Taxonomic Revision of Pygmy Devil Genera Almacris, Ginixistra, Tegotettix, and Xistra, with Comments on Xistrella (Orthoptera: Tetrigidae) †
Abstract
1. Introduction
2. Materials and Methods
2.1. Fieldwork for Almacris alleochroa gen. et sp. nov. and Tegotettix derijei in Mindanao
2.2. Type Depository
2.3. Taxonomy and Nomenclature
2.4. Terminology
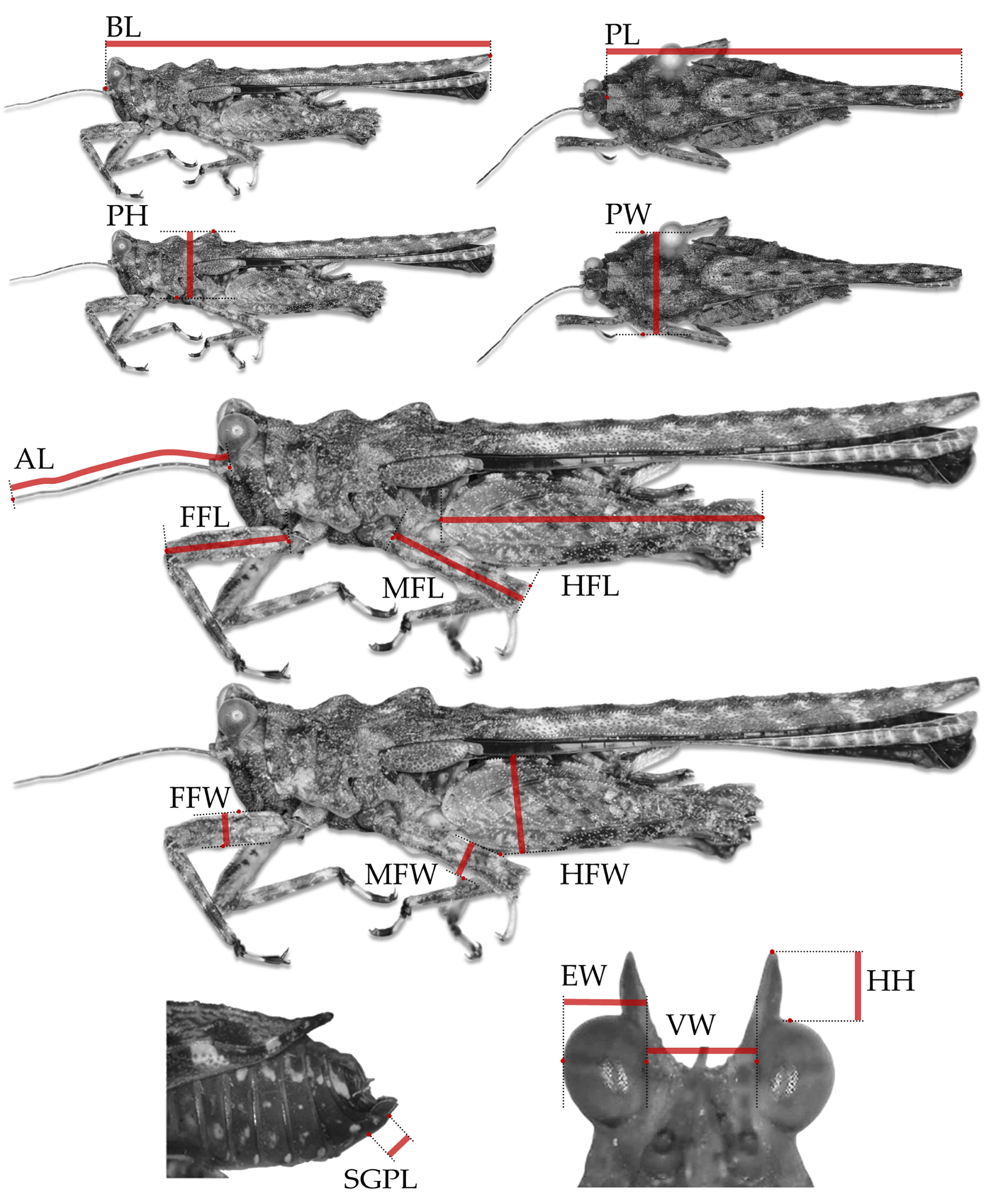
2.5. Measurements
2.6. Structure of the Study
3. Results
- Taxonomy
- Family Tetrigidae Rambur, 1838 [61]
- Genus Almacris Skejo, Patano, Škorput et Kasalo gen. nov.
- urn:lsid:zoobank.org:act:40B31705-536F-4ED7-9C27-3EB86A1916D3
- Subfamily assignment. Without subfamily assignment.
- Type species. Almacris alleochroa sp. nov., here designated.
- Etymology. Named after Prof. Dr. Alma Baguhin Mohagan, a Philippine zoologist who greatly contributed to tetrigidology of the Philippines, and to the zoological studies in the country in general (e.g., [62,63,64,65], and a good mentor and a friend of ours. The team of Dr. Mohagan discovered and described Xistra (Tegoxistra) derijei (Patano, Mohagan, Tumbrinck, Amoroso et Skejo, 2021) comb. nov. a few years ago and recognized the Xistra problem [24]. The genus name is derived from “Alma”, Ma’am Mohagan’s name, and “acris”, a Latinized Greek word (ἀκρίς, akris) for grasshopper. The genus name is a feminine gender noun of the third Latin declension (Nominative Almacris, Genitive Almacridis).
- Descriptive diagnosis. Although the placement of this genus within the higher taxonomy of Tetrigidae cannot be confidently resolved at this time, the following set of characters differentiates it from all the other known genera. Tegmina and wings are absent. Pronotum colorful, with green and reddish tints. Two-horned vertex, formed by both the lateral and secondary carinae being weakly elevated. Frontal costa bifurcation at the middle of the eye height. Paired ocelli at the lower quarter of the eye height. The top margin of the antennal grooves is at the level of the bottom margin of the eyes. Weakly bilobate vertex anteriorly as seen in dorsal view. Vertex is very slightly narrowing anteriorly. Antennae with around 15 segments. A large crest formed by the median carina in the anterior part of the pronotum. Ovipositor minute.
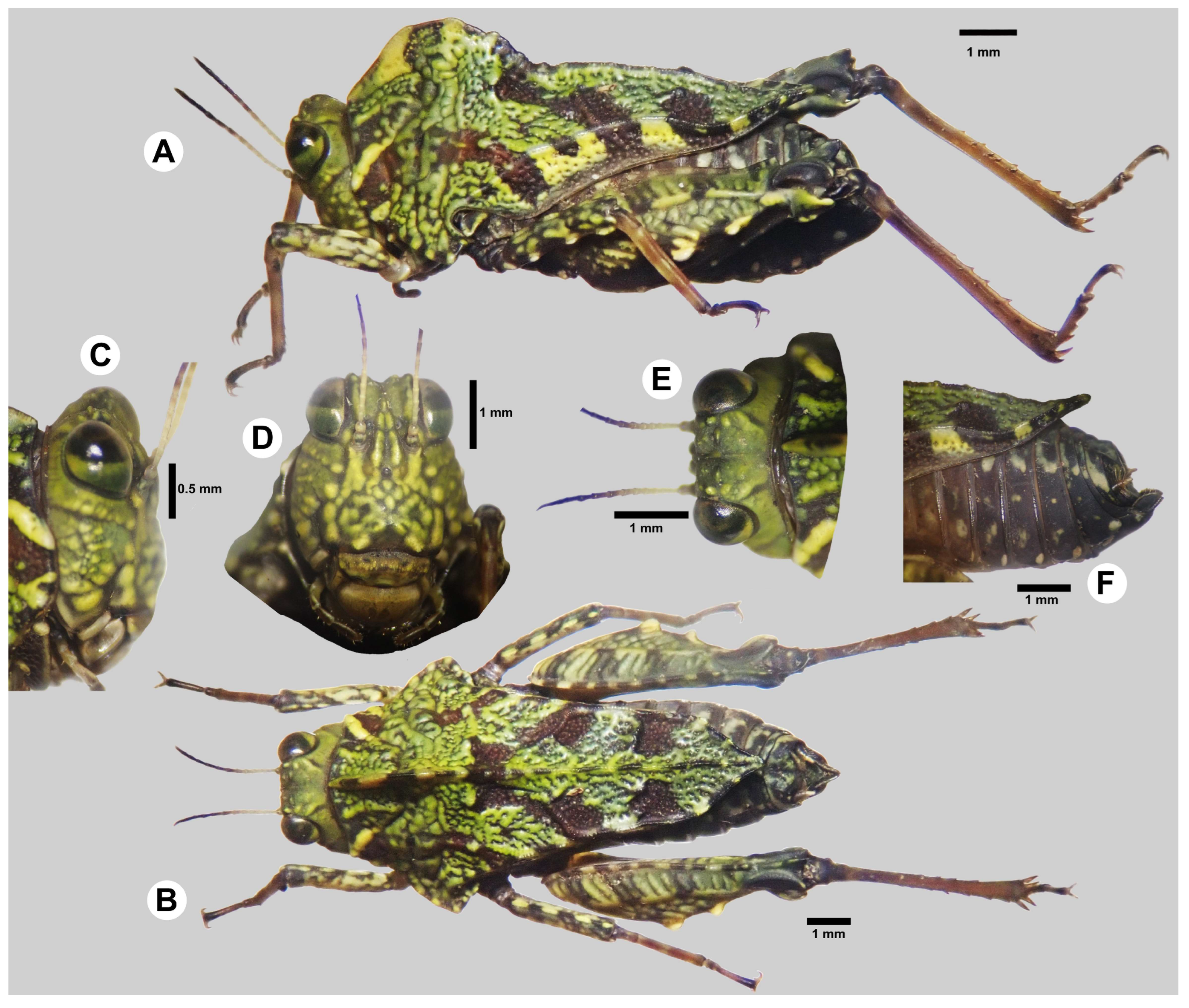
- Composition. Monotypic, including only A. alleochroa sp. nov.
- Distribution. Endemic to Mindanao Island (the Philippines), known only from Mt. Balatukan
- Species Almacris alleochroa Skejo, Patano, Škorput et Kasalo gen. et sp. n.
- urn:lsid:zoobank.org:act:DE7E971A-2014-4C19-A7B9-C6212B4F0AA2



- Type material. 1♀ holotype PHILIPPINES: Mindanao: Misamis Oriental: Barangay Lunutan, Gingoog City, Mount Balatukan (montane forest) 1210 m a.s.l. 22.XII.2022. leg. R. Patano, Jr.. (CMU); 1♀ paratype PHILIPPINES: Mindanao: Misamis Oriental: Barangay Lunutan, Gingoog City, Mount Balatukan (montane forest) 1210 m a.s.l. 22.XII.2022. leg. R. Patano, Jr.. (CMU); 1♂ paratype PHILIPPINES: Mindanao: Misamis Oriental: Barangay Lunutan, Gingoog City, Mount Balatukan (montane forest) 1200 m a.s.l. 20.I.2023. leg. R. Patano, Jr.. (CMU); 2♂♂ paratypes PHILIPPINES: Mindanao: Misamis Oriental: Barangay Lunutan, Gingoog City, Mount Balatukan (Agroforest) 1080–1110 m a.s.l.; 29.-30.X.2023. leg. R. Patano, Jr. (CMU).
- Type locality. Montane forest of the Mt. Balatukan (PHILIPPINES: Mindanao: Misamis Oriental: Barangay Lunutan, Gingoog City).
- Type depository. Central Mindanao University (CMU) Orthoptera Collection, Mindanao, Philippines.
- Etymology. The specific epitheton is a feminine adjective, New Latin, alleochrous, alleochroa, alleochroum, based on the Latinized Ancient Greek adjective, ἀλλοιόχροος ον, όα/alloiochroos, on, oa/, meaning “of varying color”, “colorful”.
- Diagnosis. The new species is easily distinguished from all known Tetrigidae. Species diagnosis is currently inseparable from the generic diagnosis (see above).
- Description.
- Head
- Frontal view. The vertex is nearly two times wider than an eye. The surface of the vertex is a little below the level of the upper margin of the eyes. Lateral carinae elevated, forming short horns. Secondary carinae (between the lateral and the medial carina) are elevated, forming horns a little shorter than the lateral ones. There is a V-shaped incision between the lateral and secondary horns, as well as between the secondary horns. The medial carina and frontal costa are not elevated and thus barely visible. Frontal costa bifurcation at the middle of the eye height. Facial carinae are moderately divergent ventrally. Paired ocelli at the lower quarter of the eye height. The top margin of the antennal grooves is at the level of the bottom margin of the eyes.
- Lateral view. Lateral carina protruding above the eyes. The entire lateral carina is raised, with an additionally elevated medial segment in the shape of a moderately wide horn.
- Dorsal view. Vertex anteriorly truncated, very slightly narrowing anteriorly. Medial carina indrawn into the vertex, giving it a bilobate look anteriorly. The medial carina is visible only in the anterior third of the vertex.
- Antennae
- Filiform. 15 segments visible. The proximal half of the flagellum is white, and the apical region progressively darkens.
- Pronotum
- Lateral view. Median carina elevated in the anterior part of the pronotum, forming a semi-circular crest that extends from the anterior margin to the level of humeral angles. Median carina is slightly elevated past this point up to the pronotal apex, where it is present but not elevated. Pronotal apex upturned. Lateral carinae are visibly elevated. Sulci are well visible across the pronotal disk. Lateral lobes are blunt and rectangular. Ventral sinus semi-oval. The infrascapular area is well-developed, extending from the ventral sinus to just before the apex; widest anteriorly, slightly narrows towards the level of the midpoint of hind femora length, then sharply narrows and continues as a thin strip towards the apex.
- Dorsal view. Anterior margin semi-oval, weakly produced forwards. Prozonal carinae long, weakly converging dorsally. Lateral lobes flaring outwards. Humeral angles blunt. Interhumeral carinae unclear; short and slightly converging dorsally, connected with many sulci. Pronotum slightly narrows dorsally up to the apex, where it moderately narrows, forming a blunt spine.
- Wings
- Tegmina and alae absent.
- Legs
- Anterior femora smooth, slightly expanded proximally. Tibiae smooth. Middle femora with slightly undulate dorsal and ventral margins. Tibiae smooth. Hind femora with slightly wavy dorsal margins. Antegenicular tooth (the one before the hind knee) weak, while the genicular one (the one on the hind knee) strong. Outer margins with two tubercles. Tibiae with weak teeth. Pulvilli blunt. Tarsal claw a little shorter than the first tarsal segment.
