Comparison of Derivatization Methods for Groomed Latent Print Residues Analysis via Gas Chromatography
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Latent Print Residue Collection
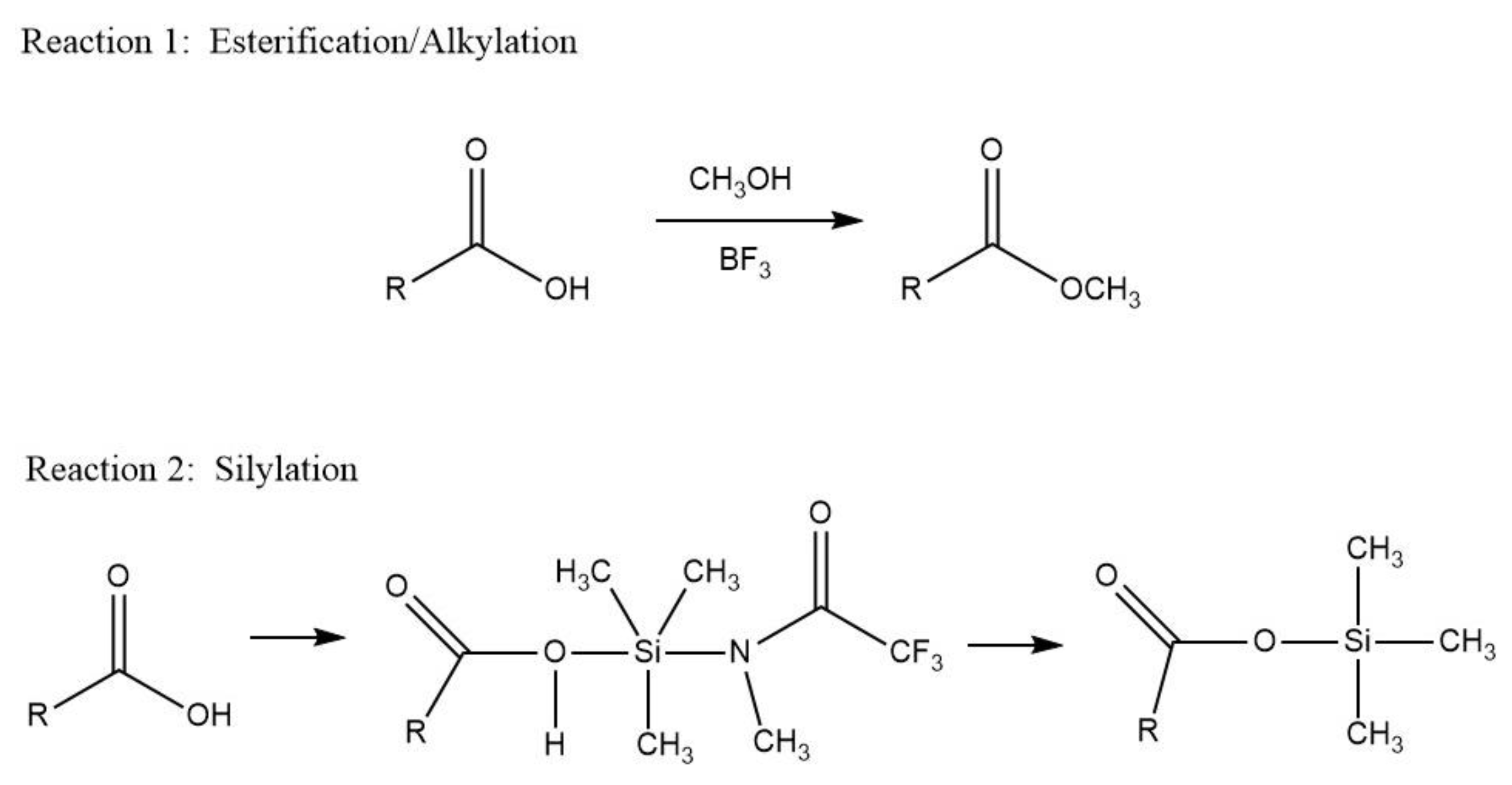
2.2.2. Derivatization Sample Preparation Protocols
Esterification Derivatization Process
Silylation Derivatization Process
Non-Derivatization Sample Preparation
2.2.3. Extraction Recovery
2.2.4. Instrumental Parameters
2.2.5. Data Analysis
3. Results and Discussion
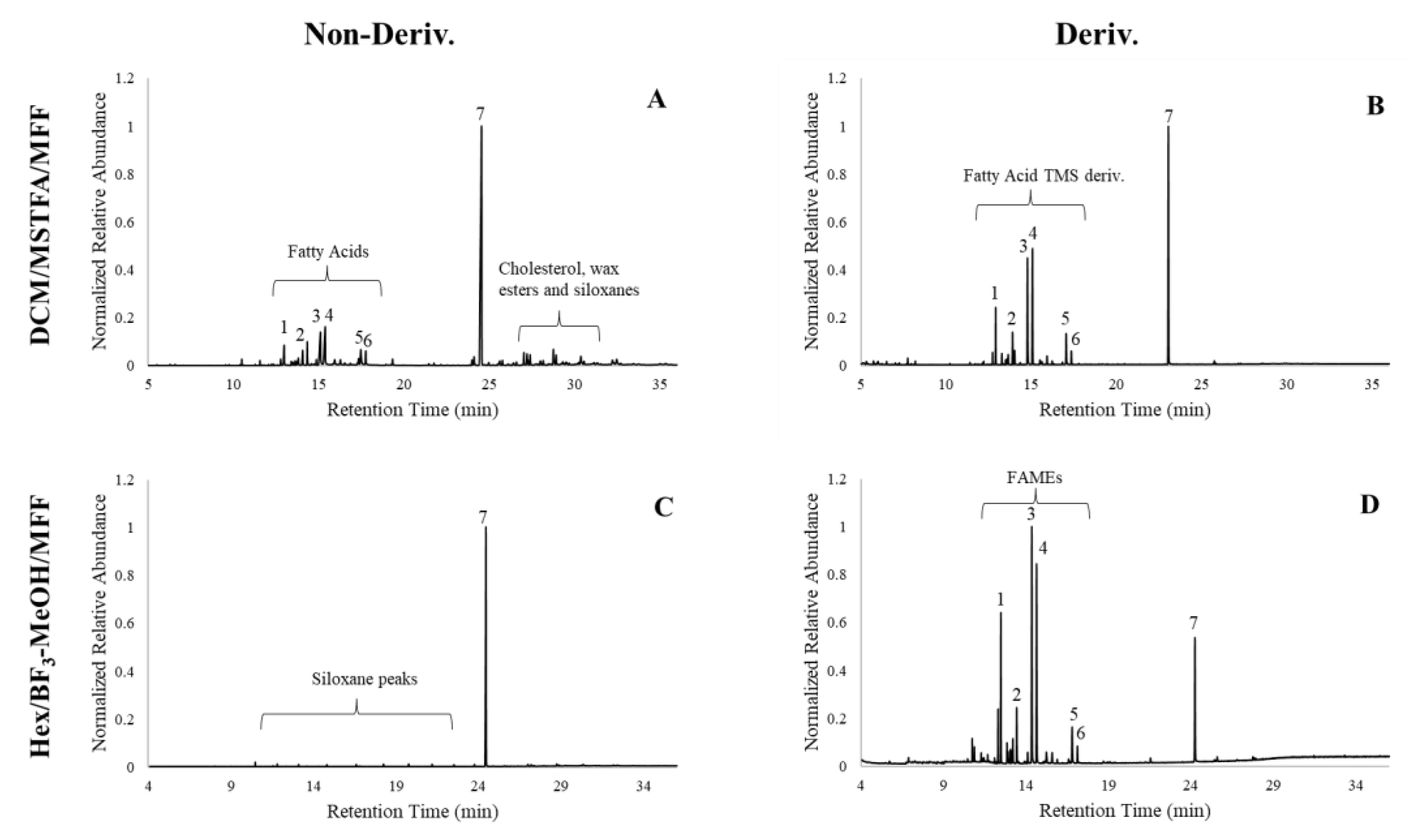
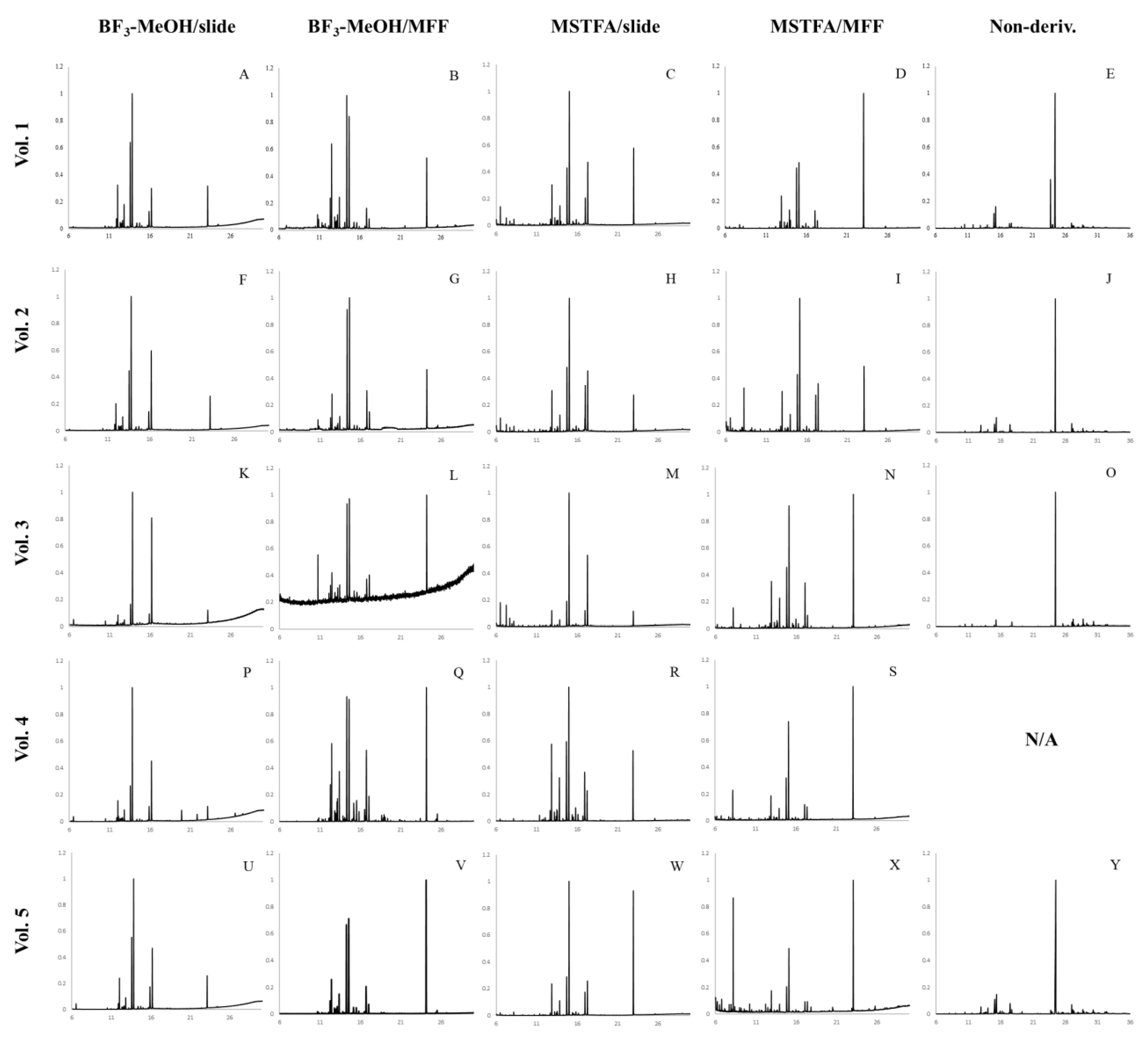
3.1. BF3 Derivatization
3.1.1. Solid Surfaces
3.1.2. Porous Surfaces
3.2. MSTFA Derivatization
3.2.1. Solid Surfaces
3.2.2. Porous Surfaces
3.3. Best Derivatization Method for Each Substrate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Girod, A.; Weyermann, C. Lipid composition of fingermark residue and donor classification using GC/MS. Forensic Sci. Int. 2014, 238, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Weyermann, C.; Roux, C.; Champod, C. Initial Results on the Composition of Fingerprints and its Evolution as a Function of Time by GC/MS Analysis. J. Forensic