcohol and other psychoactive substances tested negative. The overall picture suggested a sudden cardiac death of arrhythmic origin, most likely precipitated by acute cannabis exposure.
2.3. Case C
The third case involved a 37-year-old man who was found deceased at home in November 2019. He was previously healthy and no relevant medical history was available. Circumstantial data and external examination excluded any traumatic origin or the involvement of third parties. At autopsy, the heart showed regular shape, with regular representation of subepicardial fat, measuring 13.5 × 11 × 4.5 cm and weighing 425 g. The whole cardiovascular system showed no coronary obstruction, myocardial infarction, or congenital malformations, no valvular alterations. Histological examination Figure 1c of the samples revealed thickening of the supporting fibrous network, as well as perivascular areas of myofiber necrosis with foci of hypercontracted myocytes, with rupture of the myofibrillar apparatus and the formation of transverse hypereosinophilic bands, and segmentation of the myocardial fibers. Toxicological screening confirmed the presence of THC in biological fluids (hematic concentration 72.67 ng/mL, urine 35.36 ng/mL), while other drugs and alcohol were absent. Considering the absence of structural cardiac pathology and the toxicological profile, the death was classified as sudden arrhythmic cardiac death, with cannabis consumption representing the most plausible trigger.
3. Discussion
The present case series describes three young men who died suddenly and unexpectedly, in whom forensic autopsy and subsequent investigations excluded traumatic, infectious, coronary, and congenital causes of death. In all cases, histological examination revealed a polymorphic myocardial picture characterized by interstitial edema, fiber segmentation, and myocytolysis, consistent with arrhythmic sudden cardiac death. Toxicological analyses confirmed the presence of Δ9-tetrahydrocannabinol (THC) in blood and urine, thus supporting a temporal and causal relationship between cannabis consumption and the fatal arrhythmic events.
The findings align with previous forensic reports that describe cannabis as a potential precipitating factor for sudden cardiac death, particularly in young adults without structural cardiac disease [7,17,18,19]. While cannabis has traditionally been considered a substance with low acute toxicity, growing evidence suggests that it exerts relevant cardiovascular effects, mediated by both acute and chronic exposure [6,13,20,21].
From a pathophysiological perspective, the activation of cannabinoid receptors plays a central role. CB1 receptor stimulation, which prevails in chronic cannabis users and smokers, promotes sympathetic activation leading to tachycardia, increased myocardial oxygen demand, coronary vasospasm, and a reduction in coronary flow reserve [9,11,13]. In addition, CB1-mediated mechanisms contribute to endothelial dysfunction, a pro-thrombotic state, and arrhythmogenesis [8,12,14]. Conversely, CB2 receptor activation is associated with vasoprotective and anti-inflammatory effects, but its role appears less prominent in habitual cannabis consumers [11,12]. The predominance of CB1-driven responses therefore explains the heightened cardiovascular vulnerability observed in regular users [8,11,12].
The biphasic cardiovascular effects of cannabis further complicate the clinical picture. At lower doses, THC induces sympathetic stimulation and norepinephrine release, whereas higher doses are associated with parasympathetic activation, bradycardia, and vasodilation with reflex tachycardia [19,20]. This dose-dependent duality may underlie the heterogeneity of arrhythmic presentations, ranging from atrial fibrillation to ventricular fibrillation and Brugada-like patterns, as previously documented [7,18,20]. The arrhythmogenic substrate is further amplified by THC’s direct inhibitory action on cardiac sodium and potassium channels, which may destabilize cardiac conduction [17,18]. In the cases presented in this manuscript, in fact, a common and widespread repertoire was that of contraction band necrosis (CBN) which may indicate sympathetic overdrive and associated arrhythmogenic supersensitivity [21,22].
Epidemiological data suggest that marijuana users experience arrhythmias at approximately double the rate of non-users, with heavy and chronic users being at greater risk than occasional consumers [6,7,19]. Notably, atrial fibrillation has been reported as the most frequent arrhythmia associated with cannabis use, followed by malignant ventricular arrhythmias and sudden death [7,17,20,23].
