Challenges and (Un)Certainties for DNAm Age Estimation in Future
Abstract
1. Introduction
2. DNA Methylation (DNAm): An Epigenetic Mechanism
3. Underling Mechanisms of DNAm Changes with Age
4. Methodologies for DNAm Evaluation
5. Epigenetic Models for Age Estimation Based on DNAm Changes
5.1. Tissue-Specific APMs
5.2. Multi-Tissue APMs
6. Future Direction in DNAm Age Research
6.1. Intrinsic Influences
6.2. Environmental Factors
6.3. Technical Aspects of DNAm Evaluation
7. Implementation of DNAm Age in Forensic Cases
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cunha, E.; Baccino, E.; Martrille, L.; Ramsthaler, F.; Prieto, J.; Schuliar, Y.; Lynnerup, N.; Cattaneo, C. The problem of aging human remains and living individuals: A review. Forensic Sci. Int. 2009, 193, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Franklin, D.; Flavel, A.; Noble, J.; Swift, L.; Karkhanis, S. Forensic age estimation in living individuals: Methodological considerations in the context of medico-legal practice. Res. Rep. Forensic Med. Sci. 2015, 5, 53–66. [Google Scholar] [CrossRef]
- Parson, W. Age Estimation with DNA: From Forensic DNA Fingerprinting to Forensic (Epi) Genomics: A Mini-Review. Gerontology 2018, 64, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Nuzzolese, E.; Di Vella, G. Legal background of age estimation for the dead and the living. In Age Estimation: A Multidisciplinary Approach; Adserias-Garriga, J., Ed.; Elsevier: Oxford, UK, 2019; pp. 17–24. [Google Scholar]
- Cho, S.; Jung, S.E.; Hong, S.R.; Lee, E.H.; Lee, J.H.; Lee, S.D.; Lee, H.Y. Independent validation of DNA-based approaches for age prediction in blood. Forensic Sci. Int. Genet. 2017, 29, 250–256. [Google Scholar] [CrossRef]
- Shi, L.; Jiang, F.; Ouyang, F.; Zhang, J.; Wang, Z.; Shen, X. DNA methylation markers in combination with skeletal and dental ages to improve age estimation in children. Forensic Sci. Int. Genet. 2018, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Mahlke, N.S.; Reckert, A.; Eickhoff, S.B.; Ritz-Timme, S. Age estimation based on different molecular clocks in several tissues and a multivariate approach: An explorative study. Int. J. Leg. Med. 2020, 134, 721–733. [Google Scholar] [CrossRef]
- Baccino, E.; Cunha, E.; Cattaneo, C. Aging the Dead and the Living; Elsevier: Amsterdam, The Netherlands, 2013; pp. 42–48. [Google Scholar]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef]
- Weidner, C.I.; Lin, Q.; Koch, C.M.; Eisele, L.; Beier, F.; Ziegler, P.; Bauerschlag, D.O.; Jöckel, K.H.; Erbel, R.; Mühleisen, T.W.; et al. Aging of blood can be tracked by DNA methylation changes at just three CpG sites. Genome Biol. 2014, 15, R24. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, J.; Hou, J.; Fu, X.; Li, L.; Hou, Y. Developing a DNA methylation assay for human age prediction in blood and bloodstain. Forensic Sci. Int. Genet. 2015, 17, 129–136. [Google Scholar] [CrossRef]
