Utility of Osteoarthritis as an Indicator of Age in Human Skeletal Remains: Validating the Winburn and Stock (2019) Method
Abstract
1. Introduction
A Brief Review of Osteoarthritis
2. Materials and Methods
2.1. The Hypothesis and Study Sample
2.2. Data Collection
3. Results
3.1. Age Distributions
3.2. GLM-Probit Analyses
3.3. Estimation of Age at Death: Applying the Winburn and Stock [33] Method
3.4. Interobserver Error
3.5. Intraobserver Error
4. Discussion
4.1. Validating Winburn and Stock [33]
4.2. Inter- and Intraobserver Error
4.3. Methodological Recommendations
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goad, G. Expanding humanitarian forensic action: An approach to US cold cases. Forensic Anthropol. 2020, 3, 50. [Google Scholar] [CrossRef]
- Kimmerle, E.H.; Falsetti, A.; Ross, A.H. Immigrants, undocumented workers, runaways, transients and the homeless: Towards contextual identification among unidentified decedents. Forensic Sci. Policy Manag. 2010, 1, 178–186. [Google Scholar] [CrossRef]
- Winburn, A.; Jennings, A.; Steadman, D.; DiGangi, E. Ancestral diversity in skeletal collections: Perspectives on African American body donation. Forensic Anthropol. 2022, 5, 141–152. [Google Scholar] [CrossRef]
- Geronimus, A.T. The weathering hypothesis and the health of African-American women and infants: Evidence and speculations. Ethn. Dis. 1992, 2, 207–221. [Google Scholar]
- Simons, R.L.; Lei, M.K.; Klopack, E.; Beach, S.R.; Gibbons, F.X.; Philibert, R.A. The effects of social adversity, discrimination, and health risk behaviors on the accelerated aging of African Americans: Further support for the weathering hypothesis. Soc. Sci. Med. 2021, 282, 113169. [Google Scholar] [CrossRef]
- Gravlee, C.C. How race becomes biology: Embodiment of social inequality. Am. J. Phys. Anthropol. 2009, 139, 47–57. [Google Scholar] [CrossRef]
- Krieger, N. Embodiment: A conceptual glossary for epidemiology. J. Epidemiol. Community Health 2005, 59, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Kubzansky, L.D.; Seeman, T.E.; Glymour, M.M. Biological pathways linking social conditions and health. Soc. Epidemiol. 2014, 14, 512–561. [Google Scholar]
- Godde, K.; Gough Courtney, M.; Roberts, J. Health Insurance Coverage as a Social Determinant of Osteoporosis Diagnosis in a Population-Based Cohort Study of Older American Adults. J. Appl. Gerontol. 2023, 42, 302–312. [Google Scholar] [CrossRef]
- Gough Courtney, M.; Quintero, Y.; Godde, K. Assessing the roles of demographic, social, economic, environmental, health-related, and political factors on risk of osteoporosis diagnosis among older adults. Arch. Osteoporos. 2021, 16, 177. [Google Scholar] [CrossRef]
- Walkup, T.N.; Winburn, A.P. Does structural violence impact forensic anthropological age estimation? Investigating Skeletal Indicators of Biological “Weathering” in Modern U.S. Individuals. In Proceedings of the American Academy of Forensic Sciences 74th Annual Scientific Conference, Seattle, WA, USA, 25 February 2022. [Google Scholar]
- Burt, N.M. Identification and Interpretation of Joint Disease in Paleopathology and Forensic Anthropology; Charles C Thomas: Springfield, IL, USA, 2013. [Google Scholar]
- Reynard, L.N.; Loughlin, J. Genetics and epigenetics of osteoarthritis. Maturitas 2012, 71, 200–204. [Google Scholar] [CrossRef]
- Winburn, A.P. Validation of the acetabulum as a skeletal indicator of age at death in modern European-Americans. J. Forensic Sci. 2019, 64, 989–1003. [Google Scholar] [CrossRef]
- Felson, D.T. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol. Clin. North Am. 2004, 42, 1–9. [Google Scholar] [CrossRef]
- Hashimoto, M.; Nakasa, T.; Hikata, T.; Asahara, H. Molecular network of cartilage homeostasis and osteoarthritis. Med. Res. Rev. 2008, 28, 464–481. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Eckstein, F. Exercise and osteoarthritis. J. Anat. 2009, 214, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Lajeunesse, D.; Reboul, P.; Pelletier, J.P. The role of subchondral bone in osteoarthritis. In Osteoarthritis: A Companion to Rheumatology; Sharma, L., Berenbaum, F., Eds.; Mosby, Inc. Elsevier: Amsterdam, The Netherlands, 2007; pp. 15–32. [Google Scholar]
- Camarero-Espinosa, S.; Rothen-Rutishauser, B.; Foster, E.J.; Weder, C. Articular cartilage: From formation to tissue engineering. Biomater. Sci. 2016, 4, 734–767. [Google Scholar] [CrossRef] [PubMed]
- Aigner, T.; Soeder, S.; Haag, J. IL-1ß and BMPs-Interactive players of cartilage matrix degradation and regeneration. Eur. Cell. Mater. 2006, 12, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Arden, N.; Nevitt, M.C. Osteoarthritis: Epidemiology. Best Practice and Research. Clin. Rheumatol. 2006, 20, 3–25. [Google Scholar]