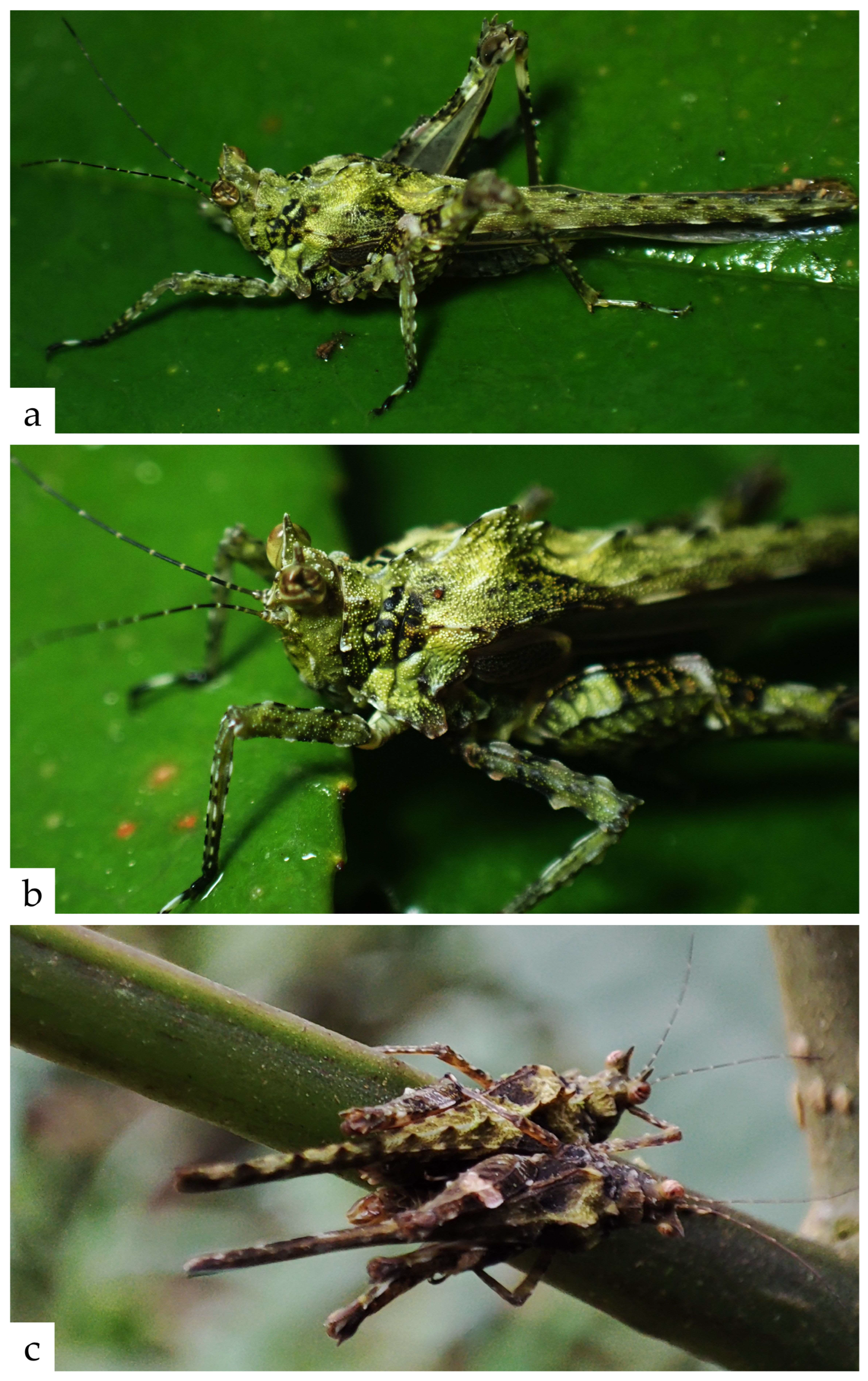
- Antennae white in the proximal half, black in the distal half, with a smooth transition between them. Head predominantly green with several yellowish spots on the fastigium. Occipital area marked by a yellow and a black line. Base color of the pronotum scarlet-brownish. Anterior part of the pronotal crest yellowish, posterior to it the median carina with alternating yellowish-green and brown spots/lines. Anterior third of the pronotal surface covered by a labyrinthine green pattern that resembles algal outgrowth. Posterior two thirds mainly brown, with three large blotches of green patterns that expand outwards from the median carina. Femora brown, dotted with yellowish-green spots. Tarsi pale brown, with yellowish stripes faintly visible along them.
- Distribution: Endemic to Balatukan Mt. in the Island of Mindanao (the Philippines) (Figure 6).
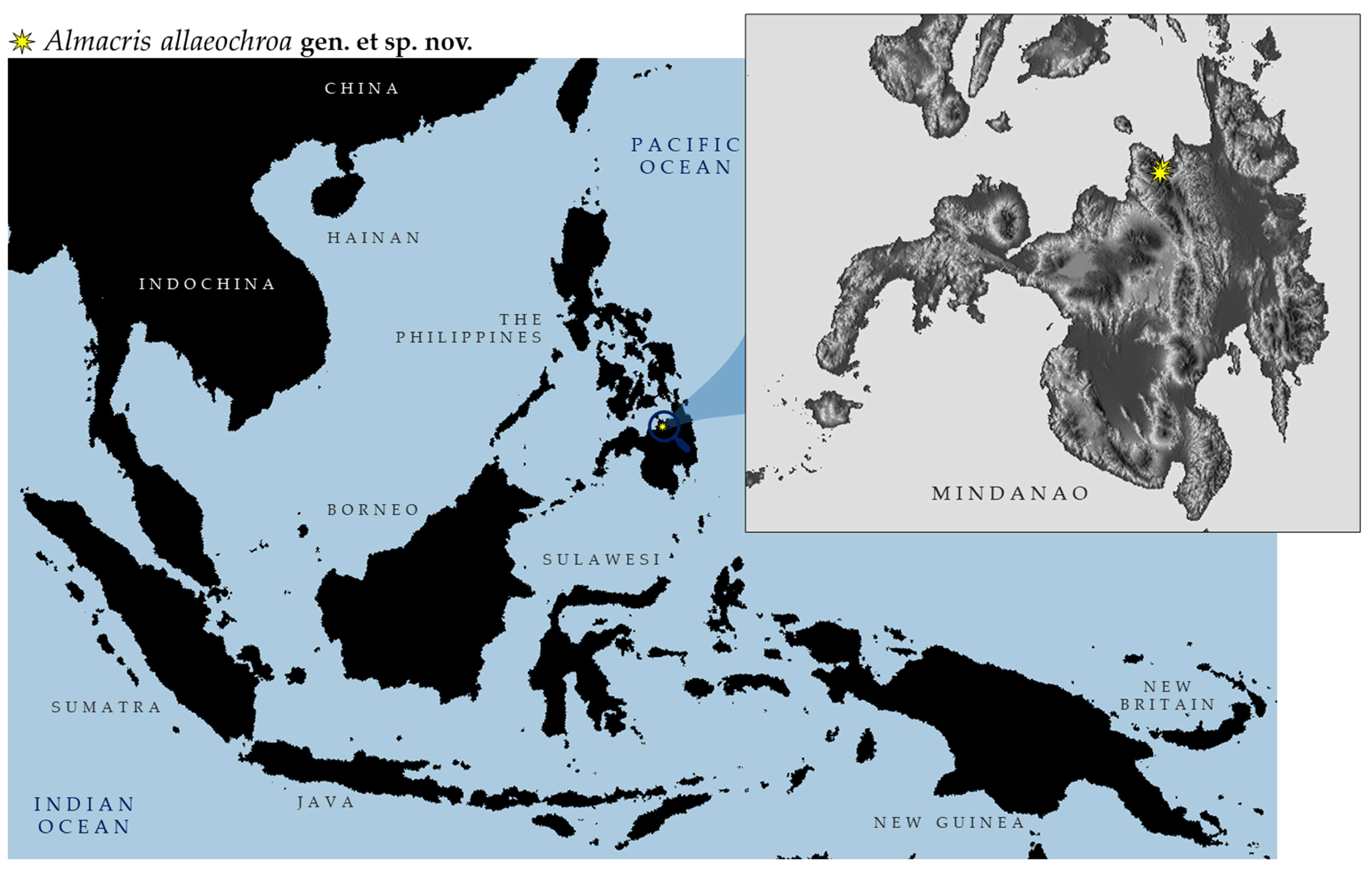
- Habitat and Ecology (Figure 7 and Figure 8). Almacris alleochroa gen. et. sp. nov. is only known so far from Mount Balatukan, Gingoog City, Misamis Oriental (Figure 7 and Figure 8). It is located on Mindanao’s northern island and is one of those declared Key Biodiversity Areas (KBAs) of the Philippines. It is a massive, possibly active compound stratovolcano. In the Misamis Oriental province, it is the highest peak. Although the volcano has never erupted before, it does display fumarolic activity. Specimens of the new genus were observed along the lower montane and agroforest ecosystems of the mountain.


| ♀ Holotype | ♀ Paratype | ♂ Paratype | ♂ Paratype | |
|---|---|---|---|---|
| BL | 12.70 | 12.50 | 11.0 | 11.30 |
| PL | 9.70 | 9.60 | 7.70 | 9.80 |
| PW | 5.50 | 5.30 | 4.60 | 5.50 |
| PH | 4.50 | 4.50 | 4.30 | 4.40 |
| FFL | 3.00 | 2.90 | 2.60 | 2.80 |
| FFW | 0.50 | 0.55 | 0.50 | 0.60 |
| MFL | 3.70 | 3.55 | 3.0 | 3.40 |
| MFW | 0.60 | 0.60 | 0.60 | 0.55 |
| HFL | 6.60 | 6.45 | 5.50 | 6.50 |
| HFW | 2.50 | 2.50 | 2.20 | 2.40 |
| VW | 1.10 | 1.10 | 1.00 | 1.00 |
| EW | 0.70 | 0.70 | 0.70 | 0.70 |
| AL | 3.30 | 3.20 | 2.90 | 3.10 |
| SGPL | 0.60 | 0.60 | 0.60 | 0.60 |
- Genus Ginixistra Kasalo, Tumbrinck et Skejo gen. nov.
- urn:lsid:zoobank.org:act:80E6DD1C-7210-4726-B4A6-7C552ABEFFDE
- Subfamily assignment. This new genus is assigned to the tribe Exanimini Kasalo, Tumbrinck, and Skejo, 2024 [66], which is without subfamily placement within Tetrigidae. New genus is assigned to this tribe based on head morphology similarity with Exanimus and Fijixistra, recently described from Fiji [66]. This is the first winged member of Exanimini.
- Type species. Ginixistra novaeguineae (Günther, 1938) [18] (=Tegotettix novaeguineae Günther, 1938), here designated.
- Etymology. “Gini-”, a simplified transliteration of Guinea, used by many Papuan languages, and “-xistra”, referring to the similarity of this new genus to Xistra from the Philippines and Fijixistra from Fiji. The genus name is of feminine gender, as it is derived from the root xistra.
- Diagnosis. Ginixistra gen. nov. is easily distinguished from other members of Exanimini by its long pronotum and by the presence of tegmina and alae. Other Exanimini members are characterized by a nanopronotal (short pronotum) and wingless morphology.
- Description.
- Head
- In frontal view vertex about as wide as one eye; while in dorsal view vertex 1.35 to 1.5 times as wide as an eye. In frontal view, vertex depressed and lowest in the mid part. Lateral carinae forming horns height of which is species-specific. Frontal costa long, bifurcating between the eyes or lower. Facial carinae form scutellum narrower than scapus. Paired ocelli situated between the lower margins of the compound eyes. Top margin of the antennal groove situated below the ventral margins of the compound eyes for at least one antennal groove diameter. In lateral view, head slightly exserted above the level of pronotal discus. Fastigium not visible in lateral view. Frontal costa rounded, slightly projected forwards in lateral view. Occipital area visible. Anterior margin of vertex truncated, slightly indrawn in the mid. In dorsal view, medial carina visible in the anterior half of the vertex length. Lateral and transverse carinae of the vertex forming acute angle.
- Pronotum
- In lateral view median carina of the pronotum continuous from the anterior margin to the tip of pronotum; forming two humps (lower one between prozona and metazona, and higher one above the tegmina), and 4–5 low undulations behind; infrascapular area narrow; lateral area wide; tip of the lateral lobe of pronotum with truncated apex; tegminal sinus shallow, rectangular; ventral sinus deep, triangular. In dorsal view anterior margin of pronotum straight; prozonal carina short, parallel; lateral lobes of pronotum directed sidewards; shoulders slightly widened, forming obtuse angle; interhumeral carinae short; pronotal apex truncated or slightly bilobate.
- Wings
- Tegmina and alae present. Tips of alae touching tips of pronotum (macropronotal state) or exceeding the tip (pauropronotal state).
- Legs
- Fore and mid femora with undulated margins. Mid femur with rounded or triangular projections on the ventral margin. Fore and mid tibiae rectangular in cross section. Proximal tarsal segment of the fore and mid legs much shorter than the distal one. Hind femora elongated; 3.3 to 3.55 times as long as wide. Dorsal and ventral margins without lappets. First segment of the hind tarsi visibly longer than the third. Tarsal pulvilli elongated and angular, but without apical teeth.
- Composition. Three species, Ginixistra novaeguineae, G. davorkae sp. nov., and G. novaebritanniae sp. nov.
- Distribution. Endemic to New Guinea (G. novaeguineae, G. davorkae sp. nov.) and New Britain (G. novaebritaniae) (Figure 9).
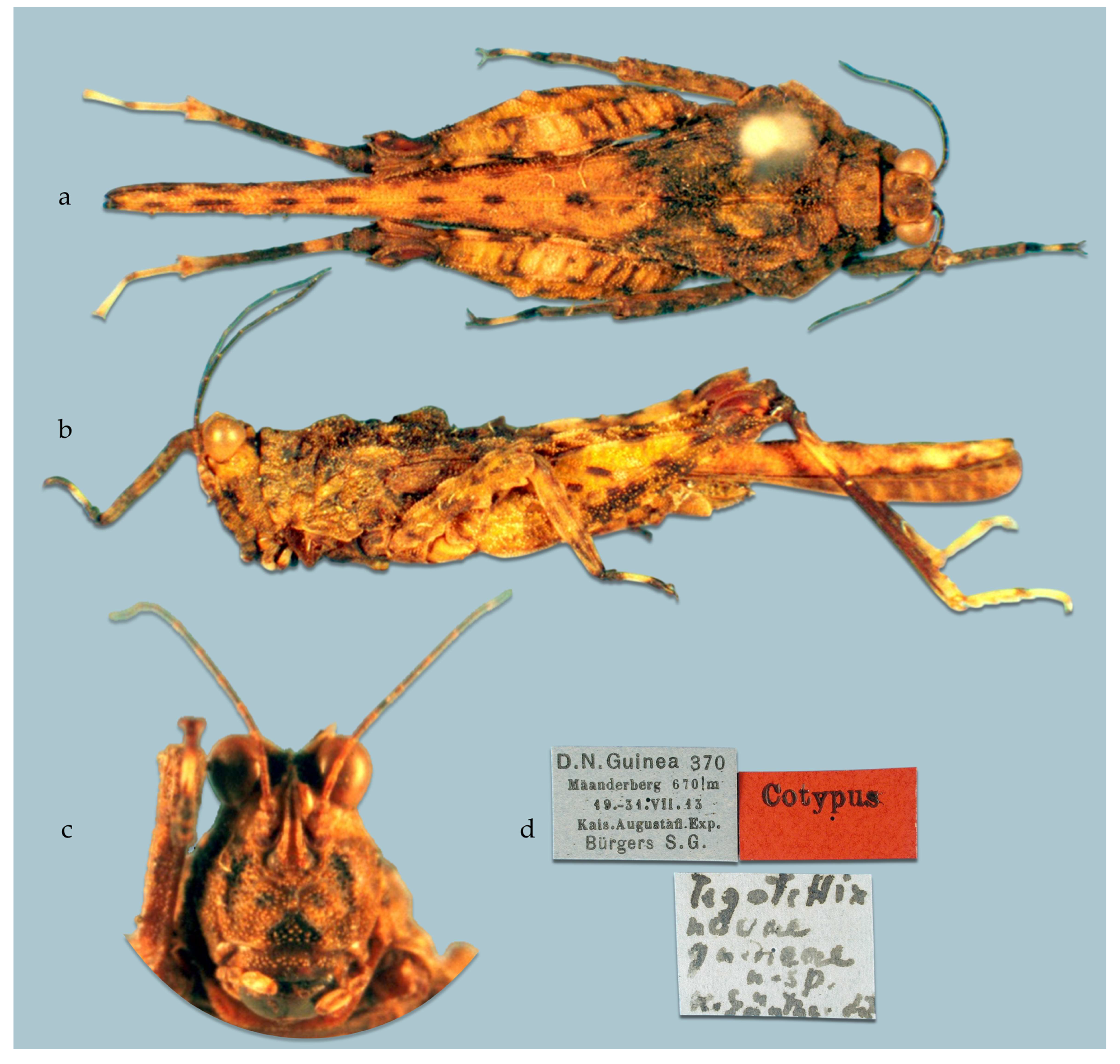
- Taxonomic remarks. The original type series of the species Tegotettix novaeguineae Günther, 1938 [18] consists of 4 specimens mentioned in the original description [18]. Three syntypes are known (1♀ syntype deposited at SMTD, 1♀ syntype, and 1♂ syntype deposited at MfN) and available on the OSF. The location of the last one is unknown. There are further recorded non-type specimens available on the OSF [5]. These and additional herein-published specimens are used as a basis for the description of two further species and the definition of the new genus. The genus, as delimited here, consists of three species distributed in geographically separate areas (New Britain Island, the northern and southern parts of New Guinea Island, separated by the New Guinean Highlands). The species are defined based on their differences, i.e., diagnoses, and the characters that are not mentioned are considered to conform to the diagnosis of the genus.

- Type locality. PAPUA NEW GUINEA: West Sepik: Toricelli Mountains; 3.379400 S, 142.239200 E; 600 to 780 m above sea level.
- Type depository. The lectotype is deposited at SMTD, and the 2 paralectotypes at MfN.
