The Andronowski Skeletal Collection for Histological Research: A Modern Anatomical Contribution
Abstract
:1. Introduction
1.1. Skeletal Collections for Histological Research
1.1.1. Melbourne Femur Research Collection
1.1.2. Ericksen Femur Collection
1.1.3. Skeletal Tissue Collections at the National Museum of Health and Medicine
1.1.4. Andronowski Skeletal Collection for Histological Research
2. Description of ASCHR Curatorial Processes and Ethical Considerations
2.1. Acquisition of Skeletal Material for ASCHR
2.2. Processing and Curation
3. ASCHR Demographic Information and Digital Data
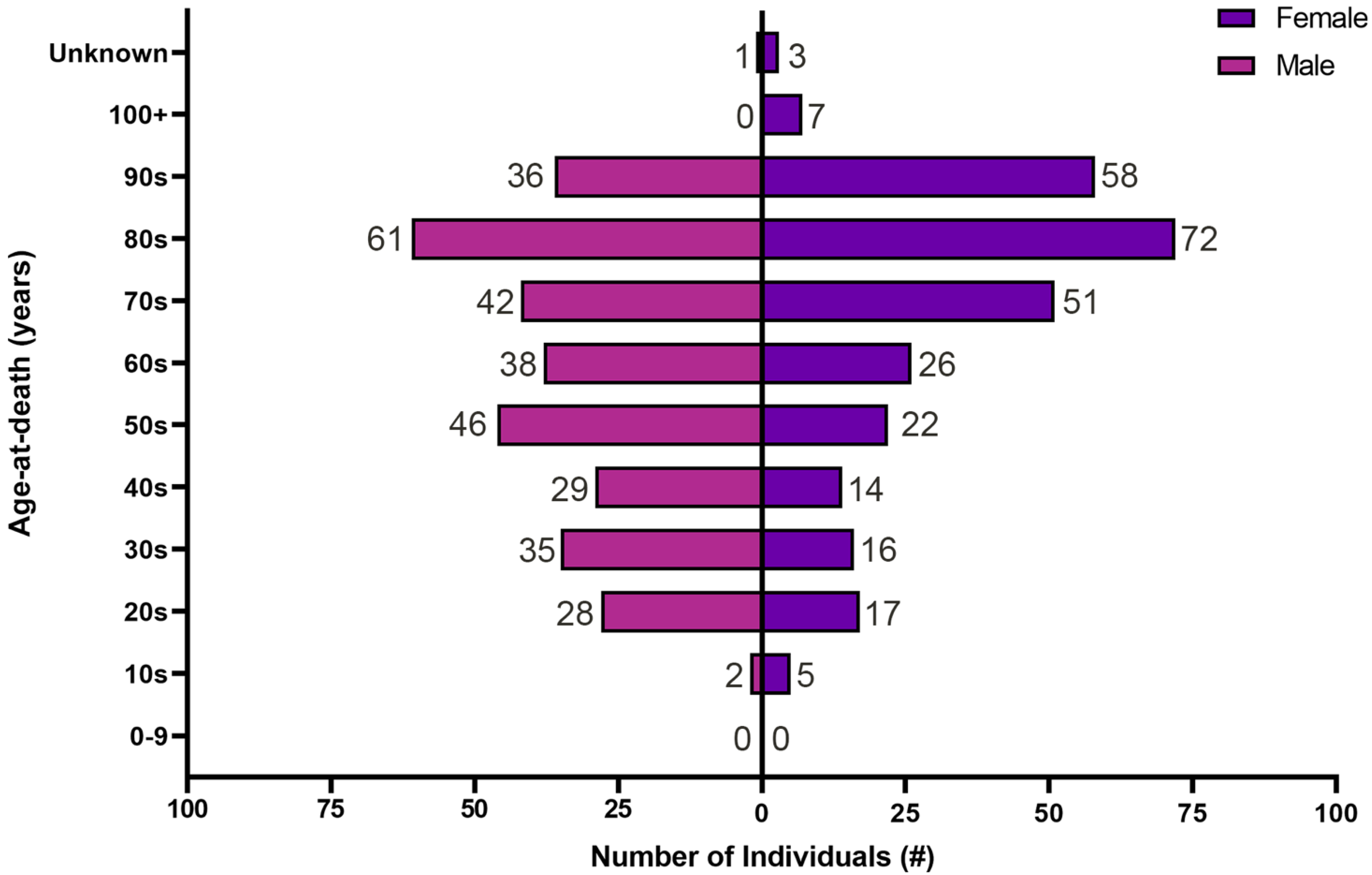
3.1. Demographic Information
3.2. Histological and Imaging Data
