Abstract
In Yucatán state, Mexico, Triatoma dimidiata (Latreille, 1811) is the primary vector of Trypanosoma cruzi, the parasite that causes Chagas disease. The vector population presents diverse forms and colorations. Therefore, this study was designed to determine the morphotypes of T. dimidiata based on the taxonomy of the body and external genitalia. Between March 2023 and April 2025, 902 triatomines from 15 municipalities were examined. Three main morphotypes were characterized (I to III). Morphotype II was the most abundant (62.86%) and most distributed in the study area (12 of 15 municipalities), with a notable presence in forests and caves. Morphotypes I and III were found primarily outside houses and in chicken coops. Within the characterized specimens of T. dimidiata sensu lato, morphotype II displays more prominent morphological and structural characteristics. They are smaller compared to morphotypes I and III. In morphotype II, the spiracles are covered by a black spot that extends from the connexival plate to the urosternites. Males had short and robust parameres. The median process of the pygophore is long and slender compared to morphotypes I and III. The female tergite VIII has six sides. The taxonomy should be complemented by a study of the life cycle of each morphotype and analysis of its genome.
1. Introduction
In Central America, Triatoma dimidiata sensu lato (Latreille, 1811) (Hemiptera, Reduviidae) is one of the main blood-sucking insects that spreads Trypanosoma cruzi, which causes Chagas disease. The historical burden of disease is estimated to be that of over 7 million individuals worldwide, primarily in Latin America, resulting in over 10,000 fatalities annually [1]. Triatoma dimidiata is responsible for around 13% of T. cruzi infections in Mexico [2]. Its vectorial competence is significant because it can harbor and transmit the six discrete typing units of the hemoflagellate parasite (TCI-TCVI) [3]. Although it feeds mainly on humans, it can also feed on wild and synanthropic animals [3]. The hematophagous habit of males and females of T. dimidiata is crucial for the dispersal of the parasite.
The genus Triatoma is the most diverse of the Triatomini tribe (Hemiptera: Reduviidae). It currently includes 81 of the 158 described species of the Triatominae subfamily [4,5]. However, for some triatomines, their identity has not been defined, and they are part of a complex [6]. The morphology and evolutionary history of T. dimidiata are intensely debatable, and a neotype with Peruvian origins was designated [7].
In the early studies, the T. dimidiata complex was divided into four lineages, which include one species sensu stricto (s. str.) and two affinis (aff.) lineages [8,9,10]. Groups 1 and 2 include Triatoma dimidiata s. str. Thereafter, morphological, morphometric, and molecular (ITS-2 and cytB) analyses of the Triatoma complex were used to describe Triatoma mopan Dorn, Justi, and Dale, 2018 (at first named Triatoma sp. aff. dimidiata cave in group 4) and Triatoma huehuetenanguensis Lima-Cordón and Justi, sp. n. 2019 (at first named Triatoma sp. aff. dimidiata in group 3) [11,12].
Triatoma nitida Usinger, 1939, and T. dimidiata s.l. are species that have been found in Yucatan [13]. It is thought that T. huehuetenanguensis is also there, but its morphology has not been confirmed [8,9,12,14]. Triatoma dimidiata has been documented in 96 of the 106 municipalities of Yucatán [15] and is considered the main vector of T. cruzi in the state. This hematophagous insect has morphological, structural, and behavioral variations. Thus, it is critical to identify the most common morphotype in the area, as some may have better vectorial competence [2]. Pech-May and colleagues [2] described three haplogroups of T. dimidiata (Hg1–Hg3) in Mexico, but only Hg1 and Hg2 occur in Yucatan. Another study performed in Yucatán found that the T. dimidiata population is divided into two genetic groups (ITS-2) and a hybrid [16]. Unfortunately, molecular methods are not always available due to the high cost of equipment, reagents, and personnel trained to routinely perform species identification. Thus, it is critical to have taxonomic descriptions of T. dimidiata morphotypes to determine the most common one. We examined external genitalia to complement the distinction between morphotypes. Previous studies have highlighted their taxonomic value in confirming triatomine identity [17,18,19,20,21,22].
2. Materials and Methods
2.1. Triatomine Collection
This entomological study was conducted in Yucatan, in southeastern Mexico. Field work was carried out from 3 March 2023, to 5 April 2025. Adult triatomines were captured in houses in the rural community of Hubilá (20°55′26″ N 89°20′04″ W), Tixkokob, Yucatán, Mexico. Triatomines were collected through active searching in the home environment (inside, outside, and peridomicile) in approximately 45 visits to the community. The specimens were transported alive to the Laboratorio de Biología Celular at the Universidad Autónoma de Yucatán (UADY), Mexico. Additionally, inhabitants from various municipalities in Yucatan brought triatomine insects to the laboratory to confirm the identity of T. dimidiata and the infection status with parasites. These triatomines were included in the present study. Triatomines were grouped according to the site (niche) where they were collected. The project was reviewed and approved by the Research Advisory Committee and the Research Ethics Committee of the Centro de Investigaciones Regionales “Dr. Hideyo Noguchi”, UADY, and was registered with the number CIRB-2021-0008.
2.2. Morphotypes of T. dimidiata
Specimens were sexed as male and female and identified under a stereoscope (Leica S9i model, Leica Microsystems, Cd. Mex., Mexico), with the help of dichotomous taxonomic keys. We identified the species based on the original description by Lent and Wygodzinsky [13] and the neotype by that of Justi and Dale [7]. In this work, specimens of T. dimidiata were differentiated by their phenotype and called morphotypes I, II, and III. Morphotype I was designated by the original description and neotype [7,13]. We designated morphotype III following the partial description of the work of Sandoval-Ruiz and collaborators [23]. We added the description of morphotype II (Figure 1C,D).