- Diagnosis. Easily distinguished from other species of the genus by the following set of characters: (1) vertex a little below the level of the eyes (noticeably below the level of the eyes in the other two species); (2) lateral carinae of the vertex barely elevated, forming barely perceptible horns (horns low in G. davorkae sp. nov., moderately high in G. novaebritanniae sp. nov.); (3) frontal costa bifurcation a little below the middle level of the eye height (at the level of the lower third of the eye height in the other two species); (4) tegmina rectangular: width/length ratio of tegmen 0.5 (0.37 or lower in the other two species); and (5) ovipositor short: dorsal valve 0.9 mm or shorter, same as in G. novaebritanniae, longer than 1.2 in G. davorkae.

- Re-description.
- Head
- Frontal view. Vertex between the eyes a bit wider than one eye. Mid part of vertex slightly lower than compound eyes. Lateral carinae forming very low horns only slightly projected above the eyes. Frontal costa long, bifurcated between the compound eyes. Facial carinae weakly diverging, forming scutellum narrower than scapus. Paired ocelli situated between the lower margins of the compound eyes. Top margin of the antennal groove below the ventral margins of the compound eyes.
- Lateral view. Head only slightly exserted above the level of the pronotum. Fastigium not visible. Frontal costa rounded, slightly projected forwards. Lateral carinae of the vertex are visible above the eyes as very low horns. Occipital area visible, as wide as a third of the compound eye length.
- Dorsal view. Vertex about 1.5 times wider than a compound eye. Anterior margin of vertex truncated, slightly indrawn in tip of the medial carina. Medial carina visible in the anterior half of the vertex length. Lateral and transverse carinae of the vertex forming acute angle.
- Pronotum
- Lateral view. Median carina of the pronotum continuous from the anterior margin to the tip of pronotum; forming two triangular humps-lower between prozona and metazona and higher just above the tegmina. Furthermore there, there are 4–5 very low elevations in metazona towards the tip. Infrascapular area narrow, with parallel margins. Lateral area wider than infrascapular, finely granulated. Tip of the lateral lobe of pronotum triangular, with truncated apex. Tegminal sinus evident, rectangularly shaped. Ventral sinus deeper than tegminal, triangularly shaped.
- Dorsal view. Anterior margin of pronotum truncated, straight. Prozonal carina short, parallel. Lateral lobes of pronotum directed sidewards, with truncated apex. Shoulders slightly widened, not armed with teeth, forming obtuse angle. Interhumeral carinae present, short and a bit curved in the mid part. Pronotal apex truncated, slightly bilobate.
- Wings
- Tegmina and alae present. Tegmina elongated, oval, with width/length ratio about 0.5. Alae not exceeding the tip of pronotum, or slightly exceeding it (for up to 0.2 mm).
- Legs
- Fore legs. Fore femora with undulated margins. Fore tibiae rectangular in cross section. Second tarsal segment significantly longer than the proximal one.
- Mid legs. Mid femora with less undulated dorsal margin and with more undulated ventral margin, bearing three rounded projections. Mid tibiae rectangular in cross section. Second tarsal segment significantly longer than the proximal one.
- Hind: legs. Hind femora elongated; about 3.3 times as long as wide. Dorsal and ventral margins without lappets. Genicular and antegenicular teeth triangular.
- Ovipositor
- Ovipositor short, dorsal valve 0.9 mm or shorter.
- Distribution. Known only from the northern part of the island of New Guinea, north of the Highlands.
- Habitat and Ecology. Not known.
- Measurements. See Table 3.
| G. novaeguineae | G. davorkae sp. nov. | G. novaebritanniae sp. nov. | |||
|---|---|---|---|---|---|
| Specimen | ♀ Lectotype | ♀ Holotype | ♂ Paratype | ♀ Non-Type | ♀ Holotype |
| Locality | Toricelli | Tekadu | Lakekamu | Aru Isl. | New Britain Isl. |
| Depository | SMDT | CJT | CJT | CJT | CJT |
| BL | 14.60 | 14.40 | 13.00 | 15.45 | 15.90 |
| PL | 13.70 | 13.25 | 12.00 | 13.85 | 14.80 |
| PW | 3.85 | 3.90 | 3.35 | 4.10 | 4.10 |
| PH | 2.40 | 2.45 | 1.85 | 2.85 | 2.20 |
| FFL | 2.30 | 2.30 | 2.05 | 2.55 | 3.20 |
| FFW | 0.60 | 0.55 | 0.55 | 0.70 | 0.87 |
| MFL | 2.75 | 2.60 | 2.50 | 2.90 | 3.15 |
| MFW | 0.65 | 0.65 | 0.70 | 0.75 | 0.60 |
| HFL | 6.10 | 5.90 | 5.50 | 6.25 | 6.80 |
| HFW | 1.85 | 1.70 | 1.55 | 1.75 | 1.90 |
| VW | 0.65 | 0.55 | 0.45 | 0.60 | 0.60 |
| EW | 0.65 | 0.60 | 0.55 | 0.60 | 0.60 |
| AL | 5.25 | 4.80 | broken | 5.35 | broken |
| HH | 0.10 | 0.15 | 0.10 | 0.15 | 0.20 |
- Taxonomic remarks. In the OSF [5], there are two nymphs from Yapen Island assigned to this species. In each, a tall pronotal crest is visible, which reduces in size as specimens go through molts [68]. It is possible that the Yapen population represents a new species of Ginixistra, but nothing can be said with certainty until adults are discovered. Yapen Island has been shown to be a place of high endemism in many animal and plant groups (e.g., [69,70,71]), but many species are also shared between mainland Papua and the island (e.g., [71,72,73]).
- Species Ginixistra davorkae Kasalo, Tumbrinck et Skejo sp. nov.
- urn:lsid:zoobank.org:act:042B3454-AA9F-467D-ABE2-436D47DC228A
- Additional material (see Taxonomic remarks below). 1♀ (Figure 14) INDONESIA: Aru Isl. leg. Kühne, H. (NMW), OSF collection object 1586194; code ee31b32c-cfcf-4fba-886a-ea75a0344b44.
- Type locality. PAPUA NEW GUINEA: Morobe Province, Tekadu; S 7.633, E 146.567
- Type depository. Currently CJT, Wassenberg, Germany.
- Diagnosis. Easily distinguished from other species of the genus by the following set of characters: (1) vertex noticeably below the level of the eyes (same in G. novaebritanniae, higher in G. novaeguineae); (2) lateral carinae of the vertex elevated, forming low horns (horns nearly absent in G. novaeguineae, of medium height in G. novaebritanniae); (3) frontal costa bifurcation at the level of the lower third of the eye height (same in G. novaebritanniae, a little below the middle level in G. novaeguineae); (4) tegmina elongated: width/length ratio of tegmen about 0.35 (similar in G. novaebritanniae, 0.5 in G. novaeguineae); (5) ovipositor long: dorsal valve longer than 1.2 mm (0.9 mm or shorter in the other two species).
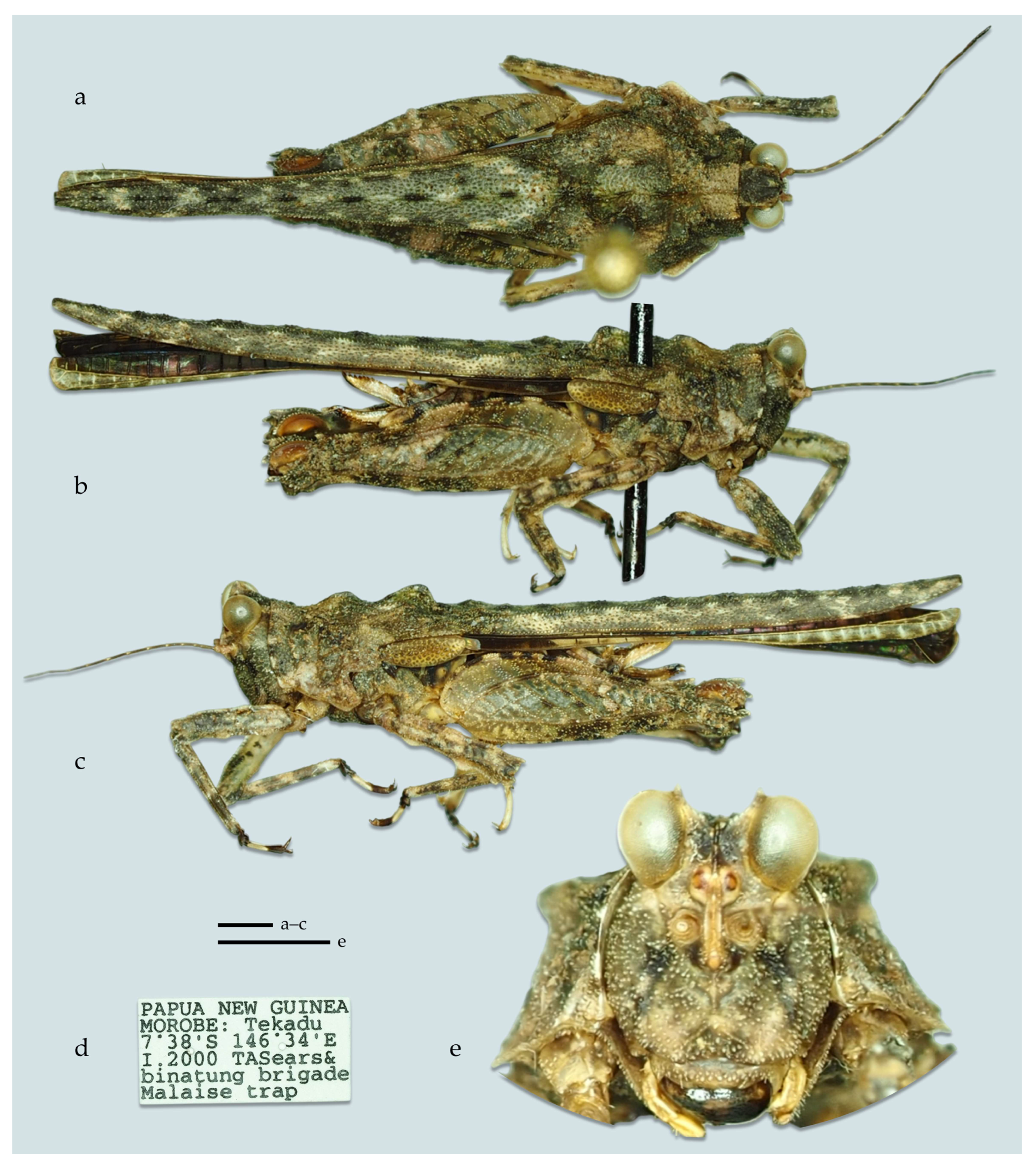

- Description.
- Head
- Frontal view. Vertex between the eyes a bit narrower than one eye. Mid part of vertex lower than compound eyes. Lateral carinae forming low horns only slightly projected above the eyes. Frontal costa long, bifurcated in the lower fourth of the compound eyes height. Facial carinae weakly diverging, forming scutellum narrower than scapus. Paired ocelli situated between the lower margins of the compound eyes. Top margin of the antennal groove below the ventral margins of the compound eyes.
- Lateral view. Head only slightly exserted above the level of pronotum. Fastigium not visible. Frontal costa rounded, slightly projected forwards. Lateral carinae of the vertex visible above the eyes as low horns. Occipital area visible, as wide as a third of the compound eye length.
- Dorsal view. Vertex 1.4 times as wide as a compound eye. Anterior margin of vertex truncated, slightly indrawn in tip of the medial carina. Medial carina visible in the anterior half of the vertex length. Lateral and transverse carinae of the vertex forming acute angle.
- Pronotum
- Lateral view. Median carina of the pronotum continuous from the anterior margin to the tip of pronotum; forming two triangularly rounded humps-lower one between prozona and metazona, and a bit higher one just above the tegmina; and 4–5 low elevations in metazona towards the tip. Infrascapular area narrow, with parallel margins. Lateral area wider than infrascapular, finely granulated. Tip of the lateral lobe of pronotum triangular, with truncated apex. Tegminal sinus evident, rectangularly shaped. Ventral sinus deeper than tegminal, triangularly shaped.
- Dorsal view. Anterior margin of pronotum truncated, straight. Prozonal carina short, parallel. Lateral lobes of pronotum directed sidewards, with truncated apex. Shoulders slightly widened, not armed with teeth, forming obtuse angle. Interhumeral carinae present, short and a bit indrawn in the mid part. Pronotal apex truncated, slightly bilobate.
- Wings
- Tegmina and alae present. Tegmina elongated, oval, with width/length ratio about 0.35. Alae not exceeding the tip of pronotum or slightly exceeding it (for up to 0.2 mm).
- Legs
- Fore legs. Fore femora with undulated margins. Fore tibiae rectangular in cross section. Second tarsal segment significantly longer than the proximal one.
- Mid legs. Mid femora with less undulated dorsal margin, and with more undulated ventral margin, bearing three circular projections. Mid tibiae rectangular in cross section. Second tarsal segment significantly longer than the proximal one.
- Hind legs. Hind femora elongated; 3.45 to 3.55 times as long as wide. Dorsal and ventral margins without lappets. Genicular and antegenicular teeth triangular and sharp. First tarsal segment visibly longer than the third. Pulvilli low ang elongated, angular, but without apical teeth.
- Ovipositor
- Ovipositor long, dorsal valve longer than 1.2 mm.
- Distribution. Known only from the southeastern part of the island of New Guinea, south of the Highlands. And from Aru? (see Taxonomic remarks below).
- Habitat and Ecology. Not known.
- Measurements. See Table 3.
- Taxonomic remarks. In the OSF, there is a specimen from Aru that matches the diagnosis of G. davorkae sp. nov. Considering the distance from the type locality, it is possible that the Aru population represents a separate species, but there is currently no morphological proof for this. It is also possible that G. davorkae sp. nov. is a widely distributed species south of the Highlands.
- Ginixistra novaebritanniae Kasalo, Tumbrinck et Skejo sp. nov.
- urn:lsid:zoobank.org:act:757FB116-16AF-42B4-8A37-D24628AA2393

- Type material. 1♀ holotype PAPUA NEW GUINEA: Islands Region: Bismarck Archipelago: New Britain Isl.: Warongoi Valley (CJT).
- Type locality. PAPUA NEW GUINEA: Islands Region: Bismarck Archipelago: New Britain (in Tok Pisin language Niu Briten): Gazelle Peninsula: Warongoi Valley, 100 m a.s.l.
- Type depository. Currently CJT, Wassenberg, Germany.
- Etymology. Genitive of Latinized “New Britain”, Nova Britannia.
- Diagnosis. Easily distinguished from other species of the genus by the following set of characters: (1) vertex noticeably below the level of the eyes (same in G. davorkae, higher in G. novaeguineae); (2) lateral carinae of the vertex elevated, forming horns of medium height (horns nearly absent in G. novaeguineae, low in G. davorkae); (3) frontal costa bifurcation at the level of the lower third of the eye height (same in G. davorkae, a little below the middle level in G. novaeguineae); (4) tegmina elongated: width/length ratio of tegmen about 0.37 (similar in G. davorkae, 0.5 in G. novaeguineae); (5) ovipositor short: dorsal valve shorter than 0.9 mm (same is G. novaeguineae, longer than 1.2 mm in G. davorkae).
- Description.
- Head
- In frontal view. Vertex between the eyes as wide as one eye. Mid part of vertex lower than compound eyes. Lateral carinae forming horns projected above the eyes. Frontal costa long, bifurcated in the lower third of the compound eyes height. Facial carinae weakly diverging, forming scutellum narrower than scapus. Paired ocelli situated between the lower margins of the compound eyes. Top margin of the antennal groove below the ventral margins of the compound eyes.
- In lateral view. Head a little bit exserted above the level of pronotum. Fastigium not visible. Frontal costa rounded, slightly projected forwards. Lateral carinae of the vertex visible above the eyes as pointed horns. Occipital area visible, as wide as a half of the compound eye length.
- In dorsal view. Vertex 1.35 times as wide as a compound eye. Anterior margin of vertex truncated, slightly indrawn in tip of the medial carina. Medial carina visible in the anterior half of the vertex length. Lateral and transverse carinae of the vertex forming acute angle.
- Pronotum
- In lateral view. Median carina of the pronotum continuous from the anterior margin to the tip of pronotum; forming two high humps-lower one between prozona and metazona, and higher one just above the tegmina; and 4–5 lower humps in metazona towards the tip. Infrascapular area narrow, with parallel margins. Lateral area wider than infrascapular, finely granulated. Tip of the lateral lobe of pronotum triangular. Tegminal sinus evident, rectangularly shaped. Ventral sinus deeper than tegminal, acute.