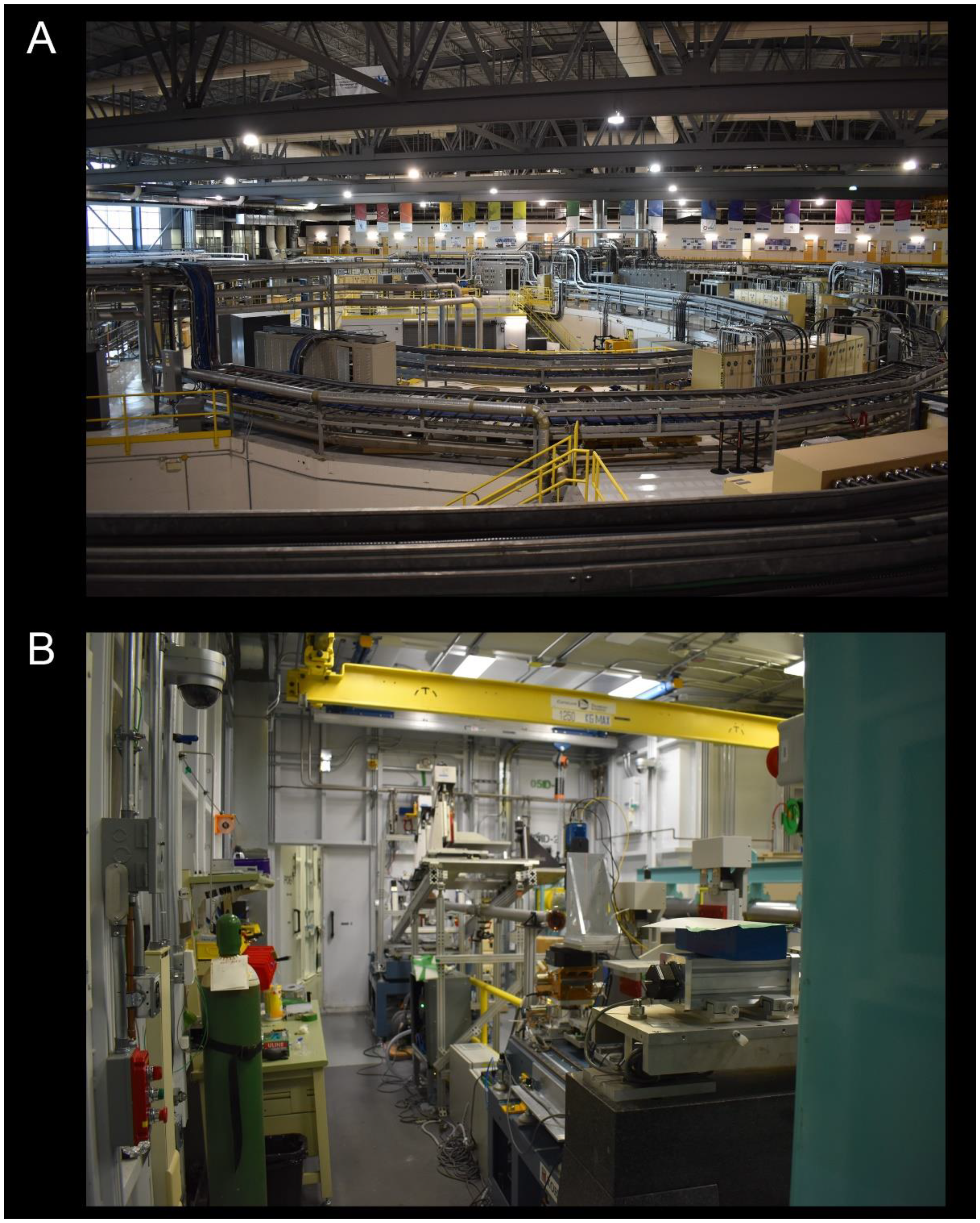
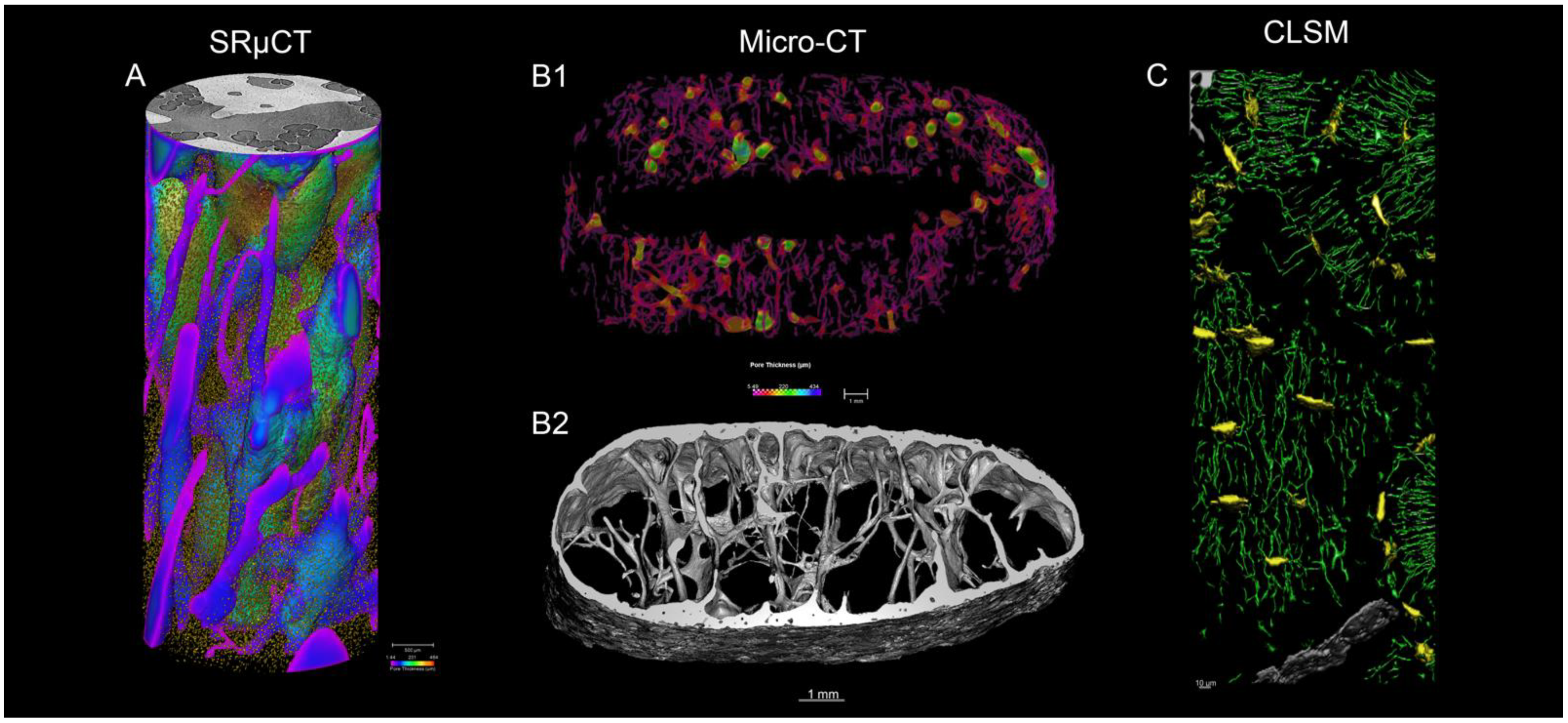
3.2.1. Synchrotron Radiation Micro-Computed Tomography
3.2.2. Laboratory micro-Computed Tomography
3.2.3. Confocal Microscopy
4. ASCHR Scientific Contributions
4.1. Protocols for Research Requests
4.2. Significance of Collection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Işcan, M.A. Comparison of the Hamann–Todd and Terry Collections. Anthropologie 1992, 30, 35. [Google Scholar]
- Hunt, D.R.; Albanese, J. History and demographic composition of the Robert J. Terry anatomical collection. Am. J. Phys. Anthropol. 2005, 127, 406. [Google Scholar] [CrossRef] [PubMed]
- Alves-Cardoso, F.; Campanacho, V. The Scientific Profiles of Documented Collections via Publication Data: Past, Present, and Future Directions in Forensic Anthropology. Forensic Sci. 2022, 2, 37–56. [Google Scholar] [CrossRef]
- Petaros, A.; Caplova, Z.; Verna, E.; Adalian, P.; Baccino, E.; de Boer, H.H.; Cunha, E.; Ekizoglu, O.; Ferreira, M.T.; Fracasso, T.; et al. Technical Note: The Forensic Anthropology Society of Europe (FASE) Map of Identified Osteological Collections. Forensic Sci. Int. 2021, 11, 110995. [Google Scholar] [CrossRef]
- Imbler, S. Can Skeletons Have a Racial Identity? The New York Times, 19 October 2021. Available online: https://www.nytimes.com/2021/10/19/science/skeletons-racism.html(accessed on 16 February 2022).
