Feasibility and Reliability of Ammer–Coelho Computational Tool for Sex Estimation: A Pilot Study on an Elderly Scottish Sample
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Observer Error
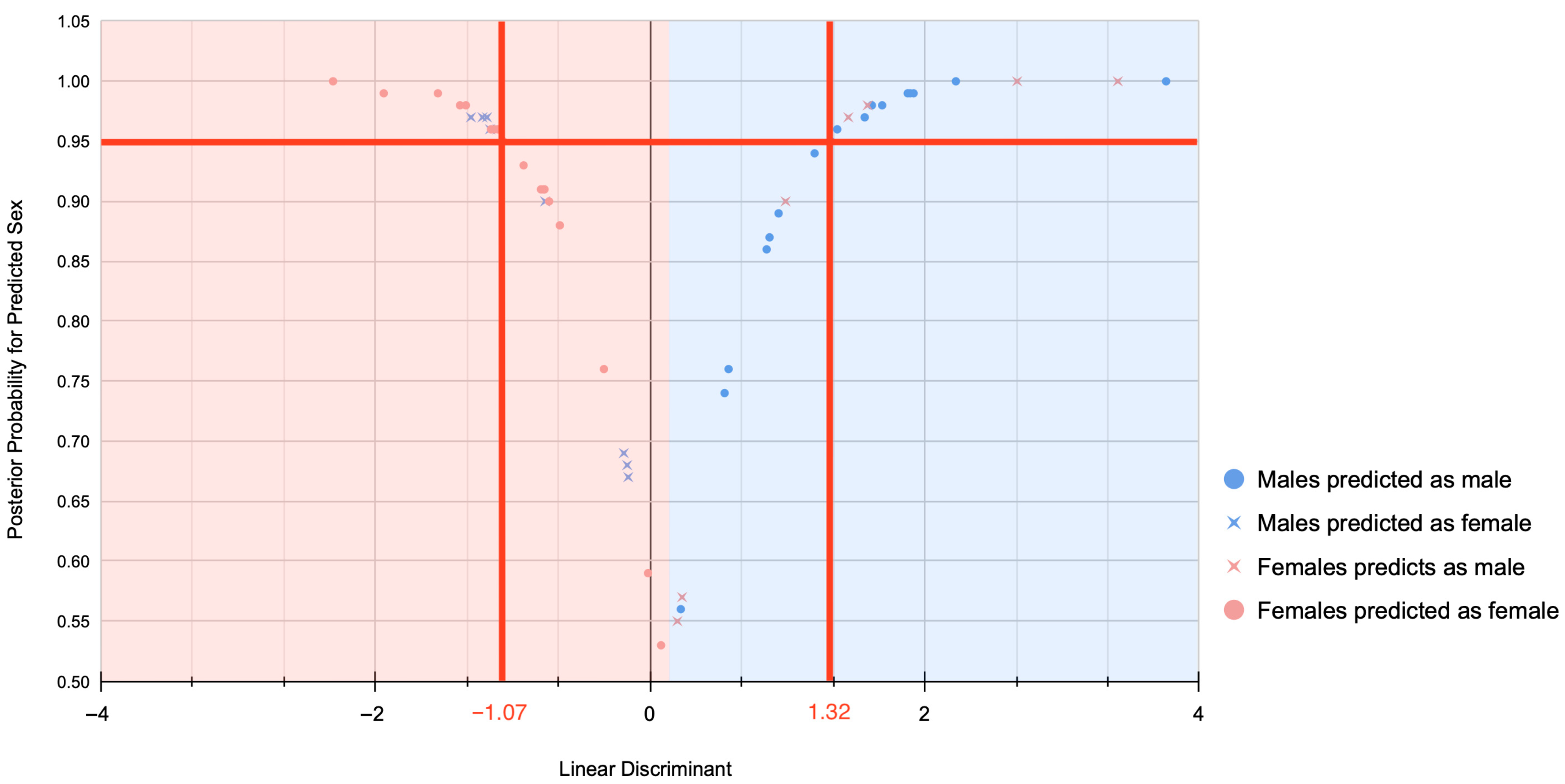
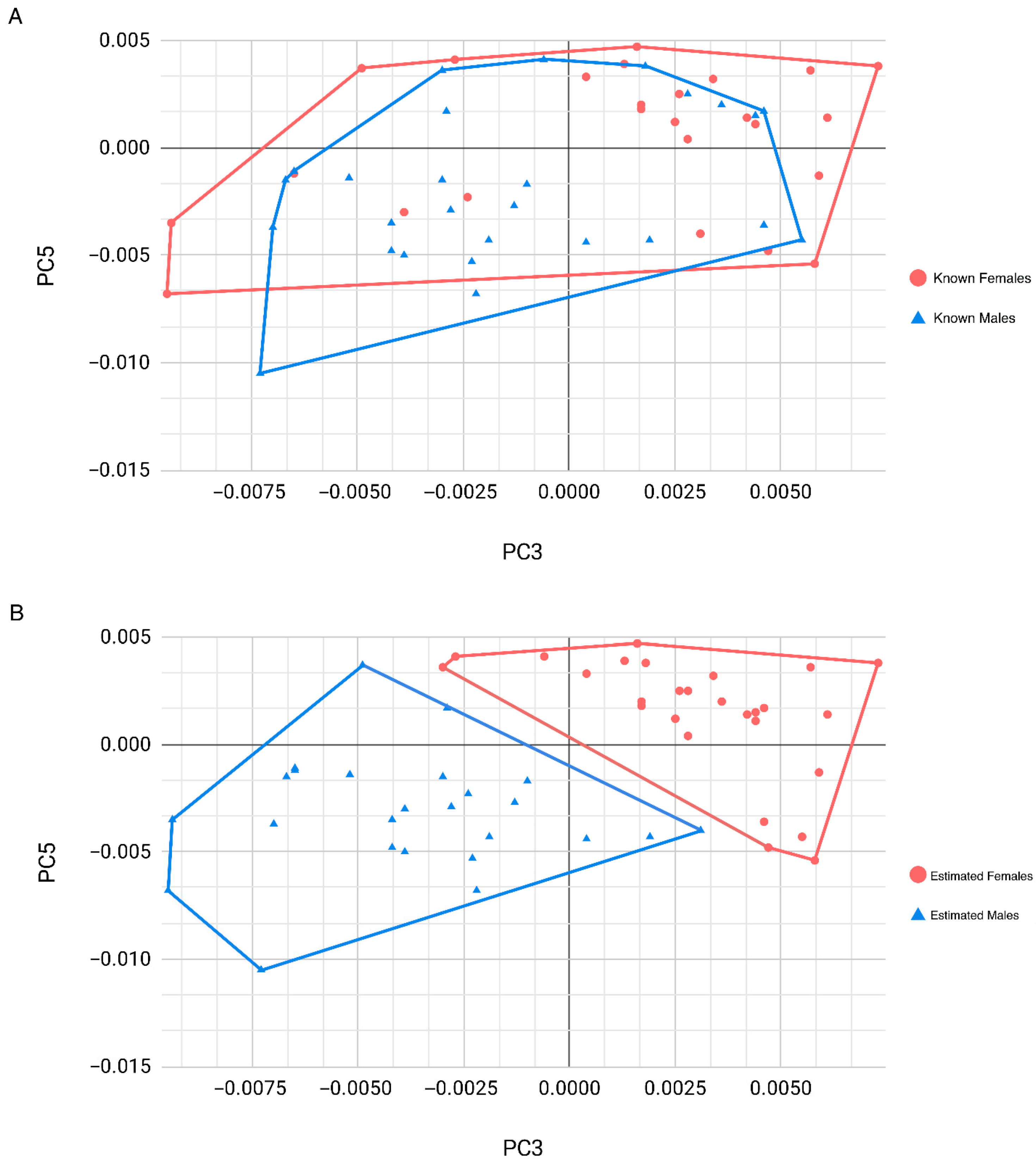
3.2. Ammer–Coelho Performance on the Scottish Sample
4. Discussion
4.1. Intra- and Inter-Observer Error
4.2. Ammer–Coelho for Sex Estimation on the Scottish Sample
4.3. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandrakanth, H.V.; Kanchan, T.; Krishan, K. Osteometric Analysis for Sexing of Modern Sternum—An Autopsy Study from South India. Leg. Med. 2014, 16, 350–356. [Google Scholar] [CrossRef]
- Kemkes-Grottenthaler, A. The Reliability of Forensic Osteology—A Case in Point. Forensic Sci. Int. 2001, 117, 65–72. [Google Scholar] [CrossRef]
- Krishan, K.; Chatterjee, P.M.; Kanchan, T.; Kaur, S.; Baryah, N.; Singh, R.K. A Review of Sex Estimation Techniques during Examination of Skeletal Remains in Forensic Anthropology Casework. Forensic Sci. Int. 2016, 261, 165.e1–165.e8. [Google Scholar] [CrossRef]
- Hefner, J.T. Cranial Nonmetric Variation and Estimating Ancestry. J. Forensic Sci. 2009, 54, 985–995. [Google Scholar] [CrossRef]
- Klales, A.R. MorphoPASSE: Morphological Pelvis and Skull Sex Estimation Program. In Sex Estimation of the Human Skeleton; Elsevier: Amsterdam, The Netherlands, 2020; pp. 271–278. [Google Scholar] [CrossRef]
- Curate, F.; d’Oliveira Coelho, J.; Silva, A.M. CalcTalus: An Online Decision Support System for the Estimation of Sex with the Calcaneus and Talus. Archaeol. Anthropol. Sci. 2021, 13, 74. [Google Scholar] [CrossRef]
- Curate, F.; Navega, D.; Cunha, E.; d’Oliveira Coelho, J. DXAGE 2.0—Adult Age at Death Estimation Using Bone Loss in the Proximal Femur and the Second Metacarpal. Int. J. Leg. Med. 2022, 136, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.K. Sex Estimation and Assessment. In Research Methods in Human Skeletal Biology; DiGangi, E., Moore, M.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 91–114. [Google Scholar]
