The results of this review have been divided in three sections: Biological Studies, Animal Studies, and Human Studies.
3.1. Biological Studies
The core of years of misinterpretations and inconsistencies in TCA analysis stems from a general lack of knowledge regarding the biology of cementum, from its development to its daily mechanisms. Understanding its biology should be the first milestone to a significant improvement of the technique.
This necessary step was identified by a few authors at the early beginning of the TCA application. One of those was Lieberman, who did not only urge future research to address this issue, but also provided one of the first summaries on cementum biology (Lieberman and Meadow [
10]; Lieberman [
12]), drawing the attention on the existence of different types of cementum, their composition, and their structure.
3.1.1. Cementum: The Tissue
Histologically, teeth consist of three main tissues: enamel, dentine, and cementum. Despite sharing some features (i.e., the incremental growth), dental tissues are significantly different in terms of function, microstructure, cellular morphology, and behaviour.
The cementum in humans coats the external surface of the root and can be broadly divided into acellular extrinsic fibre cementum (AEFC) (ca. coronal half of the root) and cellular intrinsic fibre cementum (CIFC) (ca. apical half of the root) (Nanci and Bosshardt [
23]). However, other scholars identified further types and varieties of cementum other than the two above-mentioned (i.e., afibrillar cementum, extrinsic fibre cementum, intrinsic fibre cementum, and mixed fibre cementum (Hillson [
24]); cellular mixed stratified cementum (CMSC), acellular afibrillar cementum (AAC), and intermediate cementum (Yamamoto et al. [
25]).
As suggested by the names, differences are found in presence/absence of cells/cementocytes, type of fibres found, and co-existence of one or more types of cementum.
When assessing age and season at death, it is always highly recommended to base the assessments on the analysis of the acellular extrinsic fibre cementum, which is normally more regular and “reliable” because it is less subjected to the activity of the cells reacting to tooth movements and damages. Therefore, for the purpose of this review, a deeper look into the characteristics of this specific type of cementum will follow.
The AEFC (also called primary or acellular cementum) can vary from 50 to 200 μm in thickness, according to age (Yamamoto et al. [
25]) and develops slowly, leaving its forming cells right on top of its surface rather than entrapped in the matrix as in the cellular cementum (Nanci and Bosshardt [
23]). It is ca. 45–60% mineralised with its inner layers less mineralised than the outer ones (Nanci and Bosshardt [
23]). Besides the absence of cementocytes, its main features are the presence of extrinsic fibres of the periodontal ligament (PDL) (also called Sharpey’s fibres) that runs perpendicular to the cementodentinal junction, and the presence of growth incremental lines (Yamamoto et al. [
25]; Hillson [
24] and Nanci and Bosshardt [
23]).
3.1.2. Composition, Structure, and Properties
The cementum has been often regarded as a “bone-like” tissue, even though, as rightly pointed out by Yamamoto et al. [
25], the “cementum is avascular, does not undergo dynamic remodelling, and increases in thickness throughout life. On these points, cementum is markedly different from bone.” The parallel with bone tissues, however, mostly relates to its composition. In fact, contrary to enamel and dentine (whose inorganic components are ca. 96% and 70%, respectively), the cementum is the only dental tissue to also be made of a consistent organic part. Despite scholars not always agreeing on the exact percentages of organic and inorganic material in the cementum, it can be said that they all reported values between 65–50% for the inorganic part and 50–30% for the organic one (Nanci and Bosshardt [
23]; Berkovitz et al. [
26]; Colard et al. [
27]; Perez-Berberia et al. [
28]).
About 90% of the organic part consists of collagen fibres, and more specifically collagen type I (COL1). Other types of collagen found in cementum are type III (COL3A1) (which is highly found during development and regeneration of mineralised tissues) and type XII (COL12A1) (which is linked to fibres) (Nanci and Bosshardt [
23]). More recently, another study (Lira Dos Santos et al. [
29]) also identified the presence of collagen type XIV (COL14A1), which is usually found in association with high mechanical stress tissues (such as tendons and skin). The rest of the organic component, instead, consists of non-collagenous matrix proteins such as sialoproteins, dentin matrix protein 1 (DMP1), dentine sialoprotein (DSPP), fibronectin (FINC), osteocalcin (OC), osteonectin (SPARC), osteopontin (OPN), tenascin (TENA), proteoglycans, proteolipids, and several growth factors (e.g., cementum growth factor, which seems to be an insulin-like growth factor molecule) (Nanci and Bosshardt [
23]). The presence of enamel-related proteins has been proposed by some scholars (Berkovitz et al. [
26]) as important for cementoblast-inducing activity, but it is at present still controversial (Yamamoto et al. [
25]).
The inorganic component of cementum, instead, is mostly made of calcium and phosphate in the form of thin and plate-like hydroxyapatite crystals (on average 55 nm wide and 8 nm thick) (Berkovitz et al. [
26]) with traces of other elements, such as copper, fluorine, iron, lead, potassium, silicon, sodium, and zinc (Perez-Berberia et al. [
28]). According to Edinborough et al. [
30], the atomic average Ca/P ratio in the cementum (up to 1.65 for the majority of their sample) is below the average ratio in standard bioapatite (1.69–1.71), concluding that the cementum is overall hypomineralised.
Structurally, the main features of the cementum is the type and organisation of collagen fibres, which seem to be radial in acellular cementum and woven in radial and circumferential fibres in the cellular cementum (Ho et al. [
31]; Aboulfadl and Hulliger [
32]).
Finally, according to Ho et al. [
31], the elastic modulus of cementum is between 14–17 GPa, which would set the cementum between the cortical bone (20.7–18.6 GPa) and the trabecular bone (14.8–10.4 GPa) in terms of elasticity (Rho et al. [
33]).
3.1.3. Lines of Salter
The incremental lines in the cementum have been called in numerous ways, often leading to confusion and misinterpretations. However, they are already known in the medical literature as “lines of Salter” (Berkovitz et al. [
26]; Kumar [
34]), a term that would be more appropriate to adopt hereafter in the studies of dental cementum.
The lines of Salter indicate the so-called resting lines, darker in colour, which are alternated with lighter bands of tissue when seen under transmitted light microscopy. Their nature and the mechanism behind their deposition is still debated. Amongst the reasons behind the banding phenomenon, scholars have ventured:
- (1)
A change in collagen orientation due to consumption of a hard/soft/less or more nutritious diet (Lieberman [
12]).
- (2)
A change in the degree of mineralisation of each band, where the darker band (in these papers also called “incremental line” or “resting line”) is more mineralised than the lighter one (also called “growth band”) (Klezeval [
9]; Nanci and Bosshardt [
23]; Yamamoto et al. [
25]; Colard et al. [
27]; Mani-Caplazi et al. [
35]).
- (3)
A multi-approach including changes in collagen orientation, degree of mineralisation, density of cementocytes lacunae, density of collagen fibres versus extrinsic fibres or ground substance, etc. (Hillson [
24]). A similar approach was taken by Berkovitz et al. [
26] when they suggested that the banding phenomenon might be correlated not only to differences in the degree of mineralisation, but also in matrix composition, for which, according to these authors, the lines of Salter would be rich in OPN, whereas the matrix would be rich in sialoproteins.
- (4)
A change in the shape or orientation of the mineral crystals (Cool et al. [
36]). In this study, the authors found that demineralisation was the only process that altered the pattern, and composition did not seem to change between one layer and the other.
- (5)
An optical effect. Berkovitz et al. [
26] hypothesised that the extrinsic fibres might have an unmineralised core, which, during preparation of ground sections, might be lost causing that to be replaced by air or debris and resulting in an internal reflection of the light.
- (6)
A difference in elemental composition. A study carried out on great apes (Dean et al. [
37]) showed that Ca, Zn, and Sr mapping presented evidence of incremental growth layers, whereas the concentration of P was constant. They also claimed that peaks in Zn and Ca and in carbonated hydroxyapatite coincided with the brighter bands.
3.1.4. Brief Summary of Dental Development
Dental development starts already in the embryo, when at ca. 28 days an ectodermal epithelial thickening appears where the future maxillary and mandibular arches are going to form. When they finally join in the midline, a week after, they keep developing in arch-shaped plates, where the primary epithelial bands are seen. From these, the vestibular and dental laminae will develop (Cunningham et al. [
38]).
Following these preliminary formations, ten swellings (called enamel organs) will form from each dental lamina during the bud stage. The enamel organs consist of an epithelial component, under which mesenchyme is aggregated to later form the dental papilla during the cap stage. At week 10, the now-tooth germ is surrounded by the dental follicle, in which ameloblasts and odontoblasts are already working on the formation of their respective tissues (i.e., enamel and dentine) during the bell stage. The late and last bell stage already sees the main component of the future deciduous tooth formed (Cunningham et al. [
38]).
3.1.5. Dental Root Development
The first dental tissue to be deposited is the dentine (Hillson [
24]; Cunningham et al. [
38]) and, more specifically its organic matrix on which the later differentiated ameloblasts will start depositing the enamel. Once the crown is completed, the development of the root can begin, which, in time, will eventually push the tooth through the dental crypt and finally into occlusion.
A double layer of epithelial cells, known as Hertwig’s epithelial root sheath (HERS), plays a major role in the development of the root. It forms from the external and internal enamel epithelia at the cervical loop of the enamel organ, and it proliferates apically in the shape of the future root (Berkovitz et al. [
26]). The elongation of the epithelial sheath happens within the dental follicle (from which cementum, periodontal ligament—PDL—and probably alveolar bone are formed) until it eventually encloses the dental papilla entirely except for the area of the future apical foramen (Berkovitz et al. [
26]).