- In dorsal view. Anterior margin of pronotum truncated, straight. Prozonal carina short, almost parallel but slightly diverging. Lateral lobes of pronotum directed sidewards, with truncated apex. Shoulders slightly widened, not armed with teeth, forming obtuse angle. Interhumeral carinae present, short and parallel. Pronotal apex blunt.
- Wings
- Tegmina and alae present. Tegmina elongated, oval, with width/length ratio about 0.37. Alae exceeding the tip of pronotum for about 0.2 mm.
- Legs
- Fore legs. Fore femora with undulated margins. Fore tibiae rectangular in cross section. Second tarsal segment significantly longer than the proximal one.
- Mid legs. Mid femora with moderately undulated dorsal margin bearing two low teeth, and with strongly undulated ventral margin, bearing two triangular projections. Mid tibiae rectangular in cross section. Second tarsal segment significantly longer than the proximal one.
- Hind legs. Hind femora elongated, around 3.55 times as long as wide. Dorsal and ventral margins without lappets. Genicular and antegenicular teeth triangular and sharp.
- Ovipositor
- Ovipositor short, dorsal valve shorter than 0.9 mm
- Distribution. Known only from the island of New Britain.
- Habitat and Ecology. Not known. We can assume that the species’ habitat is probably similar, if not the same, to the habitat surveyed for Odonata diversity and depicted in [79]. These are wet rainforest habitats rich in creeks.
- Measurements. See Table 3.
- Genus Tegotettix Hancock, 1913 [2]
- Subfamily assignment. Scelimeninae: Discotettigini
- Redefinition. Here, Tegotettix is defined as a genus gathering Scelimeninae, Discotettigini species with a vertex more than two times wider than a compound eye in dorsal view; with armed, wide shoulders; and without ventrolateral projections on the lateral lobes of the pronotum. The genus from here on includes only the species that Patano et al. [24] assigned to the Tegotettix (armatus) species group, as only these belong to the Scelimeninae and Discotettigini.
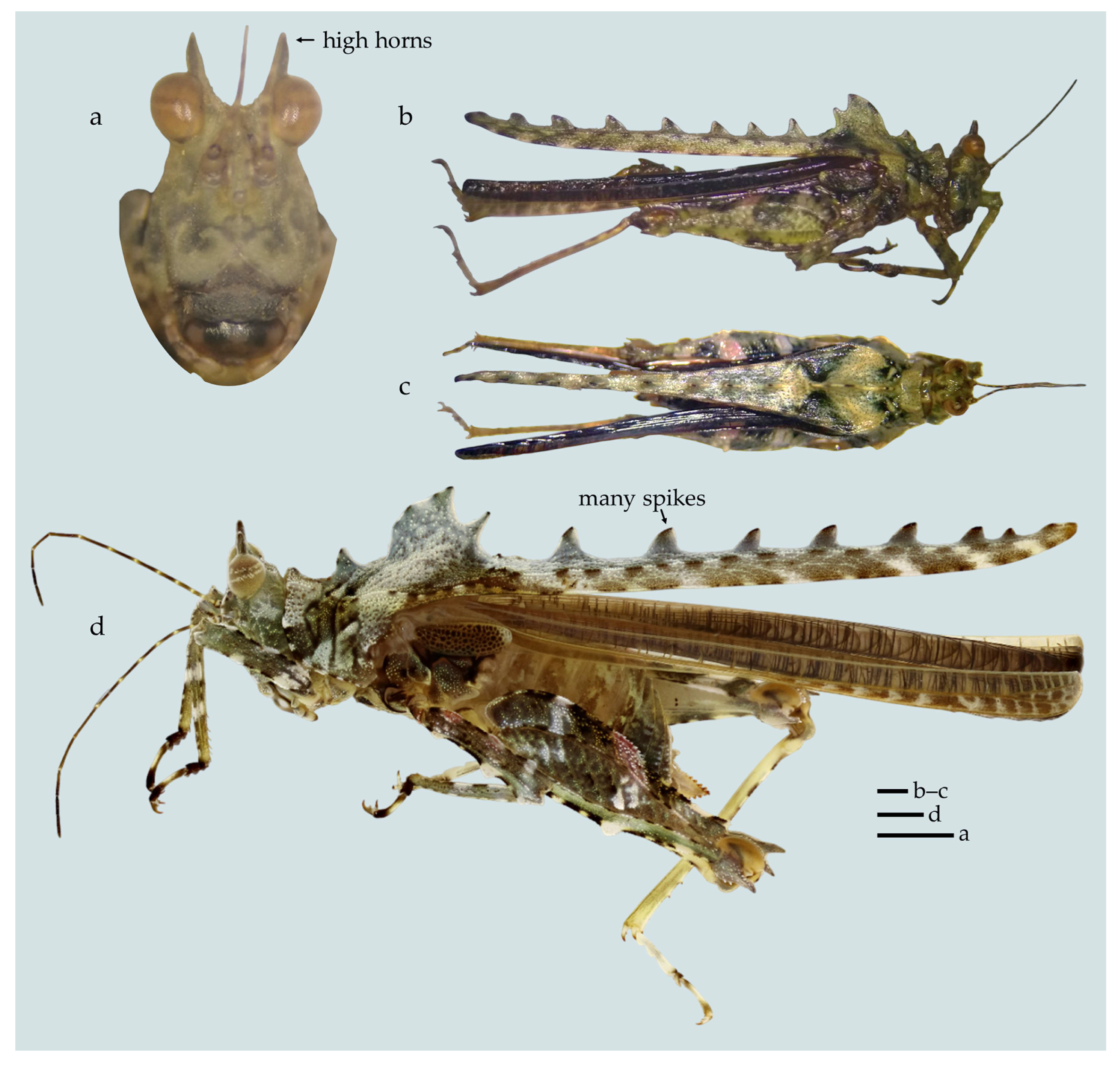
- Descriptive diagnosis (after [24]). Tegotettix is a typical Discotettiginae genus, easily distinguished from other Scelimeninae: Discotettiginae by the lack of spikes (ventrolateral projections) on the lateral lobes of pronotum. The genus bears superficial similarity to Xistra and Ginixistra gen. nov., whose members were previously included within Tegotettix. A detailed comparison with Xistra and Ginixistra gen. nov. is given under the Ginixistra gen. nov. diagnosis. Fastigial horns are very high and spiky. Paired ocelli are placed at the same level as the lower margins of the compound eyes. In the frontal view, the frontal costa is long and distinct, almost as long as half of the compound eye height. Facial carinae after the bifurcation diverge and have a concavity in their upper third. Prozona and prozonal carinae are well developed. Shoulders (the humeral part of the pronotum) are robust. Pronotum bears many projections on the median carina, on the discus, and on the lateral carinae. All femora are armed with teeth. Chitinous surfaces are very rough and tuberculated. Apex of the lateral lobe directed outwards but lacking VL spine.
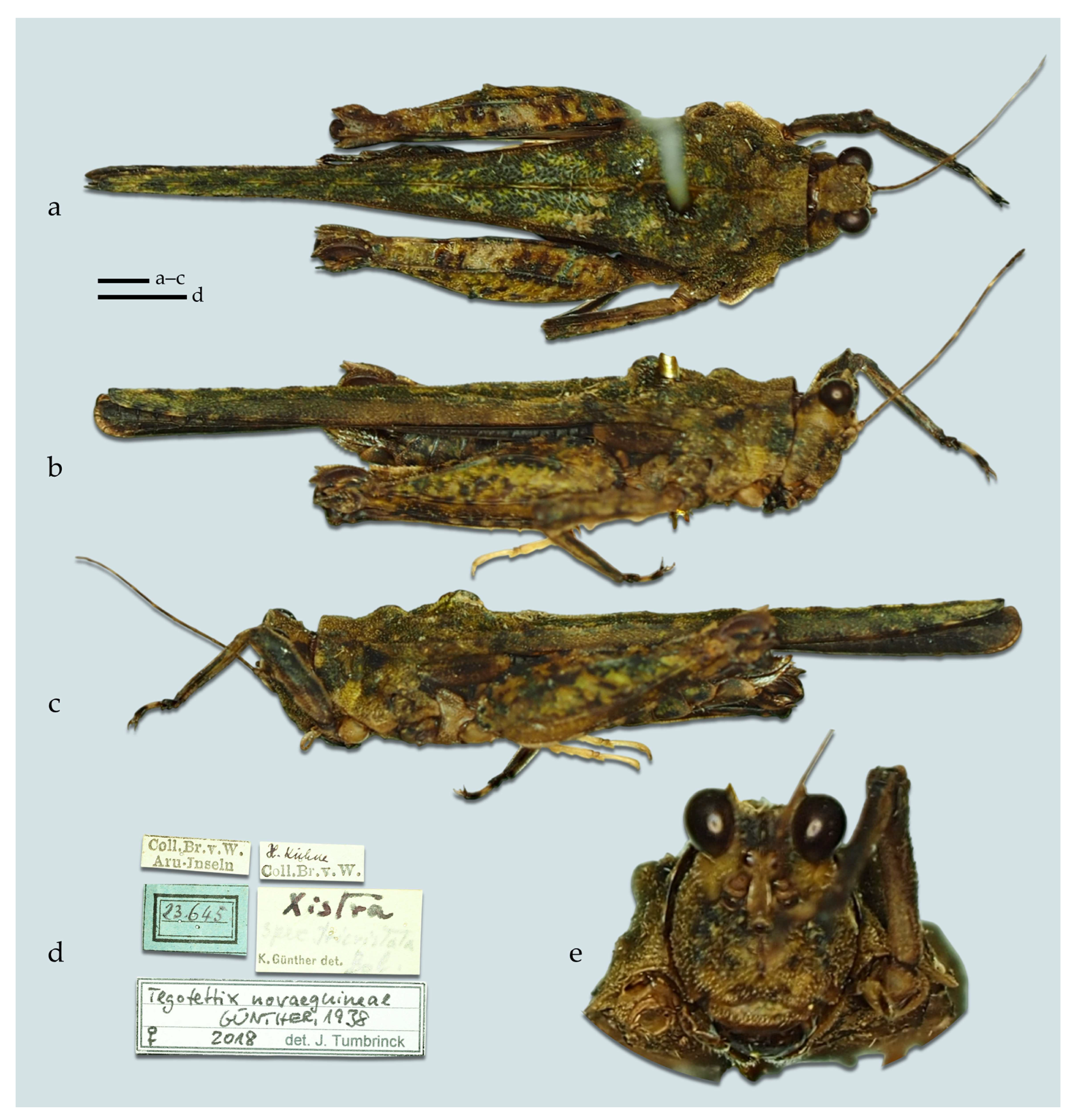
- Composition. The genus Tegotettix currently includes the following five species: (1) T. armatus Hancock, 1913 [2]; (2) T. bufocrocodil (Storozhenko et Dawwrueng, 2015) [81]; (3) T. celebensis Günther, 1937 [17]; (4) T. siebersi Günther, 1938 [18] [Borneo]; (5) T. tuberculatus (Bolívar, 1887) [1] [Sulawesi].
- Taxonomic remarks. After the update in the definition of Xistra and after the establishment of Ginixistra gen. nov., five species are removed from the genus Tegotettix, which now might represent a monophyletic unit. However, revision of this interesting corticolous genus is needed in the future when more material is available for study. The discovery of more new species is expected, as already evidenced by a new undescribed species from Mindanao (Mt. Kiamo and Mt. Kitanglad) in [24].
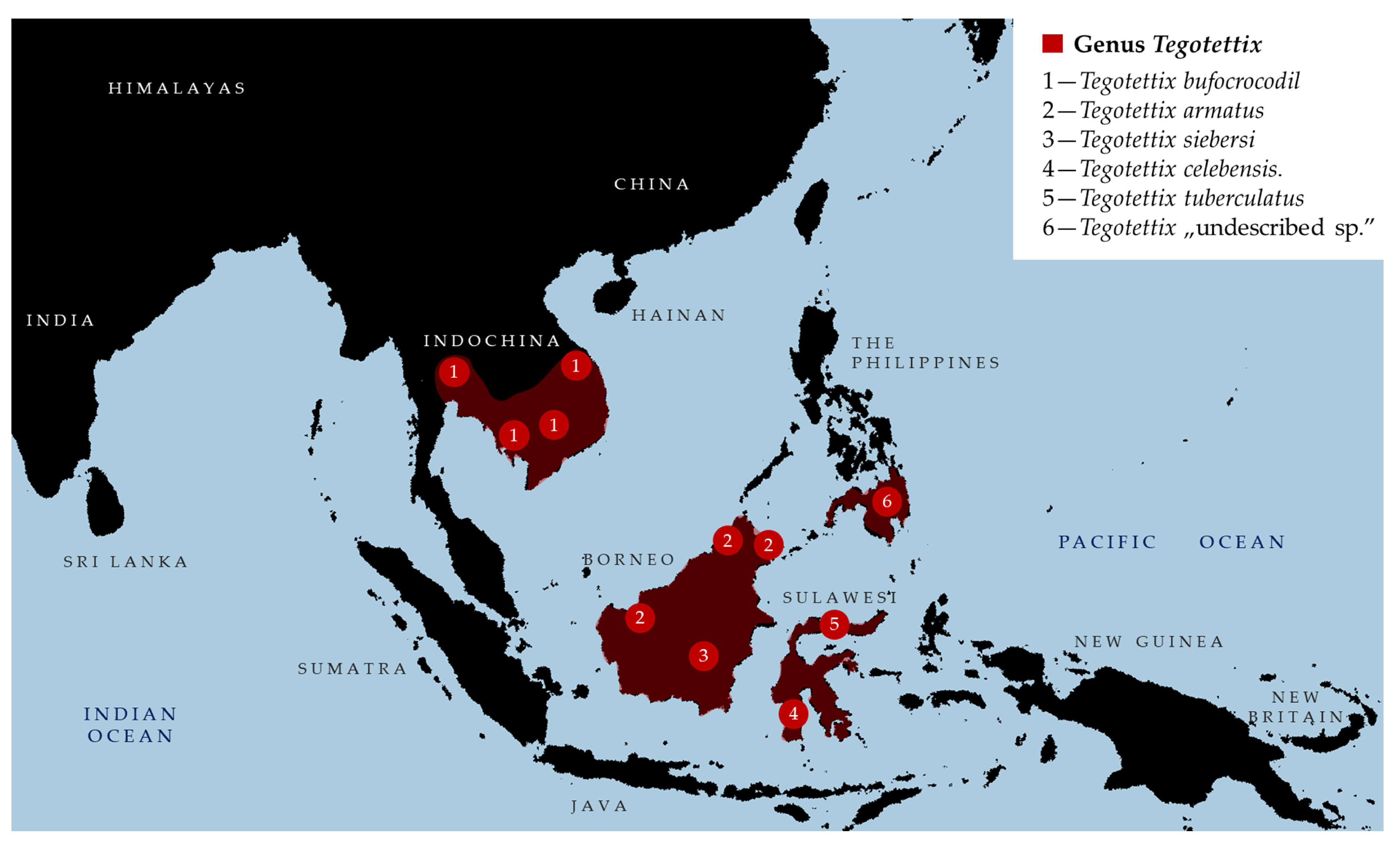
- Measurements. Measurements of all Tegotettix species are shown in Table 4.
| T. bufocrocodil | T. armatus | T. siebersi | T. tuberculatus | T. celebensis | ||
|---|---|---|---|---|---|---|
| Specimen | ♀ Holotype | ♀ Non-Type | ♀ Syntype | ♂ Syntype | ♀ Holotype | ♀ Syntype |
| Locality | Indochina | Borneo | Borneo | Sulawesi | Sulawesi | |
| Depository | ZIN | CJT | SMTD | SMTD | MNCN | MfN |
| BL | 18.95 | 20.05 | 18.00 | 17.65 | 21.45 | 24.75 |
| PL | 17.00 | 18.15 | 17.15 | 16.80 | 20.50 | 23.75 |
| PW | 5.80 | 6.30 | 4.10 | 4.20 | 4.80 | 5.35 |
| PH | 3.65 | 4.75 | 3.15 | 3.00 | 3.55 | 4.80 |
| FFL | 3.10 | 3.35 | 2.65 | 2.35 | 2.60 | 2.85 |
| FFW | 1.20 | 1.20 | 0.85 | 0.65 | 0.70 | 0.95 |
| MFL | 3.60 | 4.10 | 3.10 | 2.70 | 2.65 | 3.40 |
| MFW | 1.40 | 1.60 | 0.65 | 0.75 | 0.70 | 1.05 |
| HFL | 7.55 | 8.30 | 6.50 | 5.60 | 6.80 | 7.45 |
| HFW | 2.45 | 2.50 | 1.80 | 1.75 | 2.10 | 2.40 |
| VW | 1.00 | 1.15 | 0.80 | 0.65 | 0.70 | 1.00 |
| EW | 0.60 | 0.60 | 0.55 | 0.65 | 0.60 | 0.75 |
| AL | 7.30 | 7.30 | broken | 5.65 | broken | 5.85 |
| HH | 0.15 | 0.20 | 0.25 | 0.25 | 0.10 | 0.25 |
- Genus Xistra Bolívar, 1887 [1]
- Subfamily assignment. Orthoptera Species File [5] assigns this genus to Metrodorinae, but we do not agree, as members of Metrodorinae have a compressed medial carina of the vertex directed forwards, and for now, they are known only to inhabit the Americas [82]. Until more evidence is presented on the taxonomic position of Xistra, the genus should be without a subfamily assignment
- Redefinition. Head slightly dorsoventrally elongated; compound eyes and the tips of vertex above the level of the pronotum. In frontal view, medial carina of the vertex positioned just between the compound eyes, and with lateral sides of the vertex elevated. In the frontal view frontal costa bifurcation very high, with frontal costa itself being nearly invisible; X. cristifera being an exception with its short but visible frontal costa. Tip of the eyes visibly above the vertex. Lateral carinae projected as horns, which are in some species very low (X. sagittaria), while in others very high (X. corniculata, X. cristifera, X. derijei). Antennal grooves positioned below the lower margins of the compound eyes. Paired ocelli below the middle level of the eyes, position varies depending on the degree to which the vertex is lowered below the eyes. Scutellum slightly (X. sagittaria) or conspicuously widened (X. derijei).