- Hernigou, P.; Delambre, J.; Quiennec, S.; Poignard, A. Human bone marrow mesenchymal stem cell injection in subchondral lesions of knee osteoarthritis: A prospective randomized study versus contralateral arthroplasty at a mean fifteen year follow-up. Int. Orthop. 2021, 45, 365–373. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, J.; Zhen, G.; Hu, Y.; An, S.; Li, Y.; Zheng, Q.; Chen, Z.; Yang, Y.; Wan, M.; et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis. J. Clin. Investig. 2019, 129, 1076–1093. [Google Scholar] [CrossRef] [PubMed]
- Myszka, A.; Piontek, J.; Tomczyk, J.; Lisowska-Gaczorek, A.; Zalewska, M. Relationships between osteoarthritic changes (osteophytes, porosity, eburnation) based on historical skeletal material. Ann. Hum. Biol. 2020, 47, 263–272. [Google Scholar] [CrossRef]
- Rogers, J. The palaeopathology of joint disease. In Human Osteology in Archaeology and Forensic Science; Cambridge University Press: Cambridge, UK, 2000; pp. 163–182. [Google Scholar]
- Waldron, T.; Rogers, J. Inter-observer variation in coding osteoarthritis in human skeletal remains. Int. J. Osteoarchaeol. 1991, 1, 49–56. [Google Scholar] [CrossRef]
- Weiss, E. Reading the Bones: Activity, Biology, and Culture; University Press of Florida: Gainesville, FL, USA, 2017. [Google Scholar]
- Alves-Cardoso, F.; Assis, S. Can osteophytes be used as age at death estimators? Testing correlations in skeletonized human remains with known age at death. Forensic Sci. Int. 2018, 288, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Brennaman, A.L.; Love, K.R.; Bethard, J.D.; Pokines, J.T. A bayesian approach to age-at-death estimation from osteoarthritis of the shoulder in modern North Americans. J. Forensic Sci. 2017, 62, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Calce, S.E.; Kurki, H.K.; Weston, D.A.; Gould, L. The relationship of age, activity, and body size on osteoarthritis in weight-bearing skeletal regions. Int. J. Paleopathol. 2018, 22, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Calce, S.E.; Kurki, H.K.; Weston, D.A.; Gould, L. Effects of osteoarthritis on age-at-death estimates from the human pelvis. Am. J. Phys. Anthropol. 2018, 167, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Winburn, A.P.; Stock, M.K. Reconsidering osteoarthritis as a skeletal indicator of age at death. Am. J. Phys. Anthropol. 2019, 170, 459–473. [Google Scholar] [CrossRef]
- Falys, C.G.; Prangle, D. Estimating age of mature adults from the degeneration of the sternal end of the clavicle. Am. J. Phys. Anthropol. 2015, 156, 203–214. [Google Scholar] [CrossRef]
- Boldsen, J.L.; Milner, G.R.; Konigsberg, L.W.; Wood, J.W. Transition analysis: A new method for estimating age from skeletons. In Paleodemography: Age Distributions from Skeletal Samples; Hoppa, R.D., Vaupel, J.W., Eds.; Cambridge University Press: Cambridge, UK, 2002; pp. 73–106. [Google Scholar]
- Milner, G.R.; Boldsen, J.L. Transition analysis: A validation study with known-age modern American skeletons. Am. J. Phys. Anthropol. 2012, 148, 98–110. [Google Scholar] [CrossRef]
- Epel, E.S.; Blackburn, E.H.; Lin, J.; Dhabhar, F.S.; Adler, N.E.; Morrow, J.D.; Cawthon, R.M. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA 2004, 101, 17312–17315. [Google Scholar] [CrossRef] [PubMed]
- Kuszel, L.; Trzeciak, T.; Richter, M.; Czarny-Ratajczak, M. Osteoarthritis and telomere shortening. J. Appl. Genet. 2015, 56, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Deo, M.E. Why BIPOC fails. Va. L. Rev. Online 2021, 107, 115. [Google Scholar]
- Vidoli, G.M.; Steadman, D.W.; Devlin, J.B.; Jantz, L.M. History and development of the first anthropology research facility, Knoxville, Tennessee. In Taphonomy of Human Remains: Forensic Analysis of the Dead and the Depositional Environment: Forensic Analysis of the Dead and the Depositional Environment; Wiley Online Library: Hoboken, NJ, USA, 2017; pp. 461–475. [Google Scholar]
- Jurmain, R. Paleoepidemiology of a central California prehistoric population from CA-ALA-329: II. Degenerative disease. Am. J. Phys. Anthropol. 1990, 83, 83–94. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing (Version 4.1.0, “Camp Pontanezen”). 2021. Available online: http://www.Rproject.org/ (accessed on 6 August 2021).
- Nelder, J.A.; Wedderburn, R.W.M. Generalized linear models. J. R. Stat. Soc. Ser. A Gen. 1972, 135, 370–384. [Google Scholar] [CrossRef]
- Yee, T.W. Vector Generalized Linear and Additive Models: With an Implementation in R; Springer: New York, NY, USA, 2015. [Google Scholar]
- Bera, A.K.; Jarque, C.M.; Lee, L.-F. Testing the normality assumption in limited dependent variable models. Int. Econ. Rev. 1984, 25, 563–578. [Google Scholar] [CrossRef]
- Johnson, P.A. A test of the normality assumption in the ordered probit model. Metron 1996, 54, 213–221. [Google Scholar]
- Konigsberg, L. Transition Analysis (& Other Stuff). Available online: http://faculty.las.illinois.edu/lylek/TransAna/TransAna.html (accessed on 7 March 2023).