HERS is therefore a double epithelial layer found in between the undifferentiated mesenchyme of the dental papilla on the inside and the dental follicle on the outside, which also is divided in layers; the most internal one (adjacent to the HERS) is of ectomesenchymal origin (neural crest cells), and the remaining two (intermediate and outer ones) are of mesodermal origin. The cells of the internal layer of HERS induce the dental papilla to differentiate into odontoblasts, whereas the mesenchymal cells of the dental follicle differentiate into cementoblasts (Berkovitz et al. [
26]). Interestingly, cementoblasts seem to differentiate from the ectomesenchymal inner layer of the dental follicle (Berkovitz et al. [
26]; Hye-Sun Kim et al. [
39]), whereas the other two layers will later differentiate into the fibroblasts of the PDL after cementogenesis has begun (Berkovitz et al. [
26]). For all these differentiation processes to happen, the HERS needs to eventually disintegrate by local apoptosis into a mesh network first (Li et al. [
40]) and, finally, into clusters called epithelial cell rests of Malassez, which remain in the PDL (Yamamoto et al. [
25]).
The real role of HERS, however, is still debated. The idea that it merely stands as a “cast” for the future root (Luan et al. [
41]) seems to have been overall abandoned by the majority of scholars. The presence of organelles capable of synthesis and secretion of bioactive molecules (e.g., endoplasmic reticulum, Golgi material, and mitochondria) in HERS cells, in fact, would point to a more active role of HERS in the development of the root (Berkovitz et al. [
26]). More recent studies (Li et al. [
40]) are suggesting that HERS might be more significantly involved in these cellular differentiation processes, acting as a sort of “signalling centre” for the development of the root and the periodontium. Li et al. [
40] suggested that HERS might actually be promoting the differentiation of both odontoblasts (when HERS interacts with the CNC (cranial neural crest-derived mesenchyme cells) and cementoblasts (by allowing the interaction between dentine and dental follicle cells—FCs —during HERS’s initial perforation, also in Hye-Sun Kim et al. [
39]). Similarly, Wu et al. [
42] claimed that in their in vitro and in vivo experiments, they observed how dNCPs (dentine non-collagenous proteins) were key to induce the DFCs to differentiate into cementoblasts, which was only possible during HERS’s apoptosis. On this, Hye-Sun Kim et al. [
39] expanded by saying that dNCPs not only contribute to the differentiation of cementoblasts, but also to their mineralisation, as this in cementoblasts would take place not by matrix vesicles but by specific soluble factors or signalling molecules secreted by the odontoblasts. In their study, dNCPs were found to increase the production of important proteins for mineralisation, such as OC, ALP, and BSP mRNA in cementoblasts.
3.1.6. Cementogenesis
As previously mentioned, cementum is broadly distinguished in two main types: acellular and cellular, which are also respectively called primary and secondary, referring to their time of formation. Berkovitz et al. [
26] gave a comprehensive description of the known steps of cementogenesis. Keys to the onset of cementogenesis are the perforation of HERS and the formation of the hyaline layer between dentine and cementum. As already summarised, the perforation of HERS in a mesh network allows the interaction between epithelial and mesenchymal cells, which will eventually initiate the differentiation processes of the main cellular types of the periodontium: odontoblasts, cementoblasts, and fibroblasts.
The formation of the acellular cementum is preceded by the differentiation of the odontoblasts from the dental papilla that start secreting the organic matrix of the root predentine. This first secretion appears, once mineralised, as a structureless and “glass-like” layer, called “hyaline layer (of Hopewell-Smith)” (Berkovitz et al. [
26]). The role of this first layer (to be found between the granular layer of Tomes in the dentine and the first lines of Salter in the cementum) is paramount to the onset of acellular cementogenesis. At this point, the cementoblasts (a different type of fibroblasts-like cells) differentiate from the dental follicle and, through HERS, lie on the surface of the still unmineralised hyaline layer (Berkovitz et al. [
26]). These cells produce collagen fibrils that will connect to the fibrils already present in the hyaline layer on one side, and to those of the PDL on the other side, thus creating a particularly strong bond among these three structures, especially once mineralisation starts (Berkovitz et al. [
26]). Mineralisation begins somewhat in the middle of the hyaline layer by matrix vesicles and later spreads both inwards and outwards (Berkovitz et al. [
26]). However, mineralisation of the acellular cementum does not proceed by matrix vesicles (Berkovitz et al. [
26]; Hye-Sun Kim et al. [
39]) and might instead be influenced by the presence of hydroxyapatite crystals in the dentine, that of alkaline phosphatase in the PDL, and/or the presence of non-collagenous proteins (e.g., BSP) on its surface (Berkovitz et al. [
26]). Cementogenesis also proceeds in alternating periods of activity and rest, which are associated with the formation of the lines of Salter.
Once the cementogenesis of the acellular cementum has started and the root formation carries out apically, a different type of cementoblasts (more cuboidal in shape and with more cytoplasmic processes) appears on the surface of the root. They are responsible for the increase in speed deposition and the formation of intrinsic fibres (Berkovitz et al. [
26]). Due to these characteristics, the cellular (or secondary) cementum can be visually recognised by the presence of cementocytes (cells that have been entrapped in the matrix) and the more widely spaced lines of Salter.
3.1.7. Back to the Cells
At a cellular level, the cementoblasts producing acellular and cellular cementum seem to have phenotypical differences not only in terms of their shape, but also in terms of their secretory activity (
Table 1). The reason why two different types of cementoblasts are both differentiating from the dental follicle is still unclear (Berkovitz et al. [
26]).
The classification shown in
Table 1 is not completely agreed upon. Dean et al. [
37], for example, also suggested that cementoblasts forming the acellular cementum might derive from or be influenced by the epithelial root sheath, but, contrary to Berkovitz et al. [
26], they claimed that this type of cementoblast might actually be able to express cytokeratins, parathormone (PTH) receptors, TGFβ, and IGF.
Furthermore, because of the parallel with osteocytes, the cementocytes (being cementoblasts entrapped in the cellular cementum) have always been thought to be inactive (or destined to inactivity). However, more recent studies have highlighted a new perspective of their role within the periodontal system. The observation made so far by Salmon et al. [
43] of the activation of a cementocyte metabolism during experimentally induced apposition, suggested that cementocytes might actually have an active role in neocementogenesis, dental cementum apposition, and mineralisation. Additionally, Lira Dos Santos et al. [
29] showed the mechanoresponsive nature of these cells by experimentally inducing controlled orthodontic movements (OTM). In these experiments, the authors found an increase in the nuclear size and proportion of euchromatin (which indicates cellular activity); a different protein expression from the control sample (with COL14A1 and several extracellular matrix proteins, including decorin (DCN), biglycan (BGN), asporin (ASPN), and periostin (POSTN) being downregulated during OTM); and finally 11 keratins, which were significantly increased by OTM and mainly localised on the epithelial remnants of Hertwig’s epithelial root sheath (Lira Dos Santos et al. [
29]).
3.1.8. Molecular Signalling Pathways
The understanding of root formation has become increasingly important, especially in the field of regenerative dentistry, as scientists try to figure out a successful therapy to promote the regeneration of periodontal tissues, when these deteriorate over time or following pathological conditions. To this regard, the “riddle” of cementogenesis is a major setback. Root formation and cementogenesis have therefore been addressed also from a different point of view, which is that of genes and molecular signalling pathways.
In their review on the signalling pathways in root formation, Wang and Feng [
44] reported the importance of the coordinated interaction amongst Wnt-β catenin, Osterix (Osx), and Hedgehog (Hh) signalling pathways. They also highlighted the role of NFIC, as the “master gene” of dental root formation, as it expresses NFIC (nuclear factor IC), whose downstream molecule is osterix (also known as Sp7), a zinc finger transcription factor associated with skeletal and cementum formation (Wang and Feng [
44]). The importance of Osx in root development stands in its ability of interrupting Wnt-β catenin signalling by attaching to β catenin. Similarly, Hh signalling plays a role in this process as it can also inhibit cementum apposition by repressing Wnt-β catenin and Osx expression (Choi et al. [
45]). A negative feedback loop has been recognised for Hh-Smo and Wnt-β catenin signalling, for which a minimum level of Hh-Smo is needed for proper cellular cementum apposition (Choi et al. [
45]). Nottmeier et al. [
46] also found that Wnt 1 ligand also influences alveolar and cementum formation. Its expression, in fact, increases both acellular and cellular cementum and, if prolonged, brings to the formation of ectopic cementum-like calcification in the pulp and destruction of the PDL.
From the literature, it seems like the main pathway connected to cementum formation is that of Osx-Wnt/β catenin. An alternative route has been identified in the transcription factor 7-like 2 (TCF7L2), which is the transcription factor able to stimulate the mineralisation of the cementum by activating the nuclear factor kappa B (NF-kB) signalling pathway (Zhao et al. [
47]).
From a genetic point of view, circular RNA low-density lipoprotein receptor-related protein 6 (circLRP6) seems to regulate the expression of runt-related transcription factor 2 (RUNX2), OPN, and bone sialoprotein (BSP) via the miR-145-5p/Zeb2 axis to promote cementoblasts differentiation and mineralisation. More in detail, RUNX2 is found in precementoblasts and cementoblasts but not in cementocytes, and it is strictly connected to tooth development; OPN is an extracellular matrix protein found in mineralised connective tissues and secreted by cementoblasts; BSP is also an extracellular matrix protein found in cementum and alveolar bone (Li et al. [
48]).