- Kader, F.; Ghai, M. DNA methylation and application in forensic sciences. Forensic Sci. Int. 2015, 249, 255–265. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, S.D.; Shin, K.-J. Forensic DNA methylation profiling from evidence material for investigative leads. BMB Rep. 2016, 49, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Zubakov, D.; Liu, F.; Kokmeijer, I.; Choi, Y.; van Meurs, J.B.J.; van Ijcken, W.F.J.; Uitterlinden, A.G.; Hofman, A.; Broer, L.; van Duijn, C.M.; et al. Human age estimation from blood using mRNA, DNA methylation, DNA rearrangement, and telomere length. Forensic Sci. Int. Genet. 2016, 24, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Jylhävä, J.; Pedersen, N.L.; Hägg, S. Biological age predictors. EBioMedicine 2017, 21, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Freire-Aradas, A.; Phillips, C.; Lareu, M.V. Forensic individual age estimation with DNA: From initial approaches to methylation tests. Forensic Sci. Rev. 2017, 29, 121–144. [Google Scholar] [PubMed]
- Vidaki, A.; Kayser, M. Recent progress, methods and perspectives in forensic epigenetics. Forensic Sci. Int. Genet. 2018, 37, 180–195. [Google Scholar] [CrossRef]
- Williams, G. The emerging field of forensic epigenetics. Forensic Sci. Int. 2018, 290, e24–e25. [Google Scholar] [CrossRef]
- Zolotarenko, A.D.; Chekalin, E.V.; Bruskin, S.A. Modern Molecular Genetic Methods for Age Estimation in Forensics. Russ J Genet. 2019, 55, 1460–1471. [Google Scholar] [CrossRef]
- Hanafi, M.G.; Soedarsono, N.; Auerkari, E.I. Biological age estimation using DNA methylation analysis: A systematic review. Sci Dent. J. 2021, 5, 1–11. [Google Scholar]
- Roberti, A.; Valdes, A.F.; Torrecillas, R.; Fraga, M.F.; Fernandez, A.F. Epigenetics in cancer therapy and nanomedicine. Clin. Epigenetics 2019, 11, 81. [Google Scholar] [CrossRef]
- Liu, X.; Jiao, B.; Shen, L. The Epigenetics of Alzheimer’s Disease: Factors and Therapeutic Implications. Front. Genet. 2018, 9, 579. [Google Scholar] [CrossRef]
- Lardenoije, R.; Latrou, A.; Kenis, G.; Kompotis, K.; Steinbusch, H.W.M.; Mastroeni, D.; Coleman, P.; Lemere, C.A.; Hof, P.R.; van den Hove, D.L.A.; et al. The epigenetics of aging and neurodegeneration. Prog. Neurobiol. 2015, 131, 21–64. [Google Scholar] [CrossRef] [PubMed]
- Schübeler, D. Function and information content of DNA methylation. Nature 2015, 517, 321–326. [Google Scholar] [CrossRef]
- Jiang, S.; Guo, Y. Epigenetic Clock: DNA Methylation in Aging. Stem Cells Int. 2020, 2020, 1047896. [Google Scholar] [CrossRef]
- Heyn, H.; Li, N.; Ferreira, H.J.; Moran, S.; Pisano, D.G.; Gomez, A.; Diez, J.; Sanchez-Mut, J.V.; Setien, F.; Javier Carmona, F.; et al. Distinct DNA methylomes of newborns and centenarians. Proc. Natl. Acad. Sci. USA 2012, 109, 10522–10527. [Google Scholar] [CrossRef] [PubMed]
- Florath, I.; Butterbach, K.; Müller, H.; Bewerunge-Hudler, M.; Brenner, H. Cross-sectional and longitudinal changes in DNA methylation with age: An epigenome-wide analysis revealing over 60 novel age-associated CpG sites. Human Mol. Genet. 2014, 23, 1186–1201. [Google Scholar] [CrossRef]
- Zampieri, M.; Ciccarone, F.; Calabrese, R.; Franceschi, C.; Bürkled, A.; Caiafa, P. Reconfiguration of DNA methylation in aging. Mech. Ageing Dev. 2015, 151, 60–70. [Google Scholar] [CrossRef]
- Jones, M.J.; Goodman, S.J.; Kobor, M.S. DNA methylation and healthy human aging. Aging Cell 2015, 14, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.H.; Kong, Q.P.; Perry, B.; He, Y.H. Progress on the role of DNA methylation in aging and longevity. Brief Funct. Genom. 2016, 15, 454–459. [Google Scholar] [CrossRef][Green Version]
- Garagnani, P.; Bacalini, M.G.; Pirazzini, C.; Gori, D.; Giuliani, C.; Mari, D.; Di Blasio, A.M.D.; Gentilini, D.; Vitale, G.; Collino, S.; et al. Methylation of ELOVL2 gene as a new epigenetic marker of age. Aging Cell 2012, 11, 1132–1134. [Google Scholar] [CrossRef]