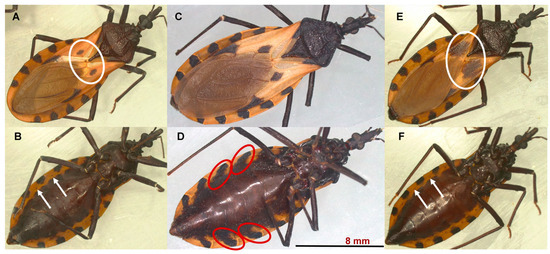
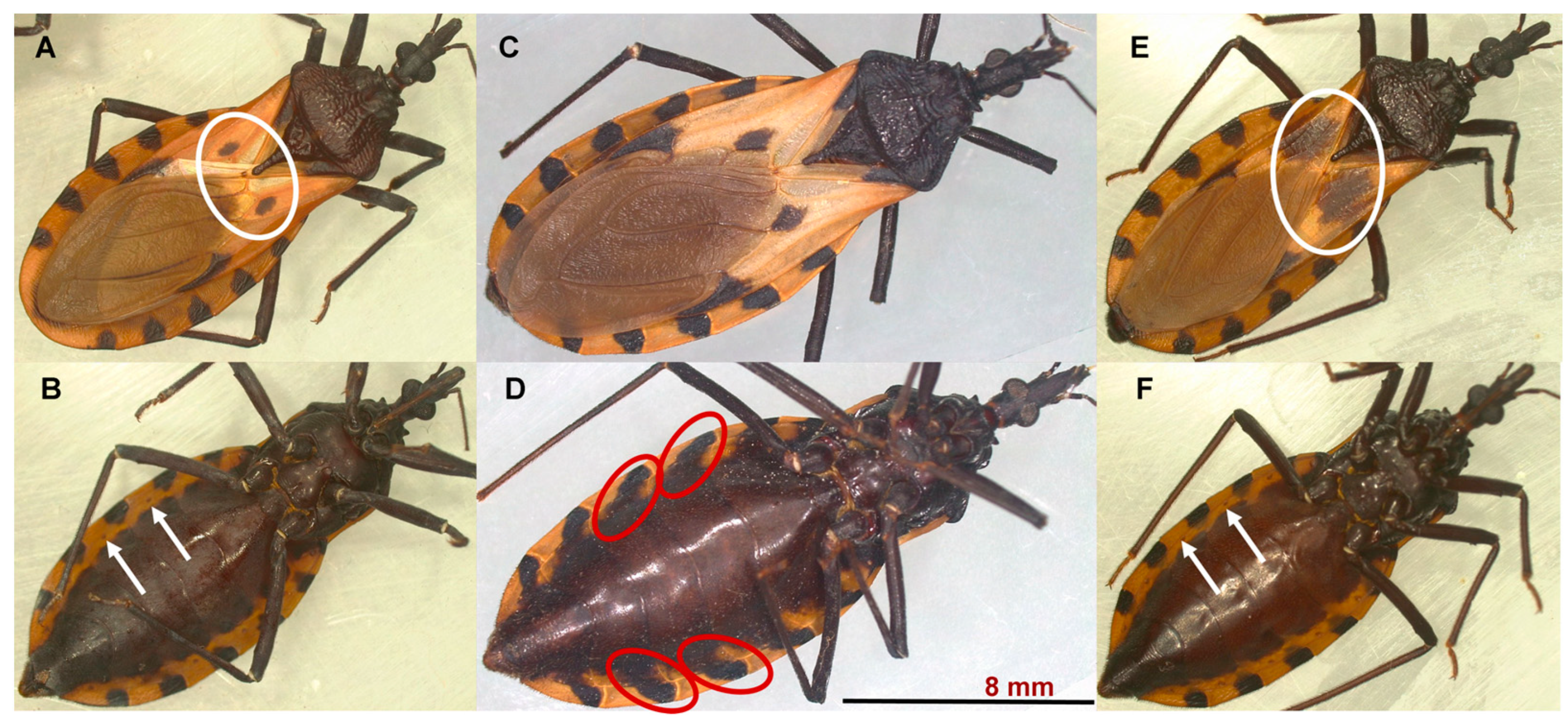
Figure 1.
Females of the morphotypes I (A,B), II (C,D), and III (E,F) of T. dimidiata. The corium of morphotype I (A) has a small central dark spot (white oval); in morphotype III (E), the central dark spot is very extensive, forming an almost complete transverse band across the hemelytron (white oval). In morphotype I and III specimens, each segment of the abdomen has a black spot that is limited to the connective suture and connexivum, and it does not extend to the abdominal sternites. This arrangement allows the spiracles to be observed (white arrows in (B,F)) in ventral view. In morphotype II (D), each abdominal segment has a black spot that extends from the connective suture and the connexivum to the sternites of the abdomen (red oval). This characteristic makes the spiracles difficult to observe in a ventral view.
2.3. Morphometric Parameters of the T. dimidiata Morphotypes
In 17 specimens of each morphotype, 16 metric characters were measured [12] using a stereoscope (Leica S9i) equipped with a graduated eyepiece micrometer and a coupled camera.
2.4. External Genitalia of the T. dimidiata Morphotypes
The segments VIII to X were sliced open on microscope slides using a drop of 65% saline solution.
The pygophore and the parameres are the external parts of the male genitalia that were measured and described. The pygophore is the genital capsule that holds the internal genitalia. The parameres are the club-shaped appendages that hold the female terminalia in place during copulation.
The gonocoxites and the gonapophysis are the external parts of the female genitalia that were measured and described. The gonocoxites are the ventral sclerites that provide structural support to the gonapophyses. The gonapophyses are the ventral sclerites that frame the orifice of the vagina. Genitalia measurements are given in millimeters, and the names of morphological structures were taken from Chiang and Chiang [19] and Tellez-Garcia and collaborators [20].