Interestingly, the observations made by Lira Dos Santos et al. [
29] on the mechanoresponsive nature of cementocytes seemed to have been found also in another study (Yun Wu et al. [
49]) where changes caused by mechanical forces were noticed on the miRNAs of PDL cells, which resulted in a higher expression of osteogenic/cementoblastic genes and proteins, and in the upregulation of RUNX2, SATB2, and MSX2 genes after mechanical loading.
3.1.9. Proteomic Studies
A few studies addressed the issue of cementogenesis by focusing on the expression of its proteins. Phosphoproteins and glycoproteins (such as BSP, OPN, DMP1), mineralisation-regulating enzyme tissue-nonspecific alkaline phosphatase (TNAP), proteoglycans (such as DCN, BGN, fibromodulin (FMOD), ASPN, lumican (LUM), osteomodulin/osteoadherin (OSAD)), POSTN, vimentin (VIM), TNA, and vitronectin (VTN), were all identified in dental cementum (Salmon et al. [
43]). Other 12 proteins were seen specifically during experimentally induced apposition, whose main four secreted factors were serpin 1a (A1AT), procollagen C-proteinase enhancer-1 (PCPE1), tenascin X (TNX), and ASPN (Salmon et al. [
43]). Differences were also seen in the proteins expressed in permanent and deciduous dental cementum, with only 50.8% of the identified proteins being shared between the two (Giovani et al. [
50]). Similarly, differences in the secretory activity of the cells in acellular and cellular cementum should be considered as, for instance, DSPP, FINC, and TENA, and proteoglycans such as versican (CSPG2), DCN, and BGN are present in cellular cementum but not acellular cementum (Berkovitz et al. [
26]).
Salmon et al. [
51] also added that most of the dental cementum proteins were involved in cell communication and signal transduction. Of these, they found extracellular superoxide dismutase (SOD3) to be present in all their human subjects, especially lining the cervical root and around the most apical cementocytes, which made them hypothesise that SOD3 might play a protective role in cementum formation and maintenance.
Other studies (Carmona-Rodriguez et al. [
52]; Hoz et al. [
53]), instead, agreed on the role of CEMP1 (cementum protein 1) as cellular promoter and differentiation regulator of PDL cells. CEMP1 has been found to be expressed by cementoblasts, PDL cells, and mesenchymal stem cells (found in the PDL). When it was transfected into “non-mineralising cells” (i.e., fibroblasts), it resulted in the production of ALP, OCN, BSP, cementum-derived attachment protein (CAP), the transcription factor Cbfa1 (responsible for mineralisation of bone and cartilage) (Carmona-Rodriguez et al. [
52]; Hoz et al. [
53]), and the formation of mineralised nodules and calcium deposition (Carmona-Rodriguez et al. [
52]). Similar results were found by Arroyo et al. [
54], where it was found that CEMP1-p4 (CEMP1 C-terminal-derived synthetic 15 amino acid-long peptide) also promotes the differentiation of the HOMSCs (human oral mucosa stem cells) into a mineralizing-like phenotype by activating β catenin signalling cascade.
Another protein was also identified that is exclusive to cementum (and not to be found in bones): CAP was mentioned by Carmona-Rodriguez et al. [
52] as one of the products of the CEMP1 transfection into fibroblasts, but also considered to be, in Berkovitz et al. [
26], a collagenous protein located in the matrix of mature cementum and in its cementoblasts.
3.1.10. PPi and Cementum
The work of Foster et al. [
55] on the importance of pyrophosphate (PPi) especially in connection with acellular cementum formation inspired a number of research studies (Thumbigere-Math et al. [
56]; Almeida et al. [
57]; Chu et al. [
58]) that further explored the link between PPi homeostasis mechanisms and cementum formation.
The link between cementum and PPi was firstly found in relation to pathological conditions, such as hypophosphatasia (HPP) (Foster et al. [
55]) and hypercementosis (Thumbigere-Math et al. [
56]). Foster et al. [
55] also claimed that the levels of PPi mostly affects acellular cementum rather than cellular cementum. In their experiments on genetically mutated mice, in fact, they observed that an increase in PPi inhibited the development of AEFC, whereas a reduced PPi produced a significantly thicker AEFC. Experiments in vitro suggested that PPi regulates cementoblast mineralisation and associated genes, and more specifically that a loss of TNAP (tissue-nonspecific alkaline phosphatase) causes underdevelopment/absence of AEFC, whereas loss of ANK (progressive ankylosis protein) or ENPP1 (ectonucleotide pyrophosphatase phosphodiesterase-1) causes loss of control in the apposition of the cementum, resulting in hypercementosis (Foster et al. [
55]). Later investigations between hypercementosis and GACI (generalised arterial calcification of infancy) condition suggested they are both caused by a mutation of the ENPP1 gene (Thumbigere-Math et al. [
56]). Similarly, the loss of ANK also resulted in a thicker cementum (Thumbigere-Math et al. [
56]), possibly suggesting its role as a redundant mechanism of cementum growth, along with ENPP1 and PPi levels (Chu et al. [
58]).
As already mentioned for the histology, composition, and genetics of cementocytes, differences have been highlighted also in their response to inorganic phosphate (Pi) levels. In a study conducted by Almeida et al. [
57], cementocytes treated with Pi were found to express more DMP1 and OPN, with further differences in the expression of OCN and TNAP mRNA between cementoblasts and cementocytes treated with Pi. These findings would again suggest that cementocytes play a different role from that of cementoblasts, and that they might actively be involved in periodontal homeostasis and regeneration (Almeida et al. [
57]).
3.1.11. Parathyroid Hormones
Parathormone (PTH) also seems to be strictly connected to calcium and phosphate homeostasis (Xu et al. [
59]), and its presence (as well as that of parathormone receptor proteins) has already been confirmed in osteoblasts as well as cementoblasts (Berkovitz et al. [
26]). Experimental administration of PTH showed interesting results in terms of cementogenesis. When continuously administered, it seemed to regulate phosphate homeostasis by downregulating DMP1; whereas, when administered in an intermittent fashion, it seemed to promote cementogenesis by increasing the expression of OCN, BSP, and COL1 (Xu et al. [
59]).
Gotz et al. [
60] also explored the presence of components of the insulin growth factor (IGF) system in the periodontium, which is stimulated by the growth hormone (in chondrocytes), and by PTH (in osteoblasts) (Lombardi et al. [
61]). The IGF system plays an important role in the development, homeostasis, and regeneration of the periodontium. More specifically, in the cementum, IGFs were found to be connected to roles of homeostasis and attachment; in the acellular cementum, only IGF-II (who mostly plays a role during prenatal development) was detected; and finally, the PDL cells seemed to function as a reservoir for IGFs, which worked in a paracrine manner (Gotz et al. [
60]).
3.1.12. The Role of Vitamin D
Last but not least, the major role that vitamin D plays in the maintenance of the skeleton is currently very well-known and recognized, and that is true for bones as well as for teeth (Botelho et al. [
62]). Vitamin D is likewise strictly linked to phosphate and calcium homeostasis and can act as a paracrine and autocrine agent (Swapna and Abdulsalam [
63]). In cases of severe vitamin D deficiencies, for instance, the resulting hypophosphatemia and hypocalcemia (which, in response, also causes secondary hyperparathyroidism) are addressed by the organism with bone turnover that then allows the levels of serum Ca
2+ to increase and the levels of serum Pi to decrease. Furthermore, vitamin D receptors (VDR) (ligand-activated transcription elements) can also activate a signalling pathway capable of controlling gene expression (Swapna and Abdulsalam [
63]).
Since vitamin D seems to have an influence and/or a connection to phosphate/calcium homeostasis, PTH, and to also have a potential action as a signalling pathway, further research addressing the role that it plays on cementum and cementogenesis might also be significant in the understanding of this tissue.
3.2. Animal Studies
The analyses of incremental growth in teeth started on animal dentition already in the 1950s with the work of Laws [
1] on the dentine of South Atlantic elephant seals (only later to be followed by that on the cementum of crabeater seals—Laws et al. [
64]).
The animal studies selected for this review (
Supplementary Materials Table S2), mostly focused on age assessments and seasonality (here also achieved with the so-called “traditional methods”). A small portion of them (7 out of 28) tried to investigate the biology of the tissue and understand the mechanisms behind the formation of the incremental layers.
Supplementary Materials Table S2 gives a summary of methods and materials adopted in these studies as well as their outcome. Similarly to what was discussed for the human section of this review, these studies showed a variety of approaches (i.e., mineralised/demineralised; stained or not; longitudinal/transverse sections; etc.), which again reflects the confusion and inconsistencies of the academic approach to the TCA, really only agreeing on the basic principles of this phenomenon (i.e., yearly forming increments made of two 6-month seasonal sub-increments). To this regard, the issue concerning the seasonal interpretation of the bands’ colour is unresolved also in the animal studies. From the collection of those selected for this review, it seems like the majority agrees in interpreting the light-coloured bands as indicative of the summer season and the dark ones of the winter season. However, a few of them agree, instead, with Klezeval’s [
9] interpretation (Beasley et al. [
65]; Burke and Castanet [
66]; Pagh et al. [
67]; Cipriano [
68]).
A closer look to the research addressing the biological formation of the incremental lines is in order, especially if we are to truly understand this phenomenon. Fundamental to the discussion regarding the biology of cementum are the works of Lieberman and Meadow [
10] and Lieberman [
11,
12], which still lead the conversation on this topic. Especially interesting to this discussion is Lieberman [
11], where the author carried out several “feeding experiments” on goats to study the effect of diet on the cementum. From this study, Lieberman concluded that the banding phenomenon is a consequence of the variations in collagen fibres’ orientation and mineralisation strictly connected to the hardness and protein/mineral content of the food consumed by the animals, and this is associated with the seasonality of the diet.