- Giles, E.; Elliot, O. Sex Determination by Discriminant Function Analysis of Crania. Am. J. Phys. Anthropol. 1963, 21, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Nikitovic, D. Sexual Dimorphism (Humans). In The International Encyclopedia of Biological Anthropology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Bonczarowska, J.H.; Bonicelli, A.; Papadomanolakis, A.; Kranioti, E.F. The Posterior Portion of the Ilium as a Sex Indicator: A Validation Study. Forensic Sci. Int. 2019, 294, 216.e1–216.e6. [Google Scholar] [CrossRef]
- Frayer, D.W.; Wolpoff, M.H. Sexual Dimorphism. Annu. Rev. Anthropol. 1985, 14, 429–473. [Google Scholar] [CrossRef]
- Ubelaker, D.H.; DeGaglia, C.M. Population Variation in Skeletal Sexual Dimorphism. Forensic Sci. Int. 2017, 278, 407.e1–407.e7. [Google Scholar] [CrossRef]
- Gonzalez, P.N.; Bernal, V.; Perez, S.I. Geometric Morphometric Approach to Sex Estimation of Human Pelvis. Forensic Sci. Int. 2009, 189, 68–74. [Google Scholar] [CrossRef]
- Bookstein, F.L. Size and Shape Spaces for Landmark Data in Two Dimensions. Stat. Sci. 1986, 1, 181–222. [Google Scholar] [CrossRef]
- Bookstein, F.L. Morphometric Tools for Landmark Data; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Mitteroecker, P.; Gunz, P.; Bernhard, M.; Schaefer, K.; Bookstein, F.L. Comparison of Cranial Ontogenetic Trajectories among Great Apes and Humans. J. Hum. Evol. 2004, 46, 679–698. [Google Scholar] [CrossRef]
- Steyn, M.; Pretorius, E.; Hutten, L. Geometric Morphometric Analysis of the Greater Sciatic Notch in South Africans. HOMO 2004, 54, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Kimmerle, E.H.; Ross, A.; Slice, D. Sexual Dimorphism in America: Geometric Morphometric Analysis of the Craniofacial Region. J. Forensic Sci. 2008, 53, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Kalbouneh, H.; Mubarak, N.; Daradkeh, S.; Ashour, O.; Alkhatib, A.M.; Suboh, L.; Nofal, A.; Mahafzah, W.; Alsalem, M. Estimation of Sex Based on Metrics of the Sternum in a Contemporary Jordanian Population: A Computed Tomographic Study. Medicine 2021, 100, e28169. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.E.; Garvin, H.M. A Morphometric Outline Analysis of Ancestry and Sex Differences in Cranial Shape. J. Forensic Sci. 2018, 63, 1001–1009. [Google Scholar] [CrossRef]