- Schuessler, J. What Should Museums Do with the Bones of the Enslaved? The New York Times, 25 August 2021. Available online: https://www.nytimes.com/2021/04/20/arts/design/museums-bones-smithsonian.html(accessed on 16 February 2022).
- Williams, S.E.; Ross, A.H. Ethical dilemmas in skeletal collection utilization: Implications of the Black Lives Matter movement on the anatomical and anthropological sciences. Anat. Rec. 2021, 10, ar.24839. [Google Scholar] [CrossRef]
- Labrash, S.; Lozanoff, S. Standards and guidelines for willed body donations at the John A. Burn. Sch. Med. Hawaii Med. J. 2007, 66, 72. [Google Scholar]
- Mann, R.W.; Labrash, S.; Lozanoff, S. Medical School Hotline: A New Osteological Resource at the John A. Burns School of Medicine. Hawaii J. Health Soc. Welf 2020, 79, 202. [Google Scholar] [PubMed]
- Andronowski, J.M.; Crowder, C.; Martinez, M.S. Recent advancements in the analysis of bone microstructure: New dimensions in forensic anthropology. Forensic. Sci. Res. 2018, 3, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andronowski, J.M.; Cole, M.E. Current and emerging histomorphometric and imaging techniques for assessing age-at-death and cortical bone quality. WIREs Forensic Sci. 2021, 3, e1399. [Google Scholar] [CrossRef]
- Andronowski, J.M.; Davis, R.A.; Tubo, G.; Cooper, D.M.L. Application of Synchrotron Micro-Computed Tomography and Confocal Laser Scanning Microscopy to Evaluate Sex-Related Differences in the Human Osteocyte Lacunar-Canalicular Network across the Lifespan. Am. Assoc. Phys. Anthropol. 2019. Available online: https://www.researchgate.net/publication/335310785_Application_of_Synchrotron_micro-Computed_Tomography_and_Confocal_Laser_Scanning_Microscopy_to_Evaluate_Sex-Related_Differences_in_the_Human_Osteocyte_Lacunar-Canalicular_Network_Across_the_Lifespan (accessed on 16 February 2022).