In 1995, instead, Burke and Castanet [
66] attempted the TCA analyses on samples processed with different protocols (i.e., decalcified and non-decalcified sections) and observed through different technologies (i.e., polarised, transmitted, and reflected light microscopy, SEM and microradiography), finally concluding that the cemental lines were visible in each sample subgroup.
More recent studies addressed questions such as the rate of deposition of the cementum, such as in Pérez-Barbería et al. [
28], where the authors found that the rate is not constant in every part of the root, but it is on the inter-radicular pads of red deer molars with an increase of 0.26 mm per year. They also claimed that the dentine thickness had no effect on the cementum, concluding that the dental cementum deposition is independent of tooth wear other than that associated with age. In the same year, Akbulut et al. [
69] also ran one of the few taphonomic experiments on the cementum where they found that in comparison to enamel (which showed signs of abrasion in the group of animals collected between 30 to 90 days postmortem), the cementum did not present such signs at any of the PMI intervals here tested (0 h; 7 days; 15 days; 30 days; 60 days; 90 days).
On a deeper level, Cipriano [
68] in their study of the TCA in wild and captive apes, highlighted a couple of key points that are extremely noteworthy regarding the biology of the tissue. First, they noticed that the cemental lines were more irregular in the captive individuals than in the wild ones, flagging the possible effect of “domestication” on this phenomenon. Secondly, they observed how a particularly cold winter showed on the TCA of each captive individual as a wider white band coinciding with the relative chronological year. This finding led the author to hypothesise that a hormonal response (i.e., thyroid hormones T3 and T4 and catecholamines) to the cold stress and consequent calcium metabolism might play an important role in the deposition of the dental cementum.
3.2.1. TCA on Animals: A Less Problematic Issue
TCA analysis for age and season estimations on animals is more accepted and applied as a technique, especially for wildlife management. This is so for several reasons: from an easier visualisation of the increments in animal specimens (as opposed to the human ones) to a greater availability of samples, less ethical implications and, above all, no forensic involvement, which is really the field that requires most of the rigorousness for any technique to be accepted as evidence in court.
As shown here, the research attempted the visualisation of the cemental increments in numerous different species and again followed different protocols for preparation and processing of the samples. Some of them in the US employed a specialised service (Matson’s Laboratory (
https://matsonslab.com/the-science/cementum-aging/ (accessed on 9 February 2022) (here for their protocol:
https://matsonslab.com/the-science/cementum-aging/ [accessed on 9 February 2022])), which is an example of how the application of the TCA analysis on animals is more widely accepted and less problematic.
3.2.2. A Protocol for Each Tooth
It is the authors’ opinion that animal teeth do not need one standardised protocol to be processed for TCA analysis. The variability of animal dentition and their internal microstructure, in fact, should suggest a species-specific or, even better, a tooth-specific protocol rather than a single standardised one, as is the case for humans.
Dentitions can be categorised by the number of dental sets developed during the animal’s life: monophyodont (1 set), diphyodont (2 sets: deciduous and permanent), and polyphyodont (2+ sets) (Ungar [
70]; Berkovitz and Shellis [
71]).
However, they can also be distinguished by whether they attach to the:
A further difference can be drawn between non-ever-growing teeth (i.e., they stop growing once they reach their final shape, as in humans) or ever-growing teeth (i.e., also called hypselodont in Berkovitz and Shellis [
71], they keep growing in response to high-wear functional movements, as in rodents) (Klezeval [
9]; Ungar [
70]).
Animal dentition can be also categorised according to their teeth morphology from a general point of view (homologous dentition (homodont or isodont, same type of teeth) and heterogeneous dentition (heterodont, different types of teeth as in humans: incisors, canines, premolars, and molars)), to a more form-specific subdivision (as shown in
Table 2) (Ungar [
70]; Berkovitz and Shellis [
71]).
Considering all the above-mentioned differences, a proper understanding of the type of dentition to be analysed and its microstructural anatomy (i.e., location and typologies of cementum) is truly important for the application of an appropriate TCA protocol.
For instance, in the majority of the studies shown in
Supplementary Materials Table S2, the samples consisted of hypsodont teeth, which were processed in longitudinal sections (contrarily to the more common and also advised transverse sections produced for human teeth). In fact, being the cementum both above and below the gum line, this sectioning choice allows a complete overview of the tissue, leading the research to select the most visible and suitable region of interest (ROI) for analysis.
3.2.3. Evolutionary Considerations
As listed in
Supplementary Materials Table S2, TCA analyses have been applied on a variety of species from domesticated animals, such as pigs, horses, and cats, to wilder ones, such as deer and boars, including also fossils and archaeological remains, such as
Titanosaurus (Dinosauria; Sauropoda) (Garcìa and Zurriaguz [
72]).
This wide application reflects an aspect of dental biology unfortunately often overseen, which is our shared evolutionary background with the rest of the animal world. The fact that our periodontal structure behaves somehow in similar ways to that of so many other animal species and with similar timings (seasonal banding phenomenon) as well implies several interesting considerations on the evolution of this tissue.
Teeth have an incredible history. Several organisms, even evolutionarily far away from each other, have these “feeding” structures that might share microstructural elements and/or morphological features. Not only mammals, but also fish and molluscs can have them. Theories about their evolution are currently two: the outside-in and the inside-out theory. The first hypothesises that teeth developed from original placoid scales (as seen on sharks’ skin), which eventually migrated within the mouth after the development of the jaw. The second theory, instead, links our teeth to extinct eel-like animals (conodonts) that presented organised tooth-like elements within their throat, which only after the development of the jaw migrated to its edges (suggesting a development of teeth first and jaws after). Evidence is found for both theories. In fact, different organisms showed traits typical of each specific theory and others, such as the
Loganellia scotica (with dermal scales on their skins and pharyngeal denticles in their throats), presented both kinds of features simultaneously. Interestingly, all of these elements (dermal skins, pharyngeal denticles, and teeth) are serial homologues, meaning that they are formed by the same set of genetic controls (Ungar [
70]; Donoghue and Rücklin [
73]).
However similar and made of similar tissues, a direct evolutionary line between the human dentition and that of all these other “toothed” organisms cannot be drawn nor proved.
Our teeth are considered to be evolutionarily different and pertaining to the same subgroup of mammalian teeth. The common ancestor of mammalian teeth is found in a vertebrate that sets our teeth apart from other animal species because of its embryonic development. The main difference of vertebrate and mammalian teeth in comparison to other organisms, in fact, resides in the involvement of the neural crest cells (which are specific to vertebrates) in the making of teeth (Ungar [
70]).
Additionally to this and especially from a TCA perspective, it is noteworthy to highlight that, by definition, a mammal is considered so also by the presence of a “true mammalian” temporo-mandibular joint (TMJ), as we know it in humans (Ungar [
70]). This is so because the evolution of endothermic organisms has been closely linked to the development of this specific form of dentitions and masticatory apparatus. Given that mammals are endothermic, they have a higher need of fuel (i.e., more complex brain, improved eyesight and hearing, capacity of function at night, etc.) that is provided by a better mastication-breakage of the food consumed. The “true mammalian” TMJ and a high specialised dentition (in terms of tissue-resistant and heterodont specialisation) provide for that need by supplying the animal with specific “tools” and musculature for a strong but also precise and more effective bite (i.e., anatomical functionality of the temporalis, masseter and pterygoids muscles as well as perfect occlusive dental surfaces) (Ungar [
70]; Berkovitz and Shellis [
71]).
Whether the cementum and the TCA phenomenon are inherently connected to this specific oral biology or not should be the topic of further investigations and comparisons between tooth attachments in mammalian and non-mammalian vertebrates first and between vertebrates and invertebrates after.
The presence and concurrent seasonal timing of the banding phenomenon in different mammals would suggest that the TCA might be strictly correlated to the thecodont arrangement of their dentition (which is shared by all mammals). Although the study on the growth increments in the Titanosaurus tooth sample (Garcìa and Zurriaguz [
72]) would hint a different answer, the fact that crocodilians are presently the only non-mammal vertebrates to also have their roots embedded in sockets and covered by cementum (Berkovitz and Shellis [
71]) again would suggest that there might be a relationship between thecodont teeth and TCA.
3.3. Human Studies
A summary of the main studies carried out on human samples can be found in
Table S1. This may help the reader navigate the abundant and varied literature regarding TCA and will also allow a quick access and comparison of the main features/information collected from each study. Specifics include details about the purpose of the study; sample type, size, condition/preservation and provenance; data availability; approach, methods, and histological procedure; final results and statistical significance (when provided).
3.3.1. A Matter of Protocol
A standardised protocol that is followed by all researchers dealing with the same technique (and, most of the time, the same research questions) is essential to the reproducibility and thus success and improvement of a technique. Despite this remark being made already during the early life of the TCA investigations, and despite the current presence of at least three published protocols (Wittewer-Backofen [
74]; Naji et al. [
75]; Colard et al. [
6]) for TCA analysis on human teeth, the vast majority of researchers still do not fully comply with them and keep trying alternative methodologies. A possible reason behind this, likewise the general confusion and misunderstanding/misinterpretation of the technique, might still reside in the overall lack of scientific knowledge when it comes to the biology of the cementum. However, until that knowledge is reached, to experiment randomly might actually be counterproductive, jeopardizing the chances of learning from each other on one hand and, on the other, risking a definitive halt to the adoption of a technique that bears a significant potential. Having said so, the authors of this review are also fully aware of the necessity of “readjusting” the protocol, as often equipment and materials available are not always the same in different laboratories.