- Descriptive diagnosis. Members of the genus Xistra are visually similar to members of Xistrella, but can easily be distinguished by the following traits: (1) Xistrella members have rectangular vertex with low horns, while Xistra members have V or wide U shape vertex with high horns; (2) Xistrella members have oval eyes in frontal view, while Xistra members have rounded eyes; (3) occipital area narrow in Xistrella, wide and elongated in Xistra; (4) tegminal sinus in Xistrella reduced, while in Xistra deep; and (5) elongated proximal segment of fore and mid tarsi in Xistrella, almost a half of the distal segment length (about a third of the distal segment length in Xistra).
- Composition. Genus is divided into two subgenera and comprises five species in total. Nominotypical subgenus Xistra includes two species (X. (Xistra) gogorzae Bolívar, 1887 and X. (Xistra) sagittaria Bolívar, 1887 [1] comb. resurr.) while Tegoxistra subgen. nov. includes three species (X. (Tegoxistra) corniculata (Stål, 1877) [40] comb. resurr., X. (T.) derijei (Patano, Mohagan, Tumbrinck, Amoroso et Skejo, 2021) [24] comb. nov. 5) X. (T.) cristifera Günther, 1935 [16] comb. resurr.).
- An annotated identification key to Xistra species
- (1A) Horns of vertex low. Upper margins of antennal grooves situated slightly under the lower margins of eyes. Pronotal crest absent or very low. subgenus Xistra…2
- (1B) Horns of vertex high. Top margins of the antennal grooves visibly below the bottom margins of the eyes. Pronotal crest(s) clearly visible. subgenus Tegoxistra…3
- (2A) In frontal view, vertex V-shaped, narrow. In lateral view, horns of vertex visible above eyes. Pronotum is almost completely flat. X. (Xistra) gogorzae (Figure 19)
- (2B) In frontal view, vertex C-shaped, wide. In lateral view, horns of vertex not visible or hardly visible as a dot above eye. Pronotum has an undulation above the tegmina. X. (Xistra) sagittaria (Figure 20)
- (3A) Highest pronotal projection visibly higher than horns of vertex. In lateral view, dorsum of pronotum is rough. Genicular tooth large…4
- (3B) Highest pronotal projection as high as horns of vertex. There is one in lateral view, dorsum of pronotum smooth. Genicular tooth small. X. (Tegoxistra) corniculata comb. resurr. (Figure 21)
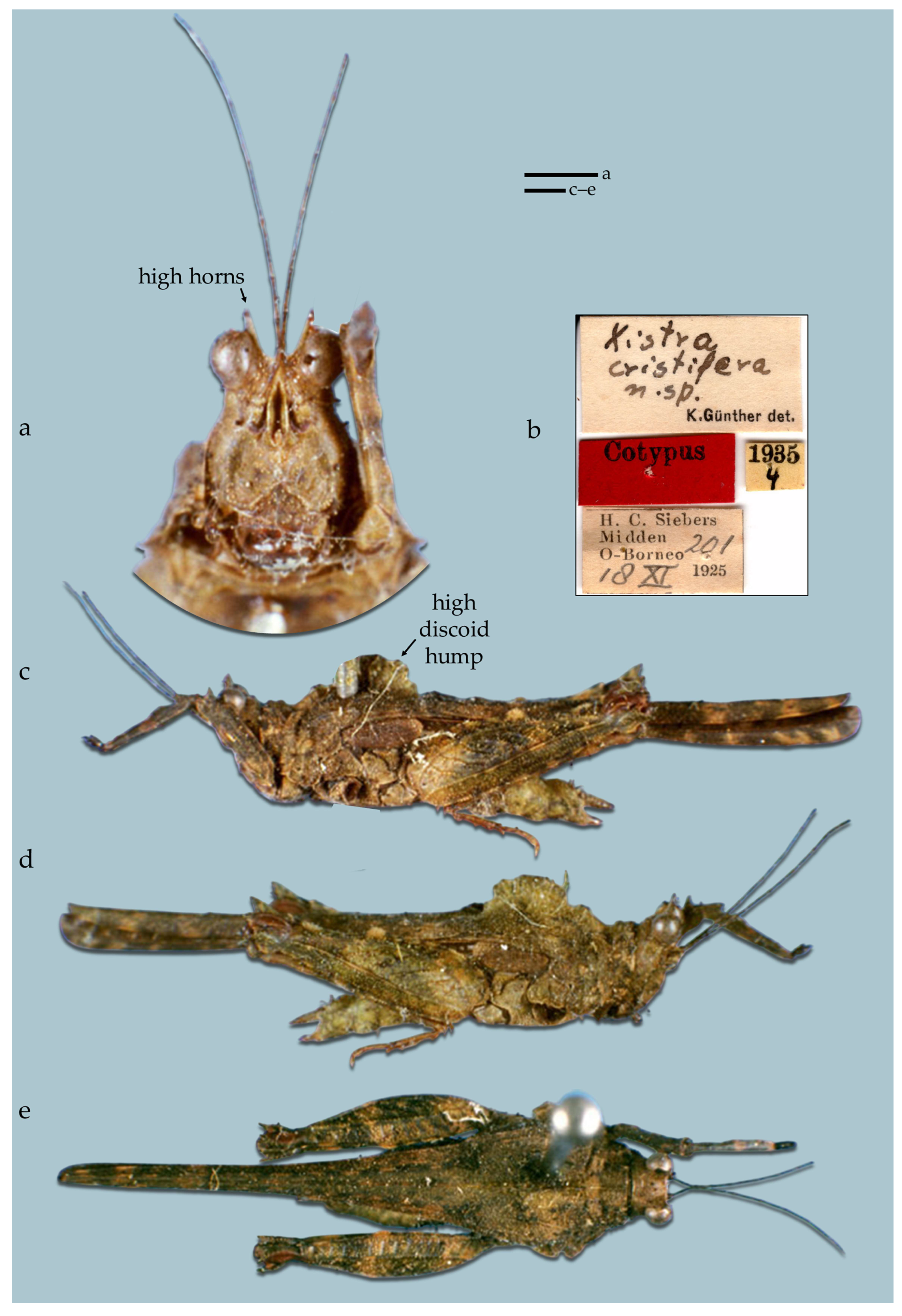
- (4A) One high semicircular crest (projection) formed by the median carina of the pronotum visible in lateral view above the tegmina. X. (Tegoxistra) cristifera comb. resurr. (Figure 22)
- Taxonomic remarks. The diagnoses presented in this study define the genus Xistra and its two subgenera in a falsifiable way. The diagnoses are based on highly similar facial morphologies, as the pronotal and leg characters are very similar among different genera but can significantly vary within them. Such characters are the presence and shape of the pronotal crest, the presence of numerous pronotal projections, the development of lateral, prozonal, and interhumeral carinae of the pronotum, sulcation of the legs, and tarsal morphologies, for which it is still unclear at which taxonomic level they could be useful. This study does not aim to resolve the ocean of problems that lie beyond Xistra; thus, the diagnoses are kept short and are expected to be expanded once the genera listed further in the text are revised.
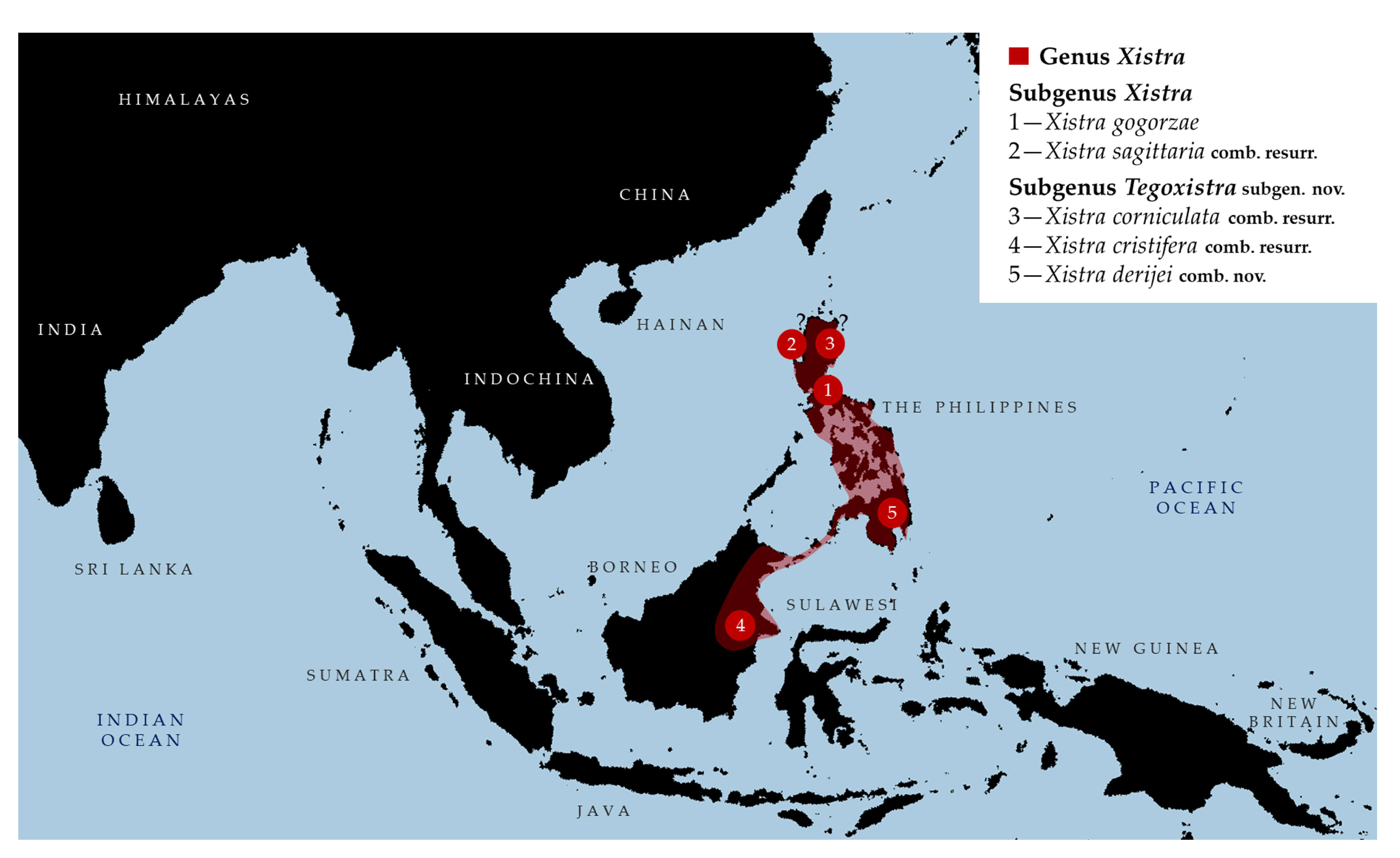
- Subgenus Xistra Bolívar, 1887 [1]
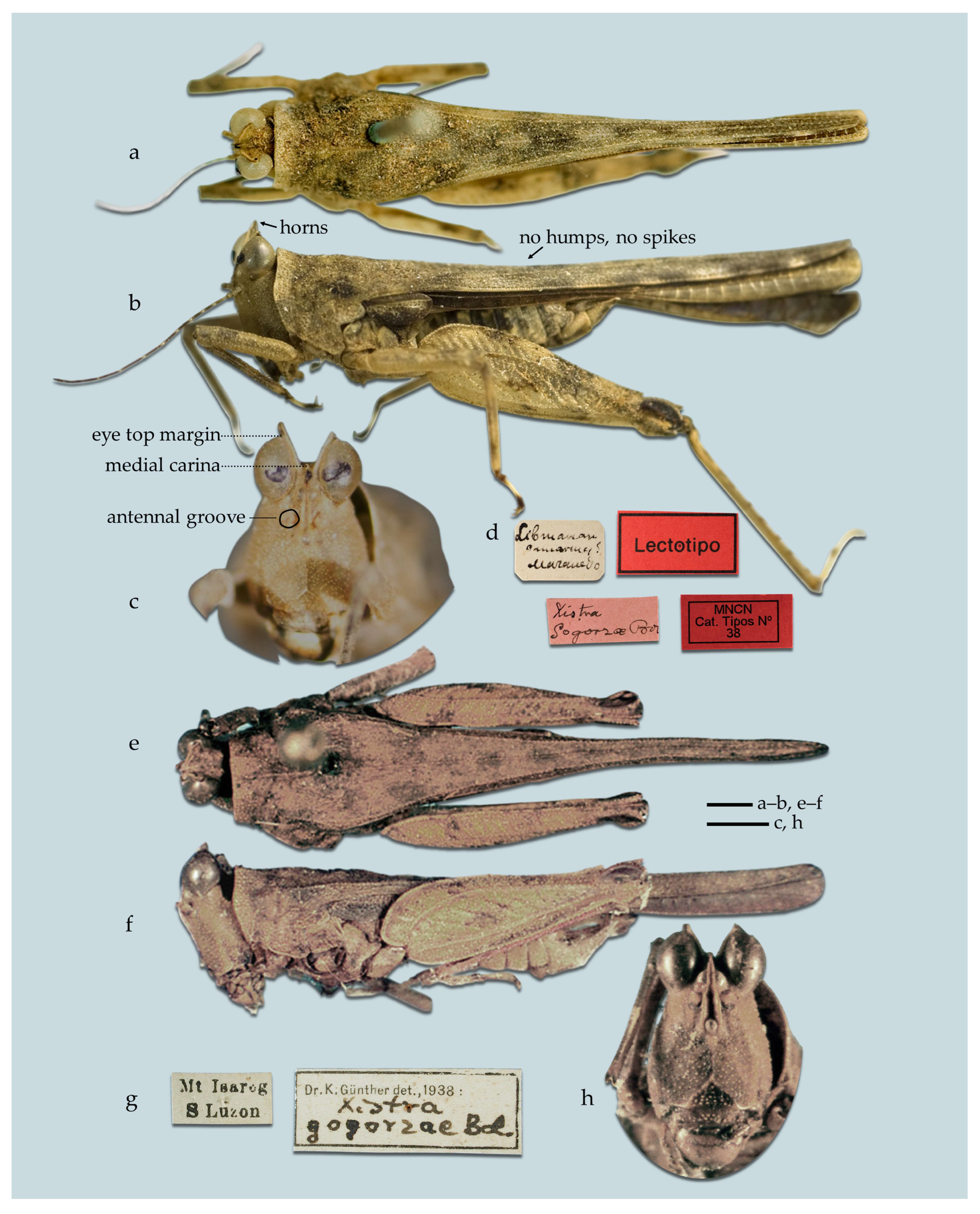
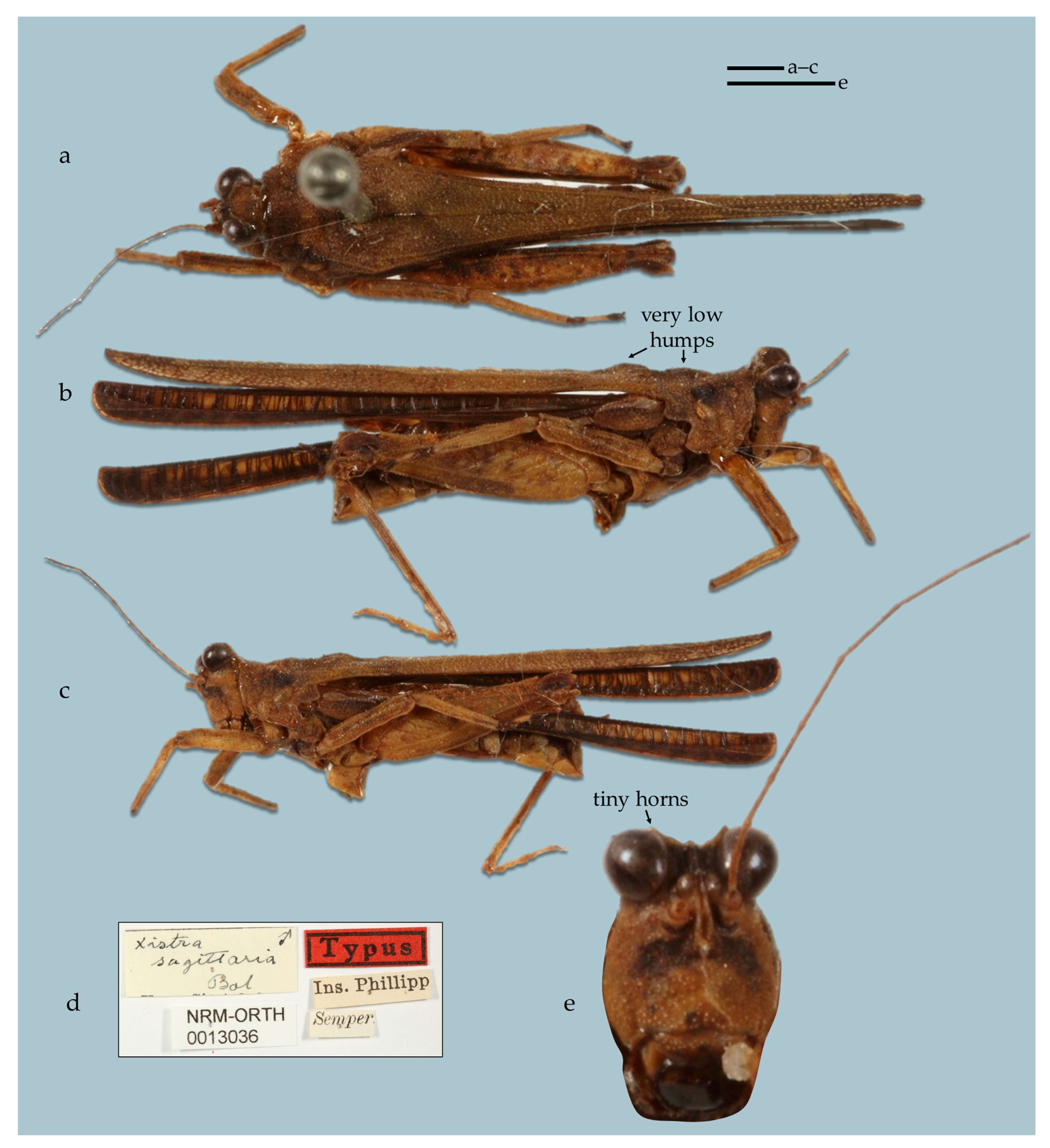
- Diagnosis of the subgenus Xistra. Horns of the vertex weakly to moderately developed. Bifurcation of the frontal costa at the top of the face in anterior view, frontal costa nearly invisible. Top margins of the antennal grooves a little below the bottom margins of the eyes. The crest in the anterior part of the pronotum is absent or weakly developed.