2.5. Data Analysis
All statistical analyses were conducted in R (version 4.2.2) using RStudio (version 2024.12.0). Statistical significance was set at p ≤ 0.05. A linear discriminant analysis based on different body measurements was performed to classify three T. dimidiata morphotypes. The analysis estimated the prior probabilities of groups, the centroids of each group (group means), and Fisher’s discriminant functions using the respective coefficients. The genital measurements of male and female T. dimidiata were assessed for normality and variance homogeneity using the Shapiro–Wilk and Levene tests, respectively. Data that followed normal distribution were analyzed using one-way ANOVA, while data that did not meet this requirement were analyzed using the Kruskal–Wallis test. When a statistically significant difference was found, Holm’s post hoc test was used to identify which morphotypes had differences in genital measurements. The raw data from the statistical analysis can be found in supplementary materials (https://www.mdpi.com/article/10.3390/taxonomy5040061/s1).
3. Results
3.1. Abundance and Distribution
In total, 902 triatomines collected in 15 municipalities in Yucatán were examined, and three morphotypes were characterized. Triatomines of morphotype II were the most abundant in the study, representing 62.86% (567/902). More females (n = 368) were collected from this morphotype compared to males (n = 199). The remaining collection was represented by 20.06% (181/902) of morphotype I and 17.07% (154/902) of morphotype III. Similar sex frequencies were observed in the triatomines of morphotype I (85 females vs. 96 males) and III (76 females vs. 78 males). Triatomines of morphotype II were found in 12 of 15 municipalities, morphotype I in 8 municipalities, while morphotype III was only collected in Tixkokob and Mérida (Table 1).

Table 1.
Abundance of Triatoma dimidiata by municipality in the state of Yucatan between March 2023 and April 2025.
Specimens of morphotype II were found in all niches, although 85.36% (484/567) were found principally outside homes, in the forest, and in caves. Triatomines of morphotypes I and III were found mainly outside dwellings and in poultry coops (Table 2).

Table 2.
Number of triatomines collected per niche between March 2023 and April 2025.
3.2. Body Measurements and Morphology per Morphotypes
Morphotypes I, II, and III were represented by the average body lengths of 26.19 ± 1.44 mm, 25.77 ± 1.76 mm, and 26.78 ± 1.10 mm, respectively (Table 3). The model estimated two discriminant functions to classify T. dimidiata morphotypes; the first discrimination ratio was 0.77 (LD1), and the second was 0.23 (LD2). The model estimated an error of 7.84% when classifying specimens by morphotype. The discriminant model indicated that the length and width of the pronotum (LOP/WOP) and the length of the antennal segments 2 and 5 are distinctive characteristics of morphotype I. In morphotype II, the length of the ante- and post-ocular region (AOR/POR), the antennal segment 3, and the labial segment 3 are distinctive characteristics that separate it from the other morphotypes. Finally, the total length of the body (TL), labial segments 2 and 3, and the length of antennal segment 4 are distinctive characteristics of morphotype III (Appendix: Table A1 and Figure A1).