The following comparison (
Table 3) was made amongst studies that reported a precise sample size and a quantitative outcome, expressed in terms of correlation between real and estimated age/season (r), statistical significance (
p) and margin of error—or mean standard deviation of the estimated age/season from the real one. This analysis aimed to identify the most successful studies on the basis of quantitative results and to propose them as guidelines to future research, highlighting key points of the protocol that should not be changed. Studies that wish to change these “key points” should clearly state their reasons for doing so, explain how the protocol has been changed, list the limitations encountered and the improvements achieved with the modified protocol, and assess their reliability accordingly.
Out of the 55 studies selected for this section of the review, only 31 presented a quantitative outcome (
Table 3) and only 2 proved to have obtained the most significant results in their research: these being Wittewer-Backofen et al. [
20] and Bertrand et al. [
76], who presented the highest correlation between real and estimated age with a relative low margin of error (1–5 years) on a significantly wide sample size (respectively, 363 and 400 teeth). A further comparison of these two studies is presented in
Table 4 with the aim of highlighting similarities and differences in their methodologies.
In both studies, the authors adopted the traditional approach for age estimation (i.e., direct count of the incremental pairs added to the mean age of tooth eruption or root completion).
As for the sample specifics, both studies preferred single-rooted teeth, whereas the real age, if pathological or not, if modern or archaeological, did not seem to significantly affect the results.
Coming to the histological preparation and processing of the samples, the two protocols agree on the necessity of embedding the roots (even if Wittwer-Backofen specifies that in case of fresh teeth, the embedding might not be necessary) and the production of consecutive non-decalcified cross-section between 70–100 μm from the middle cervical part of the root. As for the option of staining the sections, Wittewer-Backofen clearly explains how teeth were stained with the aim to assess the level of periodontal diseases present in their samples and not to improve the visualisation of the TCA. Similarly, Bertrand did not stain the sections, as he claimed to have followed the ISO-9001 protocol (Colard et al. [
6]), thus not only validating that methodology, but also the one of Naji et al. [
75], which was a sort of preface to the final ISO-9001 and eventually became a major contribution to it.
It is also worth mentioning other studies that adopted this same protocol and still claimed positive results (even if sometimes partial) on smaller sample sizes, such as Jankauskas et al. [
86]; Gauthier and Schutkowski [
81]; Gocha and Schutkowski [
83]; de Broucker et al. [
79]; Lanteri et al. [
90].
In conclusion, the key points resulting from this comparison are:
single rooted teeth;
embedding in resin (avoidable in fresh specimens);
non-decalcified sections;
non-stained sections;
transverse cross-sections;
production of 3 to 5 sequential sections from the mid-cervical part of the root;
thickness of the sections: 70 to 100 μm.
Worthy of note are also the results obtained by Goutham et al. [
84]. Despite reporting a higher margin of error (ca. 8–9 years), they still claimed a high correlation between real and estimated age as well as a positive statistical significance. Whereas they agreed with most of the above-mentioned protocols, they only differed in the choice of the sections’ orientation, which were here cut longitudinally rather than transversally.
3.3.2. Planes and Methods of Sectioning. Longitudinal or Transverse?
As good practice, all the successful above-mentioned protocols recommend the production of multiple consecutive cross-sections from the same tooth. The rationale behind this sectioning procedure is that, first of all, producing sequential cross-sections allows total coverage of the acellular cementum and, secondly, it assures a certain margin of security by increasing the chances of obtaining sections suitable for analysis. This has also been reported by Naji et al. [
75], where they performed multiple sections (4–6 per tooth) and evaluated multiple areas (4–6 per section) to “stabilise the average number of lines by identifying outlier counts within the series produced by phenomenon such as the “doubling effect””. In this way, they averaged the number of lines and added this number to the age of tooth eruption to produce a more precise estimate.
One of the major contributions in terms of sectioning techniques was given by Maat et al. [
104]. In their work, the authors suggested that adjusting the angle of cut of the sections would improve the visualisation of the increments under light microscopy. Given that the cementum is a mineralised tissue and given that the TCA phenomenon is also possibly related to the orientation/size of its crystals (Cool et al. [
36]), adopting an appropriate procedure for the sectioning becomes paramount in order to avoid artefacts and/or undesired optical effects (e.g., “doubling phenomenon”). What Maat et al. [
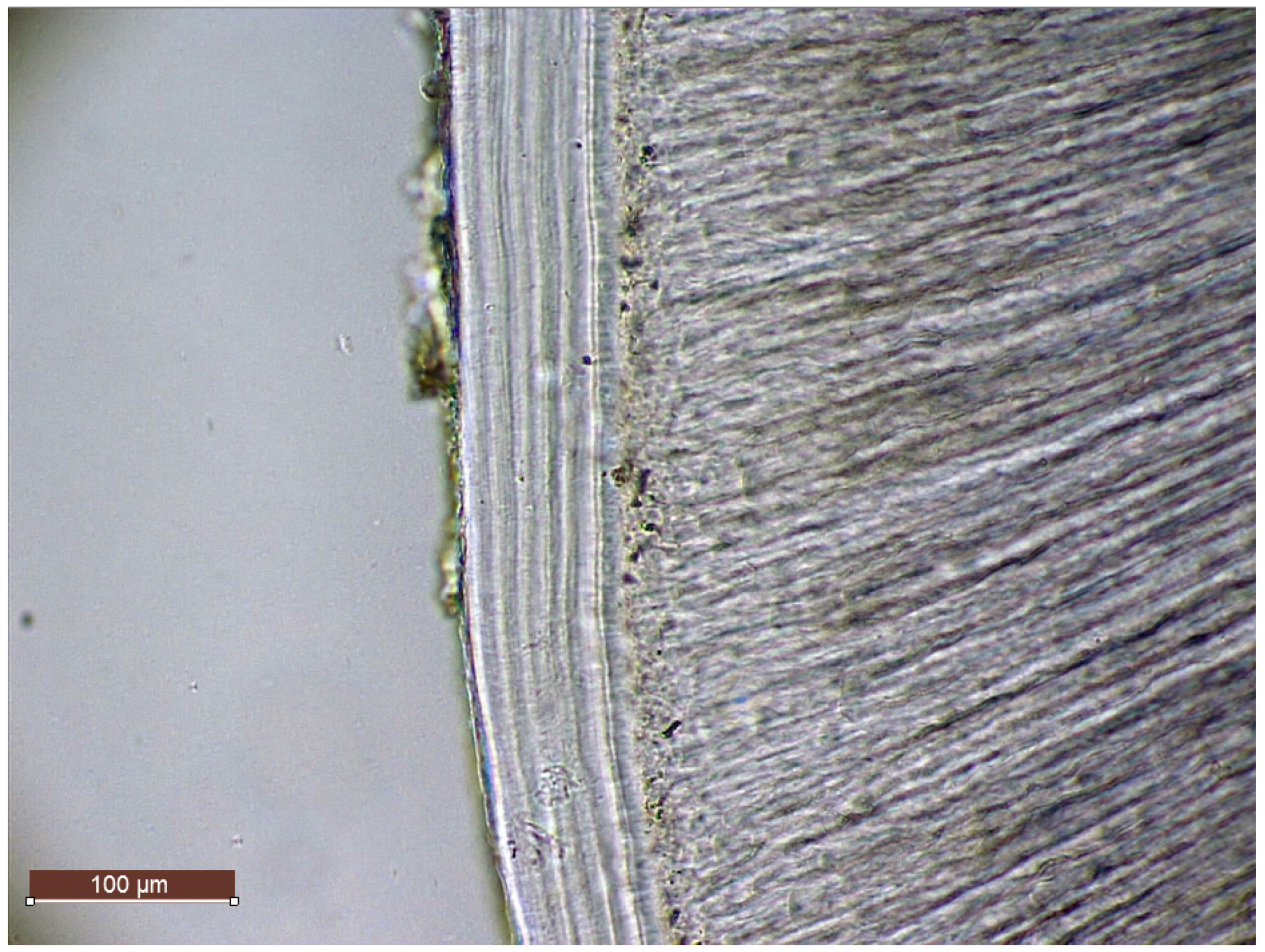
104] proposed (which was then included also in the ISO-9001 protocol) was to cut transverse sections perpendicular to the external surface of the root, rather than to its long axis (as shown in
Figure 1).
Despite numerous recommendations indicating that transverse sections are more suitable for the TCA analysis on human teeth, the literature has been bouncing between longitudinal and transverse sectioning since the very beginning of the TCA investigations (Stott et al. [
5]; Charles et al. [
7]; Condon et al. [
8]). Longitudinal sections were most commonly used for TCA analysis on animal teeth (
Supplementary Materials Table S2). The reason behind this different choice was most probably related to the fact that some animal teeth do not share the same characteristics of humans. More specifically, the cementum in some animal species (e.g., horses) grows alongside the enamel (Hillson [
24]), instead of coating only the root surface like in the human dentition. That being the case, a longitudinal section would indeed be more appropriate as it would allow a wider surface of investigation. Since the technique of TCA was originally devised on animal dentition, this might explain why some scholars originally adopted some of the same protocols on human teeth. The studies that have more recently tried longitudinal sections on human teeth (Kasetty et al. [
88]; Schug et al. [
99]; Goutham et al. [
84]; Geetha et al. [
82]; Kulkarni et al. [
89]; Mohan et al. [
94]; Swetha et al. [
101]; Mahalakshmi Loganathan et al. [
92]) show a variety of results (see
Table 3 and
Supplementary Materials Table S1). Amongst the ones listed here, the most successful seem to be those where sections were not stained or demineralised (Goutham et al. [
84]; Geetha et al. [
82]; Mallar et al. [
93]). When Mallar et al. [
93] state that in their study the
p value between actual and estimated age was more significant in longitudinal sections than in cross sections, it is necessary to also consider their sectioning procedure. This, in fact, could have been easily biased by the fact that sections were produced manually and not by using a microtome, thus affecting their evenness and thickness.