Taken together, defining a definitive toxic blood concentration for Δ9-tetrahydrocannabinol is challenging, as the compound exhibits low intrinsic acute lethal toxicity, and clinical data supporting an LD50 (Lethal Dose) based on blood concentration in humans is lacking [24,25]. Consequently, toxicological focus shifts primarily to behavioral toxicity and acute adverse health effects associated with elevated THC levels. Following smoked cannabis, plasma THC concentrations peak rapidly, with mean maximum concentrations in controlled studies reaching ranges such as 7.0 to 18.1 ng/mL after a single cigarette [26,27]. These peak levels are associated with acute effects including impaired motor coordination, altered judgment, paranoia, and psychosis in high doses [28]. Pharmacodynamic models have estimated that blood concentrations in the range of 7-29 ng/mL are required for 50% of the maximal subjective "high" effect [29]. Crucially, impairment in driving-related skills is often observed at lower concentrations. For instance, in real-life cases of suspected impaired driving, drivers judged to be impaired had a statistically significant higher median blood THC concentration (2.5 ng/mL) compared to those judged not impaired (1.9 ng/mL), with concentrations above 3 ng/mL increasing the risk of being assessed as impaired [30]. Acute exposure also carries physiological risks, as THC is associated with dose-dependent adverse cardiovascular effects, including tachycardia [31]. The cardiovascular effects of cannabis are dose-dependent. Human studies demonstrate a rapid, dose-related rise in heart rate after smoking, with effects that may persist for up to 2–3 h [32,33,34]. At low inhaled doses, sympathetic predominance typically manifests as tachycardia within minutes of exposure; with higher or sustained exposures, a biphasic autonomic response becomes apparent, including bradycardia and hypotension related to parasympathetic activation and reflex mechanisms [32,33,34,35]. Pharmacokinetic studies confirm that inhaled THC produces rapid systemic absorption and near-immediate cardiovascular effects, consistent with fast effect-site equilibration after inhalation [19].
Controlled administration studies provide more precise quantification of these effects. In a randomized crossover trial, smoking or vaporizing cannabis containing 10 mg THC increased heart rate by approximately 11 bpm (smoked) and 23 bpm (vaporized), whereas 25 mg vaporized THC caused a mean increase of nearly 27 bpm, with peak effects occurring within 10 min and persisting up to 3–4 h [35]. Similarly, pharmacokinetic/pharmacodynamic modeling confirmed that higher systemic exposure to THC and its active metabolite 11-OH-THC is directly associated with dose-dependent increases in heart rate, with measurable effects sustained for several hours after administration [36]. Oral administration further demonstrates that THC dose-dependently elevates heart rate and reduces high-frequency HRV, indicating autonomic imbalance; these effects are more prolonged compared with inhalation due to first-pass metabolism [30].
Epidemiologically, cannabis use is associated with an increased arrhythmic burden, with chronic and heavy users displaying a nearly twofold higher incidence of arrhythmias compared with non-users [37]. These observations highlight the clinical and forensic importance of accounting for dose thresholds and exposure patterns (e.g., binge use, high-potency concentrates) when evaluating cannabis-related fatalities.
Beyond THC, other phytocannabinoids warrant consideration, although cardiovascular safety data remain limited. Cannabidiol (CBD) exerts vasorelaxant and anti-inflammatory actions; in a randomized crossover trial, a single oral dose of CBD reduced systolic blood pressure in healthy volunteers [38], and ex vivo studies confirmed endothelium-dependent relaxation in human arteries [39]. At higher doses, however, CBD can inhibit cytochrome P450 isoenzymes, raising the potential for drug–drug interactions with antiarrhythmic or anticoagulant therapies [40]. Cannabigerol (CBG) displays α2-adrenergic agonism and 5-HT1A antagonism in vitro, suggesting possible pressor effects in preclinical models, although no human cardiovascular data exist [41]. Cannabinol (CBN), a degradation product of THC, shows weak CB1 receptor activity and sedative properties; robust data on cardiovascular impact are lacking, and any bradycardic effects remain speculative [42]. Cannabichromene (CBC) demonstrates CB2 receptor agonism and immunomodulatory properties, but its hemodynamic and electrophysiological effects remain essentially undefined [43]. Taken together, while these non-THC cannabinoids are often marketed as safer alternatives, the absence of rigorous cardiovascular studies—particularly when co-administered with THC—requires caution in both clinical and forensic interpretation.