Table 3.
Average length of morphological characters of Triatoma dimidiata.
Morphotype I and III specimens are similar in coloration; the body has a light black background, and the connexivum and corium are pale yellow to orange-yellow (Figure 1A,B,E,F). However, the corium of morphotype III triatomines has a very extensive central dark spot, forming an almost complete transverse band across the hemelytron (see the white oval in Figure 1E). The body of morphotype II specimens has a deep black background color (Figure 1C,D); the connexivum and corium are pale yellow. The spiracles are located near the connexival suture. Each abdominal segment has a black spot that extends from the connective suture to the sternites of the abdomen. This characteristic makes the spiracles difficult to observe (see the red oval in Figure 1D).
3.3. External Genitalia per Morphotypes
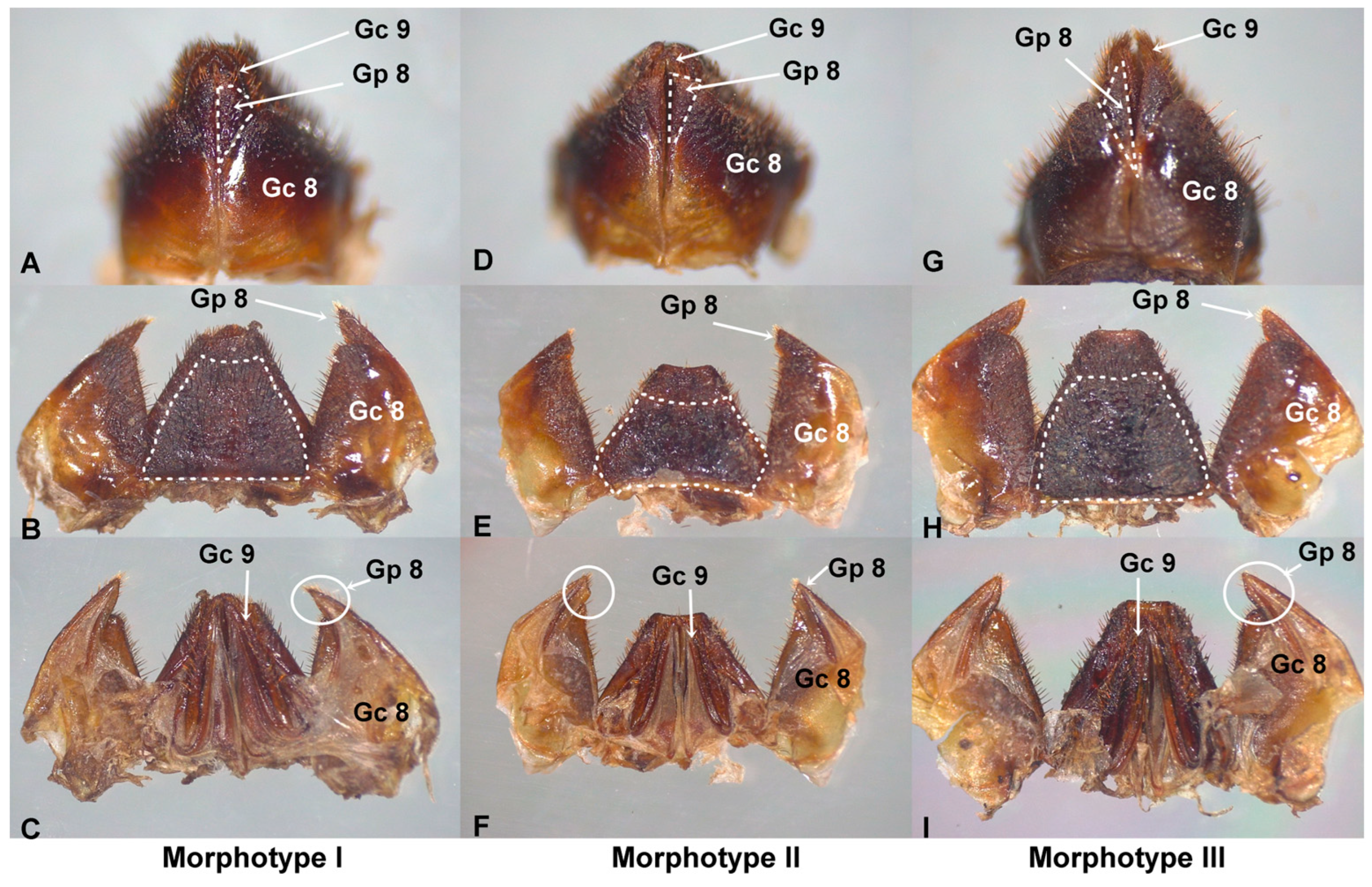
The female genitals of morphotype II are smaller compared to those of I and III, and the difference in lengths was statistically significant (Table 4). In the dorsal view of morphotype II, the base of tergite VIII and the distal part of tergite IX are curved (Figure 2E), while in morphotypes I (Figure 2B) and III (Figure 2H), they are straight. In morphotype II, tergite VIII has six sides (2E), while in morphotypes I and II, it has four sides (Figure 2B,H).

Table 4.
Measurements of the external genitalia of Triatoma dimidiata females (mean ± standard deviation value).
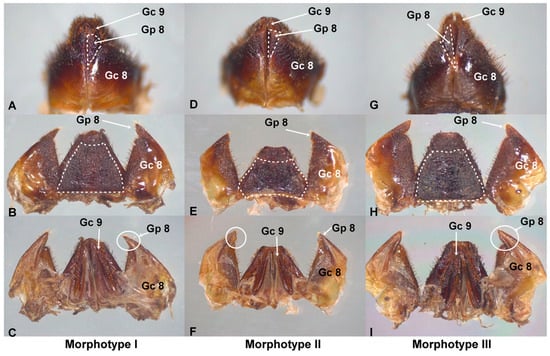
Figure 2.
External female genitalia of T. dimidiata. In morphotype I (A), gonapophysis VIII (Gp8) is cone-shaped and extends beyond gonocoxite VIII (Gc8); gonapophysis VIII (Gp8) ends in a point (B,C). In morphotype II, Gp8 is an inverted isosceles triangle and ends at the level of Gc8 (D); gonapophysis VIII (Gp8) ends in a straight plane (E,F). In morphotype III, Gp8 is very long, shaped like a scalene triangle (G), and extends beyond Gc8; gonapophysis VIII (Gp8) ends in a blunt point (I). Tergite VIII has 6 sides in morphotype II (E) and 4 sides in I and III (B,H). Scale bar: 2 mm.
In the ventral view of morphotype I, gonapophysis VIII (Gp8) is cone-shaped and larger than gonocoxite VIII (Gc8) (Figure 2A). In dorsal (Figure 2B) and ventral (Figure 2C) views, the distal part of Gp8 ends in a point. In the ventral view of morphotype II, Gp8 is shaped like an inverted isosceles triangle (Figure 2D) and ends at the level of Gc8. In dorsal (Figure 2E) and ventral (Figure 2F) views, Gp8 ends in a straight plane. In the ventral view of morphotype III, Gp8 is very long and has the shape of a scalene triangle (Figure 2G). Its size exceeds Gc8. Gonapophysis VIII (Gp8) ends in a blunt point (Figure 2H,I). Gonocoxyte IX (Gc9) is well developed and expands beyond the abdomen.