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.; Hughes, G.; Sadda, S.; Klotzle, B.; Bibikova, M.; Fan, J.-B.; Gao, Y.; et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 2013, 49, 359–367. [Google Scholar] [CrossRef]
- Bocklandt, S.; Lin, W.; Sehl, M.E.; Sanchez, F.J.; Sinsheimer, J.S.; Horvath, S.; Vilain, E. Epigenetic predictor of age. PLoS ONE 2011, 6, e14821. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tyler, J.K. Epigenetics and aging. Sci. Adv. 2016, 2, e1600584. [Google Scholar] [CrossRef] [PubMed]
- Fraga, M.F.; Ballestar, E.; Paz, M.F.; Ropero, S.; Setien, F.; Ballestar, M.L.; Heine-Suner, D.; Cigudosa, J.C.; Urioste, M.; Benitez, J.; et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl. Acad. Sci. USA. 2005, 102, 10604–10609. [Google Scholar] [CrossRef]
- Tan, Q.; Heijmans, B.T.; Hjelmborg, J.V.; Soerensen, M.; Christensen, K.; Christiansen, L. Epigenetic drift in the aging genome: A ten-year follow-up in an elderly twin cohort. Int. J. Epidemiol. 2016, 45, 1146–1158. [Google Scholar] [CrossRef]
- Hasnain, S.E. Forensic Epigenetic Analysis: The Path Ahead. Med. Princ. Pract. 2019, 28, 301–308. [Google Scholar] [CrossRef]
- Goel, N.; Karira, P.; Garg, V.K. Role of DNA methylation in human age prediction. Mech. Ageing Dev. 2017, 166, 33–41. [Google Scholar] [CrossRef]
- Bergsma, T.; Rogaeva, E. DNA Methylation Clocks and Their Predictive Capacity for Aging Phenotypes and Healthspan. Neurosci. Insights 2020, 21, 2633105520942221. [Google Scholar] [CrossRef] [PubMed]
- Correia Dias, H.; Cordeiro, C.; Pereira, J.; Pinto, C.; Corte Real, F.; Cunha, E.; Manco, L. DNA methylation age estimation in blood samples of living and deceased individuals using a multiplex SNaPshot assay. Forensic Sci. Int. 2020, 311, 110267. [Google Scholar] [CrossRef]
- Dias, M.H.C. DNA methylation as an age predictor in living and deceased individuals. Ph.D. Thesis, Universidade de Coimbra, Coimbra, Portugal, 2021. [Google Scholar]
- Jung, S.E.; Lim, S.M.; Hong, S.R.; Lee, E.H.; Shin, K.J.; Lee, H.Y. DNA methylation of the ELOVL2, FHL2, KLF14, C1orf132/MIR29B2C, and TRIM59 genes for age prediction from blood, saliva, and buccal swab samples. Forensic Sci. Int. Genet. 2019, 38, 1–8. [Google Scholar] [CrossRef]
- Eipel, M.; Mayer, F.; Arent, T.; Ferreira, M.R.P.; Birkhofer, C.; Gerstenmaier, U.; Costa, I.G.; Ritz-Timme, S.; Wagner, W. Epigenetic age predictions based on buccal swabs are more precise in combination with cell type-specific DNA methylation signatures. Aging 2016, 8, 1034–1048. [Google Scholar] [CrossRef]
- Naue, J.; Sänger, T.; Hoefsloot, H.C.J.; Lutz-Bonengel, S.; Kloosterman, A.D.; Verschure, P.J. Proof of concept study of age-dependent DNA methylation markers across different tissues by massive parallel sequencing. Forensic Sci. Int. Genet. 2018, 36, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Correia Dias, H.; Manco, L.; Corte Real, F.; Cunha, E. A Blood-Bone-Tooth Model for Age Prediction in Forensic Contexts. Biology 2021, 10, 1312. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S.; Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 2018, 19, 371–384. [Google Scholar] [CrossRef]
- Liu, Z.; Leung, D.; Levine, M. Comparative analysis of epigenetic aging clocks from CpG characteristics to functional Associations. BioRxiv 2019, 512483. [Google Scholar]