In addition to inhaled forms, edible cannabis represents an emerging concern due to distinct pharmacokinetic properties. Oral THC undergoes slower and more variable absorption compared with smoking, with onset of effects typically delayed, sometimes up to several hours after ingestion. Furthermore, first-pass metabolism in the liver leads to the production of 11-hydroxy-THC (11-OH-THC), a metabolite that displays psychoactive potency at least comparable to THC and may contribute to more intense and prolonged effects [25,44]. These characteristics predispose users to “dose stacking,” in which additional doses are consumed before the initial effects manifest, thereby increasing the risk of intoxication and cardiovascular complications. Clinical and forensic case reports have documented severe adverse outcomes linked to edibles, including myocardial infarction, life-threatening arrhythmias, and fatalities in susceptible individuals [45,46,47,48,49].
Taken together, the available evidence indicates that cannabis-related cardiovascular toxicity is shaped by both the dose and the formulation. THC remains the principal arrhythmogenic agent, but the role of non-THC phytocannabinoids and edible preparations requires closer scrutiny. These findings emphasize the need for clinicians and forensic specialists to consider cannabis exposure as a potential independent risk factor for sudden cardiac death.
Moreover, the present observations support the hypothesis of a concurrent causal role of cannabis in sudden cardiac death, particularly in predisposed individuals or under conditions of acute high exposure. Although this study is limited by the absence of advanced genetic investigations to exclude primary channelopathies, the concordance of autopsy, histological, and toxicological findings with literature data strengthens the association between cannabis use and fatal arrhythmias.
Future research should aim to better delineate the mechanistic pathways linking cannabis to sudden cardiac death, incorporating genetic, molecular, radiological, and electrophysiological analyses [8,13,50,51,52]. In addition, public health awareness should be raised regarding the underestimated cardiovascular risks of cannabis, especially in the context of increasing legalization and widespread use among young adults [1,2,6].
4. Limitations
The definitive diagnosis of poisoning in these cases was made according to forensic toxicological methodology of four crucial evidentiary criteria, which is very different from an etiopathological relationship in the clinical medical field [24,53,54]. These criteria form a structured, multidisciplinary approach essential for establishing a causal link between substance exposure and impairment or death: (1) Circumstantial Findings involve evidence gathered from the scene, such as suicide notes, drug paraphernalia, empty prescription bottles, or the presence of poisons, which suggests exposure as rolled cannabis cigarettes in the three cases [55]; (2) Clinical and Anamnestic Findings include medical history and the sudden onset of symptoms or death that suggest a toxic syndrome [28,56]; (3) Anatomical, Histopathological Findings are derived from autopsy and microscopy, documenting organ damage (as heart examination) that excludes traumatic or natural causes leaving the suspected toxic agent, though many poisons leave non-specific changes [57]; (4) Chemical and Toxicological Findings provide the ultimate confirmation through the unequivocal identification and quantification of the toxic agent in biological specimens [24,53].
Therefore, although the scientific framework of the cases in this manuscript remains valid, further studies are necessary to confirm the abstraction from the particular cases to the general thesis, which at present remains strongly suggested.
5. Conclusions
This case series describes three young adults who died suddenly in the absence of significant pathological history, with autopsy, histological, and toxicological investigations converging toward cannabis-related cardiac arrhythmogenesis. The systematic integration of our findings with current evidence reinforces the role of cannabinoids in modulating cardiovascular function through CB1 receptor activation on vascular endothelium and cardiomyocytes, promoting vasospasm, reduced coronary reserve, and arrhythmic vulnerability [8,9,10,11]. Chronic exposure, particularly in smokers, has further been associated with endothelial dysfunction and a pro-thrombotic state, amplifying the risk of myocardial ischemia and sudden death [12,13,14].