- Phenice, T.W. A Newly Developed Visual Method of Sexing the Os Pubis. Am. J. Phys. Anthropol. 1969, 30, 297–301. [Google Scholar] [CrossRef]
- Walker, P.L. Sexing Skulls Using Discriminant Function Analysis of Visually Assessed Traits. Am. J. Phys. Anthropol. 2008, 136, 39–50. [Google Scholar] [CrossRef]
- Washburn, S.L. Sex Differences in the Pubic Bone. Am. J. Phys. Anthropol. 1948, 6, 199–208. [Google Scholar] [CrossRef]
- Mostafa, E.M.; El-Elemi, A.H.; El-Beblawy, M.A.; Dawood, A.E.-W.A. Adult Sex Identification Using Digital Radiographs of the Proximal Epiphysis of the Femur at Suez Canal University Hospital in Ismailia, Egypt. Egypt. J. Forensic Sci. 2012, 2, 81–88. [Google Scholar] [CrossRef]
- Hemy, N.; Flavel, A.; Ishak, N.-I.; Franklin, D. Sex Estimation Using Anthropometry of Feet and Footprints in a Western Australian Population. Forensic Sci. Int. 2013, 231, 402.e1–402.e6. [Google Scholar] [CrossRef]
- Kotěrová, A.; Velemínská, J.; Dupej, J.; Brzobohatá, H.; Pilný, A.; Brůžek, J. Disregarding Population Specificity: Its Influence on the Sex Assessment Methods from the Tibia. Int. J. Leg. Med. 2017, 131, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Tallman, S.D.; Blanton, A.I. Distal Humerus Morphological Variation and Sex Estimation in Modern Thai Individuals. J. Forensic Sci. 2020, 65, 361–371. [Google Scholar] [CrossRef]
- Abdelghani, N.; Barut, C.; Ogut, E. The Investigation of Cranial Fossae in the Intracranial Cavity of Fixed Cadaveric Skull Bases: Associations with Sex, Laterality, and Clinical Significance. Surg. Radiol. Anat. 2024, 46, 1305–1329. [Google Scholar] [CrossRef]
- Grabiner, M. The Elbow and Radioulnar Joints. In Kinesiology and Applied Anatomy; Lea & Febiger: London, UK, 1989; pp. 136–150. [Google Scholar]
- Rogers, T. A Visual Method of Determining the Sex of Skeletal Remains Using the Distal Humerus. J. Forensic Sci. 1999, 44, 57–60. [Google Scholar] [CrossRef]
- Vance, V.L.; Steyn, M.; L’Abbé, E.N. Nonmetric Sex Determination from the Distal and Posterior Humerus in Black and White South Africans: Nonmetric Sex Estimation from Distal Humerus. J. Forensic Sci. 2011, 56, 710–714. [Google Scholar] [CrossRef]
- Vance, V.L.; Steyn, M. Geometric Morphometric Assessment of Sexually Dimorphic Characteristics of the Distal Humerus. HOMO 2013, 64, 329–340. [Google Scholar] [CrossRef]