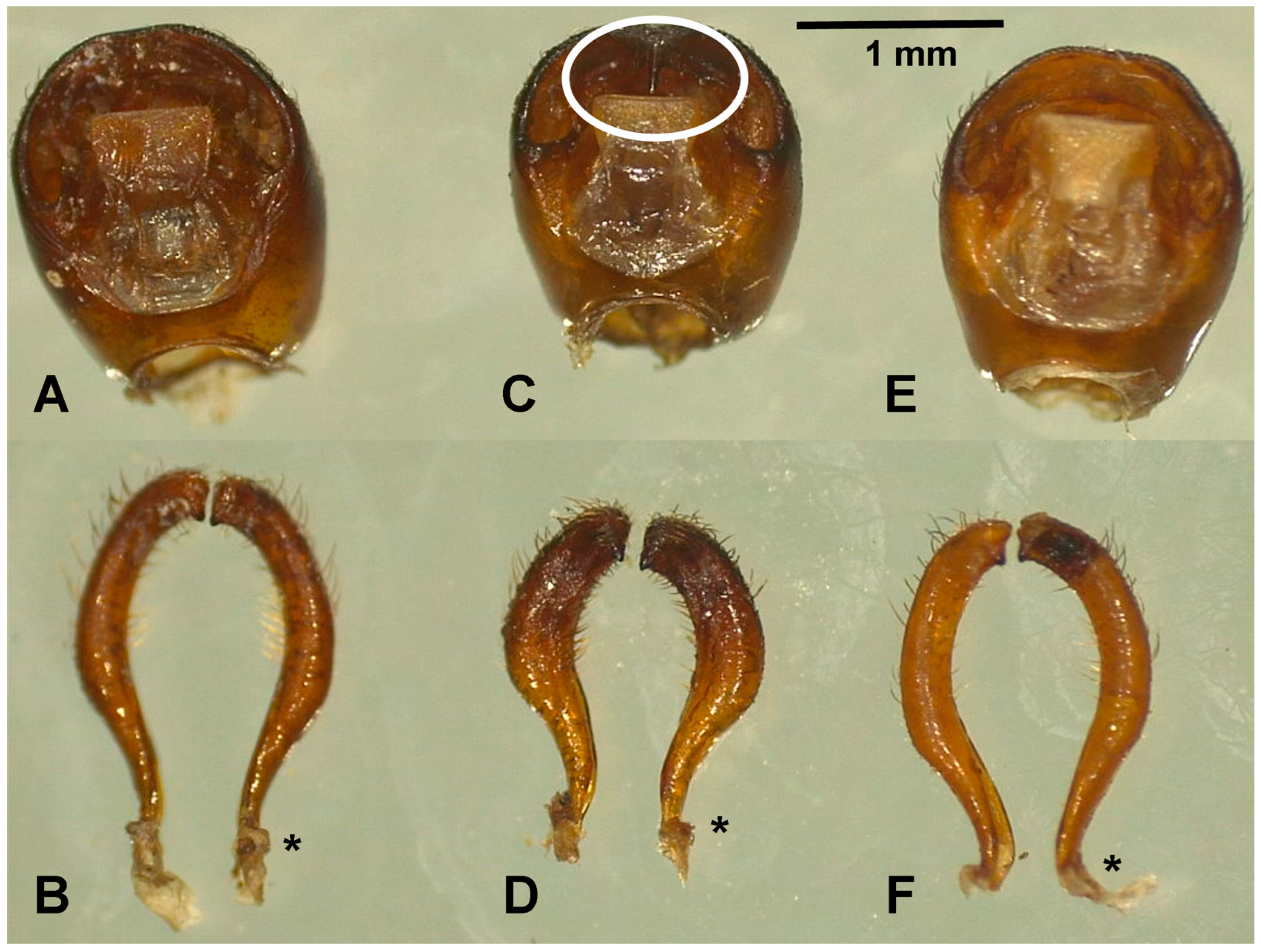
A statistically significant difference was found in the mean length and width of the external genitalia of males among the different morphotypes (Table 5). The pygophore and paramere of morphotype II were shorter in length and width compared to morphotypes I and III, and this difference was significant (p = 0.001). Notably, the median process of the pygophore (Figure 3C) is longer and thinner in morphotype II than in the other morphotypes. Furthermore, the parameres are shorter and more robust in morphotype II (Figure 3D).

Table 5.
Measurements of the external genitalia of Triatoma dimidiata males (mean ± standard deviation value).
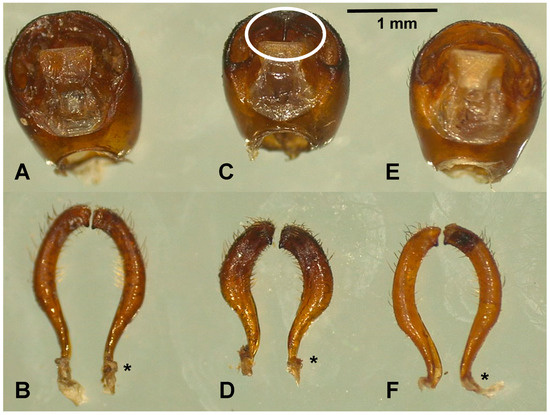
Figure 3.
External male genitalia of T. dimidiata. Pygophore of morphotypes I (A), II (C), and III (E). Dorsal view of the left and right of morphotypes I (B), II (D), and III (F) parameres. The asterisks indicate the sites where the parameres articulate with the pygophore. The median process of the pygophore is long and thin in morphotype II (white circle, Figure 3C).
4. Discussion
In this work, we document morphotype II of T. dimidiata as the most abundant, exhibiting very distinctive morphological and structural characteristics. The study’s shortcomings include the lack of sample size estimations for triatomine collection and the absence of similar sampling efforts in each niche and municipality throughout Yucatán. The true abundance and distribution patterns of the morphotypes in Yucatán cannot be estimated due to these limitations. We used two methodologies to obtain triatomines. The greatest collection effort was made in the municipality of Tixkokob, while specimens from other municipalities in Yucatán were donated. However, using both methodologies, morphotype II was the most abundant and widely distributed. This discovery opens up ecological, entomological, and epidemiological perspectives because previous studies carried out in Yucatán considered T. dimidiata as a species sensu lato that inhabits different ecotopes [16,24,25,26,27,28,29,30,31].
Triatoma dimidiata was recently documented to occur in 88.07% of Yucatán municipalities [15]. Pending systematic and broader-scope studies, morphotype II is likely to be more widespread in the state. In the present study, we characterized it in 12 of Yucatán’s 15 municipalities. This finding is also supported by the fact that morphotype II was found in all niches, with a significant presence in the wild (forests and caves). In contrast, morphotypes I and III were found in chicken coops and outside homes. Peridomiciliary triatomines also contribute to the infestation of homes, although, based on the results of the present study, we hypothesize that morphotype II from the wild areas contributes significantly to the reinfestation of human settlements. Gene flow between triatomines from wild and domestic ecotopes has been documented [16]. Previous studies have suggested that the invasion of wild triatomines re-infests homes and limits their eradication [24,27,28,29,30]. This behavior influences entomological and epidemiological indices. In the late 1970s, an entomological study was conducted in 116 homes in seven municipalities of Yucatán, and high rates of dispersion (95%) and infestation (65%) were estimated [31]. In contrast, the colonization rate was only 25%. Other entomological studies conducted in rural communities in Yucatán also reported low colonization rates, ranging from 14% to 27.7% [24,32].