- Andronowski, J.M.; Davis, R.A.; Holyoke, C.W. A Sectioning, Coring, and Image Processing Guide for High-Throughput Cortical Bone Sample Procurement and Analysis for Synchrotron Micro-CT. J. Vis. Exp. 2020, 10, e61081. [Google Scholar] [CrossRef] [PubMed]
- Carter, Y.; Suchorab, J.L.; Thomas, C.D.L.; Clement, J.G.; Cooper, D.M.L. Normal variation in cortical osteocyte lacunar parameters in healthy young males. J. Anat. 2014, 225, 328. [Google Scholar] [CrossRef]
- Ericksen, M.F. Histologic estimation of age at death using the anterior cortex of the femur. Am. J. Phys. Anthropol. 1991, 84, 171. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.W.; Das, R.; Cleary, P.W.; Hunter, P.J.; Thomas, C.D.L.; Clement, J.G. Using smooth particle hydrodynamics to investigate femoral cortical bone remodelling at the Haversian level. Int. J. Numer. Methods Biomed. Eng. 2013, 29, 129. [Google Scholar] [CrossRef] [PubMed]
- Lerebours, C.; Thomas, C.D.L.; Clement, J.G.; Buenzli, P.R.; Pivonka, P. The relationship between porosity and specific surface in human cortical bone is subject specific. Bone 2015, 72, 109. [Google Scholar] [CrossRef] [PubMed]
- Spatola, B.F.; Damann, F.E.; Ragsdale, B.D. Bone Histology Collections of the National Museum of Health and Medicine. In Bone Histology; Crowder, C., Stout, S., Eds.; CRC Press: Boca Raton, FL, USA, 2012; Chapter 12; p. 313. [Google Scholar]
- Thomas, C.; Clement, J.G. The Melbourne Femur Collection: How a Forensic and Anthropological Collection Came to Have Broader Applications. In Bone Histology; Crowder, C., Stout, S., Eds.; CRC Press: Boca Raton, FL, USA, 2012; Chapter 13; p. 327. [Google Scholar]
- Wang, X.; Thomas, C.D.L.; Clement, J.G.; Das, R.; Davies, H.; Fernandez, J.W. A mechanostatistical approach to cortical bone remodelling: An equine model. Biomech. Model. Mechanobiol. 2016, 15, 29. [Google Scholar] [CrossRef]
- Crowder, C. Estimation of Age at Death Using Cortical Bone Histomorphometry; National Institute of Justice, U.S. Department of Justice: Washington, DC, USA, 2013. Available online: https://nij.ojp.gov/library/publications/estimation-age-death-using-cortical-bone-histomorphometry (accessed on 16 February 2022).
- Andronowski, J.M. Evaluating Age-related Bone Loss in the Ericksen Femur Collection. In Proceedings of the American Association of Physical Anthropologists 83rd Annual Meeting, Calgary, AB, Canada, 8–12 April 2014. [Google Scholar]
- Crowder, C.M.; Dominguez, V.M. A New Method for Histological Age Estimation of the Femur. In Proceedings of the American Academy of Forensic Sciences 64th Annual Scientific Meeting, Altanta, GA, USA, 20–25 February 2012. [Google Scholar]
- Kevey, D. Melbourne Femur Research Collection. 2017. Available online: https://dental.unimelb.edu.au/research/melbourne-femur-research-collection (accessed on 16 February 2022).
- Kerley, E.R. The Microscopic Determination of Age in Human-Bone. Am. J. Phys. Anthropol. 1965, 23, 149. [Google Scholar] [CrossRef] [PubMed]
- Crowder, C. Rib Histomorphometry for Adult Age Estimation. In Forensic Microscopy for Skeletal Tissues: Methods and Protocols; Bell, L.S., Ed.; Humana Press: Totowa, NJ, USA, 2012; Chapter 7; p. 109. [Google Scholar]
- Alblas, A.; Greyling, L.M. Geldenhuys, Composition of the Kirsten Skeletal Collection at Stellenbosch University. S. Afr. J. Sci. 2018, 114, 110. [Google Scholar]
- Andronowski, J.M.; Davis, R.A.; Stephen, H.E. Inferring bone attribution to species through micro-Computed Tomography: A comparison of third metapodials from Homo sapiens and Ursus americanus. J. Forensic. Radiol. Im. 2019, 18, 11. [Google Scholar] [CrossRef]
- Hicks, T.; Andronowski, J.M.; Davis, R. Age-Related Changes to Bone Microarchitecture in a Non-Weight Bearing Bone. Bachelor’s Thesis, The University of Akron, Akron, OH, USA, 2019; p. 871. Available online: https://ideaexchange.uakron.edu/honors_research_projects/871/ (accessed on 16 February 2022).
- Muakkassa, L. Assessment of Sex-Related Differences of the Osteocyte Lacunar-Canaliculaar Network across the Human Lifespan Using Synchrotron Micro-Computed Tomography. Bachelor’s Thesis, The University of Akron, Akron, OH, USA, 2019; p. 947. Available online: https://ideaexchange.uakron.edu/honors_research_projects/947/ (accessed on 16 February 2022).