Sci. 2011, 56, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Koenig, A.; Girod, A.; Weyermann, C. Identification of Wax Esters in Latent Print Residues by Gas Chromatography-Mass Spectromertry and Their Potential Use as Aging Parameters. J. Forensic Ident. 2011, 61, 652. [Google Scholar]
- Frick, A.; Chidlow, G.; Lewis, S.; van Bronswijk, W. Investigations into the initial composition of latent fingermark lipids by gas chromatography–mass spectrometry. Forensic Sci. Int. 2015, 254, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Croxton, R.S.; Baron, M.; Butler, D.; Kent, T.; Sears, V.G. Development of a GC-MS Method for the Simultaneous Analysis of Latent Fingerprint Components. J. Forensic Sci. 2006, 51, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Cadd, S.J.; Mota, L.; Werkman, D.; Islam, M.; Zuidberg, M.; de Puit, M. Extraction of fatty compounds from fingerprints for GCMS analysis. Anal. Methods 2015, 7, 1123–1132. [Google Scholar] [CrossRef]
- Croxton, R.S.; Baron, M.G.; Butler, D.; Kent, T.; Sears, V.G. Variation in amino acid and lipid composition of latent fingerprints. Forensic Sci. Int. 2010, 199, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Pleik, S.; Spengler, B.; Schäfer, T.; Urbach, D.; Luhn, S.; Kirsch, D. Fatty Acid Structure and Degradation Analysis in Fingerprint Residues. J. Am. Soc. Mass Spectrom. 2016, 27, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Knapp, D.R. Handbook of Analytical Derivatization Reactions; Wiley: New York, NY, USA, 1979. [Google Scholar]
- Blau, K.; King, G.S. Handbook of Derivatives for Chromatography; Reprinted with corrections. ed.; Heyden: London, UK, 1978. [Google Scholar]
- Orata, F. Derivatization reactions and reagents for gas chromatography analysis. In Advanced Gas Chromatography—Progress in Agricultural, Biomedical and Industrial Applications; InTech: Rijeka, Croatia, 2012; pp. 83–156. [Google Scholar]
- Archer, N.E.; Charles, Y.; Elliott, J.A.; Jickells, S. Changes in the lipid composition of latent fingerprint residue with time after deposition on a surface. Forensic Sci. Int. 2005, 154, 224–239. [Google Scholar] [CrossRef] [PubMed]