In a transmission dynamics study, it was determined that T. dimidiata from domestic habitats had a higher prevalence of infection compared to triatomines from jungle origin. The authors propose that anthropogenic disturbances are associated with a higher risk of T. cruzi transmission [26]. Likewise, Rebollar-Tellez and collaborators [25] reported a prevalence of infection of 3.7% in the wild population of T. dimidiata with T. cruzi. Other studies have reached similar conclusions, stating that animal shelters (chicken coops, dog houses, and animal and bird nests) in the peridomiciliary area are significantly associated with the occurrence of T. dimidiata and triatomines infected with T. cruzi [24,28]. This statement is supported because T. dimidiata feeds primarily on humans, dogs, and cows, which are hosts that inhabit domestic environments [27]. We identified morphotypes I and III, mainly in domestic environments (outside houses and chicken coops), so their vectorial competence to transmit the causal agent of Chagas disease must be evaluated. Conversely, T. dimidiata is divided into three haplogroups on a genetic basis. The maximum rate of infection with T. cruzi is observed in haplogroups 1 and 3 [2]. It is imperative to provide a comprehensive morphological description of the haplogroups to identify them without the use of molecular methods.
The identity of T. dimidiata in the state of Yucatán has been a taxonomic problem. Early studies referred to hybrid populations and later to haplogroups [2,16]. Regardless of the approach, evidence points to morphological and genetic differences. For example, the antennal phenotype indicates differences in the abundance of each sensillum type among haplogroups [33]. Likewise, the morphometric study of shapes (elliptic Fourier descriptors) also classified and validated the three haplogroups of T. dimidiata [34]. Taxonomic complexity has led to the suggestion that T. huehuetenanguensis is present in the state [8,9,12,14]. However, there is no morphological evidence to support this claim. We reviewed 902 triatomines and characterized three main morphotypes. Therefore, we believe that T. huehuetenanguensis might be a genetic misidentification.
Body morphometry and genitalia showed statistically significant differences between morphotypes. Notably, specimens of morphotype II present a combination of structural characteristics necessary to be considered a species. For example, in both sexes, the spiracles are covered by a black spot that extends from the connexival plate to the urosternites. The genitalia of the female morphotype II also showed significant differences from the other morphotypes, one of which was the presence of six sides to the tergite VIII. This structure has not been documented in the T. dimidiata complex. Triatoma jatai has six sides to the tergite VIII, but it is wider than it is long [21]. The gonapophysis VIII of Gp8 is like that of T. huehuetenanguensis; both are more long than wide and end at the same level as gonocoxyte VIII Gc 8. One difference between them is that the base of tergite VIII in T. huehuetenanguensis is slightly concave [12]. The external morphology of the genitalia has taxonomic value for species identification and is increasingly used. Dos Rodrigues and collaborators [22] described the external genitalia of 26 triatomine species. They mention that tergite IX is the structure of greatest taxonomic value for species distinction, although for us, it was the combination of morphological characteristics and measurements.
In males of morphotype II, the parameres are short and robust. The median process of the pygophore is visible, presenting a long and slender structure compared to morphotypes I and III. The shape and length of the median process of the pygophore has been used as a taxonomic characteristic to separate the species of Rhodnius nasutus, Rhodnius neglectus, Rhodnius prolixus, and Rhodnius robustus [35]. It has also been used to separate Belminus rugulosus and Belminus correri [36].
5. Perspective
Males and females of morphotype II have morphological and structural characteristics that might represent an independent species, and taxonomic modifications within the group are a possibility. Furthermore, they primarily inhabit wild areas. This assertion must be confirmed by genetic analysis. While NAD4 and ITS2 indicate a genetic difference in T. dimidiata, future studies should sequence more genes, thus strengthening the conclusion. Furthermore, this claim should be complemented by a study of the life cycle of each morphotype and crossbreeding to determine the offspring. Finally, it is essential to determine the infection rate of T. cruzi in T. dimidiata morphotypes captured in the field and perform experimental infection studies to assess how these morphotypes’ vector competence relates to the DTUs of T. cruzi present in the region to identify which morphotype is most efficient at transmitting the parasite.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/taxonomy5040061/s1, Table S1: Body and genitalia measurements.
Author Contributions
Conceptualization, N.C.-T. and C.M.B.-B.; methodology, W.A.C.-C. and F.Á.-L.; validation, J.I.C.-P.; formal analysis, I.M.-C.; investigation, A.P.-M.; data curation, J.C.T.-D. and I.Y.C.-M.; writing—original draft preparation, J.E.G.-R. and N.C.-T.; writing—review and editing, K.Y.A.-V.; supervision, C.M.B.-B.; project administration, N.C.-T.; funding acquisition, C.M.B.-B. All authors have read and agreed to the published version of the manuscript.
Funding
The investigation is part of the project: “Training children in entomology and inventorying the local entomofauna of Yucatan: with an emphasis on medical entomology” CONAHCYT Grant: CF-2023-I-678, and SISTPROY Registration: CIRB-2023-0015.
Data Availability Statement
Raw data on body and genitalia measurements of the Triatoma dimidiata morphotypes are available as supplementary material.
Acknowledgments
We thank the inhabitants of the Hubilá community for allowing us access to their homes and the inhabitants of various municipalities in Yucatán who were kind enough to bring the triatomines to the Cell Biology Laboratory. We also thank biologist Andrea N. Zapata Pantoja for participating in the collection and measurement of the triatomines.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Coefficients of discriminant functions.
Table A1.
Coefficients of discriminant functions.
| Variable | LD1 | LD2 |
|---|---|---|
| TL | 0.0746642 | −0.248155 |
| LOP/WOP | −1.052806 | −0.889712 |
| AOR/POR | 3.2371953 | 0.9324774 |
| SYN | 10.852682 | 13.463025 |
| HL/WOH | −3.268681 | 0.0726561 |
| WE | 5.371945 | 10.239435 |
| TS | −0.85398 | 2.6913045 |
| POS | 1.8417671 | −4.239118 |
| ANTENNAl.SEG.1 | 3.5317678 | 4.461134 |
| ANTENNAl.SEG.2 | −2.902931 | −3.888127 |
| ANTENNAl.SEG.3 | 1.5002307 | 0.197906 |
| ANTENNAl.SEG.4 | 1.0978322 | 1.872826 |
| ANTENNAl.SEG.5 | −0.849266 | −1.495951 |
| S..LABIAL.1 | 3.153749 | −1.397268 |
| S.LABIAL.2 | −3.035265 | 3.3296145 |
| S.LABIAL.3 | −14.96195 | 1.5103849 |
Coefficients in bold contributed significantly to the classification of morphotypes.