- Distribution of the subgenus Xistra. Endemic to the Philippines.

- Subgenus Tegoxistra Skejo, Patano, Škorput et Kasalo subgen. nov.
- urn:lsid:zoobank.org:act:7EA3C37F-CC3D-40EA-883A-CE6AD0F57046
- Type species. Tegotettix derijei Patano, Mohagan, Tumbrinck, Amoroso et Skejo, 2021 [24] (=X. (Tegoxistra) derijei comb. nov.), here designated. Because it is the species for which the largest amount of material is available for research, we decided to designate X. derijei comb. nov. as the type species of this new subgenus.
- Diagnosis of the subgenus Tegoxistra subgen. nov. Horns of the vertex are highly developed. Bifurcation of the frontal costa at the top of the face in anterior view or a little lower (in X. cristifera). Top margins of the antennal grooves visibly below the bottom margins of the eyes. The crest in the anterior part of the pronotum moderately developed.
- Composition. Three species, X. corniculata, X. derijei, and X. cristifera.
- Distribution. Endemic to the Philippines (X. corniculata, X. derijei), and Borneo (X. cristifera).
- Measurements. Comparative measurements of all Xistra species are shown in Table 5.
| Subgenus Xistra | Subgenus Tegoxistra | |||||||
|---|---|---|---|---|---|---|---|---|
| X. gogorzae | X. sagittaria | X. derijei | X. corniculata | X. cristifera | ||||
| Specimen | ♂ LT | ♂ Non-Type | ♂ HT | ♀ HT | ♂ PT | ♀ PT | ♂ HT | ♀ ST |
| Locality | Camarines Sur: Libmanan | Luzon: Isarog | Philippines: No Locality | Philippines: Mindanao: Davao: Marilog: Mount Malambo | Philippines: No Locality | Borneo: Central East Part | ||
| Depository | MNCN | MfN | NHRS | CMU | CMU | CMU | NHRS | SMTD |
| BL | 14.45 | 15.55 | 13.50 | 16.00 | 15.25 | 17.30 | 13.90 | 14.80 |
| PL | 13.05 | 13.40 | 12.20 | 14.50 | 13.60 | 15.6 | 12.70 | 13.00 |
| PW | 3.15 | 2.95 | 3.15 | 3.00 | 2.90 | 4.00 | 3.10 | 3.75 |
| PH | 2.30 | 2.15 | 1.85 | 2.10 | 2.85 | 2.95 | 2.10 | 2.40 |
| FFL | 2.45 | broken | 2.35 | 3.60 | 2.40 | 2.60 | 2.45 | 2.85 |
| FFW | 0.45 | broken | 0.50 | 0.40 | 0.40 | 0.65 | 0.45 | 0.75 |
| MFL | 2.85 | broken | 2.60 | 3.70 | 2.70 | 3.35 | 2.80 | broken |
| MFW | 0.65 | broken | 0.50 | 0.70 | 0.55 | 0.70 | 0.65 | broken |
| HFL | 6.40 | 6.05 | 5.05 | 5.90 | 5.70 | 6.70 | 5.70 | 6.10 |
| HFW | 1.80 | 1.80 | 1.45 | 2.00 | 1.30 | 2.00 | 1.40 | 1.70 |
| VW | 0.40 | 0.40 | 0.85 | 0.95 | 0.80 | 0.85 | 0.50 | 0.50 |
| EW | 0.75 | 0.70 | 0.70 | 0.80 | 0.70 | 0.65 | 0.55 | 0.65 |
| AL | broken | broken | 4.65 | 6.80 | 5.00 | 6.50 | broken | 6.00 |
| HH | 0.30 | 0.35 | 0.05 | 0.45 | 0.30 | 0.60 | 0.25 | 0.35 |
- Tribe Xistrellini Skejo, Storozhenko, Tumbrinck et Kasalo trib. nov.
- urn:lsid:zoobank.org:act:8115B788-393B-492B-A405-0437C19977DA
- Composition. The tribe consists of 11 genera:
- (1)
- Afrosystolederus Devriese et Husemann, 2023 [95] [Africa: Liberia];
- (2)
- Bannatettix Zheng, 1993 [96] [PR China; Nepal];
- (3)
- Kanakacris Kasalo et Skejo, 2025 [97] [New Caledonia];
- (4)
- Pseudoparatettix Günther, 1937 [17] [islands of SE Asia and Papua; Nepal];
- (5)
- (6)
- (7)
- (8)
- Synalibas Günther, 1939 [4] [India, Nepal, PR China, Papua];
- (9)
- (10)
- (11)
- Distribution. Mostly Asia (Bannatettix, Pseudoparatettix, Phaesticus, Pseudoxistrella, Synalibas, Systolederus, Xistrella) but also Madagascar (Pseudosystolederus), New Caledonia (Kanakacris), Africa (Afrosystolederus), and Southern America (Teredorus). Most of the aforementioned genera require a comprehensive revision.
- Description
- Moderately large pygmy grasshoppers. Short and rounded vertex; in dorsal view never projected. Antennae long and filiform (most genera), or with flattened subapical antennomeres (Phaesticus). Dorsal margins of the antennal grooves’ placement genus-specific; situated between the eyes, or at the level of the lower margins of the compound eyes, or lower. Bifurcation of the frontal costa usually around the middle of the eye height, sometimes lower. Long and relatively narrow frontal costa never forming wide scutellum. Vertex usually narrower than an eye (many Xistrella, Pseudoparatettix, Phaesticus, and all Systolederus, Synalibas, Kanakacris, Pseudosystolederus, Afrosystolederus, Teredorus), sometimes as wide as an eye or wider. Pronotal carinae, especially prozonal, reduced in many genera. First tarsal segment of the fore and mid tarsi elongated. Pulvilli of the hind tarsi are obliquely angular, without apical teeth. Nymphs of several genera (Xistrella, Phaesticus, Pseudoxistrella, Pseudoparatettix) with striped coloration.
- Diagnosis. The new tribe is visually similar most similar to Tetriginae and especially to Tetrigini, a specious subfamily and Tribe whose members exhibit wide range of morphological traits. Xistrellini members are similar to Tetriginae in their slender and usually smooth appearance; paranota directed downwards; and pauropronotal state (hind wings longer than pronotum). However, Xistrellini members can generally be distinguished from Tetriginae by (1) short and rounded vertex (usually wide, rectangular or triangular in Tetriginae), (2) elongated proximal tarsal segments of fore and mid legs (not elongated in Tetriginae), (3) reduced prozonal carinae; (4) the lack of apical teeth on the pulvilli of hind tarsi, and (5) enlarged compound eyes.
- Remarks on the nymphal morphology. Members of Phaesticus (e.g., iNaturalist observations 255283114, 235071700, 235459474, 236842858, 237243682, 228412504, 226344612, 224981610, 224600328), Pseudoxistrella (e.g., iNaturalist observations 147581446, 147581440, 253289656, 239757201, 214499626, 194731007), and Pseudoparatettix (e.g., iNaturalist observation 177970395) have very similar-looking nymphs, and it seems clear that they are related. Xistrella nymphs have a somewhat similar pronotal apex, but the median carina is not so uniformly elevated throughout its length, the anterior part of the pronotum does not protrude, and the vertex reaches nearly to the level of the eyes.
4. Discussion
4.1. Non-Binary Traits That Led to Confusion
4.2. The Problematics of Xistrellini Taxonomy
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANSP | Academy of Natural Sciences of Philadelphia, Philadelphia, USA |
| CJT | Collection Josef Tumbrinck, Wassenberg, Germany |
| CMU | Zoological Section, Central Mindanao University, the Philippines |
| MfN | Museum für Naturkunde, Berlin, Germany |
| MNCN | Museo Nacional de Ciencias Naturales, Madrid, Spain |
| NHRS | Naturhistoriska riksmuseet, Stockholm, Sweden |
| OSF | Orthoptera Species File |
| SMTD | Staatliches Museum für Tierkunde, Dresden, Germany |
| ZIN | Russian Academy of Sciences, Zoological Institute, St. Petersburg, Russia |
References
- Bolívar, I. Essai sur les Acridiens de la tribu des Tettigidae. Ann. Soc. Ent. Belg. 1887, 31, 175–313. [Google Scholar]
- Hancock, J.L. Studies of Tetriginae (Acrydinae) from Sarawak Museum, Borneo. Sarawak Mus. J. 1913, 1, 39–54. [Google Scholar]
- Bolívar, I. Nouvelles espèces d’Acridiens du Musée de Genève. Bol. R. Soc. Esp. Hist. Nat. 1909, 9, 393–403. [Google Scholar]
- Günther, K. Revision der Acrydiinae (orthoptera), III. Sectio Amorphopi (Metrodorae Bol. 1887, aut.). Abh. Berichte Staatllichen Mus. Tierkd. Völkerkunde Dresd. (Reihe A Zool.) (N. F.) 1939, 20, 16–335. [Google Scholar]
- Cigliano, M.M.; Braun, H.; Eades, D.C.; Otte, D. Orthoptera Species File. Version 5.0/5.0. 2025. Available online: http://Orthoptera.SpeciesFile.org (accessed on 30 September 2025).