- Cohen, J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Cohen, J. Weighted kappa: Nominal scale agreement provision for scaled disagreement or partial credit. Psychol. Bull. 1968, 70, 213–220. [Google Scholar] [CrossRef]
- Morrison, L.W. Observer error in vegetation surveys: A review. J. Plant Ecol. 2016, 9, 367–379. [Google Scholar] [CrossRef]
- Zhang, T. Iteratively reweighted least squares with random effects for maximum likelihood in generalized linear mixed effects models. J. Stat. Comput. Simul. 2021, 91, 3404–3425. [Google Scholar] [CrossRef]
- Becker, S.K.; Goldstein, P.S. Evidence of osteoarthritis in the Tiwanaku colony, Moquegua, Peru (AD 500–1100). Int. J. Osteoarchaeol. 2018, 28, 54–64. [Google Scholar] [CrossRef]
- Eng, J.T. A bioarchaeological study of osteoarthritis among populations of northern China and Mongolia during the Bronze Age to Iron Age transition to nomadic pastoralism. Quat. Int. 2016, 405, 172–185. [Google Scholar] [CrossRef]
- Klaus, H.D.; Spencer Larsen, C.; Tam, M.E. Economic intensification and degenerative joint disease: Life and labor on the postcontact north coast of Peru. Am. J. Phys. Anthropol. 2009, 139, 204–221. [Google Scholar] [CrossRef] [PubMed]
- Molnar, P.; Ahlstrom, T.P.; Leden, I. Osteoarthritis and activity—An analysis of the relationship between eburnation, Musculoskeletal Stress Markers (MSM) and age in two Neolithic hunter–gatherer populations from Gotland, Sweden. Int. J. Osteoarchaeol. 2011, 21, 283–291. [Google Scholar] [CrossRef]
- Palmer, J.L.; Hoogland, M.H.; Waters-Rist, A.L. Activity reconstruction of post- medieval Dutch rural villagers from upper limb osteoarthritis and entheseal changes. Int. J. Osteoarchaeol. 2016, 26, 78–92. [Google Scholar] [CrossRef]
- Schrader, S.A. Activity patterns in New Kingdom Nubia: An examination of entheseal remodeling and osteoarthritis at Tombos. Am. J. Phys. Anthropol. 2012, 149, 60–70. [Google Scholar] [CrossRef]
- Zhang, H.; Merrett, D.C.; Jing, Z.; Tang, J.; He, Y.; Yue, H.; Yue, Z.; Yang, D.Y. Osteoarthritis, labour division, and occupational specialization of the Late Shang China-insights from Yinxu (ca. 1250–1046 BC). PLoS ONE 2017, 12, e0176329. [Google Scholar]