Appendix B
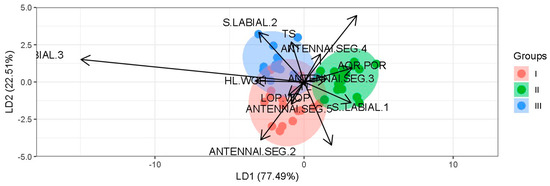
Figure A1.
The classification of T. dimidiata morphotypes is based on discriminant functions.
Figure A1.
The classification of T. dimidiata morphotypes is based on discriminant functions.
References
- World Health Organization (WHO). Chagas disease (American trypanosomiasis). Available online: https://www.who.int/health-topics/chagas-disease#tab=tab_1 (accessed on 15 September 2025).
- Pech-May, A.; Mazariegos-Hidalgo, C.J.; Izeta-Alberdi, A.; López-Cancino, S.A.; Tun-Ku, E.; Cruz-Félix, K.D.L.; Ibarra-Cerdeña, C.N.; Ittig, R.E.G.; Ramsey, J.M. Genetic variation and phylogeography of the Triatoma dimidiata complex evidence a potential center of origin and recent divergence of haplogroups having differential Trypanosoma cruzi and DTU infections. PLoS. Negl. Trop. Dis. 2019, 13, e0007044. [Google Scholar] [CrossRef]
- Becker, I.; Miranda-Ortiz, H.; Fernández-Figueroa, E.A.; Sánchez-Montes, S.; Colunga-Salas, P.; Grostieta, E.; Juárez-Gabriel, J.; Lozano-Sardaneta, Y.N.; Arce-Fonseca, M.; Rodríguez-Morales, O.; et al. The Low Variability of Tc24 in Trypanosoma cruzi TcI as an Advantage for Chagas Disease Prophylaxis and Diagnosis in Mexico. Pathogens 2023, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Alevi, K.; Oliveira, D.J.; Rocha, D.D.S.; Galvão, C. Trends in Taxonomy of Chagas Disease Vectors (Hemiptera, Reduviidae, Triatominae): From Linnaean to Integrative Taxonomy. Pathogens 2021, 10, 1627. [Google Scholar] [CrossRef]
- Téllez-Rendón, J.; Esteban, L.; Rengifo-Correa, L.; Díaz-Albiter, H.; Huerta, H.; Dale, C. Triatoma yelapensis sp. nov. (Hemiptera: Reduviidae) from Mexico, with a Key of Triatoma Species Recorded in Mexico. Insects 2023, 14, 331. [Google Scholar] [CrossRef] [PubMed]
- Carcavallo, R.U.; Jurberg, J.; Lent, H.; Noireau, F.; Galvão, C. Phylogeny of the Triatominae (Hemiptera: Reduviidae). Proposals for taxonomic arrangements. Entomol. Vect. 2000, 7, 1–99. [Google Scholar]
- Justi, S.A.; Dale, C. Designation of the neotype of Triatoma dimidiata (Latreille, 1811) (Hemiptera, Reduviidae, Triatominae), with full integrated redescription including mitogenome and nuclear ITS-2 sequences. Zookeys 2021, 1076, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Bargues, M.D.; Klisiowicz, D.R.; Gonzalez-Candelas, F.; Ramsey, J.M.; Monroy, C.; Ponce, C.; Salazar-Schettino, P.M.; Panzera, F.; Abad-Franch, F.; Sousa, O.E.; et al. Phylogeography and genetic variation of Triatoma dimidiata, the main Chagas disease vector in Central America, and its position within the genus Triatoma. PLoS. Negl. Trop. Dis. 2008, 2, e233. [Google Scholar] [CrossRef]
- Dorn, P.L.; de la Rúa, N.M.; Axen, H.; Smith, N.; Richards, B.R.; Charabati, J.; Suarez, J.; Woods, A.; Pessoa, R.; Monroy, C.; et al. Hypothesis testing clarifies the systematics of the main Central American Chagas disease vector, Triatoma dimidiata (Latreille, 1811), across its geographic range. Infect. Genet. Evol. 2016, 44, 431–443. [Google Scholar] [CrossRef]
- Justi, S.A.; Cahan, S.; Stevens, L.; Monroy, C.; Lima-Cordón, R.; Dorn, P.L. Vectors of diversity: Genome wide diversity across the geographic range of the Chagas disease vector Triatoma dimidiata sensu lato (Hemiptera: Reduviidae). Mol. Phylogenet. Evol. 2018, 120, 144–150. [Google Scholar] [CrossRef]
- Dorn, P.L.; Justi, S.A.; Dale, C.; Stevens, L.; Galvão, C.; Lima-Cordón, R.; Monroy, C. Description of Triatoma mopan sp. n. from a cave in Belize (Hemiptera, Reduviidae, Triatominae). Zookeys 2018, 775, 69–95. [Google Scholar] [CrossRef]
- Lima-Cordón, R.A.; Monroy, M.C.; Stevens, L.; Rodas, A.; Rodas, G.A.; Dorn, P.L.; Justi, S.A. Description of Triatoma huehuetenanguensis sp. n., a potential Chagas disease vector (Hemiptera, Reduviidae, Triatominae). Zookeys 2019, 820, 51–70. [Google Scholar] [CrossRef]
- Lent, H.; Wygodzinsky, P.W. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas’ disease. Bull. Am. Mus. Nat. Hist. 1979, 163, 123–520. [Google Scholar]
- Cigarroa-Toledo, N.; Baak-Baak, C.M.; Chan-Pérez, J.I.; Hernandez-Mena, D.I.; Amaya-Guardia, K.C.; Ocaña-Correa, M.F.; Pech-May, A.; Acosta-Viana, K.Y. Dataset of assembly and annotation of the mitogenomes of Triatoma dimidiata and Triatoma huehuetenanguensis captured from Yucatán, Mexico. Data Br. 2024, 52, 109866. [Google Scholar] [CrossRef]