The results are consistent with prior literature reporting a biphasic cardiovascular response to cannabis, whereby low doses predominantly enhance sympathetic activity and tachycardia, while higher doses induce parasympathetic stimulation, bradycardia, and reflex tachycardia [6,11,17]. Furthermore, epidemiological data suggest that regular cannabis users display a higher incidence of arrhythmias, particularly atrial fibrillation and ventricular fibrillation, as well as conduction disturbances resembling Brugada-like patterns [7,15,16,17,18].
Taken together, these findings highlight the underestimated cardiovascular toxicity of cannabis, especially in young individuals with no other apparent risk factors. The cases presented underscore the importance of thorough postmortem investigations, including targeted histopathological and toxicological analyses, in elucidating the role of cannabis in sudden unexpected death. From a preventive perspective, clinicians and public health authorities should recognize cannabis consumption as a potential independent risk factor for sudden cardiac death, warranting both clinical vigilance and stronger awareness strategies.
Author Contributions
Conceptualization, G.B.; methodology, G.B. and M.F.; investigation, M.F.; paper collection, V.M.G. and M.C.; paper selection, N.P. and A.D.F.; writing—original draft preparation, V.M.G.; writing—review and editing, A.D.F.; supervision, G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The present experimental study concerns samples taken from deceased bodies. The trial, the Italian legislation on this kind of subjects did not require the ethics committee to express a binding opinion in relation to this type of trial, as it was a study that did not directly intervene in modifying diagnostic or therapeutic pathways on living subjects.
Informed Consent Statement
Since these were corpses, subjected to a judicial autopsy by the Prosecutor, consent to publication was obtained from all entitled subjects. The study was conducted in accordance with the Declaration of Helsinki and informed consent was obtained from anyone who could exercise the right. The processing of the data reported in this paper is covered by the general authorization to process personal data for scientific research purposes granted by the Italian Data Protection Authority (1 March 2012 as published in Italy’s Official Journal no. 72 dated 26 March 2012) since the data do not entail any significant personalized impact on data subjects.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| THC | Δ9-tetrahydrocannabinol |
| CBD | cannabidiol |
| SCD | Sudden cardiac death |
References
- United Nations Office on Drugs and Crime. World Drug Report; United Nations Publication: New York, NY, USA, 2025; Available online: https://www.unodc.org/unodc/data-and-analysis/world-drug-report-2025.html (accessed on 21 October 2025).
- Hoch, E.; Volkow, N.D.; Friemel, C.M.; Lorenzetti, V.; Freeman, T.P.; Hall, W. Cannabis, cannabinoids and health: A review of evidence on risks and medical benefits. Eur. Arch. Psychiatry Clin. Neurosci. 2025, 275, 281–292. [Google Scholar] [CrossRef]
- Sideris, A.; Doan, L.V. An Overview of Cannabidiol. Anesth. Analg. 2024, 138, 54–68. [Google Scholar] [CrossRef]
- Meiri, E.; Jhangiani, H.; Vredenburgh, J.J.; Barbato, L.M.; Carter, F.J.; Yang, H.-M.; Baranowski, V. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr. Med. Res. Opin. 2007, 23, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Christo, P.J.; Vortsman, E.; Gharibo, C.; LeQuang, J.A.K.; Pergolizzi, J.V. Considering Long-Acting Synthetic Cannabidiol for Chronic Pain: A Narrative Review. Cureus 2025, 17, e81577. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, P.K.; Odom, E.C.; Patel, R.; Loustalot, F.; Coleman King, S. Nonmedical Marijuana Use and Cardiovascular Events: A Systematic Review. Public Health Rep. 2021, 137, 62–71. [Google Scholar] [CrossRef]