- Heidari, B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Casp. J. Intern. Med. 2011, 2, 205. [Google Scholar]
- Meehan, J.P.; Danielsen, B.; Tancredi, D.J.; Kim, S.; Jamali, A.A.; White, R.H. A population-based comparison of the incidence of adverse outcomes after simultaneous- bilateral and staged-bilateral total knee arthroplasty. JBJS 2011, 93, 2203–2213. [Google Scholar] [CrossRef]
- Jaffar, A.A.; Abass, S.J.; Ismael, M.Q. Biomechanical aspects of shoulder and hip articulations: A comparison of two ball and socket joints. Al-Khwarizmi Eng. J. 2006, 2, 1–14. [Google Scholar]
- Wood, J.W.; Milner, G.R.; Harpending, H.C.; Weiss, K.M.; Cohen, M.N.; Eisenberg, L.E.; Hutchinson, D.L.; Jankauskas, R.; Cesnys, G.; Česnys, G.; et al. The osteological paradox: Problems of inferring prehistoric health from skeletal samples [and comments and reply]. Curr. Anthropol. 1992, 33, 343–370. [Google Scholar] [CrossRef]
- Hens, S.M.; Godde, K. A Bayesian Approach to Estimating Age from the Auricular Surface of the Ilium in Modern American Skeletal Samples. Forensic Sci. 2022, 2, 682–695. [Google Scholar] [CrossRef]
- Bowleg, L. The problem with the phrase women and minorities: Intersectionality—An important theoretical framework for public health. Am. J. Public Health 2012, 102, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Galtung, J. Violence, peace, and peace research. J. Peace Res. 1969, 6, 167–191. [Google Scholar] [CrossRef]
- Khanlou, N.; Vazquez, L.M.; Pashang, S.; Connolly, J.A.; Ahmad, F.; Ssawe, A. 2020 syndemic: Convergence of COVID-19, gender-based violence, and racism pandemics. J. Racial Ethn. Health Disparities 2021, 9, 2077–2089. [Google Scholar] [CrossRef]
- Panter-Brick, C. Health, risk, and resilience: Interdisciplinary concepts and applications. Annu. Rev. Anthropol. 2014, 43, 431–448. [Google Scholar] [CrossRef]
- Casagrande, K. Understanding Barriers to Whole-Body Donation to Forensic Anthropology Facilities: Implications for Criminal Investigations. Unpublished Thesis, Texas State University, San Marcos, TX, USA, 2020. [Google Scholar]