- Ammer, S.; d’Oliveira Coelho, J.; Cunha, E.M. Outline Shape Analysis on the Trochlear Constriction and Olecranon Fossa of the Humerus: Insights for Sex Estimation and a New Computational Tool. J. Forensic Sci. 2019, 64, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Navega, D.; Coelho, C.; Vicente, R.; Ferreira, M.T.; Cunha, E. AncesTrees: Ancestry Estimation with Randomized Decision Trees. Int. J. Leg. Med. 2015, 129, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.R.; Pilloud, M.A.; Navega, D.; d’Oliveira Coelho, J.; Cunha, E.; Irish, J.D. rASUDAS: A New Web-Based Application for Estimating Ancestry from Tooth Morphology. Forensic Anthropol. 2018, 1, 18–31. [Google Scholar] [CrossRef]
- İşcan, M.Y.; Loth, S.R.; Wright, R.K. Metamorphosis at the Sternal Rib End: A New Method to Estimate Age at Death in White Males. Am. J. Phys. Anthropol. 1984, 65, 147–156. [Google Scholar] [CrossRef]
- Haas, J.; Buikstra, J.E.; Ubelaker, D.H. Standards for Data Collection from Human Skeletal Remains: Proceedings of a Seminar at the Field Museum of Natural History Organized by Jonathan Haas, Réimpression; Arkansas Archeological Survey Research Series; Arkansas Archeological Survey: Fayetteville, AR, USA, 1994. [Google Scholar]
- Human Tissue (Scotland) Act 2006 (asp 4). An Act of the Scottish Parliament to Make Provision in Relation to Activities Involving Human Tissue. 2006. Available online: https://www.legislation.gov.uk/asp/2006/4/contents (accessed on 29 September 2025).
- Scottish Government. Body Donation in Scotland: Guidance. 2023. Available online: https://www.gov.scot/publications/body-donation-in-scotland-guidance/ (accessed on 29 September 2025).
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef]
- Tang, W.; Hu, J.; Zhang, H.; Wu, P.; He, H. Kappa Coefficient: A Popular Measure of Rater Agreement. Shanghai Arch. Psychiatry 2015, 27, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Fleiss, J.L.; Levin, B.A.; Paik, M.C. Statistical Methods for Rates and Proportions, 3rd ed.; Wiley Series in Probability and Statistics; Wiley-Interscience: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Trevethan, R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front. Public Health 2017, 5, 307. [Google Scholar] [CrossRef] [PubMed]