- Storozhenko, S.Y. A new species of the genus Xistra Bolívar, 1887 (Orthoptera: Tetrigidae) from Cambodia. Far East. Entomol. 2021, 430, 1–6. [Google Scholar] [CrossRef]
- Zhang, R.-J.; Deng, W.-A.; Li, J.-J.; Lin, L.-L. Pygmy grasshoppers of the genus Xistra Bolívar, 1887 (Orthoptera: Tetrigidae: Metrodorinae). Zootaxa 2022, 5162, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Storozhenko, S.Y. New and little-known species of the genus Zhengitettix (Orthoptera: Tetrigidae: Scelimeninae) from Southeast Asia. Zoosyst. Ross. 2013, 22, 204–223. [Google Scholar] [CrossRef]
- Tumbrinck, J. Taxonomic revision of the Cladonotinae (Orthoptera: Tetrigidae) from the islands of South-East Asia and from Australia, with general remarks to the classification and morphology of the Tetrigidae and descriptions of new genera and species from New Guinea and New Caledonia. In Biodiversity, Biogeography and Nature Conservation in Wallacea and New Guinea; Entomological Society of Latvia: Riga, Latvia, 2014; Volume 2, pp. 345–396. ISBN 978-9984-9768-7-7. [Google Scholar]
- Tan, M.K.; Tumbrinck, J.; Baroga-Barbecho, J.B.; Yap, S.A. A new species and morphometric analysis of Cladonotella (Tetrigidae: Cladonotinae). J. Orthoptera Res. 2019, 28, 129–135. [Google Scholar] [CrossRef]
- Bruner, L. Preliminary catalogue of the Orthopteroid insects of the Philippine Islands. Univ. Nebr. Stud. 1915, 15, 195–281. [Google Scholar]
- Tan, M.K.; Tumbrinck, J.; Rivera, R.R.; Nuñeza, O.M. A new genus and a new species of Cladonotinae (Orthoptera: Tetrigidae) from Mindanao, Philippines. Zootaxa 2024, 5506, 194–204. [Google Scholar] [CrossRef]
- Brunner von Wattenwyl, C. Révision du système des Orthoptères et déscription des espèces rapportées par M. Leonardo Fea de Birmanie. Ann. Mus. Civ. Stor. Nat. 1893, 13, 1–230. [Google Scholar]
- Hancock, J.L. Orthoptera Fam. Acridiidae. Subfam. Tetriginae. In Genera Insectorum; V. Verteneuil & L. Desmet: Bruxelles, Belgium, 1907; Volume 48, pp. 1–79. [Google Scholar]
- Bolívar, I. Contributions à l’étude des Acridiens espèces de la Faune indo et austro-malaisienne du Museo Civico di Storia Naturale di Genova. Ann. Mus. Civ. Stor. Nat. 1898, 19, 66–101. [Google Scholar]
- Günther, K. Acrydiinen (Orth. Acrididae) aus dem mittleren Ostborneo gesammelt von H. C. Siebers 1925. Arb. Morphol. Taxon. Ent. Berlin-Dahlem 1935, 2, 257–263. [Google Scholar]
- Günther, K. Orthoptera celebica sarasiniana, Fam. Acrididae, Subfam. Acrydiinae. Treubia 1937, 16, 165–195. [Google Scholar] [CrossRef]
- Günther, K. Revision der Acrydiinae, I. Sectiones Tripetalocerae, Discotettigiae, Lophotettigiae, Cleostrateae, Bufonidae, Cladonotae, Scelimenae verae. Mitt. Zool. Mus. Berl. 1938, 23, 299–437. [Google Scholar]
- Hancock, J.L. The Tettigidae of Ceylon. Spolia Zeylan. 1904, 2, 97–157. [Google Scholar]
- Yin, X.-C.; Shi, J.-P.; Yin, Z. A Synonymic Catalogue of Grasshoppers and Their Allies of the World (Orthoptera: Caelifera); China Forestry Publishing House: Beijing, China, 1996; ISBN 7-5038-1478-0. [Google Scholar]
- Otte, D. Orthoptera Species File 6. Tetrigoidea and Tridactyloidea (Orthoptera: Caelifera) and Addenda to OSF Vols. 1-5; The Orthopterists’ Society and The Academy of Natural Sciences of Philadelphia: Philadelphia, PA, USA, 1997. [Google Scholar]
- Lu, Y.-Z.; Zha, L.-S. A new species of the genus Lamellitettigodes (Orthoptera: Tetrigidae) from PR China, with taxonomic notes on the genus. Zootaxa 2020, 4851, 338–348. [Google Scholar] [CrossRef]
- Tumbrinck, J. Taxonomic and Biogeographic Revision of the Genus Lamellitettigodes (Orthoptera: Tetrigidae) with Description of Two New Species and Additional Notes on Lamellitettix, Probolotettix, and Scelimena. J. Orthoptera Res. 2019, 28, 167–180. [Google Scholar] [CrossRef]
- Patano, R.R., Jr.; Mohagan, A.B.; Tumbrinck, J.; Amoroso, V.B.; Skejo, J. Horned and spiky: Tegotettix derijei sp. n. (Orthoptera: Tetrigidae) is a peculiar new pygmy grasshopper species from Mindanao. Zootaxa 2021, 4933, 198–210. [Google Scholar] [CrossRef]
- Kevan, D.K.M. Some Orthoptera-Caelifera from the Philippine, Bismarck and Solomon Islands, with a few interesting records from New Guinea and the Moluccas. Entomol. Meddelelser 1966, 34, 375–420. [Google Scholar]
- Deng, W.-A. Taxonomic Study of Tetrigoidea from China. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2016. [Google Scholar]
- Blackith, R.E. Tetrigidae (Insecta; Orthoptera) of Southeast Asia: Annotated Catalogue with Partial Translated Keys and Bibliography; JAPAGA: Wicklow, Ireland, 1992; pp. i–liv, 1–248. [Google Scholar]
- Zheng, Z.-M. Orthoptera: Tetrigidae. In Insects of Mt. Namjagbarwa Region of Xizang; Science Press: Beijing, China, 1988; pp. 45–52. [Google Scholar]
- Zheng, Z.-M. Fauna of the Tetrigoidea from Western China; Science Press: Beijing, China, 2005. [Google Scholar]
- Zheng, Z.-M. Key to the species of Xistra Bolívar and Teredorus Hancock from China with descriptions of two new species. J. Shangqiu Norm. Univ. 2015, 31, 1–9. [Google Scholar]
- Liang, G.-Q.; Jiang, G. Four new species of Tetrigoidea from Tianlin County, Guangxi, south China (Orthoptera). Acta Zootaxonomica Sin. 2004, 29, 115–120. [Google Scholar]
- Zheng, Z.-M.; Shi, F. Five new species of Tetrigoidea from Jiangxi province of China (Orthoptera). Acta Entomol. Sin. 2009, 34, 572–577. [Google Scholar]
- Zheng, Z.-M.; Ou, X.-H. A survey of Tetrigoidea from Yuanjiang Nature Reserve, Yunnan Province, China (Orthoptera). J. Shaanxi Normal Univ. (Nat. Sci.) 2010, 38, 60–70. [Google Scholar]
- Zheng, Z.-M.; Zeng, H.-H. A review of the genus Xistra Bolivar 1887 (Orthoptera: Tetrigidae) with description of two new species. J. Nat. Hist. 2011, 45, 1895–1904. [Google Scholar] [CrossRef]
- Liang, G.-Q.; Chen, Y.-Q. Two new species of the genus Xistra (Orthoptera: Metrodoridae) from Yunnan, China. Entomotaxonomia 2010, 32, 13–17. [Google Scholar]
- Li, X.; Deng, W.-A.; Zheng, Z.-M.; Lin, M.; Lu, C.-W. Two new species of the genus Xistra Bolivar (Orthoptera: Tetrigidae) from China. Neotrop. Entomol. 2014, 43, 209–217. [Google Scholar] [CrossRef]
- Zheng, Z.-M.; Mao, B.-Y.; Xu, J.-S. A preliminary survey of Tetrigoidea from southwestern Yunnan Province. J. Dali Univ. 2010, 9, 1–12. [Google Scholar]
- Bai, Q.-X.; Long, M.; Deng, W.-A. A new species of the genus Xistra (Orthoptera: Tetrigoidea: Metrodorinae) with comments on the characters of mitochondrial genome. Zootaxa 2024, 5447, 373–384. [Google Scholar] [CrossRef]
- Ingrisch, S. Orthoptera of the Nepal expeditions of Prof. J. Martens (Mainz). Senck. Biol. 2001, 81, 147–186. [Google Scholar]
- Stål, C. Orthoptera nova ex Insulis Philippinis descripsit. Öfversigt Af Kongliga Vetensk. Akad. Förhandlinger 1877, 34, 33–58. [Google Scholar]
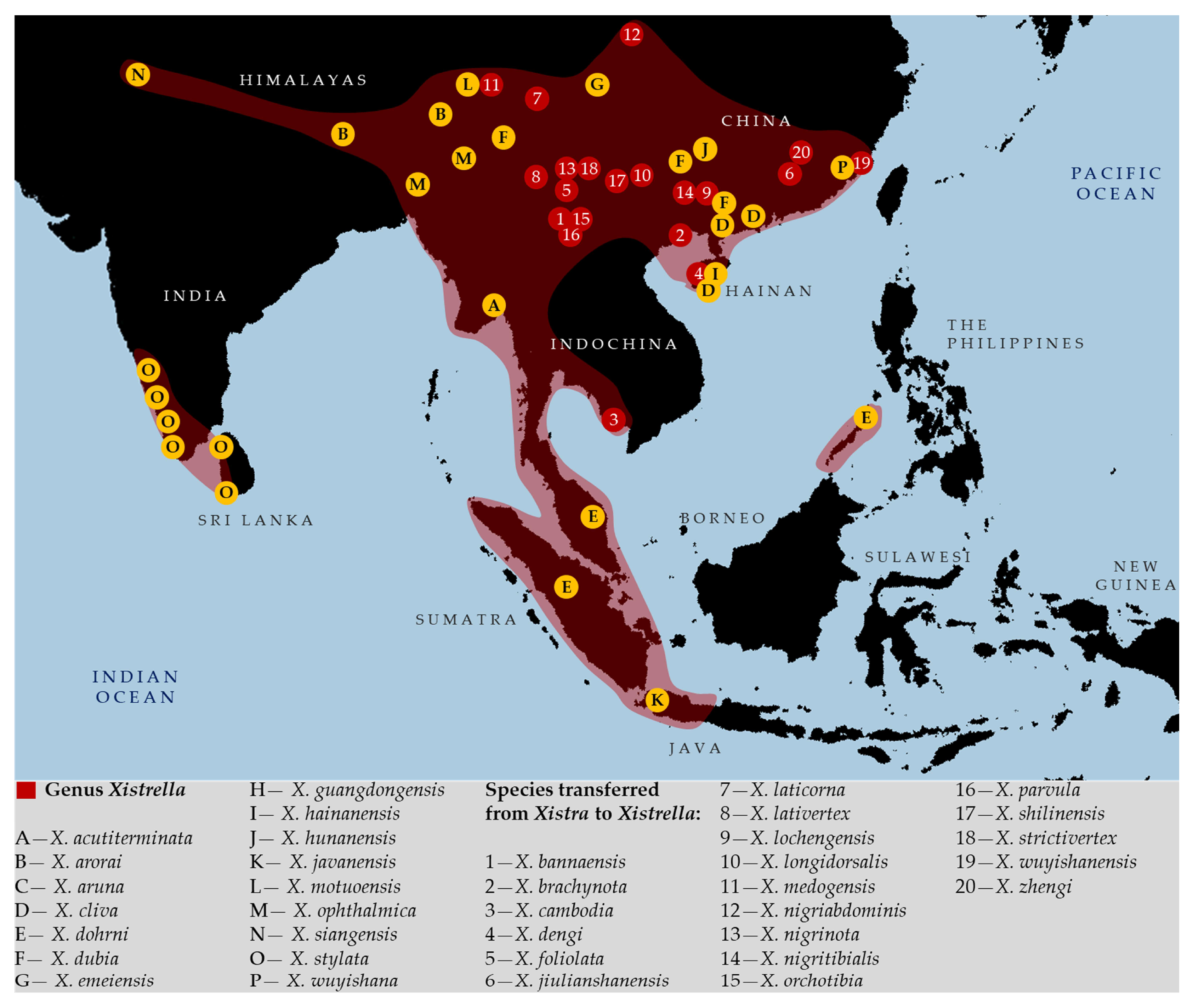
- Xin, L.; Deng, W.-A. A new species and a new record of Xistrella from China (Orthoptera: Tetrigidae). Acta Entomol. Musei Natl. Pragae 2019, 59, 453–462. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, M.-Q.; Mao, B.-Y.; Li, M. Two new species of Xistra Bolivar, 1887 (Orthoptera: Tetrigidae: Metrodorinae) from China, with a key to species of the genus. Orient. Insects 2022, 56, 408–427. [Google Scholar] [CrossRef]
- Ichikawa, A. A revision of the family Tetrigidae (Orthoptera) of the Ryukyu Islands, southern Japan, with descriptions of new species and subspecies. (Part 1). Jpn. J. Entomol. 1994, 62, 457–474. [Google Scholar]
- Jiang, G.-F.; Zheng, Z.-M. Grasshoppers and Locusts from Guangxi; Guangxi Normal University Press: Guilin, China, 1998. [Google Scholar]
- Wang, Y.-Z. A new species of the genus Xistrelia Bolivar from Wuling Mountains area, China (Orthoptera: Tetrigidae). Acta Entomol. Sin. 1999, 42, 199–201. [Google Scholar]
- Yin, X.-C. Grasshoppers and Locusts from Qinghai-Xizang Plateau of China; Science Press: Beijing, China, 1984. [Google Scholar]
- ICZN. The International Trust for Zoological Nomenclature 1999. In International Code of Zoological Nomenclature, 4th ed.; Ride, W.D.L., Cogger, H.G., Dupuis, C., Kraus, O., Minelli, A., Thompson, F.C., Tubbs, P.K., Eds.; The Natural History Museum: London, UK, 2000. [Google Scholar]
- ICZN. Proposed Amendment of Articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Bull. Zool. Nomencl. 2008, 65, 265–275. [Google Scholar]
- ICZN. Amendment of Articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. ZooKeys 2012, 219, 1–10. [Google Scholar] [CrossRef] [PubMed]
- ICZN. Amendment of Articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Zootaxa 2012, 3450, 1–7. [Google Scholar] [CrossRef]
- ICZN. Amendment of Articles 8, 9, 10, 21 and 78 of the International Code of Zoological nomenclature to expand and refine methods of publication. Bull. Zool. Nomencl. 2012, 69, 161–169. [Google Scholar]
- Devriese, H. Contribution à l’étude des Tetrigidae de Madagascar (Orthoptera). Bull. Ann. De La Société R. Belg. D’entomologie 1991, 127, 119–131. [Google Scholar]
- Devriese, H. Bijdrage tot de systematiek, morfologie en biologie van de West-Palearktische Tetrigidae. Nieuwsbr. Saltabel 1996, 15, 2–38. [Google Scholar]
- Devriese, H. Revision des Xerophyllini d’Afrique (Orthoptera, Tetrigidae). Belg. J. Entomol. 1999, 1, 21–99. [Google Scholar]
- Hancock, J.L. Tettigidae of North America; The Lakerside Press, R.R. Donnelley & Sons Company: Chicago, IL, USA, 1902. [Google Scholar]
- Pavón-Gozalo, P.; Manzanilla, J.; García-París, M. Taxonomy and morphological characterization of Allotettix simoni (Bolívar, 1890) and implications for the systematics of Metrodorinae (Orthoptera: Tetrigidae). Zool. J. Linn. Soc. 2012, 164, 52–70. [Google Scholar] [CrossRef]
- Zha, L.-S.; Wu, X.-M.; Ding, J.-H. Two new species of the genus Formosatettix Tinkham, 1937 (Orthoptera, Tetrigidae) from Guizhou and Chongqing, PR China. ZooKeys 2020, 936, 61–76. [Google Scholar] [CrossRef]
- Skejo, J.; Pushkar, T.I.; Kasalo, N.; Pavlović, M.; Deranja, M.; Adžić, K.; Tan, M.K.; Rebrina, F.; Muhammad, A.A.; Abdullah, N.A.; et al. Spiky pygmy devils: Revision of the genus Discotettix (Orthoptera: Tetrigidae) and synonymy of Discotettiginae with Scelimeninae. Zootaxa 2022, 5217, 1–64. [Google Scholar] [CrossRef]
- Devriese, H.; Nguyen, E.; Husemann, M. An identification key to the genera and species of Afrotropical Tetrigini (genera Paratettix, Leptacrydium, Hedotettix, Rectitettix nov. gen., and Alienitettix nov. gen.) with general remarks on the taxonomy of Tetrigini (Orthoptera, Tetrigidae). Zootaxa 2023, 5285, 511–556. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Rambur, J.P. Orthoptères. Faune Entomol. L’andalousie 1838, 2, 12–94. [Google Scholar]
- Mangaoang, C.C.; Mohagan, A.B. Odonata diversity at University of Southern Mindanao, Kabacan, Cotabato. Asian J. Biodivers. 2016, 7, 868. [Google Scholar] [CrossRef]
- Lee, Y.J.; Mohagan, A.B. A new species of Oncotympana Stål, 1870 (Hemiptera: Cicadidae: Oncotympanini) from Negros, Philippines. J. Asia Pac. Biodivers. 2021, 14, 651–653. [Google Scholar] [CrossRef]
- Beleno, J.V.I.; Mohagan, A.B. The diversity and status of bats in Quarry Cave, Poblacion, Kitaotao Bukidnon, Philippines. Asian J. Biodivers. 2023, 14, 1–13. [Google Scholar] [CrossRef]
- Rukmane-Bārbale, A.; Acal, D.A.; Mohagan, A.B. Four new species of the genus Homalocyrtus Heller, 1912 (Coleoptera: Curculionidae: Pachyrhynchini) from the Philippines. Baltic J. Coleopterol. 2024, 24, 115–129. [Google Scholar]
- Kasalo, N.; Tumbrinck, J.; Pavlović, M.; Skejo, J. Atlas of Fijian pygmy grasshoppers (Orthoptera: Tetrigidae) with new taxa descriptions and an identification key. Ann. Zool. 2024, 74, 43–70. [Google Scholar] [CrossRef]
- Steinmann, H. The Tetricidae (Orthoptera) of the Notogea. Opusc. Zool. Bp. 1970, 10, 155–164. [Google Scholar]
- Kasalo, N. Metamazarredia is Rosacris is Mazarredia (Orthoptera: Tetrigidae)—A long-awaited sequel to a taxonomic dilemma. Zootaxa 2022, 5200, 495–500. [Google Scholar] [CrossRef]
- Hughes, M.; Barber, S.; Heatubun, C.D.; Gagul, J. Begonia yapenensis (sect. Symbegonia, Begoniaceae), a new species from Papua, Indonesia. Eur. J. Taxon. 2015, 119, 1–6. [Google Scholar] [CrossRef]
- Atkins, H.J.; Heatubun, C.D.; Galloway, L.; Bramley, G.L.C. Two new species, Cyrtandra bungahijau and C. vittata, and notes on Cyrtandra (Gesneriaceae) from Yapen Island, Indonesia. Kew Bull. 2019, 74, 29. [Google Scholar] [CrossRef]
- Diamond, J.; Bishop, K.D. Origins of the upland avifauna of Yapen Island, New Guinea region. Bull. Br. Ornithol. Club 2020, 140, 423–448. [Google Scholar] [CrossRef]
- Keim, A.P. Pandanaceae of the island of Yapen, Papua (West New Guinea), Indonesia, with their nomenclature and notes on the rediscovery of Sararanga sinuosa, and several new species and records. Blumea 2009, 54, 255–266. [Google Scholar] [CrossRef]
- Daawia; Rahareng, E.F.; Ramandey, E.; Morib, G.; Yando, H.; de Vos, R.; Sinnema, S.; Sinnema-Bloemen, J.; Guimarães Cruz, M.; Zumkehr, P.; et al. A faunistic overview of the moth species recorded from Yapen Island, Papua, Indonesia (Lepidoptera). SUGAPA 2024, 15, 102–120. [Google Scholar]
- Kitonić, D. Put po Melaneziji: Rituali i Običaji Papue Nove Gvineje; Online Book, Attribution Non-Commercial (BY-NC) Licence. 2017. Available online: https://kupdf.net/download/put-po-melaneziji-rituali-i-obicajipapue-nove-gvineje_5b08056ee2b6f51e62dfe8ba_pdf (accessed on 30 September 2025).