- de la Cova, C. Marginalized bodies and the construction of the Robert J. Terry Anatomical Skeletal Collection: A promised land lost. In Bioarchaeology of Marginalized People; Mant, M., Holland, A., Eds.; Elsevier Academic Press: Cambridge, MA, USA, 2019; pp. 133–155. [Google Scholar]
- Hunt, D.R.; Albanese, J. History and demographic composition of the Robert J. Terry anatomical collection. Am. J. Phys. Anthropol. 2005, 127, 406–417. [Google Scholar] [CrossRef]
- Brandon, D.T.; Isaac, L.A.; LaVeist, T.A. The legacy of Tuskegee and trust in medical care: Is Tuskegee responsible for race differences in mistrust of medical care? J. Natl. Med. Assoc. 2005, 97, 951. [Google Scholar]
- Edgar, H.J.; Berry, S.R. NMDID: A new research resource for biological anthropology. Am. J. Phys. Anthropol. Suppl. 2019, 168, 66. [Google Scholar]
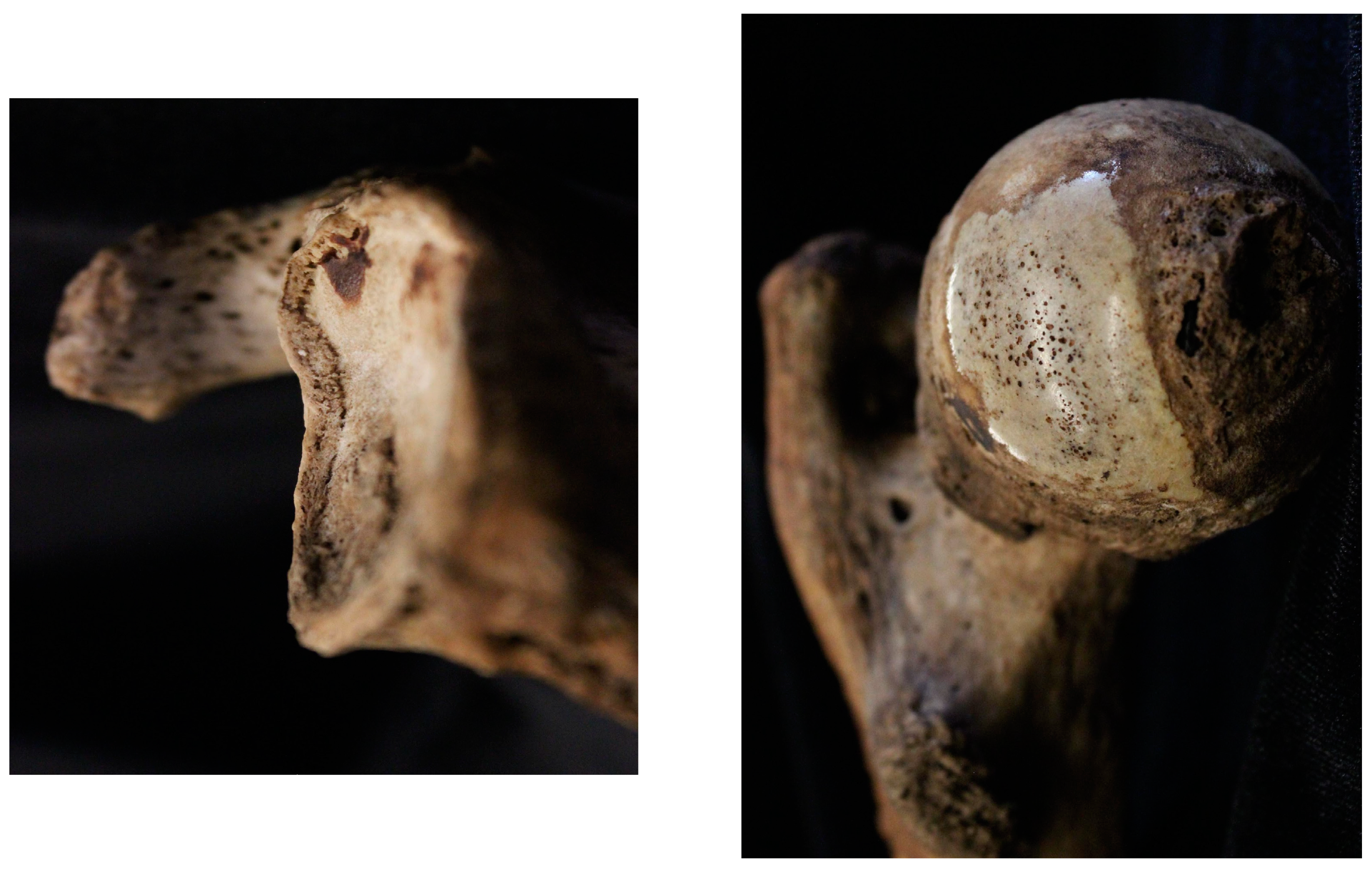

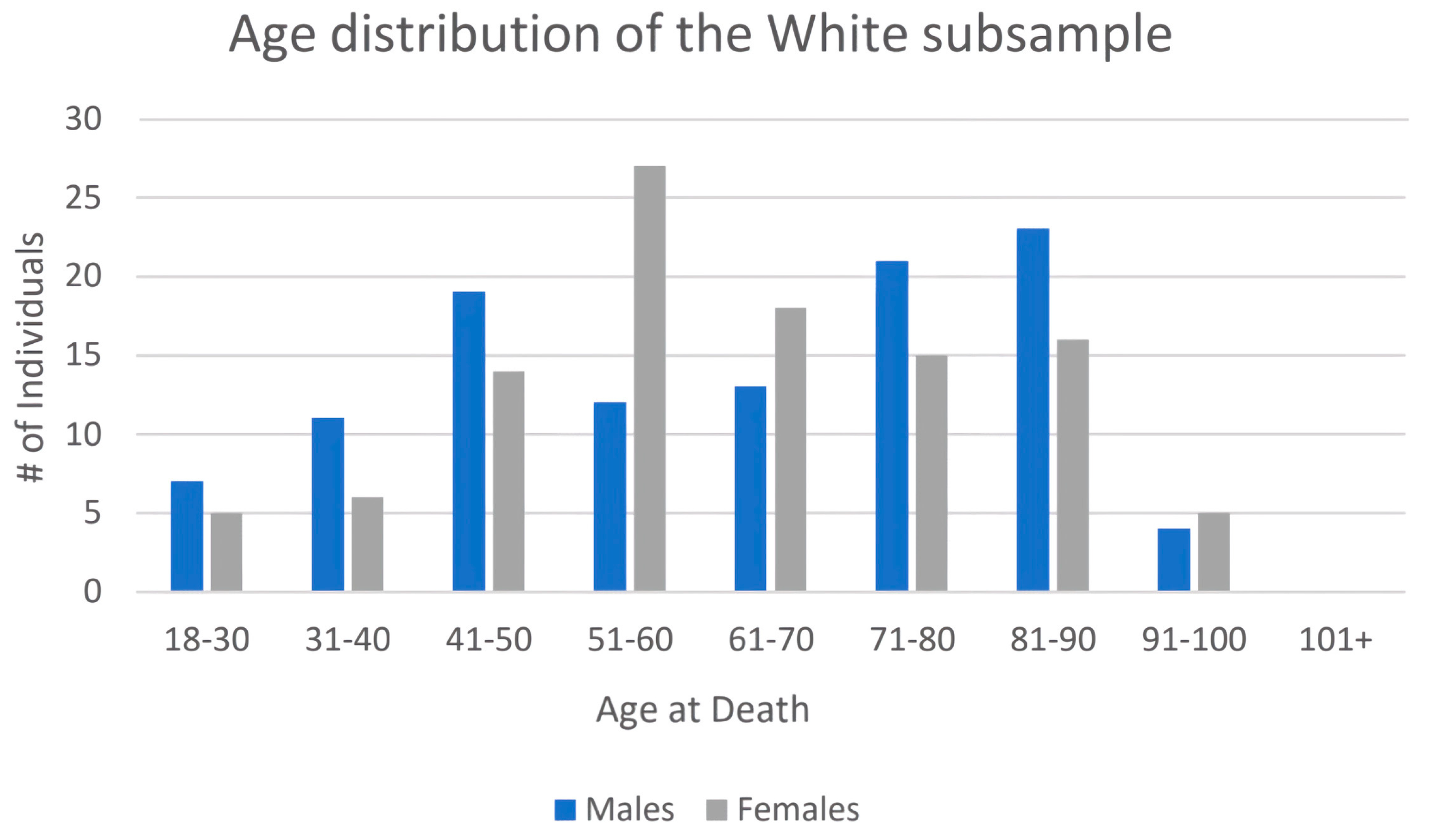
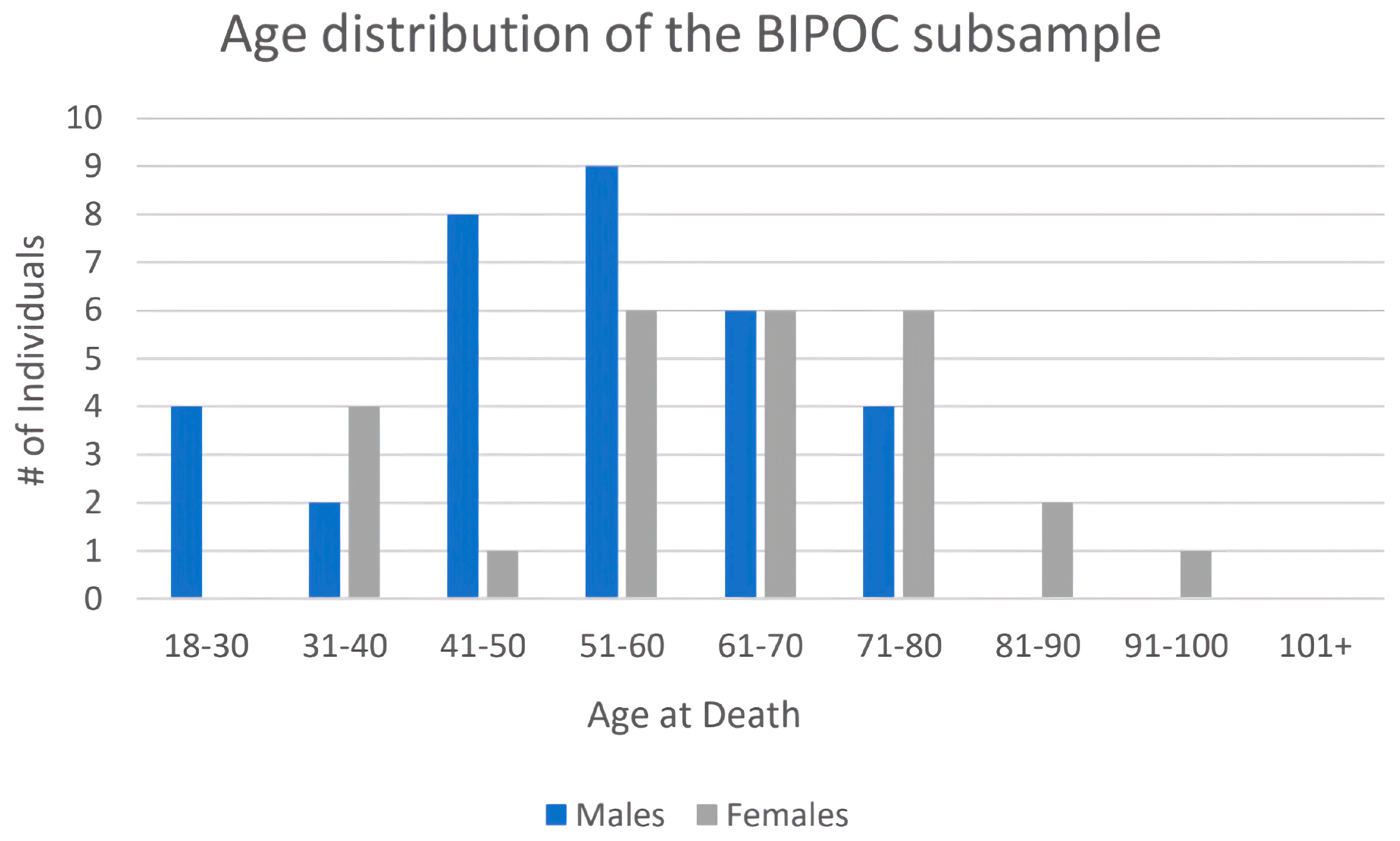
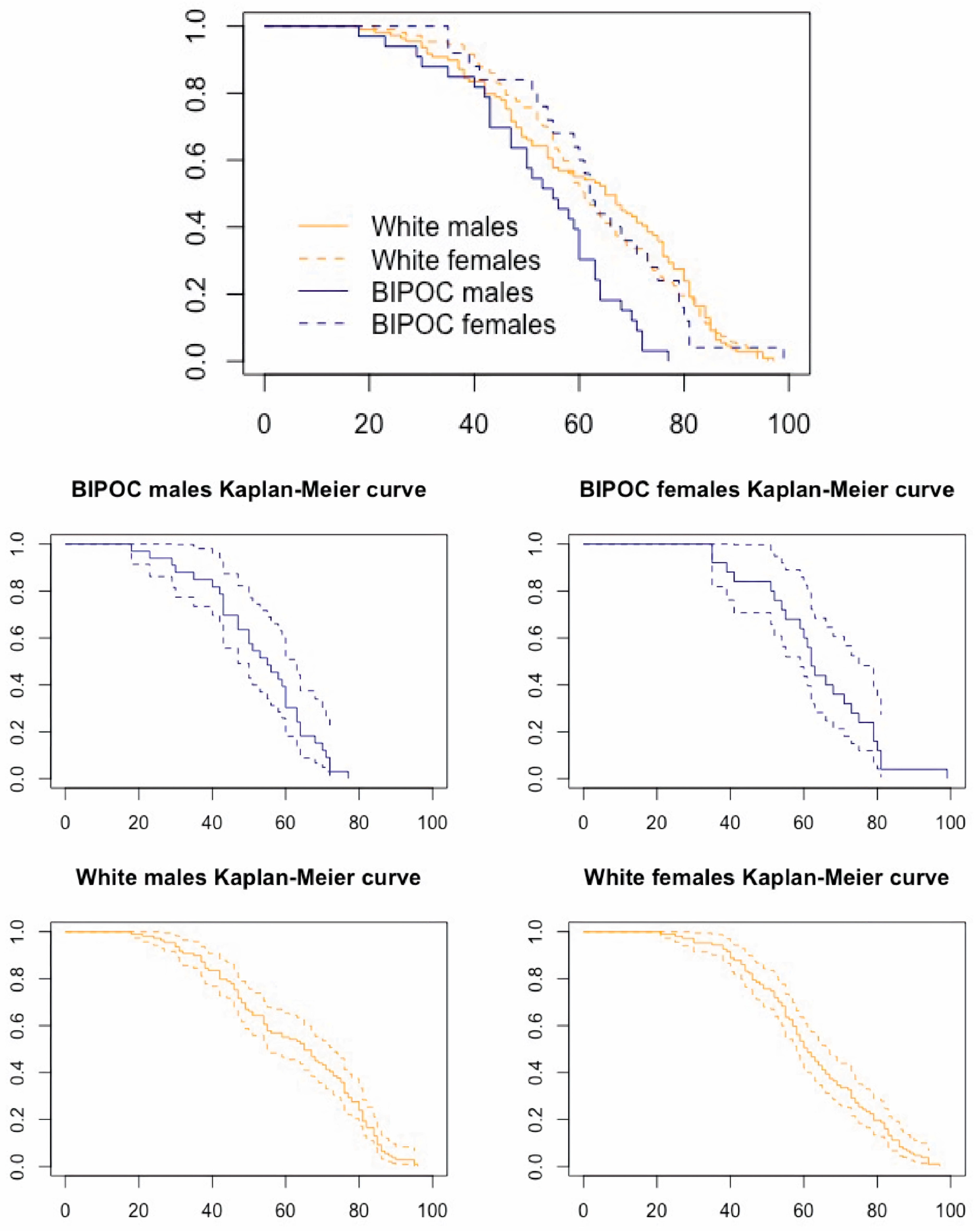
| Age at Death | # White Males | # White Females | # BIPOC Males | # BIPOC Females |
|---|---|---|---|---|
| 18–30 | 7 | 5 | 4 | 0 |
| 31–40 | 11 | 6 | 2 | 4 |
| 41–50 | 19 | 14 | 8 | 1 |
| 51–60 | 12 | 27 | 9 | 6 |
| 61–70 | 13 | 18 | 6 | 6 |
| 71–80 | 21 | 15 | 4 | 7 |
| 81–90 | 23 | 16 | 0 | 2 |
| 91–100 | 4 | 5 | 0 | 1 |
| TOTAL | 109 | 106 | 33 | 27 |
| Mean ages | 62.2 years | 62.6 years | 52.6 years | 62.3 years |
| Female | Male | Pooled Sex | ||||
|---|---|---|---|---|---|---|
| Joint | 90% | 95% | 90% | 95% | 90% | 95% |
| TMJ | 45.0 | 46.9 | 32.0 | 50.1 | 35.7 | 47.2 |
| Shoulder | Inf | Inf | 43.7 | 48.3 | 41.3 | 45.1 |
| Elbow | Inf | Inf | 18.1 * | 31.5 * | 20.5 | 29.3 |
| Wrist | 52.2 * | 56.9 * | 68.5 | 90.3 | 57.7 | 71.1 |
| Hand | 44.6 * | 53.8 * | 54.2 | 65.5 | 50.4 | 59.8 |
| Hip | Inf | Inf | 38.4 * | 42.7 * | 37.1 | 40.9 |
| Knee | NaN | NaN | 42.2 | 60.6 | 36.1 | 47.3 |
| Ankle | NaN | NaN | 45.1 | 54.0 | 41.1 | 47.9 |
| Foot | 44.6 | 53.8 | 50.7 | 61.3 | 48.3 | 57.6 |
| Female | Male | Pooled Sex | ||||