- Freire-Aradas, A.; Pośpiech, E.; Aliferi, A.; Girón-Santamaría, L.; Mosquera-Miguel, A.; Pisarek, A.; Ambroa-Conde, A.; Phillips, C.; Casares de Cal, M.A.; Gómez-Tato, A.; et al. A Comparison of Forensic Age Prediction Models Using Data From Four DNA Methylation Technologies. Front. Genet. 2020, 19, 932. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhang, Y.; Fei, J.; Chang, X.; Fan, W.; Qian, X.; Zhang, T.; Lu, D. Rapid quantification of DNA methylation by measuring relative peak heights in direct bisulfite-PCR sequencing traces. Lab. Investig. 2010, 90, 282–290. [Google Scholar] [CrossRef]
- Parrish, R.R.; Day, J.J.; Lubin, F.D. Direct bisulfite sequencing for examination of DNA methylation patterns with gene and nucleotide resolution from brain tissues. Curr. Protoc. Neurosci. 2012, 60, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Bekaert, B.; Kamalandua, A.; Zapico, S.C.; Van de Voorde, W.; Decorte, R. Improved age determination of blood and teeth samples using a selected set of DNA methylation markers. Epigenetics 2015, 10, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Bekaert, B.; Kamalandua, A.; Zapico, S.C.; Van de Voorde, W.; Decorte, R. A selective set of DNA-methylation markers for age determination of blood, teeth and buccal samples. Forensic Sci. Int. Genet. Suppl. Ser. 2015, 5, e144–e145. [Google Scholar] [CrossRef]
- Zbieć-Piekarska, R.; Spólnicka, M.; Kupiec, T.; Makowska, Ż.; Spas, A.; Parys-Proszek, A.; Kucharczyk, K.; Płoski, R.; Branicki, W. Examination of DNA methylation status of the ELOVL2 marker may be useful for human age prediction in forensic science. Forensic Sci. Int. Genet. 2015, 14, 161–167. [Google Scholar] [CrossRef]
- Zbieć-Piekarska, R.; Spólnicka, M.; Kupiec, T.; Parys-Proszek, A.; Makowska, Ż.; Pałeczka, A.; Kucharczyk, K.; Płoski, R.; Branicki, W. Development of a forensically useful age prediction method based on DNA methylation analysis. Forensic Sci. Int. Genet. 2015, 17, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Park, J.L.; Kim, J.H.; Seo, E.; Bae, D.H.; Kim, S.Y.; Lee, H.C.; Woo, K.M.; Kim, Y.S. Identification and evaluation of age-correlated DNA methylation markers for forensic use. Forensic Sci. Int. Genet. 2016, 23, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Thong, Z.; Chan, X.L.S.; Tan, J.Y.Y.; Loo, E.S.; Syn, C.K.C. Evaluation of DNA methylation-based age prediction on blood. Forensic Sci. Int. Genet. Suppl. Ser. 2017, 6, e249–e251. [Google Scholar] [CrossRef]
- Spólnicka, M.; Pośpiech, E.; Pepłońska, B.; Zbieć-Piekarska, R.; Makowska, Ż.; Pięta, A.; Karłowska-Pik, J.; Ziemkiewicz, B.; Wężyk, M.; Gasperowicz, P.; et al. DNA methylation in ELOVL2 and C1orf132 correctly predicted chronological age of individuals from three disease groups. Int. J. Leg. Med. 2017, 132, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Daunay, A.; Baudrin, L.G.; Deleuze, J.F.; How-Kit, A. Evaluation of six blood-based age prediction models using DNA methylation analysis by pyrosequencing. Sci. Rep. 2019, 9, 8862. [Google Scholar] [CrossRef]
- Pfeifer, M.; Bajanowski, T.; Helmus, J.; Poetsch, M. Inter-laboratory adaption of age estimation models by DNA methylation analysis-problems and solutions. Int. J. Leg. Med. 2020, 134, 953–961. [Google Scholar] [CrossRef]