- Drummer, O.H.; Gerostamoulos, D.; Woodford, N.W. Cannabis as a cause of death: A review. Forensic Sci. Int. 2019, 298, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Rorabaugh, B.R.; Guindon, J.; Morgan, D.J. Role of Cannabinoid Signaling in Cardiovascular Function and Ischemic Injury. J. Pharmacol. Exp. Ther. 2023, 387, 265–276. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Gao, B.; Mirshahi, F.; Sanyal, A.J.; Khanolkar, A.D.; Makriyannis, A.; Kunos, G. Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem. J. 2000, 346 Pt 3, 835–840. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Batkai, S.; Pacher, P.; Harvey-White, J.; Wagner, J.A.; Cravatt, B.F.; Gao, B.; Kunos, G. Lipopolysaccharide induces anandamide synthesis in macrophages via CD14/ MAPK/ phosphoinositide 3-kinase/NF-kappaB independently of platelet-activating factor. J. Biol. Chem. 2003, 278, 45034–45039. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Bátkai, S.; Kunos, G. Cardiovascular pharmacology of cannabinoids. Handb. Exp. Pharmacol. 2005, 168, 599–625. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weis, F.; Beiras-Fernandez, A.; Sodian, R.; Kaczmarek, I.; Reichart, B.; Beiras, A.; Schelling, G.; Kreth, S. Substantially altered expression pattern of cannabinoid receptor 2 and activated endocannabinoid system in patients with severe heart failure. J. Mol. Cell Cardiol. 2010, 48, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- D Rezkalla, S.H.; Kloner, R.A. A Review of Cardiovascular Effects of Marijuana Use. J. Cardiopulm. Rehabil. Prev. 2025, 45, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, L.; Navabzadeh, M.; Jiménez-Téllez, N.; Han, D.D.; Reagan, E.; Naughton, J.; Zhou, L.Y.; Almeida, R.; Castaneda, L.M.; Abdelaal, S.A.; et al. Association of Endothelial Dysfunction With Chronic Marijuana Smoking and THC-Edible Use. JAMA Cardiol. 2025, 10, 851–855. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Basso, C.; Aguilera, B.; Banner, J.; Cohle, S.; d’Amati, G.; de Gouveia, R.H.; di Gioia, C.; Fabre, A.; Gallagher, P.J.; Leone, O.; et al. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Arch. 2017, 471, 691–705. [Google Scholar] [CrossRef]
- Cafarelli, F.P.; Macarini, L.; Cipolloni, L.; Maglietta, F.; Guglielmi, G.; Sessa, F.; Pennisi, A.; Cantatore, S.; Bertozzi, G. Ex situ heart magnetic resonance imaging and angiography: Feasibility study for forensic purposes. Forensic Imaging 2021, 25, 200442. [Google Scholar] [CrossRef]
- Hartung, B.; Kauferstein, S.; Ritz-Timme, S.; Daldrup, T. Sudden unexpected death under acute influence of cannabis. Forensic Sci. Int. 2014, 237, e11–e13. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, V.N.; Menezes, A.R.; De Schutter, A.; Lavie, C.J. The Cardiovascular Effects of Marijuana: Are the Potential Adverse Effects Worth the High? Mo. Med. 2019, 116, 146–153. [Google Scholar] [PubMed] [PubMed Central]
- Latif, Z.; Garg, N. The Impact of Marijuana on the Cardiovascular System: A Review of the Most Common Cardiovascular Events Associated with Marijuana Use. J. Clin. Med. 2020, 9, 1925. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bachs, L.; Mørland, H. Acute cardiovascular fatalities following cannabis use. Forensic Sci. Int. 2001, 124, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Baroldi, G.; Mittleman, R.E.; Parolini, M.; Silver, M.D.; Fineschi, V. Myocardial contraction bands. Int. J. Legal Med. 2001, 115, 142–151. [Google Scholar] [CrossRef]
- Bertozzi, G.; Ferrara, M.; Maiese, A.; Delogu, G.; Fazio, N.; Di Tortorella, V. Oxidative Stress in Sepsis: A focus on cardiac pathology. Int. J. Mol. Sci. 2024, 25, 2912. [Google Scholar] [CrossRef]