|---|---|---|---|---|---|---|
| Joint | 90% | 95% | 90% | 95% | 90% | 95% |
| TMJ | 34.8 | 49.1 | 47.8 | 83.0 | 40.0 | 63.3 |
| Shoulder | 36.6 | 40.5 | 47.5 * | 56.3 * | 42.7 | 49.6 |
| Elbow | 27.9 * | 29.8 * | 21.7 | 31.7 | 26.7 | 33.3 |
| Wrist | 49.8 | 57.2 | 42.6 | 47.3 | 46.6 | 53.0 |
| Hand | 35.7 | 44.3 | 44.5 | 54.8 | 41.0 | 50.2 |
| Hip | 27.9 * | 29.8 * | 36.1 | 39.0 | 33.8 | 36.6 |
| Knee | 28.6 * | 30.4 * | 32.3 | 37.2 | 31.2 | 35.1 |
| Foot | 45.9 | 56.3 | 51.0 * | 63.6 * | 48.6 | 60.1 |
| Female | Male | Pooled Sex | ||||
|---|---|---|---|---|---|---|
| Joint | 90% | 95% | 90% | 95% | 90% | 95% |
| TMJ | 43.2 | 56.1 | 51.4 | 67.2 | 42.7 | 61.2 |
| Shoulder | 37.4 | 41.1 | 45.2 | 52.7 | 40.4 | 46.8 |
| Elbow | 31.2 | 33.0 | 32.7 | 43.8 | 28.6 | 35.5 |
| Wrist | 47.8 | 55.8 | 40.8 | 45.0 | 45.6 | 51.5 |
| Hand | 36.6 | 47.9 | 42.0 | 52.0 | 39.6 | 48.6 |
| Hip | 31.1 | 33.1 | 36.4 | 38.6 | 33.8 | 36.3 |
| Knee | 32.0 | 33.8 | 32.4 | 38.1 | 30.1 | 34.1 |
| Ankle | 60.9 | 77.4 | 33.3 | 35.7 | 45.3 | 62.5 |
| Foot | 45.3 | 57.0 | 49.0 | 59.2 | 47.3 | 59.2 |
| Female | Male | Pooled Sex | ||||
|---|---|---|---|---|---|---|
| Joint | 90% | 95% | 90% | 95% | 90% | 95% |
| TMJ | 75.3 | 85.5 | 87.0 | 94.1 | 87.4 | 97.6 |
| Shoulder | 42.2 | 47.7 | 39.1 | 42.3 | 40.2 | 44.3 |
| Elbow | 45.1 | 54.3 | 34.5 | 37.4 | 37.7 | 45.0 |
| Wrist | 54.4 | 67.5 | 43.2 | 52.2 | 47.9 | 61.4 |
| Hand | 55.9 | 64.4 | 53.6 | 62.6 | 55.8 | 66.1 |
| Hip | 41.2 | 47.1 | 32.7 | 35.4 | 33.5 | 38.5 |
| Knee | 45.1 | 56.8 | 40.3 | 49.9 | 40.6 | 53.3 |
| Ankle | 89.8 | 97.1 | 54.0 | 65.0 | 84.2 | 94.2 |
| Foot | 59.6 | 73.4 | 49.5 | 59.9 | 54.2 | 71.5 |
| Female | Male | Pooled Sex | ||||
|---|---|---|---|---|---|---|
| Joint | 90% | 95% | 90% | 95% | 90% | 95% |
| TMJ | 0.85 | 0.36 | 0.03 | 0.11 | 0.43 | 0.18 |
| Shoulder | N/A | N/A | 0.87 | 0.66 | 0.92 | 0.86 |
| Elbow | N/A | N/A | 0.04 | 0.16 | 0.25 | 0.44 |
| Wrist | 0.66 | 0.92 | 0.01 * | <0.01 * | 0.23 | 0.08 |
| Hand | 0.37 | 0.56 | 0.21 | 0.21 | 0.25 | 0.28 |
| Hip | N/A | N/A | 0.82 | 0.65 | 0.70 | 0.60 |