- Lee, H.Y.; Jung, S.-E.; Oh, Y.N.; Choi, A.; Yang, W.I.; Shin, K.-J. Epigenetic age signatures in the forensically relevant body fluid of semen: A preliminary study. Forensic Sci. Int. Genet. 2015, 19, 28–34. [Google Scholar] [CrossRef]
- Hong, S.R.; Jung, S.E.; Lee, E.H.; Shin, K.J.; Yang, W.I.; Lee, H.Y. DNA methylation based age prediction from saliva: High age predictability by combination of 7 CpG markers. Forensic Sci. Int. Genet. 2017, 29, 118–125. [Google Scholar] [CrossRef]
- Hong, S.R.; Shin, K.-J.; Jung, S.-E.; Lee, E.H.; Lee, H.Y. Platform-independent models for age prediction using DNA methylation data. Forensic Sci. Int. Genet. 2019, 38, 39–47. [Google Scholar] [CrossRef]
- Han, Y.; Franzen, J.; Stiehl, T.; Gobs, M.; Kuo, C.C.; Nikolić, M.; Hapala, J.; Koop, B.E.; Strathmann, K.; Ritz-Timme, S.; et al. New targeted approaches for epigenetic age predictions. BMC Biol. 2020, 18, 71. [Google Scholar] [CrossRef]
- Manco, L.; Dias, H.C. DNA methylation analysis of ELOVL2 gene using droplet digital PCR for age estimation purposes. Forensic Sci. Int. 2022, 333, 111206. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, A.; Heidegger, A.; Piniewska-Róg, D.; Pośpiech, E.; Xavier, C.; Pisarek, A.; Kartasińska, E.; Boroń, M.; Freire-Aradas, A.; Wojtas, M.; et al. Development of the VISAGE enhanced tool and statistical models for epigenetic age estimation in blood, buccal cells and bones. Aging 2021, 11, 6459–6484. [Google Scholar] [CrossRef]
- Vidaki, A.; Ballard, D.; Aliferi, A.; Miller, T.H.; Barron, L.P.; Syndercombe Court, D. DNA methylation-based forensic age prediction using artificial neural networks and next generation sequencing. Forensic Sci. Int. Genet. 2017, 28, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Aliferi, A.; Ballard, D.; Gallidabino, M.D.; Thurtle, H.; Barron, L.; Syndercombe Court, D. DNA methylation-based age prediction using massively parallel sequencing data and multiple machine learning models. Forensic Sci. Int. Genet. 2018, 37, 215–226. [Google Scholar] [CrossRef]
- Naue, J.; Hoefsloot, H.C.J.; Mook, O.R.F.; Rijlaarsdam-Hoekstra, L.; van der Zwalm, M.C.H.; Henneman, P.; Kloosterman, A.D.; Verschure, P.J. Chronological age prediction based on DNA methylation: Massive parallel sequencing and random forest regression. Forensic Sci. Int. Genet. 2017, 31, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Hamano, Y.; Manabe, S.; Morimoto, C.; Fujimoto, S.; Ozeki, M.; Tamaki, K. Forensic age prediction for dead or living samples by use of methylation-sensitive high resolution melting. Leg. Med. 2016, 21, 5–10. [Google Scholar] [CrossRef]
- Alghanim, H.; Antunes, J.; Silva, D.S.B.S.; Alho, C.S.; Balamurugan, K.; McCord, B. Detection and evaluation of DNA methylation markers found at SCGN and KLF14 loci to estimate human age. Forensic Sci. Int. Genet. 2017, 31, 81–88. [Google Scholar] [CrossRef]
- Correia Dias, H.; Cordeiro, C.; Corte Real, F.; Cunha, E.; Manco, L. Age estimation based on DNA methylation using blood samples from deceased individuals. J. Forensic Sci. 2020, 65, 465–470. [Google Scholar] [CrossRef]
- Correia Dias, H.; Cunha, E.; Corte Real, F.; Manco, L. Age prediction in living: Forensic epigenetic age estimation based on blood samples. Leg. Med. 2020, 47, 101763. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, H.; Xu, Y. Age Prediction of Human Based on DNA Methylation by Blood Tissues. Genes 2021, 12, 870. [Google Scholar] [CrossRef]