- Kitonić, D. Ptice Delte Neretve. 1. Stanarice; Gradsko Kulturno Središte: Metković, Croatia, 2007; pp. 1–105. [Google Scholar]
- Kitonić, D. Ptice Delte Neretve. 2. Gnjezdarice; Gradsko Kulturno Središte: Metković, Croatia, 2007; pp. 106–244. [Google Scholar]
- Kitonić, D. Ptice Delte Neretve. 3. Zimovalice; Gradsko Kulturno Središte: Metković, Croatia, 2007; pp. 245–428. [Google Scholar]
- Kitonić, D. Ptice Delte Neretve. 4. Selice; Gradsko Kulturno Središte: Metković, Croatia, 2007; pp. 429–652. [Google Scholar]
- Gassmann, D. Odonata recorded from northeastern Papua New Guinea including the Bismarck Archipelago in May to July 1997. Faun. Stud. South East Asian Pac. Isl. Odonata 2015, 10, 1–46. [Google Scholar]
- Otte, D. The primary types of Orthoptera (Saltatoria, Blattodea, Phasmatodea, and Mantodea) at the Academy of Natural Sciences of Philadelphia. Proc. Acad. Nat. Sci. Phila. 1979, 130, 26–87. [Google Scholar]
- Storozhenko, S.Y.; Dawwrueng, P. New and little-known pygmy grasshoppers (Orthoptera: Tetrigidae) from Thailand. Zootaxa 2015, 4052, 527–554. [Google Scholar] [CrossRef]
- Kasalo, N.; Yong, S.; Rebrina, F.; Skejo, J. Definition of the tribe Metrodorini (Orthoptera: Tetrigidae) with notes on biogeography and evolution of Metrodorinae and Cladonotinae. Acta Entomol. Musei Natl. Pragae 2023, 63, 187–193. [Google Scholar] [CrossRef]
- Kirby, W.F. A Synonymic Catalogue of Orthoptera (Orthoptera Saltatoria, Locustidae vel Acridiidae), 3 (2); Order of the Trustees: London, UK, 1910. [Google Scholar]
- Deng, W.-A.; Zheng, Z.-M.; Li, X.; Lin, M.; Wei, S.-Z.; Yuan, B.-D.; Lin, L.-L. The groundhopper fauna (Orthoptera: Tetrigidae) of Shiwanshan (Guangxi, China) with description of three new species. Zootaxa 2015, 3925, 151–178. [Google Scholar] [CrossRef]
- Zheng, Z.-M. A systematic study of Xistra Bolivar from China (Tetrigoidea: Metrodoridae). J. Huazhong Agric. Univ. 2005, 24, 129–132. [Google Scholar]
- Zheng, Z.-M. Taxonomic review of the genus Xistra Bolivar (Orthoptera: Metrodoridae) from China with description of a new species. Acta Entomol. Sin. 2009, 52, 296–300. [Google Scholar]
- Liang, G.-Q.; Zheng, Z.-M. Fauna Sinica, Insecta, Vol. 12. Orthoptera Tetrigoidea; Science Press: Beijing, China, 1998. [Google Scholar]
- Zheng, Z.-M.; Li, K.; Wang, Q. A new genus and two new species of Orthoptera from Xizang. Acta Zootaxonomica Sin. 2004, 29, 110–114. [Google Scholar]
- Zheng, Z.-M. A systematic study of Mazarredia Bolivar from China with descriptions of four new species (Tetrigoidea: Metrodoridae). Acta Entomol. Sin. 2005, 48, 588–593. [Google Scholar]
- Deng, W.-A.; Zheng, Z.-M.; Wei, S.-Z. A review of the genus Mazarredia Bolivar (Orthoptera: Tetrigoidea: Tetrigidae: Metrodorinae) from China. Zootaxa 2007, 1645, 47–56. [Google Scholar] [CrossRef]
- Deng, W.-A.; Zheng, Z.-M.; Wei, S.-Z. Fauna of Tetrigoidea from Yunnan and Guangxi; Guangxi Science & Technology Press: Nanning, China, 2007. [Google Scholar]
- Jiang, G.-F.; Liang, G.-Q. Fifty-three species of Tetrigoidea (Orthoptera) from Cenwang Mountain in the western Guangxi Zhuang autonomous region, China. Entomol. News 2004, 115, 201–206. [Google Scholar]
- Zheng, Z.-M.; Jiang, G.-F. Three new species of Tetrigoidea from Guangxi (Orthoptera). Acta Entomol. Sin. 2002, 45, 9–12. [Google Scholar]
- Wei, T.; Nong, C.-G.; Huang, J.-H. A preliminary study on grasshoppers in Nonggang Nature Reserve of Guangxi, China. J. Environ. Entomol. 2014, 36, 843–852. [Google Scholar]
- Devriese, H.; Husemann, M. Afrosystolederus garmsi (Orthoptera, Tetrigidae), a new genus and species from Mount Gibi (Liberia) with remarks on Systolederus, Pseudosystolederus and Teredorus. Zootaxa 2023, 5258, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-M. One new genus and three new species of Tetrigidae from Yunnan province (Orthoptera: Tetrigidae). J. Shaanxi Norm. Univ. (Nat. Sci.) 1993, 21, 46–50. [Google Scholar]
- Kasalo, N.; Brouste, D.; Desutter-Grandcolas, L.; Hervé, C.; Bogić, D.; Skejo, J. Atlas of New Caledonian Tetrigidae with preliminary suprageneric classification of Batrachideinae. Zoosystema 2025, 47, 327–397. [Google Scholar] [CrossRef]
- Uvarov, B.P. Twenty-four new generic names in Orthoptera. Ann. Mag. Nat. Hist. 1940, 6, 112–117. [Google Scholar] [CrossRef]
- Zha, L.-S.; Skejo, J.; Mao, B.-Y.; Ding, J.-H. Taxonomic revision of Phaesticus Uvarov and synonymy with Flatocerus Liang & Zheng syn. nov. (Orthoptera: Tetrigidae). Zootaxa 2021, 4965, 501–514. [Google Scholar] [CrossRef]
- Günther, K. Beitrag zur Kenntnis der Tetrigiodea (Orth. Caelifera) von Madagaskar und von Mauritius. Bull. Mus. NatlHist. Nat. Paris (Sér. 3, Zool.) 1974, 236, 937–1031. [Google Scholar]
- Devriese, H. Deux nouvelles espèces de Tetrigidae de Madagascar (Orthoptera). Bull. Ann. Soc. R. Belge Entomol. 1995, 131, 97–105. [Google Scholar]
- Liang, G.-Q. A new genus and a new species of Tetrigidae (Orthoptera) from Hainan Province, China. Orient. Insects 1991, 25, 95–97. [Google Scholar] [CrossRef]
- Storozhenko, S.Y.; Omelko, M. Review of the genus Pseudoxistrella Liang, 1991 (Orthoptera: Tetrigidae, Metrodorinae). Zootaxa 2012, 3573, 59–65. [Google Scholar] [CrossRef]
- Shishodia, M.S. Taxonomy and Zoogeography of the Tetrigidae (Orthoptera: Tetrigoidea) of North Eastern India; Zoological Survey of India: Kolkata, India, 1991; Volume 140, pp. 1–204.
- Zheng, Z.-M.; Xu, S.-Q. A review of the genus Teredorus Hancock (Orthoptera, Tetrigidae) from China and adjacent countries with the description of two new species. J. Huazhong Agric. Univ. 2010, 29, 14–20. [Google Scholar]
- Zha, L.-S.; Wen, T.-C.; Kang, J.-C.; Hyde, K.D. Records of Hedotettix and Teredorus in Thailand with the description of three new species (Orthoptera, Tetrigidae). ZooKeys 2016, 556, 83–95. [Google Scholar] [CrossRef]
- Adžić, K. Pygmy Grasshoppers (Orthoptera: Tetrigidae) of Peninsular Malaysia. Diploma Thesis, University of Zagreb, Zagreb, Croatia, 2020. [Google Scholar]
- Li, X.-J.; Liu, Y.-X.; Lin, L.-L. Comparative mitogenomes and phylogenetic analysis reveal taxonomic relationship of genera Teredorus and Systolederus (Orthoptera, Tetrigoidea). Zootaxa 2021, 5027, 127–135. [Google Scholar] [CrossRef]
- Regul, J. Sistematika Skakavaca Veslača (Insecta: Orthoptera: Scelimeninae) Temeljena na Morfološkim Svojstvima. Master’s Thesis, University of Zagreb, Zagreb, Croatia, 2022. [Google Scholar]
- Guan, D.-L.; Huang, C.-M.; Deng, W.-A. Reassessment of the phylogenetics of two pygmy grasshopper generic groups Tetrix and Systolederus through mitochondrial phylogenomics using four new mitochondrial genome assemblies. Insects 2024, 15, 174. [Google Scholar] [CrossRef]
- Storozhenko, S.Y. To synonymy of the genus Xistrella Bolívar, 1909 (Orthoptera: Tetrigidae, Metrodorinae). Far East. Entomol. 2014, 277, 47–48. [Google Scholar]
- Bolívar, I. Nuevo acridino del grupo Discotettigiae. Arch. Zool. Ital. 1931, 15, 29–33. [Google Scholar]
- Skejo, J. On the taxonomy of the genus Rosacris Bolívar, 1931 (Orthoptera: Tetrigidae). Entomol. Heute 2016, 28, 43–52. [Google Scholar]
- Liang, G.-Q. Eine neue Gattung und neue Art der Tetrigidae von China (Orthoptera: Tetrigidae). Entomol. Nachr. 1990, 34, 213–214. [Google Scholar]
- Zha, L.-S.; Yu, F.-M.; Boonmee, S.; Eungwanichayapant, P.D.; Wen, T. The subfamily Cladonotinae (Orthoptera: Tetrigidae) from China with description of a new monotypic genus. J. Nat. Hist. 2017, 51, 1479–1489. [Google Scholar] [CrossRef]
- Kasalo, N.; Skejo, J.; Husemann, M. DNA barcoding of pygmy hoppers—The first comprehensive overview of the BOLD Systems’ data shows promise for species identification. Diversity 2023, 15, 696. [Google Scholar] [CrossRef]
- Luo, J.-L.; Zhang, R.-J.; Deng, W.-A. First mitogenomic characterization of Macromotettixoides (Orthoptera, Tetrigidae), with the descriptions of two new species. ZooKeys 2024, 1195, 95–120. [Google Scholar] [CrossRef]
- Teng, C.-L.; Li, Y.-M.; Chen, Y.-Z.; Deng, W.-A. Complete mitochondrial genomes and phylogenetic relationships of two Saussurella species (Orthoptera: Tetrigidae) from China. Mitochondrial DNA Part B 2025, 10, 447–452. [Google Scholar] [CrossRef]
- Li, X.-J.; Dou, W.-L.; Lin, L. Mitogenomic phylogeny of Tetrigoidea (Insecta, Orthoptera), with a focus on the genus Zhengitettix. PeerJ 2025, 13, e19521. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-M.; Leng, S.-X.; He, J.-S.; Deng, W.-A.; Guan, D.-L. Mitochondrial phylogenomics of pygmy grasshoppers (Orthoptera, Tetrigidae, Metrodorinae): Descriptions of a new genus, two new species, and new synonyms from China. ZooKeys 2025, 1236, 249–281. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-J.; Dong, X.-M.; Lin, L.-L. New insights into the phylogeny of Tetrigoidea (Insecta, Orthoptera), with the announcement of the first mitogenome of the genus Phaesticus. Zookeys 2025, 1251, 115–130. [Google Scholar] [CrossRef] [PubMed]
| Bolívar [1,15] | Hancock [14] | Günther [16,17] | OSF [5] |
|---|---|---|---|
| Xistra gogorzae Bolívar, 1887 | Xistra gogorzae | Xistra gogorzae | Xistra gogorzae |
| Xistra corniculata (Stål, 1877) | Xistra corniculata | Tegotettix corniculatus | Tegotettix corniculatus |
| Xistra sagittaria Bolívar, 1887 | Xistra sagittaria | Tegotettix sagittarius | Tegotettix sagittarius |
| Xistra impressa Bolívar, 1887 | Xistra impressa | Lamellitettix impressus | Lamellitettix impressus |
| Xistra lurida Bolívar, 1887 | Xistra lurida | Pseudoparatettix luridus | Pseudoparatettix luridus |
| Xistra similis Bolívar, 1887 | Xistra similis | Pseudoparatettix similis | Pseudoparatettix similis |
| Xistra ochracea Bolívar, 1887 | Xistra ochracea | Pseudoparatettix ochraceus | Pseudoparatettix ochraceus |
| Xistra t. tricristata Bolívar, 1898 | Xistra t. tricristata | Lamellitettigodes contractus tricristatus | Syn. of L. contractus |
| Xistra t. sumatrana Bolívar, 1898 | Xistra t. sumatrana | Syn. of Lamellitettigodes contractus | Syn. of L. contractus |
| Paratettix sagittatus Bolívar, 1887 | Xistra sagittata | Euparatettix sagittata | Lamellitettigodes sagittatus |
| - | Xistra dubia Brunner von Wattenwyl, 1893 | Xistrella dubia | Xistrella dubia |
| - | - | Xistra cristifera Günther, 1935 | Tegotettix cristiferus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skejo, J.; Kasalo, N.; Patano, R.R., Jr.; Storozhenko, S.Y.; Tumbrinck, J.; Domazet-Lošo, T.; Amoroso, V.B.; Yap, S.A.; Škorput, J. Taxonomic Revision of Pygmy Devil Genera Almacris, Ginixistra, Tegotettix, and Xistra, with Comments on Xistrella (Orthoptera: Tetrigidae). Taxonomy 2025, 5, 60. https://doi.org/10.3390/taxonomy5040060
Skejo J, Kasalo N, Patano RR Jr., Storozhenko SY, Tumbrinck J, Domazet-Lošo T, Amoroso VB, Yap SA, Škorput J. Taxonomic Revision of Pygmy Devil Genera Almacris, Ginixistra, Tegotettix, and Xistra, with Comments on Xistrella (Orthoptera: Tetrigidae). Taxonomy. 2025; 5(4):60. https://doi.org/10.3390/taxonomy5040060
Chicago/Turabian StyleSkejo, Josip, Niko Kasalo, Romeo R. Patano, Jr., Sergey Yu. Storozhenko, Josef Tumbrinck, Tomislav Domazet-Lošo, Victor B. Amoroso, Sheryl A. Yap, and Jadranka Škorput. 2025. "Taxonomic Revision of Pygmy Devil Genera Almacris, Ginixistra, Tegotettix, and Xistra, with Comments on Xistrella (Orthoptera: Tetrigidae)" Taxonomy 5, no. 4: 60. https://doi.org/10.3390/taxonomy5040060
APA StyleSkejo, J., Kasalo, N., Patano, R. R., Jr., Storozhenko, S. Y., Tumbrinck, J., Domazet-Lošo, T., Amoroso, V. B., Yap, S. A., & Škorput, J. (2025). Taxonomic Revision of Pygmy Devil Genera Almacris, Ginixistra, Tegotettix, and Xistra, with Comments on Xistrella (Orthoptera: Tetrigidae). Taxonomy, 5(4), 60. https://doi.org/10.3390/taxonomy5040060