- Wolff, V.; Jouanjus, E. Strokes are possible complications of cannabinoids use. Epilepsy Behav. 2017, 70 Pt B, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Sewell, R.A.; Poling, J.; Sofuoglu, M. The effect of cannabis compared with alcohol on driving. Am. J. Addict. 2009, 18, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A. Human Cannabinoid Pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef]
- Huestis, M.A.; Cone, E.J. Relating blood concentrations of tetrahydrocannabinol and metabolites to pharmacologic effects and time of marijuana usage. Forensic Sci. Int. 1993, 60, 17–25. [Google Scholar] [CrossRef]
- Schwope, D.M.; Karschner, E.L.; Gorelick, D.A.; Huestis, M.A. Identification of recent cannabis use: Whole-blood and plasma free and glucuronidated cannabinoid pharmacokinetics following controlled smoked cannabis administration. Clin Chem. 2011, 57, 1406–1414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Volkow, N.D.; Baler, R.D.; Compton, W.M.; Weiss, S.R. Adverse health effects of marijuana use. N. Engl. J. Med. 2014, 370, 2099–2107. [Google Scholar] [CrossRef]
- Hjelmeland, K.; Middelkoop, G.; Mørland, J.; Høiseth, G. The relationship between clinical impairment and blood drug concentration: Comparison between the most prevalent traffic relevant drug groups. Forensic Sci. Int. 2024, 363, 112180. [Google Scholar] [CrossRef] [PubMed]
- Pabon, E.; Rockwood, F.; Norman, G.J.; de Wit, H. Acute effects of oral delta-9-tetrahydrocannabinol on autonomic cardiac activity and their relation to subjective and anxiogenic effects. Psychopharmacology 2022, 238, 3429–3440. [Google Scholar] [CrossRef]
- Chandy, M.; Nishiga, M.; Wei, T.T.; Hamburg, N.M.; Nadeau, K.; Wu, J.C. Adverse Impact of Cannabis on Human Health. Annu. Rev. Med. 2024, 75, 353–367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sidney, S. Cardiovascular consequences of marijuana use. J. Clin. Pharmacol. 2002, 42, 64S–70S. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.A.; Frishman, W.H. Marijuana use and cardiovascular disease. Cardiol Rev. 2016, 24, 158–162. [Google Scholar] [CrossRef]
- Goyal, H.; Awad, H.H.; Ghali, J.K. Role of cannabis in cardiovascular disorders. J. Thorac. Dis. 2017, 9, 2079–2092. [Google Scholar] [CrossRef] [PubMed]
- Spindle, T.R.; Cone, E.J.; Schlienz, N.J.; Mitchell, J.M.; Bigelow, G.E.; Flegel, R.; Hayes, E.; Vandrey, R. Acute effects of smoked and vaporized cannabis in healthy adults who infrequently use cannabis: A crossover trial. JAMA Netw. Open 2018, 1, e184841. [Google Scholar] [CrossRef] [PubMed]
- Wolowich, W.R.; Greif, R.; Theiler, L.; Kleine-Brueggeney, M. Pharmacokinetic/pharmacodynamic modeling of the acute heart rate effect of THC and 11-OH-THC in volunteers. Eur. J. Drug Metab. Pharmacokinet. 2025, 50, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Kloner, R.A.; Rezkalla, S. Adverse cardiovascular, cerebrovascular, and peripheral vascular effects of marijuana inhalation: What cardiologists need to know. Am. J. Cardiol. 2014, 113, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Jadoon, K.A.; Tan, G.D.; O’Sullivan, S.E. A single dose of cannabidiol reduces blood pressure in healthy volunteers in a randomized crossover study. JCI Insight 2017, 2, e93760. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.P.; Hind, W.H.; O’Sullivan, S.E. Is the cardiovascular system a therapeutic target for cannabidiol? Br. J. Clin. Pharmacol. 2015, 80, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Zendulka, O.; Dovrtělova, G.; Nosková, K.; Turjap, M.; Šulcová, A.; Hanuš, L.; Juřica, J. Cannabinoids and cytochrome P450 interactions. Curr. Drug Metab. 2016, 17, 206–226. [Google Scholar] [CrossRef] [PubMed]