- Tubo, G. Evaluating Sex Related Differences in the Osteocyte Lacunar Canalicular Network across the Lifespan: A Confocal Laser Scanning Microscopy Approach. Bachelor’s Thesis, The University of Akron, Akron, OH, USA, 2019; p. 1010. Available online: https://ideaexchange.uakron.edu/honors_research_projects/1010/ (accessed on 16 February 2022).
- Andronowski, J.M. The Andronowski Skeletal Collection for Histological Research. Mizzou Musculoskelet. Res. Symp. 2021. Available online: https://medicine.missouri.edu/centers-institutes-labs/thompson-laboratory-for-regenerative-orthopaedics/2021-mizzou-msk-research-symposium-abstracts (accessed on 16 February 2022).
- Andronowski, J.M.; Davis, R.A.; Cole, M.E. Investigating the Impact of Opioid Abuse on Intracortical Porosity and Bone Cellular Density: A Synchrotron-radiation micro-Computed Tomography Approach. In Proceedings of the American Academy of Forensic Sciences 72nd Annual Scientific Meeting, Anaheim, CA, USA, 17–22 February 2020. [Google Scholar]
- Cole, M.E.; Davis, R.A.; Taylor, J.T.; Andronowski, J.M. Automated Techniques for Cortical Bone Histological Variable Segmentation and Image Enhancement. In Proceedings of the American Academy of Forensic Sciences 73rd Annual Scientific Meeting, Virtual, 15–19 February 2021. [Google Scholar]
- Davis, R.A.; Stephen, H.; Andronowski, J.M. Inferring Species Origin Through Virtual Histology: A Comparison of Third Metapodials From From Homo sapiens and Ursus americanus Using Micro-Computed Tomography. In Proceedings of the American Academy of Forensic Sciences 71st Annual Scientific Meeting, Baltimore, MD, USA, 18–23 February 2019; 2019. [Google Scholar]
- Andronowski, J.M.; Mundorff, A.Z.; Davis, R.A.; Price, E.W. Application of X-ray photoelectron spectroscopy to examine surface chemistry of cancellous bone and medullary contents to refine bone sample selection for nuclear DNA analysis. J. Anal. Atom. Spectrom. 2019, 34, 2074. [Google Scholar] [CrossRef]
- Andronowski, J.M.; Mundorff, A.Z.; Pratt, I.V.; Davoren, J.M.; Cooper, D.M.L. Evaluating differential nuclear DNA yield rates and osteocyte numbers among human bone tissue types: A synchrotron radiation micro-CT approach. Forensic Sci. Int. Genet. 2017, 28, 211. [Google Scholar] [CrossRef] [PubMed]
- Andronowski, J.M.; Pratt, I.V.; Cooper, D.M.L. Occurrence of osteon banding in adult human cortical bone. Am. J. Phys. Anthropol. 2017, 164, 635. [Google Scholar] [CrossRef] [PubMed]
- Vogelgesang, M.; Farago, T.; Morgeneyer, T.F.; Helfen, L.; Rolo, T.d.S.; Myagotin, A.; Baumbach, T. Real-time image-content-based beamline control for smart 4D X-ray imaging. J. Synchrotron. Rad. 2016, 23, 1254. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andronowski, J.M.; Taylor, J.T. The Andronowski Skeletal Collection for Histological Research: A Modern Anatomical Contribution. Forensic Sci. 2022, 2, 175-189. https://doi.org/10.3390/forensicsci2010014
Andronowski JM, Taylor JT. The Andronowski Skeletal Collection for Histological Research: A Modern Anatomical Contribution. Forensic Sciences. 2022; 2(1):175-189. https://doi.org/10.3390/forensicsci2010014
Chicago/Turabian StyleAndronowski, Janna M., and Joshua T. Taylor. 2022. "The Andronowski Skeletal Collection for Histological Research: A Modern Anatomical Contribution" Forensic Sciences 2, no. 1: 175-189. https://doi.org/10.3390/forensicsci2010014
APA StyleAndronowski, J. M., & Taylor, J. T. (2022). The Andronowski Skeletal Collection for Histological Research: A Modern Anatomical Contribution. Forensic Sciences, 2(1), 175-189. https://doi.org/10.3390/forensicsci2010014