The studies that, instead, preferred to stain their sections (Schug et al. [
99] and Kasetty et al. [
88]) showed a lower success in the assessments.
As for the others, unfortunately, they cannot be properly assessed as they lack consistent information about their results, mostly stating a positive correlation with no further information. The undoubtedly good results in terms of correlation to real age,
p value and sample size obtained by Goutham et al. [
84] with longitudinal sections could make their protocol a valid alternative to the two previously suggested (Wittewer-Backofen et al. [
20] and Bertrand et al. [
76]) despite a slightly higher margin of error of 8–9 years, and might also suggest that the use of longitudinal or transverse sections is ultimately at the discretion of the researchers and does not affect the analysis in a significant way.
3.3.3. Demineralisation and Staining: Is It Really Necessary for the TCA?
What really seems to be more damaging to the TCA is the demineralisation and staining of the sections. As these are basic techniques in histology and are widely and commonly used when analysing tissues, they have been carried out also on the first examinations of human cementum (Condon et al. [
8]; Klezeval [
9]).
However, in our opinion, until the biological structure and composition of the tissue is better understood, it would be best not to chemically destroy (demineralisation) or alter (staining) any of it.
So far, the study of the literature has shown that annulations are visible in mineralised, demineralised, stained, and unstained sections. While this raises more questions on their biology, it is the degree of their visibility that should matter when the TCA is used for age and season assessments.
The results collected here clearly show that visibility is not as clear in demineralised and stained sections as it is in the non-demineralised and unstained ones. Thete et al. [
102], for instance, based their observations on decalcified and stained sections and reported one of the lowest r values amongst the quantitative studies here reviewed.
A visual example of the outcome obtained by staining the sections is presented by the experiments run by Petrovic et al. [
97]. The sections were here stained in standard histological Hematoxylin and Eosin (H&E), Toluidine Blue (TB), Van Gieson, Mallory Trichrome, Masson Trichrome, Periocid Acid Shiff, and Crocein Scarlet/Acid Fuchsin. Eventually, the authors claimed that through Toluidine blue “the dentinocemental line was recognizable and incremental lines were distinct and simple to count”. Similarly, Shukla et al. [
105] tested different stains on decalcified sections and concluded that cresyl violet better highlighted the increments, thus helping in the count of the annulations (on this topic, see also a review by Devi et al. [
106]).
However, by looking at their results, in our opinion, the stained sections (
Figure 2) are not in any way clearer than the natural increments (
Figure 3).
Sousa et al. [
100] also carried out a similar experiment and concluded that the best results in terms of positive correlation between estimated and real age were obtained from mineralised and unstained sections. More recently, Pradeep et al. [
98] seconded this by clearly stating that mineralised ground sections analysed in phase-contrast microscopy are more reliable in age determination, whereas (despite achieving a positive correlation: r = 0.96/0.97), they added that “there was a statistically significant difference observed between actual and calculated age in decalcified sections stained with H and E and PSR” and therefore “further studies have to be done to prove the reliability of estimating the age from PSR stained sections”.
In conclusion, it could be said that, so far, the majority of quantitative successful studies agrees in saying that mineralised and unstained transverse sections are to be preferred in TCA analysis. Promising results might also be achieved from mineralised and unstained longitudinal sections, even if assessments on longitudinal sections seem to bear a general higher margin of error in the estimation of age. A lower rate of success and an overall decrease in the visualisation of the increments appear to be linked to demineralisation and staining techniques, which should therefore be avoided in future TCA analysis.
3.3.4. Age Estimation: Age at Eruption VS Age at Root Completion
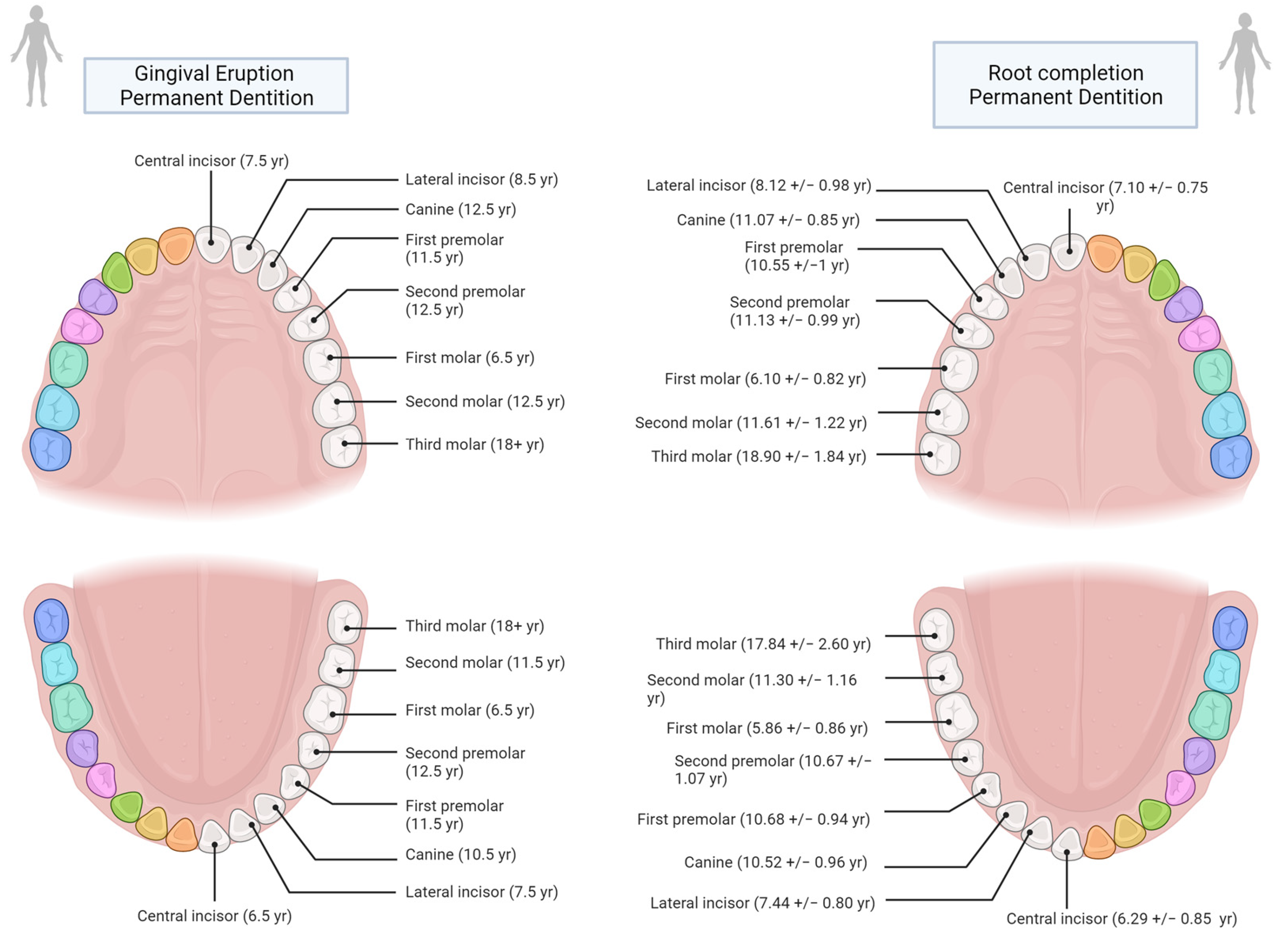
The traditional approach to the TCA studies for age estimation consists in the sum of the total annulations to the tooth-specific age of eruption or to the root completion.
It is important to understand that “eruption” is, as Liversidge [
107] says, “the process of movement of a tooth from within the jaw to a functional position in the oral cavity and subsequent movement thereafter” rather than a one-time event. Similarly, Hillson [
108] clarifies that dental eruption, despite being the most reliable ageing factor in the human skeleton (especially up to 18 years of age), is, however, also the “most variable of all the dental development stages”. If the development of the crown, for instance, follows very strictly timed steps, the time of eruption, instead, varies upon a number of different factors (diet, environment, genetics, etc.). Hillson, adding that this variability might be caused by the fact that eruption is not only the result of dental development, but also that of the surrounding jaw and soft tissues, fundamentally agrees with Liversidge in saying that dental eruption is a continuum, and as such follows a tempo of growth (term from Franz Boas [
109]) that might differ intra- and inter-population.
Having established that dental eruption is a process, then a further distinction should be made (and taken into consideration when determining age) between alveolar eruption and gingival eruption (also known as clinical eruption). While the first one describes the moment in which the alveolar crypt opens to let the tooth through (visible only in dry and archaeological specimens), the latter instead relates to the emergence of the cusps through the gum (Hillson [
108]).
In this regard, another important observation was made by Liversidge [
107], who showed that there are differences in the development of the roots at gingival emergence: “Permanent teeth erupt clinically when three-fourths (R3/4) of the root have formed (Grøn [
110]). Mandibular M1 and I1 (i.e., first molar and incisor) exhibit generally less root development (R1/2) and canines more root (Rc) at emergence than other teeth”.
As shown in
Supplementary Materials Table S1, the margin of error amongst studies that have used the age at eruption and those that have used the age at root completion does not vary significantly. This is due to the fact that there is no major difference between the two in terms of years (
Figure 4). Nonetheless, following Hillson [
108], an effort should be made to create reference tables tailored to each specific population and its sexual dimorphism.