- Cascio, M.G.; Gauson, L.A.; Stevenson, L.A.; Ross, R.A.; Pertwee, R.G. Evidence that the plant cannabinoid cannabigerol is a highly potent α2-adrenoceptor agonist and moderately potent 5-HT1A receptor antagonist. Br. J. Pharmacol. 2010, 159, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-THC, cannabidiol and Δ9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Udoh, M.; Santiago, M.; Devenish, S.; Al Yacoub, N.; Bhatia, H.; Irving, A.J. Cannabichromene is a cannabinoid CB2 receptor agonist. Br. J. Pharmacol. 2019, 176, 4537–4547. [Google Scholar] [CrossRef] [PubMed]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef] [PubMed]
- CDC. Death following ingestion of an edible marijuana product—Colorado, March 2014. Morb. Mortal. Wkly. Rep. (MMWR) 2015, 64, 771–772. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6428a6.htm (accessed on 21 October 2025). [CrossRef]
- Monte, A.A.; Shelton, S.K.; Mills, E.; Saben, J.; Hopkinson, A.; Sonn, B.; Devivo, M.; Chang, T.; Fox, J.; Brevik, C.; et al. Associated with Cannabis Use, by Route of Exposure: An Observational Study. Ann. Intern. Med. 2019, 170, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.; Stevenson, R.S. Marijuana Lollipop-Induced Myocardial Infarction. Can. J. Cardiol. 2019, 35, 229.e1–229.e3. [Google Scholar] [CrossRef] [PubMed]
- Kariyanna, P.T.; Yadav, R.; Yadav, V.; Jayarangaiah, A.; Srinivasan, M.; Chandrakumar, H.P.; McFarlane, I.M. Atrioventricular Nodal Reentrant Tachycardia Triggered by Edible Marijuana: A Case Report and Review of the Literature. Am. J. Med. Case Rep. 2020, 8, 123–127. [Google Scholar]
- Meneses, V.; Mata, D. Cannabinoid Stability in Antemortem and Postmortem Blood. J. Anal. Toxicol. 2020, 44, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmari, A.I. Method for Postmortem Quantification of Δ9-Tetrahydrocannabinol and Metabolites Using LC-MS-MS. J. Anal. Toxicol. 2019, 43, 703–719. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi, G.; Cafarelli, F.P.; Ferrara, M.; Di Fazio, N.; Guglielmi, G.; Cipolloni, L.; Manetti, F.; La Russa, R.; Fineschi, V. Sudden Cardiac Death and Ex-Situ Post-Mortem Cardiac Magnetic Resonance Imaging: A Morphological Study Based on Diagnostic Correlation Methodology. Diagnostics 2022, 12, 218. [Google Scholar] [CrossRef]
- Bertozzi, G.; Ferrara, M.; Maiese, A.; Di Fazio, A.; Morena, D.; Padovano, M.; Scopetti, M.; La Russa, R.; Fineschi, V. Post-mortem Cardiac MRI in Sudden Cardiac Death: The Interesting Intertwining of Radiology and Histology to Diagnose Arrhythmic Death or Myocardial Infarction. Curr. Med. Imaging 2025, 21, E15734056343601. [Google Scholar] [CrossRef]
- Soria, M.L. The improvements in forensic toxicology and its role in the forensic process. The interpretation of results (II). Span. J. Leg. Med. 2024, 50, 62–75. [Google Scholar] [CrossRef]
- Bertozzi, G.; Sessa, F.; Maglietta, F.; Cipolloni, L.; Salerno, M.; Fiore, C.; Fortarezza, P.; Ricci, P.; Turillazzi, E.; Pomara, C. Immunodeficiency as a side effect of anabolic androgenic steroid abuse: A case of necrotizing myofasciitis. Forensic Sci. Med. Pathol. 2019, 15, 616–621. [Google Scholar] [CrossRef]
- Lalit, P.C.; Mamta, P. Assessment and Diagnosis of Poisoning with Characteristics Features in Living or Dead. J. Forensic Sci. Crim. Investig. 2018, 10, 555796. [Google Scholar] [CrossRef]
- Lee, J.S.; Cha, Y.S.; Yeon, S.; Kim, T.Y.; Lee, Y.; Choi, J.G.; Cha, K.C.; Lee, K.H.; Kim, H. Changes in Diagnosis of Poisoning in Patients in the Emergency Room Using Systematic Toxicological Analysis with the National Forensic Service. J. Korean Med. Sci. 2021, 36, e118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Argo, A.; Zerbo, S.; Buscemi, R.; Trignano, C.; Bertol, E.; Albano, G.D.; Vaiano, F. A Forensic Diagnostic Algorithm for Drug-Related Deaths: A Case Series. Toxics 2022, 10, 152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