- Bartholdy, B.P.; Sandoval, E.; Hoogland, M.L.P.; Schrader, S.A. Getting Rid of Dichotomous Sex Estimations: Why Logistic Regression Should Be Preferred Over Discriminant Function Analysis. J. Forensic Sci. 2020, 65, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Jerković, I.; Bašić, Ž.; Anđelinović, Š.; Kružić, I. Adjusting Posterior Probabilities to Meet Predefined Accuracy Criteria: A Proposal for a Novel Approach to Osteometric Sex Estimation. Forensic Sci. Int. 2020, 311, 110273. [Google Scholar] [CrossRef]
- Lesciotto, K.M.; Klales, A.R. Sex Estimation Using Metrics of the Innominate: A Test of the DSP2 Method. J. Forensic Sci. 2025, 70, 249–257. [Google Scholar] [CrossRef]
- Kranioti, E.F.; Nathena, D.; Michalodimitrakis, M. Sex Estimation of the Cretan Humerus: A Digital Radiometric Study. Int. J. Leg. Med. 2011, 125, 659–667. [Google Scholar] [CrossRef]
- Sieber, K.S.; García-Donas, J.G. Population Affinity Estimation on a Spanish Sample: Testing the Validity and Accuracy of Cranium and Mandible Online Software Methods. Leg. Med. 2023, 60, 102180. [Google Scholar] [CrossRef]
- Reynolds, M.S.; MacGregor, D.M.; Alston-Knox, C.L.; Meredith, M.; Barry, M.D.; Schmutz, B.; Gregory, L.S. Bayesian Modeling Predicts Age and Sex Are Not Required for Accurate Stature Estimation from Femoral Length. Forensic Sci. Int. 2018, 289, 452.e1–452.e14. [Google Scholar] [CrossRef]
- Krüger, G.C.; L’Abbé, E.N.; Stull, K.E. Sex Estimation from the Long Bones of Modern South Africans. Int. J. Leg. Med. 2017, 131, 275–285. [Google Scholar] [CrossRef]
- İşcan, M.Y.; Loth, S.R.; Wright, R.K. Age Estimation from the Rib by Phase Analysis: White Males. J. Forensic Sci. 1984, 29, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- İşcan, M.Y.; Loth, S.R.; Wright, R.K. Age Estimation from the Rib by Phase Analysis: White Females. J. Forensic Sci. 1985, 30, 853–863. [Google Scholar] [CrossRef]
- Steyn, M.; İşcan, M.Y. Osteometric Variation in the Humerus: Sexual Dimorphism in South Africans. Forensic Sci. Int. 1999, 106, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Liebenberg, L.; Krüger, G.C.; L’Abbé, E.N.; Stull, K.E. Postcraniometric Sex and Ancestry Estimation in South Africa: A Validation Study. Int. J. Leg. Med. 2019, 133, 289–296. [Google Scholar] [CrossRef]