3.3.5. Counting the Increments: Old and New Approaches
Traditionally, the increments are “manually” counted either directly from the microscope eyepiece or from digital pictures. Researchers have raised a few issues concerning this, such as being time-consuming and bound to error, as the count can be tiring and subjective to the observer. A solution sometimes adopted for this (Bertrand et al. [
76]) is that the count of a random subset of the sample is performed again after an established amount of time (in this case 6 months) by the same observer to assess the intraobserver error.
A further step ahead was attempted by automatising the increments count via a software (AutoTCA) that, using Fourier analysis and algorithms, was able to analyse the TCA digital pictures and create graphs with peaks corresponding to the darker increments. The peaks count would ultimately be easier to perform and less subjective (Czermak et al. [
112]). The final outcome of this study, however, showed a general underestimation of the total number of the increments. Therefore, the software, despite resulting in a slightly better count to the one manually obtained, still needs improvements (on the use of algorithms, see also Streso and Lagona [
113]). Another digital approach was tested by Wall-Scheffler and Foley [
114] on animal teeth by using a tool called Digital Cementum Luminance Analysis (DCLA). With this method, a selected area for analysis would be graphed and given numerical values for each pixel. A minimum luminance value for low luminance (LL) bands and a maximum value for high luminance (HL) bands was also given. However, in this case the authors clearly state that this approach is not to be seen as an alternative to the traditional method for the age estimation from TCA (as in Czermak et al. [
112]), but rather a complement to it. In fact, it seems to have a correlation to the differences in seasonal temperatures, carrying a potential insight into the seasonality of this phenomenon.
This effort towards objectivity might have led more recent studies into tackling the same issue by adopting a completely different approach that relies on measurements rather than visual counting of the increments.
Some of the studies that preferred longitudinal sections (Gupta et al. [
85]; Geetha et al. [
82]; Kulkarni et al. [
89]; Mahalakshmi Loganathan et al. [
92]) also shared the same “mathematical approach”, which consists of dividing the total thickness of the cementum (from the cemento-dentinal junction, CDJ, to the external surface) by the width of two consecutive well-defined incremental lines. This would result in the total number of increments which then is added to the specific tooth age at eruption. However, as already mentioned above, the results of these studies are not always clearly presented and appear significantly inconsistent (see
Table 3 and
Table S1). Amongst these studies, only Charan Gowda et al. [
78] and Pradeep et al. [
98] showed a more positive correlation to real age (r = 0.97; r = 0.98/0.96, respectively) by using the same calculation but, contrary to the others, they were carried out on transverse sections instead of longitudinal. Due to these inconsistencies, a fair judgement on this approach and sectioning protocol cannot be currently obtained.
Another original but dubious attempt was made by Thete et al. [
102], where the authors, attesting a “positive correlation” (+0.3625) between age and cementum thickness, calculated two regression formulae, claiming that “for every 1µm increase in cementum thickness, the age increases by 0.3603 years” and “for every 1-year increase in age, the cementum thickness increases by 0.2453 µm”. Also in this case, the uncertainty regarding the biology of the tissue as well as the fact that certain life events seem to affect and cause variations in the thickness of the increments (e.g., childbirth, kidney diseases, etc.—see Kagerer and Grupe [
87], Cerrito et al. [
115], Penezić et al. [
116]) should suggest caution, and extended supporting evidence should be reported before making such a claim.
On a brighter note, the use of measurements brought some alternative solutions to the study of more challenging samples and to answer different research questions. An example is given by the works carried out by Oliveira-Santos et al. [
96] and Cerrito et al. [
115].
The first is a comparison of the traditional TCA method for age estimation and the so-called Increments Extrapolation Approach (ILE) in experimentally burnt human teeth. The ILE is similar to the mathematical approach already discussed above, but follows a different formula: ILE = (L/2) × C (where L = thickness of two contiguous lines and C = total thickness of the cementum from CDJ to its external surface). While on one side, the results of this study showed that annulations are still visible in teeth that were burnt at 400 °C (also up to 600 °C, and still visible but with decreasing quality in samples burnt at 800 °C and 1000 °C in Gocha and Schutkowski [
83]), on the other, it proved that the ILE approach estimated age with a reduced margin of error in comparison to the traditional method (which was, in this case, hindered by the dimensional changes induced by heat).
In Cerrito et al. [
115], instead, a ratio between the cementum thickness and the number of years of deposition was established. The year corresponding to each band was also calculated. This way, the authors were able to identify pathologies and stressful events through the increments and date them. Likewise, Panezić et al. [
116] used a similar approach to identify “stress layers” during the Neolithic demographic transition in Europe.
A further use of measurements consisted in the statistical prediction of age on the basis of cementum thickness. An example was given by the work of Pinchi et al. [
117] where the authors relied on the thickness of the cementum (taken at different sides of the tooth) and on regression models to predict age. Similarly, Birimiša et al. [
118] took the measurements at four different points on each section and on several levels from apex to dental cervix and by multiple statistical analyses observed that thickness can vary between males and females and can be used as a reliable tool for predicting age. However, both studies highlight the necessity to test this approach to a wider sample size, including a wider age range diversity.
3.3.6. Season at Death Estimation
Along with age, the TCA is also adopted for determining season at death. The assessment is carried out by observing the colour of the outer increment. Each colour (i.e., light or dark) corresponds to roughly six months either in the fall/winter or spring/summer period.
The first attempt in narrowing the estimation down to a shorter time interval was performed by Wedel [
103]. In this case, the author measured the increments of the same colour as the outer increment and calculated an average of growth. That average was then used to estimate a percentage of growth of the outer band. The same approach coupled with the traditional method for age estimation was attempted again in Wedel and Wescott [
21], where the authors were able to link the season at death of eleven individuals to the peak seasonal timing of an epidemic of infectious diseases. Later, Wedel and Gocha [
119] reassessed the seasonality method proposed in 2007 [
103] and found that the accuracy was 78% instead of the 99% reported before.
Additionally, Sinha et al. [
95] based their seasonal estimation on the calculation of percentage of growth, following the formula: x = b/a × 100 (where x = percentage of growth; b = outer increment thickness; a = average of the same colour increments thickness). This study also confirmed a strong correlation between the thickness of the increments and the progression into the next seasonal period.
The question of seasonality in the TCA, however, needs further clarification. Up to the most recent studies, the seasonal TCA has been applied on samples that were collected in the Northern Hemisphere, whose countries mostly share the same seasonal pattern. A few studies concerning seasonality included in their sample specimens coming from Colombia (Ralston [
120]) and Latin America (Swearinger [
121]). Ralston claimed that the geographical origin of the sample did not appear to have any impact on the determination of season-at-death, even though Colombia is still mostly part of the Northern Hemisphere, despite being closer to the Equatorial line. We believe that only samples collected from countries belonging to the Southern Hemisphere that present a proper reversed seasonality from the Northern one might give a more sensible and significant indication on the effects of seasons on the tissue.
As for Swearinger, instead, as the individuals here analysed were unidentified, they were presumed to be migrants of “Latin American origin”—a definition that anyways includes a number of different countries at different latitudes, thus not giving any further insight on this matter.
Understanding how seasons affect the cementum would also shed a better light on the direction that biological studies of the tissue should take, since it would clarify at what degree the environment plays a role in the formation of the increments. Future studies are therefore strongly advised to pursue such an investigation.
3.3.7. Analysis and Interpretation. The Use of Microscopy
The analysis and interpretation of the data is the last but not least important step in the TCA analysis.
Researchers differed in the choice of type of microscopes (i.e., transmitted or reflected light microscopes) and in the choice of illumination techniques with the bright-field microscope being the most commonly used. Interestingly, Geetha et al. [
82] ran their analyses through a light microscope, a phase contrast, polarised, and stereomicroscope. Then, by comparing the r value obtained with each one of these, they found that the best performing microscopes for TCA analyses were the polarised, light, and phase contrast microscope (respectively, r = 0.877; 0.875; 0.866). Similarly, but with different results, Goutham et al. [
84] confirmed what already said in Charan Gowda et al. [
78] by claiming that phase-contrast is to be preferred to brightfield and polarised light microscopy (respectively, r = 0.9952; 0.9760; 0.9833).
More attention should be paid to the thickness of the sections and the microscope light chosen for analysis. As a rule of thumb, materials that are transparent or semi-transparent (which allow the light to travel through them, e.g., tissues, cells, crystalline components, etc. (
https://jordilabs.com/lab-testing/technique/microscopy/tlm/ [accessed on 16 April 2021])) should be analyzed in transmitted light microscopes, whereas more opaque materials (e.g., metals, ceramics, etc. (
http://zeiss-campus.magnet.fsu.edu/articles/basics/reflected.html [accessed on 16 April 2021];
http://zeiss-campus.magnet.fsu.edu/articles/basics/contrast.html [accessed on 16 April 2021])) should be analyzed in reflected light microscopes.
In the TCA studies, both types of light have been used because both thin and thick sections have been analyzed, as also stated by Lieberman and Meadow [
10]. The implications of using different types of sections and microscope light on the TCA were brought up only by Burke and Castanet [
66], Klezeval [
9], and Hillson [
24]. These authors warned about the changing appearance of the increments’ colours according to the type of light adopted for the analysis. In the following 30 years of application, however, the vast majority of research was conducted on thin sections with transmitted light (
Supplementary Materials Table S1) and mostly agreed on the seasonal interpretation of the increments as light bands indicating the spring/summer season and dark bands indicating the fall/winter season. This caused future researchers to overlook the distinction between transmitted and reflected light, which, coupled with the use of ambiguous terminology to indicate the increments, added to following misinterpretations and confusion that led authors to disregard the TCA as unreliable.