| Knee | N/A | N/A | 0.26 | 0.02 * | 0.46 | 0.14 |
| Ankle | N/A | N/A | 0.18 | 0.05 | 0.65 | 0.16 |
| Foot | 0.94 | 0.76 | 0.86 | 0.85 | 0.92 | 0.88 |
| Female | Male | Pooled Sex | ||||
|---|---|---|---|---|---|---|
| Joint | 90% | 95% | 90% | 95% | 90% | 95% |
| TMJ | <0.01 * | <0.01 * | <0.01 * | <0.01 * | <0.01 * | <0.01 * |
| Shoulder | N/A | N/A | 0.62 | 0.53 | 0.91 | 0.93 |
| Elbow | N/A | N/A | 0.02 * | 0.48 | 0.02 * | 0.07 |
| Wrist | 0.83 | 0.34 | 0.02 * | <0.01 * | 0.34 | 0.40 |
| Hand | 0.26 | 0.33 | 0.95 | 0.80 | 0.60 | 0.57 |
| Hip | N/A | N/A | 0.50 | 0.41 | 0.66 | 0.79 |
| Knee | N/A | N/A | 0.84 | 0.31 | 0.61 | 0.55 |
| Ankle | N/A | N/A | 0.37 | 0.31 | <0.01 * | <0.01 * |
| Foot | 0.14 | 0.08 | 0.90 | 0.90 | 0.56 | 0.22 |
| Female | Male | Pooled Sex | ||||
|---|---|---|---|---|---|---|
| Joint | 90% | 95% | 90% | 95% | 90% | 95% |
| TMJ | <0.01 * | <0.01 * | N/A | N/A | N/A | N/A |
| Shoulder | 0.52 | 0.44 | 0.43 | 0.20 | 0.90 | 0.70 |
| Elbow | 0.04 | 0.01 * | 0.12 | 0.56 | 0.12 | 0.13 |
| Wrist | 0.72 | 0.27 | 0.89 | 0.48 | 0.85 | 0.39 |
| Hand | 0.02 * | 0.04 | 0.28 | 0.35 | 0.12 | 0.14 |
| Hip | 0.25 | 0.10 | 0.43 | 0.46 | 0.89 | 0.82 |
| Knee | 0.86 | 0.01 * | 0.29 | 0.15 | 0.27 | 0.04 |
| Ankle | N/A | N/A | 0.02 * | <0.01 * | N/A | N/A |
| Foot | 0.18 | <0.01 * | 0.90 | 0.93 | 0.56 | 0.22 |
| Subsample | Correct Age from 90% Ages at Transition | Correct Age from 95% Ages at Transition |
|---|---|---|
| Black (n = 32) | 97% | 88% |
| American Indian (n = 12) | 83% | 83% |
| White (n = 215) | 93% | 88% |
| BIPOC (n = 60) | 92% | 88% |
| All males (n = 143) | 92% | 89% |
| All females (n = 132) | 94% | 86% |
| American Indian female (n = 4) | 100% | 100% |
| American Indian male (n = 8) | 75% | 75% |
| Black female (n = 17) | 100% | 88% |
| Black male (n = 15) | 93% | 87% |
| White female (n = 103) | 90% | 83% |
| White male (n = 112) | 93% | 92% |
| Total | 93% | 88% |
| Interobserver Error by Joint | Intraobserver Error by Joint | |||
|---|---|---|---|---|
| Joint Surface | Error (Left) | Error (Right) | Error (Left) | Error (Right) |
| TMJ | 19.57% | 19.57% | 5.00% | 10.00% |