- Michael-Jubeli, R.; Bleton, J.; Baillet-Guffroy, A. High-temperature gas chromatography-mass spectrometry for skin surface lipids profiling. J. Lipid Res. 2011, 52, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Hartzell-Baguley, B.; Hipp, R.E.; Morgan, N.R.; Morgan, S.L. Chemical Composition of Latent Fingerprints by Gas Chromatography–Mass Spectrometry. An Experiment for an Instrumental Analysis Course. J. Chem. Educ. 2007, 84, 689. [Google Scholar] [CrossRef]
- Raghallaigh, S.N.; Bender, K.; Lacey, N.; Brennan, L.; Powell, F. The fatty acid profile of the skin surface lipid layer in papulopustular rosacea. Br. J. Dermatol. 2012, 166, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom, K.M.; Labows, J.N.; McGinley, K.J.; Leyden, J.J. Characterization of Wax Esters, Triglycerides, and Free Fatty Acids of Follicular Casts. J. Investig. Dermatol. 1986, 86, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, K.; Fukubayashi, Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J. Lipid Res. 2010, 51, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Thermo Scientific Reagents, Solvents, and Accessories. Available online: https://tools.thermofisher.com/content/sfs/brochures/BR-20535-GC-LC-MS-Reagents-Solvents-Accessories-BR20535-EN.pdf (accessed on 6 May 2018).