- Lesciotto, K.M. Daubert and the Effect on Biological Profile Research. Forensic Anthropol. 2024, 7. Available online: https://journals.upress.ufl.edu/fa/article/view/2368 (accessed on 15 July 2025). [CrossRef]
- Christensen, A.M.; Crowder, C.M.; Ousley, S.D.; Houck, M.M. Error and Its Meaning in Forensic Science. J. Forensic Sci. 2014, 59, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Klemm, M.M. Inter- and Intra-Observer Error in Forensic Anthropology. Neb. Anthropol. 2024, 35, 93–114. Available online: https://digitalcommons.unl.edu/nebanthro/12 (accessed on 1 July 2025).
- İşcan, M.Y. Human Skeleton in Forensic Medicine, 3rd ed.; Charles, C., Ed. Thomas Publisher, Limited: Springfield, IL, USA, 2013. [Google Scholar]
- Lewis, C.J.; Garvin, H.M. Reliability of the Walker Cranial Nonmetric Method and Implications for Sex Estimation. J. Forensic Sci. 2016, 61, 743–751. [Google Scholar] [CrossRef]
- Kamnikar, K.R.; Plemons, A.M.; Hefner, J.T. Intraobserver Error in Macromorphoscopic Trait Data. J. Forensic Sci. 2018, 63, 361–370. [Google Scholar] [CrossRef]
- Nakhaeizadeh, S.; Dror, I.E.; Morgan, R.M. Cognitive Bias in Forensic Anthropology: Visual Assessment of Skeletal Remains Is Susceptible to Confirmation Bias. Sci. Justice 2014, 54, 208–214. [Google Scholar] [CrossRef]
- Klales, A.R.; Kenyhercz, M.W. Non-Metric Assessment of Ancestry Through Cranial Macromorphoscopics: A Validation of the Hefner (2009) Method; American Academy of Forensic Sciences: Atlanta, GA, USA; Colorado Springs, CO, USA, 2012. [Google Scholar]
- Klales, A.R.; Kenyhercz, M.W. Morphological Assessment of Ancestry Using Cranial Macromorphoscopics. J. Forensic Sci. 2015, 60, 13–20. [Google Scholar] [CrossRef]
- Lesciotto, K.M.; Doershuk, L.J. Accuracy and Reliability of the Klales et al. (2012) Morphoscopic Pelvic Sexing Method. J. Forensic Sci. 2018, 63, 214–220. [Google Scholar] [CrossRef] [PubMed]
- d’Oliveira Coelho, J.; Navega, D. Cranial Nonmetric Traits Ancestry Estimation. Available online: https://osteomics.com/hefneR/ (accessed on 14 October 2025).