| Shoulder | 10.87% | 13.04% | 15.00% | 25.00% |
| Elbow | 6.52% | 2.17% | 0.00% | 0.00% |
| Wrist | 4.44% | 13.64% | 10.53% | 21.05% |
| Hand | 4.55% | 4.55% | 0.00% | 0.00% |
| Hip | 4.44% | 4.44% | 0.00% | 5.00% |
| Knee | 6.67% | 7.32% | 5.00% | 5.56% |
| Ankle | 18.60% | 15.38% | 5.00% | 5.00% |
| Foot | 9.09% | 4.55% | 10.00% | 10.00% |
| Average Error | 9.42% | 9.41% | 5.61% | 9.07% |
| Individual | Error |
|---|---|
| 1 | 5.56% |
| 2 | 0.00% |
| 3 | 62.86% |
| 4 | 5.56% |
| 5 | 12.12% |
| 6 | 0.00% |
| 7 | 0.00% |
| 8 | 0.00% |
| 9 | 0.00% |
| 10 | 8.57% |
| 11 | 2.78% |
| 12 | 30.56% |
| 13 | 2.86% |
| 14 | 0.00% |
| 15 | 2.78% |
| 16 | 17.14% |
| 17 | 5.71% |
| 18 | 5.71% |
| 19 | 2.78% |
| 20 | 13.89% |
| 21 | 0.00% |
| 22 | 16.67% |
| 23 | 14.71% |
| Average Error | 9.14% |
| Individual | Error |
|---|---|
| 1 | 0.00% |
| 2 | 0.00% |
| 3 | 0.00% |
| 4 | 2.78% |
| 5 | 22.22% |
| 6 | 0.00% |
| 7 | 2.78% |
| 8 | 35.48% |
| 9 | 13.89% |
| 10 | 0.00% |
| Average Error | 7.72% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strasheim, A.N.; Winburn, A.P.; Stock, M.K. Utility of Osteoarthritis as an Indicator of Age in Human Skeletal Remains: Validating the Winburn and Stock (2019) Method. Forensic Sci. 2023, 3, 205-230. https://doi.org/10.3390/forensicsci3020016
Strasheim AN, Winburn AP, Stock MK. Utility of Osteoarthritis as an Indicator of Age in Human Skeletal Remains: Validating the Winburn and Stock (2019) Method. Forensic Sciences. 2023; 3(2):205-230. https://doi.org/10.3390/forensicsci3020016
Chicago/Turabian StyleStrasheim, Ariana N., Allysha P. Winburn, and Michala K. Stock. 2023. "Utility of Osteoarthritis as an Indicator of Age in Human Skeletal Remains: Validating the Winburn and Stock (2019) Method" Forensic Sciences 3, no. 2: 205-230. https://doi.org/10.3390/forensicsci3020016
APA StyleStrasheim, A. N., Winburn, A. P., & Stock, M. K. (2023). Utility of Osteoarthritis as an Indicator of Age in Human Skeletal Remains: Validating the Winburn and Stock (2019) Method. Forensic Sciences, 3(2), 205-230. https://doi.org/10.3390/forensicsci3020016