- Lee, J.W.; Choung, C.M.; Jung, J.Y.; Lee, H.Y.; Lim, S.K. A validation study of DNA methylation-based age prediction using semen in forensic casework samples. Leg. Med. 2018, 31, 74–77. [Google Scholar] [CrossRef]
- Giuliani, C.; Cilli, E.; Bacalini, M.G.; Pirazzini, C.; Sazzini, M.; Gruppioni, G.; Franceschi, C.; Garagnani, P.; Luiselli, D. Inferring chronological age from DNA methylation patterns of human teeth. Am. J. Phys. Anthropol. 2016, 159, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Ruiz, A.B.; González-Herrera, L.; Luna, J.D.; Valenzuela, A. DNA methylation levels and telomere length in human teeth: Usefulness for age estimation. Int. J. Leg. Med. 2020, 134, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Correia Dias, H.; Corte Real, F.; Cunha, E.; Manco, L. DNA methylation age estimation from human bone and teeth. Aust. J. Forensic Sci. 2022, 54, 163–176. [Google Scholar] [CrossRef]
- Zapico, C.S.; Gauthier, Q.; Antevska, A.; McCord, B.R. Identifying Methylation Patterns in Dental Pulp Aging: Application to Age-at-Death Estimation in Forensic Anthropology. Int. J. Mol. Sci. 2021, 22, 3717. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, S.; Gaige, J.; Henn, B.M. DNA methylation-based forensic age estimation in human bone. BioRxiv 2019, 801647. [Google Scholar] [CrossRef]
- Lee, H.Y.; Hong, S.R.; Lee, J.E.; Hwang, I.K.; Kim, N.Y.; Lee, J.M.; Fleckhaus, J.; Jung, S.E.; Lee, Y.H. Epigenetic age signatures in bones. Forensic Sci. Int. Genet. 2020, 46, 102261. [Google Scholar] [CrossRef]
- Hao, T.; Guo, J.; Liu, J.; Wang, J.; Liu, Z.; Cheng, X.; Li, J.; Ren, J.; Li, Z.; Yan, J.; et al. Predicting human age by detecting DNA methylation status in hair. Electrophoresis 2021, 42, 1255–1261. [Google Scholar] [CrossRef]
- Naue, J.; Winkelmann, J.; Schmidt, U.; Lutz-Bonengel, S. Analysis of age-dependent DNA methylation changes in plucked hair samples using massive parallel sequencing. Rechtsmedizin 2021, 31, 226–233. [Google Scholar] [CrossRef]
- Koop, B.E.; Mayer, F.; Gündüz, T.; Blum, J.; Becker, J.; Schaffrath, J.; Wagner, W.; Han, Y.; Boehme, P.; Ritz-Timme, S. Postmortem age estimation via DNA methylation analysis in buccal swabs from corpses in different stages of decomposition-a “proof of principle” study. Int. J. Leg. Med. 2020, 135, 167–173. [Google Scholar] [CrossRef]
- Alsaleh, H.; McCallum, N.A.; Halligan, D.L.; Haddrill, P.R. A multi-tissue age prediction model based on DNA methylation analysis. Forensic Sci. Int. Genet. Suppl. Ser. 2017, 6, e62–e64. [Google Scholar] [CrossRef]
- He, X.; Liu, J.; Liu, B.; Shi, J. The use of DNA methylation clock in aging research. Exp. Biol. Med. 2021, 246, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.C.; Wagner, W. Epigenetic-aging-signature to determine age in different tissues. Aging 2011, 3, 1018. [Google Scholar] [CrossRef]
- Freire-Aradas, A.; Phillips, C.; Mosquera-Miguel, A.; Girón-Santamaría, L.; Gómez-Tato, A.; Casares de Cal, M.; Álvarez-Dios, J.; Ansede-Bermejo, J.; Torres-Español, M.; Schneider, P.M.; et al. Development of a methylation marker set for forensic age estimation using analysis of public methylation data and the Agena Bioscience EpiTYPER system. Forensic Sci. Int. Genet. 2016, 24, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Freire-Aradas, A.; Phillips, C.; Girón-Santamaría, L.; Mosquera- Miguel, A.; Gómez-Tato, A.; Casares de Cal, M.