An example of “ambiguous terminology” can be found in Klezeval and Shishlina [
122], where the authors claimed that under transmitted light “one annual layer consists of a rather wide opaque band bordered by a transparent incremental line” and that “the incremental line forms from late autumn to early spring and the wide band of the layer forms from spring to autumn”. In Klezeval’s main work [
9], it is also said that “A growth layer of cementum consists of a band of tissue bordered by an incremental line (…). This layer is presented on a ground section in transmitted light as a wide dark band and narrow light incremental line. In reflected light, the incremental line looks dark”, and then she adds “it is the translucent band (i.e., incremental line), which is hypercalcified” (Klezeval p. 38 [
9]).
However, as above-mentioned, numerous more recent authors claimed the opposite to be true, as in Wedel and Wescott [
21]. In fact, they claimed to have used a transmitted light microscope for seasonal estimation and interpreted the “less mineralised opaque or dark band” (also called “winter or arrested cementum increment”) for the winter season and the “translucent or light band” (also called “summer or growth increment”) for the spring/summer period.
In conclusion, more recent and quantitative results demonstrated that Wedel’s seasonal interpretation of the increments (Wedel [
103], Wedel et al. [
123], and Wedel and Wescott [
21]) is more correlated with the real season at death.
From an issue as such, it appears clear how important it is for future studies to develop an appropriate terminology to refer to the TCA and its features.
Future studies comparing the tissue under transmitted and reflected light are strongly recommended in order to ascertain and highlight this phenomenon, which, if not stressed accordingly, might easily be overlooked (as it has already happened) leading to a cascade of future misinterpretations.
3.3.8. New Tech
New innovative technologies have been adopted with the aim of avoiding the destructive procedure that TCA analysis requires. In particular, it is worth mentioning the work presented by Le Cabec et al. [
91]. In this study, 20 canines were scanned in transverse sections with a Propagation Phase contrast X-ray synchrotron μCT (PPC-SR-μCT). The overall result showed a promising correlation but a general age underestimation, which might be explained by the fact that at the synchrotron, the so-called “stress events” are more easily visible than the regular ones, thus compromising the count for ageing. However, the possibility of being able to see the cementum, one of the thinnest hard tissues of the human body, without destroying the sample is remarkable, especially when dealing with rare or archaeological specimens. In case of such exceptional specimens (if a better image quality is not reached in the meantime), age estimation could be carried out on the images obtained with the synchrotron by applying methods such as the ILE approach proposed by Oliveira-Santos et al. [
96] for severely damaged specimens.
Another interesting approach is the one proposed by Colard et al. [
27] (and further discussed in the paragraph “Lines of Salter”), where Raman spectroscopy was used to determine the mineralisation level of the cementum lines showing that smaller and darker incremental lines are associated with heavily mineralised tissues and with fall-winter periods, whereas thicker and brighter bands are correlated with lower mineralisation levels and can be attributed to spring-summer months.
3.3.9. A Decades-Long Debate: Controversial Issues
In nearly 70 years of application, the debate surrounding the TCA never stopped. On the contrary, it is still ongoing.
Numerous issues have been discussed by a few scholars that eventually agreed on the unreliability of the technique, discouraging further studies (Renz and Radlanski [
124]).
Issues mostly go from protocol difficulties such as breaking of the cementum during sectioning and grinding (Renz and Radlanski [
124]), unevenness of the sections, and saw marks (Cobb [
125]) to poor visibility (Renz and Radlanski [
124] and Roksandic et al. [
126]).
In some cases, the state of preservation of the specimens might have affected the analysis (as clarified in Roksandic et al. [
126] and also favoured by Bertrand [
127]). Unfortunately, there are not enough studies exploring taphonomic effects on the TCA, an area that undoubtedly also needs further research.
In other cases (such as in Cobb [
125]) the inexperience of the researcher(s) played a major role in the unsuccess of the analysis. As the purpose of their study was to evaluate the ease of the TCA technique for non-expert examiners, they eventually concluded by saying that an inexperienced researcher could not produce sections suitable to cementochronology, again discouraging new researchers from applying it. However, a statement as such easily goes for any other anthropological technique. As you need to have some familiarity with human anatomy in order to determine age at death from skeletal remains, likewise researchers need some practice and familiarity with teeth and histological procedures before dealing with the TCA (see again Bertrand [
127] for more on this topic).
Smith [
128] mentions a problem that, in our opinion, is closer to the real issue regarding the cementum. Besides all that has been said so far, she also refers to the fact that ignoring the rate at which the cementum is deposited makes this whole technique uncertain. With that said, the ignorance of this tissue on a basic level, from its early development to its seasonal behaviour throughout an individual’s life, remains the most considerable flaw of all. In fact, it is from this flaw that all the others stem. If we were to understand its underlying mechanisms, then we could also theorise and agree to a more efficient and standardised protocol, putting a halt to all the different and often incongruent experimentations and interpretation carried out so far (on this see also Colard et al. [
6]).
This is the reason why the future of the TCA depends on studies dealing with its biology rather than those still concerned with the assessments of age, season, and life events. Yet, even the most recent TCA studies are still “predictive studies”. A clear example of that (and also of how this debate is still current) is the latest discussion between Edinborough et al. [
129] and Panezic et al. [
130]. Here, the first criticises the latter’s previously published work (Panezic et al. [
116]), questioning how the authors were able to identify and directly connect “irregularities” in the increments’ appearance to pregnancies, when (1) it is not clear how and why the increments form and what are they affected from and (2) the main problem of the TCA is its own irregularity (i.e., the increments are rarely regular).
Other authors mentioned the potential of using the TCA to identify specific life events. However, we believe that, again, until the biology of the tissue is well understood and clarified, we should refrain from such claims and from the temptation of looking for the “Holy Grail” of biological anthropology. TCA has, in fact, great potential but it is, as all anthropological techniques, a tool to be used along with the others in a multi-approach method and not standing alone. While we crave for that one technique that answers all of our questions, what should really matter is how much a technique can help in drawing the final biological profile, how much it can say, what its limits are, and how we can complete the missing information with the other methods we already have. In fact, if we were to disregard a technique because it is not 100% accurate, then we could not perform anthropology.
3.3.10. A Note on the Terminology
To the new researcher approaching the TCA for the first time, a number of different terms are listed in the literature when referring to the colours and pattern of the cementum.
Only in the 55 papers selected for this review, the phenomenon is referred to with the following terms:
Sometimes a few different terms are used in the same study to refer to the same phenomenon.
Klezeval [
9] describes the cementum pattern as one band of tissue followed by one incremental line. This description is the closest to what is found in the clinical literature, where, instead, the increments are called “lines of Salter”: “Incremental lines called as lines of Salter are seen in cementum as during the process of cementogenesis, there are periods of rest and periods of activity. The periods of rests are associated with these lines” (Kumar [
34]).
The width of the banding phenomenon is generally defined as “wide/narrow” and/or “thicker/thinner”, while the colours might be referred to as “translucent/opaque”, “transparent/opaque”, “bright/dark” and/or “light/dark” (with the latter being the most commonly used in recent publications).
Furthermore, additional names have been introduced to refer to the technique in itself. Since the acronym “TCA” (Tooth Cementum Annulation) indicates the banding phenomenon rather than the technique, the word “Cementochronology” (Colard et al. [
6]) was created in order to talk about the use of the TCA in age estimation, whereas “Dental Cementum Increment Analysis” (or “DCIA”) was introduced to refer to the use of the same phenomenon for season estimation (Wedel and Wescott [
21]).
The variety of terms adopted to describe the phenomenon observed on the cementum has unfortunately increased confusion amongst researchers and misinterpretation of their work.
Ideally, all researchers working with the TCA should adopt the same terminology when referring to the features of the phenomenon they are observing. Especially when referring to colours, terms such as “transparent” or “opaque” should be avoided since they might not be interpreted in the same way by everybody.
A greater effort towards objectivity should be collectively made when working with the TCA. An example is given in Oliveira-Santos et al. [
96] when they adopt acronyms such as IL for Incremental Lines, despite the fact that this adds a new acronym in the field of cementochronology that could introduce confusion in regards to past publications.
In the meantime, if a terminology for the TCA that is internationally recognised and adopted is not yet present, researchers are strongly advised to include a key in their papers that would clearly describe what they are referring to and with which terms.
3.3.11. A Proposal
A proposal on how to report data and results related to the TCA is here described in the following steps:
Definition of the purpose of the study.
Sample specifications: The sample size should ideally be expressed in the number of sections, in addition to the number of teeth and individuals. In fact, having only one of these three factors would give only a partial understanding of the sample analysed. For instance, knowing the number of teeth is not indicative of how many sections have been produced per tooth, and therefore it is not indicative of how many times the count/analysis has been carried out.
Personal data. When possible, until the TCA is still to be understood, studies should be carried out on a known sample (i.e., known sex, age, pathologies, and place of origin/residence). The place of origin/residence is here included mostly in case of seasonal studies.
Protocol. It is strongly advised to follow protocols that already proved to be valid and with successful outcomes. The adoption of the same protocol from the majority of researchers would allow the collection of comparable data. If the proposed protocols are not followed, a clear description and rationale of the chosen procedure should be given and explained step by step.
Microscopes. Further details on the type of microscope and light adopted in the study should be added. This information would significantly increase our understanding of the tissue’s behaviour when visualised under different types of light.
Results should be presented in a quantitative way, clearly stating correlation value, statistical significance, and margin of error.
Terminology. Ambiguous terms should be avoided in the description of the increments’ colours or in the definition of the TCA. The adoption of the clinical name “lines of Salter” to indicate the incremental lines and/or the use of acronyms with shared meanings are suggested.