- Constantinou, C.; Nikita, E. SexEst: An Open Access Web Application for Metric Skeletal Sex Estimation. Int. J. Osteoarchaeol. 2022, 32, 832–844. [Google Scholar] [CrossRef]
- Hefner, J.; Ousley, S.; Richardson, R. MaMD Analytical. 2024. Available online: https://mamdanalytical.com/ (accessed on 15 June 2025).
- Spiros, M. 3D MMS Initiative: Cranial & Postcranial Three-Dimensional Interactive Learning Instrument. Available online: https://www.3dmms-initiative.com/ (accessed on 15 June 2025).
- Wanek, V.L. A Qualitative Analysis for Sex Determination in Humans Utilizing Posterior and Medial Aspects of the Distal Humerus. Master’s Thesis, Portland State University, Portland, OR, USA, 2002. [Google Scholar]
- Falys, C.; Schutkowski, H.; Weston, D. The Distal Humerus-A Blind Test of Rogers’ Sexing Technique Using a Documented Skeletal Collection. J. Forensic Sci. 2005, 50, 1289–1293. [Google Scholar] [CrossRef]
- Craik, K.; Collings, A.J. A Preliminary Study into the Impact of Using Three-Dimensional Models in Forensic Anthropology Learning and Teaching. Sci. Justice 2022, 62, 814–821. [Google Scholar] [CrossRef]
- Obertová, Z.; Stewart, A.; Cattaneo, C. Statistics and Probability in Forensic Anthropology; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar] [CrossRef]
- Morrison, G.S.; Weber, P.; Basu, N.; Puch-Solis, R.; Randolph-Quinney, P.S. Calculation of Likelihood Ratios for Inference of Biological Sex from Human Skeletal Remains. Forensic Sci. Int. Synerg. 2021, 3, 100202. [Google Scholar] [CrossRef]
- Brůžek, J.; Santos, F.; Dutailly, B.; Murail, P.; Cunha, E. Validation and Reliability of the Sex Estimation of the Human Os Coxae Using Freely Available DSP2 Software for Bioarchaeology and Forensic Anthropology. Am. J. Phys. Anthropol. 2017, 164, 440–449. [Google Scholar] [CrossRef]
- Pilmann Kotěrová, A.; Santos, F.; Bejdová, Š.; Rmoutilová, R.; Attia, M.H.; Habiba, A.; Velemínská, J.; Brůžek, J. Prioritizing a High Posterior Probability Threshold Leading to Low Error Rate over High Classification Accuracy: The Validity of MorphoPASSE Software for Cranial Morphological Sex Estimation in a Contemporary Population. Int. J. Leg. Med. 2024, 138, 1759–1768. [Google Scholar] [CrossRef]
- López-Lázaro, S.; Pérez-Fernández, A.; Alemán, I.; Viciano, J. Sex Estimation of the Humerus: A Geometric Morphometric Analysis in an Adult Sample. Leg. Med. 2020, 47, 101773. [Google Scholar] [CrossRef] [PubMed]
- Seldin, M.F.; Shigeta, R.; Villoslada, P.; Selmi, C.; Tuomilehto, J.; Silva, G.; Belmont, J.W.; Klareskog, L.; Gregersen, P.K. European Population Substructure: Clustering of Northern and Southern Populations. PLoS Genet. 2006, 2, e143. [Google Scholar] [CrossRef]
- Suazo Galdames, I.; Zavando, D. Age Effect in the Morphological Traits Performance for Sex Determination in Human Skulls and Mandibles. Int. J. Morphol. 2012, 30, 296–301. [Google Scholar] [CrossRef]