Á.; Álvarez-Dios, J.; Lareu, M.V. Tracking age-correlated DNA methylation markers in the young. Forensic Sci. Int. Genet. 2018, 36, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Bacalini, M.G.; Deelen, J.; Pirazzini, C.; De Cecco, M.; Giuliani, C.; Lanzarini, C.; Ravaioli, F.; Marasco, E.; van Heemst, D.; Suchiman, H.E.D.; et al. Systemic Age-Associated DNA Hypermethylation of ELOVL2 Gene: In Vivo and In Vitro Evidences of a Cell Replication Process. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1015–1023. [Google Scholar] [CrossRef]
- Bell, C.G.; Lowe, R.; Adams, P.D.; Baccarelli, A.A.; Beck, S.; Bell, J.T.; Christensen, B.C.; Gladyshev, V.N.; Heijmans, B.T.; Horvath, S.; et al. DNA methylation aging clocks: Challenges and recommendations. Genome Biol. 2019, 20, 249. [Google Scholar] [CrossRef]
- Fiorito, G.; McCrory, C.; Robinson, O.; Carmeli, C.; Rosales, C.O.; Zhang, Y.; Colicino, E.; Dugué, P.A.; Artaud, F.; McKay, G.J.; et al. BIOS Consortium; Lifepath consortium. Socioeconomic position, lifestyle habits and biomarkers of epigenetic aging: A multi-cohort analysis. Aging 2019, 11, 2045–2070. [Google Scholar] [CrossRef]
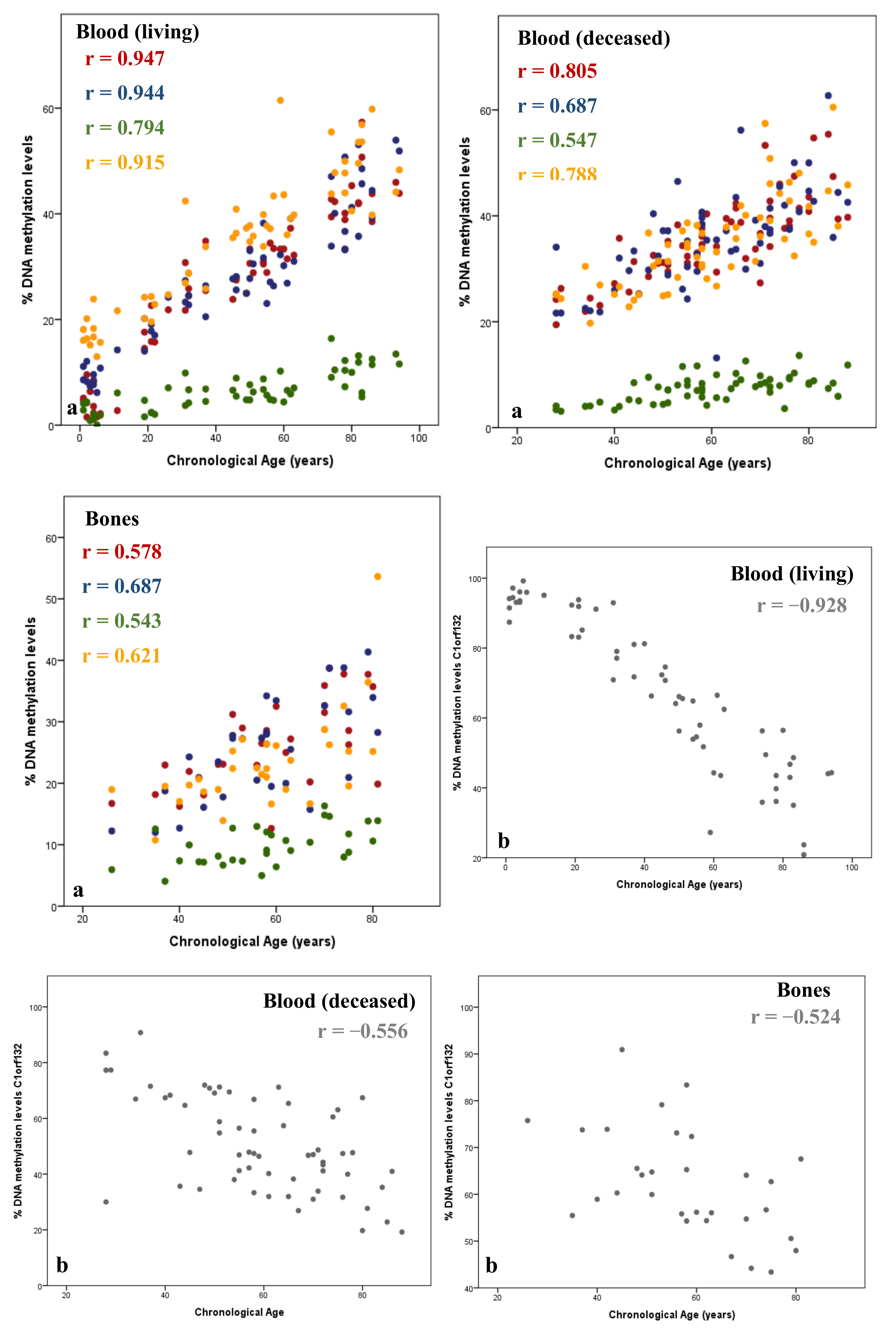
| CpGs or Genes | Main Findings | Reference |
|---|---|---|
| NPTX2, EDARADD, TOM1L1 | The first study using DNAm levels for age prediction. APM (2 CpGs) for saliva revealed an accuracy of 5.2 years. | [33] |
| ELOVL2, Clorf132, TRIM59, KLF14, FHL2 | The first age-prediction calculator available online for blood samples (www.agecalculator.ies.krakow.pl, accessed on 31 August 2022). Model with 5 CpGs revealed high accuracy with a MAD value of 3.4 years. | [54] |
| ELOVL2 | High model accuracy using only 2 CpGs from ELOVL2: MAD = 5.03 years. The first study that evaluated DNAm patterns in bloodstains, it has shown that the DNAm did not change after one-month storage as bloodstains. | [53] |
| ASPA, ELOVL2, PDE4C, EDARADD | The first study that investigated DNAm levels in blood samples from deceased individuals and dentin samples. A MAD value of 3.75 years has been obtained evaluating 4 CpGs in blood from living and deceased individuals. An accurate APM with a 4.86 years of MAD value has been developed using 7 CpGs in dentin samples. | [51] |
| ELOVL2, FHL2, PENK | The first study that evaluated DNAm levels in different layers of tooth samples (cementum: 2.45 years; dentin: 7.07 years; dental pulp: 2.25 years). | [75] |
| DDO, ELOVL2, F5, GRM2, HOXC4, KLF14, LDB2, MEIS1-AS3, NKIRAS2, RPA2, SAMD10, TRIM59, ZYG11A. | The first study that evaluated the correlation between DNAm levels and age in bone samples. The authors investigated the correlation between DNAm levels of 13 blood–age-correlated loci used in [44] and age in many samples from deceased individuals. | [44] |
| Total of 485.577 CpG sites investigated; CpGs selected are located at DDO, PRPH2, DHX8, ITGA2B and at one unknown gene with the Illumina ID number of 22398226 | Highly accurate models developed for young children (aged 6–15 years): MAE = 0.47 years (boys); MAE = 0.33 years (girls). The first study that combined anthropological and epigenetic approaches. | [6] |
| ELOVL2, FHL2, KLF14, C1orf132, TRIM59 | Tissue-specific APMs for blood (MAD = 3.17 years), buccal swabs (MAD = 3.82 years), and saliva (MAD = 3.29 years). A multi-tissue APM that is highly accurate (MAD = 3.55 years). | [42] |