- Greene, R.S.; Downing, D.T.; Pochi, P.E.; Strauss, J.S. Anatomical Variation in the Amount and Composition of Human Skin Surface Lipid. J. Investig. Dermatol. 1970, 54, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Thody, A.J.; Shuster, S. Control and function of sebaceous glands. Physiol. Rev. 1989, 69, 383–416. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Risk Management for Methylene Chloride. Available online: https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/risk-management-methylene-chloride (accessed on 8 May 2023).
- University of Pennsylvania Environmental Health & Radiation Safety. Fact Sheet: Solvent Alternatives. Available online: https://ehrs.upenn.edu/health-safety/lab-safety/chemical-hygiene-plan/fact-sheets/fact-sheet-solvent-alternatives (accessed on 8 May 2023).
- Michalski, S.; Shaler, R.; Dorman, F.L. The Evaluation of Fatty Acid Ratios in Latent Fingermarks by Gas Chromatography/Mass Spectrometry (GC/MS) Analysis. J. Forensic Sci. 2013, 58, S215–S220. [Google Scholar] [CrossRef] [PubMed]
- de Puit, M.; Ismail, M.; Xu, X. LCMS Analysis of Fingerprints, the Amino Acid Profile of 20 Donors. J. Forensic Sci. 2014, 59, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Girod, A.; Ramotowski, R.; Weyermann, C. Composition of fingermark residue: A qualitative and quantitative review. Forensic Sci. Int. 2012, 223, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Derivatization of Fatty acids to FAMEs. Available online: https://www.sigmaaldrich.com/US/en/technical-documents/protocol/food-and-beverage-testing-and-manufacturing/chemical-analysis-for-food-and-beverage/derivatization-of-fatty-acids-to-fames (accessed on 3 April 2018).
| Test | Experiment Description |
|---|---|
| Test 1 | time trials of 1 min and 45 min extractions using DCM and hexanes |
| Test 2 | squalene extraction and derivatization |
| Test 3 | extraction test post-evaporation |
| Test 4 | step-by-step extraction and derivatization test for BF3-MeOH and MSTFA
|
| Component Identification |
|---|
| Myristic acid |
| Pentadecanoic acid |
| Palmitoleic acid |
| Palmitic acid |
| Oleic acid |
| Stearic acid |
| Squalene |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kindell, J.; Bridge, C. Comparison of Derivatization Methods for Groomed Latent Print Residues Analysis via Gas Chromatography. Forensic Sci. 2023, 3, 302-315. https://doi.org/10.3390/forensicsci3020023
Kindell J, Bridge C. Comparison of Derivatization Methods for Groomed Latent Print Residues Analysis via Gas Chromatography. Forensic Sciences. 2023; 3(2):302-315. https://doi.org/10.3390/forensicsci3020023
Chicago/Turabian StyleKindell, Jessica, and Candice Bridge. 2023. "Comparison of Derivatization Methods for Groomed Latent Print Residues Analysis via Gas Chromatography" Forensic Sciences 3, no. 2: 302-315. https://doi.org/10.3390/forensicsci3020023
APA StyleKindell, J., & Bridge, C. (2023). Comparison of Derivatization Methods for Groomed Latent Print Residues Analysis via Gas Chromatography. Forensic Sciences, 3(2), 302-315. https://doi.org/10.3390/forensicsci3020023