- Selliah, P.; Martino, F.; Cummaudo, M.; Indra, L.; Biehler-Gomez, L.; Campobasso, C.P.; Cattaneo, C. Sex Estimation of Skeletons in Middle and Late Adulthood: Reliability of Pelvic Morphological Traits and Long Bone Metrics on an Italian Skeletal Collection. Int. J. Leg. Med. 2020, 134, 1683–1690. [Google Scholar] [CrossRef]
- Ahlborg, H.G.; Johnell, O.; Turner, C.H.; Rannevik, G.; Karlsson, M.K. Bone Loss and Bone Size After Menopause: Obstet. Gynecol. Surv. 2004, 59, 103–105. [Google Scholar] [CrossRef]
- Velemínská, J.; Fleischmannová, N.; Suchá, B.; Dupej, J.; Bejdová, Š.; Kotěrová, A.; Brůžek, J. Age-Related Differences in Cranial Sexual Dimorphism in Contemporary Europe. Int. J. Leg. Med. 2021, 135, 2033–2044. [Google Scholar] [CrossRef] [PubMed]
- Carvallo, D.; Retamal, R. Sex estimation using the proximal end of the femur on a modern Chilean sample. Forensic Sci. Int. Rep. 2020, 2, 100077. [Google Scholar] [CrossRef]
| Number of Individuals | Age Range | Mean Age | SD | |
|---|---|---|---|---|
| Males | 27 | 70–98 | 84.37 | 6.68 |
| Females | 25 | 77–95 | 85.64 | 5.69 |
| Total | 52 | 70–98 | 84.98 | 6.19 |
| Observer | Male Accuracy% | Female Accuracy% | Overall Accuracy% | Sex Bias% | Cohen’s Kappa Coefficient and Confidence Intervals (CI) |
|---|---|---|---|---|---|
| A | 40 | 0 | 20 | 40 | 0.348 (95% CI, 0.24 to 0.94), p = 0.26 |
| B | 100 | 0 | 50 | 100 | 0.00 |
| C | 100 | 20 | 60 | 80 | 0.286 (95% CI, 0.18–0.76), p = 0.19 |
| D | 60 | 20 | 40 | 40 | 0.348 (95% CI, 0.24 to 0.94), p = 0.26 |
| E | 80 | 0 | 40 | 80 | −0.190 (95% CI, −0.51 to 0.139), p = 0.38 |
| F | 60 | 0 | 30 | 60 | 0.348 (95% CI, 0.24 to 0.94), p = 0.26 |
| G | 80 | 0 | 40 | 80 | 0.286 (95% CI, 0.18 to 0.76), p = 0.19 |
| Average | 74 | 6 | 40 | 68.57 |
| Probability Threshold | |||
|---|---|---|---|
| >0.50 | ≥0.80 | ≥0.95 | |
| Male (n/%) | 18/100 | 15/83.33 | 11/61.11 |
| Female (n/%) | 18/100 | 15/83.33 | 9/50 |
| Overall (N/%) | 36/100 | 30/83.33 | 20/55.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todd, M.S.; García-Donas, J.G. Feasibility and Reliability of Ammer–Coelho Computational Tool for Sex Estimation: A Pilot Study on an Elderly Scottish Sample. Forensic Sci. 2025, 5, 49. https://doi.org/10.3390/forensicsci5040049
Todd MS, García-Donas JG. Feasibility and Reliability of Ammer–Coelho Computational Tool for Sex Estimation: A Pilot Study on an Elderly Scottish Sample. Forensic Sciences. 2025; 5(4):49. https://doi.org/10.3390/forensicsci5040049
Chicago/Turabian StyleTodd, Mackenzie S., and Julieta G. García-Donas. 2025. "Feasibility and Reliability of Ammer–Coelho Computational Tool for Sex Estimation: A Pilot Study on an Elderly Scottish Sample" Forensic Sciences 5, no. 4: 49. https://doi.org/10.3390/forensicsci5040049
APA StyleTodd, M. S., & García-Donas, J. G. (2025). Feasibility and Reliability of Ammer–Coelho Computational Tool for Sex Estimation: A Pilot Study on an Elderly Scottish Sample. Forensic Sciences, 5(4), 49. https://doi.org/10.3390/forensicsci5040049