| ELOVL2, PDE4C, FHL2, EDARADD, C1orf132 | The first study developed only for blood samples from deceased individuals. MAD = 6.08 years. | [71] |
| CpGs located, among other genes, at TRIM59, ELOVL2 and KLF14 | The first model developed for bones namely the “37 bone clock CpGs”, revealing an accuracy of 4.9 years (RMSE). DNAm levels of forensic samples have been evaluated, however, these were excluded. | [79] |
| ELOVL2, KLF14, C1orf132, FHL2, TRIM59 | Population-specific differences in DNAm levels. The authors applied the predictive equation developed by [37] in Korean to Portuguese living individuals obtaining a MAD value of 15.26 years. APM for Portuguese people: MAD = 4.25 years (living); MAD = 5.36 years (deceased); MAD = 4.97 years (living and deceased individuals). | [40] |
| ELOVL2, KLF14, C1orf132, FHL2, TRIM59, PDE4C, EDARADD | The second APMs developed for bones in the literature (MAD = 7.18 years, using SNaPshot; MAD = 2.56 years, using Sanger sequencing). | [77] |
| LAG3, SCGN, ELOVL2, KLF14, C1orf132, SLC12A5, GRIA2, PDE4C | The first study developed for hair samples. Accuracy of 3.68 years using 10 CpGs. | [81] |
| Year | CpGs | Main Findings | Reference |
|---|---|---|---|
| 2013 | 353 CpGs | The first multi-tissue model with different cellular tissues such as whole blood, occipital cortex, colon, peripheral blood mononuclear cells, liver, lung, saliva, buccal epithelium, among others, was developed using microarray hybridization technology, revealing an accuracy of 2.9 years. | [9] |
| 2017 | 10 CpGs | A multi-tissue model developed for whole blood, saliva, semen, menstrual blood, and vaginal secretions with methylation data captured using the Illumina Infinium HM450 platform with an accuracy of 3.8 years. | [84] |
| 2019 | 5 CpGs | APM developed in Korean people for saliva, blood, and buccal swabs. Multi-tissue with DNAm levels of ELOVL2, FHL2, KLF14, TRIM59, and C1orf132 genes developed using the SNaPshot method, revealing a MAD of 3.6 years. | [42] |
| 2021 | The first multi-tissue APMs developed including bone and tooth samples. Multi-tissue APMs developed for Portuguese individuals. | [45] | |
| 7 CpGs | A Blood–Bone–Tooth APM (BBT-APM) with an MAD of 6.06 years developed with methylation information of CpGs located at EDARADD, FHL2, ELOVL2, PDE4C, and C1orf132 genes using Sanger sequencing. | ||
| 3 CpGs | BBT-APM with a MAD of 6.49 years developed with DNAm levels of ELOVL2, KLF14, and C1orf132 genes, using the SNaPshot assay. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia Dias, H.; Cunha, E.; Corte Real, F.; Manco, L. Challenges and (Un)Certainties for DNAm Age Estimation in Future. Forensic Sci. 2022, 2, 601-614. https://doi.org/10.3390/forensicsci2030044
Correia Dias H, Cunha E, Corte Real F, Manco L. Challenges and (Un)Certainties for DNAm Age Estimation in Future. Forensic Sciences. 2022; 2(3):601-614. https://doi.org/10.3390/forensicsci2030044
Chicago/Turabian StyleCorreia Dias, Helena, Eugénia Cunha, Francisco Corte Real, and Licínio Manco. 2022. "Challenges and (Un)Certainties for DNAm Age Estimation in Future" Forensic Sciences 2, no. 3: 601-614. https://doi.org/10.3390/forensicsci2030044
APA StyleCorreia Dias, H., Cunha, E., Corte Real, F., & Manco, L. (2022). Challenges and (Un)Certainties for DNAm Age Estimation in Future. Forensic Sciences, 2(3), 601-614. https://doi.org/10.3390/forensicsci2030044