- Valdez-Tah, A.R.; Romero, D. Geografía de la enfermedad de Chagas en Yucatán: Determinantes socioeconómicos y demográficos a nivel municipal. Investig. Geográficas 2024. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=9536016 (accessed on 13 October 2025). [CrossRef]
- Herrera-Aguilar, M.; Be-Barragán, L.A.; Ramirez-Sierra, M.J.; Tripet, F.; Dorn, P.; Dumonteil, E. Identification of a large hybrid zone between sympatric sibling species of Triatoma dimidiata in the Yucatan peninsula, Mexico, and its epidemiological importance. Infect. Genet. Evol. 2009, 9, 1345–1351. [Google Scholar] [CrossRef]
- da Rosa, J.A.; Mendonça, V.J.; Gardim, S.; de Carvalho, D.B.; de Oliveira, J.; Nascimento, J.D.; Pinotti, H.; Pinto, M.C.; Cilense, M.; Galvão, C. Study of the external female genitalia of 14 Rhodnius species (Hemiptera, Reduviidae, Triatominae) using scanning electron microscopy. Parasit. Vectors 2014, 7, 17. [Google Scholar] [CrossRef]
- da Rosa, J.A.; Mendonça, V.J.; Rocha, C.S.; Gardim, S.; Cilense, M. Characterization of the external female genitalia of six species of Triatominae (Hemiptera: Reduviidade) by scanning electron microscopy. Mem. Inst. Oswaldo Cruz. 2010, 105, 286–292. [Google Scholar] [CrossRef]
- Chiang, R.G.; Chiang, J.A.; Chiang, R.G.; Chiang, J.A. Functional Anatomy of the External and Internal Reproductive Structures in Insect Vectors of Chagas Disease with Particular Reference to Rhodnius prolixus. In Biological Control of Pest and Vector Insects; Shields, V.D.C., Ed.; InTech: Rigeka, Croatia, 2017; pp. 299–324. [Google Scholar]
- Tellez-Garcia, A.A.; Bello-Bedoy, R.; Enríquez-Vara, J.N.; Córdoba-Aguilar, A.; Gutiérrez-Cabrera, A.E. Genital morphology and copulatory behavior in triatomine bugs (Reduviidae: Triatominae). Arthropod Struct. Dev. 2019, 49, 103–118. [Google Scholar] [CrossRef]
- Teves, S.C.; Gonçalves, T.C.M.; de Freitas, S.P.C.; Lopes, C.M.; Carbajal-de-la-Fuente, A.L.; dos Santos-Mallet, J.R. External female genitalia of Triatoma jatai, Triatoma costalimai and Triatoma williami (Hemiptera: Reduviidae: Triatominae). Parasit. Vectors. 2020, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- dos Rodrigues, J.M.S.; da Rosa, J.A.; Moreira, F.F.F.; Galvão, C. Morphology of the terminal abdominal segments in females of Triatominae (Insecta: Hemiptera: Reduviidae). Acta Trop. 2018, 185, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Ruiz, C.A.; Cervantesperedo, L.; Mendoza-Palmero, F.S.; Ibáñez-Bernal, S. The Triatominae (Hemiptera: Heteroptera: Reduviidae) of Veracruz, Mexico: Geographic distribution, taxonomic redescriptions, and a key. Zootaxa 2012, 3487, 1–23. [Google Scholar] [CrossRef]
- Reyes-Novelo, E.; Ruiz-Piña, H.; Escobedo-Ortegón, J.; Barrera-Pérez, M.; Manrique-Saide, P.; Rodríguez-Vivas, R.I. Triatoma dimidiata (Latreille) Abundance and Infection with Trypanosoma cruzi in a Rural Community of Yucatan, Mexico. Neotrop. Entomol. 2013, 42, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Rebollar-Téllez, E.A.; Reyes-Villanueva, F.; Escobedo-Ortegón, J.; Balam-Briceño, P.; May-Concha, I. Abundance and nightly activity behavior of a sylvan population of Triatoma dimidiata (Hemiptera: Reduviidae: Triatominae) from the Yucatan, Mexico. J. Vector Ecol. 2009, 34, 304–310. [Google Scholar] [CrossRef]
- Moo-Millan, J.I.; Hernández-Andrade, A.; May-Concha, I.J.; Montalvo-Balam, T.; Arnal, A.; Talavera-Escalante, M.J.; Amblard-Rambert, A.; Martínez-Vega, P.P.; Ramos-Ligonio, Á.; Ibarra-Cerdeña, C.N. Temporal variation of Triatoma dimidiata abundance and infection with Trypanosoma cruzi in domestic and sylvatic habitats of rural Yucatan, Mexico. Acta Trop. 2023, 248, 107038. [Google Scholar] [CrossRef]
- Moo-Millan, J.I.; Arnal, A.; Pérez-Carrillo, S.; Hernandez-Andrade, A.; Ramírez-Sierra, M.J.; Rosado-Vallado, M.; Dumonteil, E.; Waleckx, E. Disentangling Trypanosoma cruzi transmission cycle dynamics through the identification of blood meal sources of natural populations of Triatoma dimidiata in Yucatán, Mexico. Parasit. Vectors. 2019, 12, 1–11. [Google Scholar] [CrossRef]
- Koyoc-Cardeña, E.; Medina-Barreiro, A.; Escobedo-Ortegón, F.J.; Rodríguez-Buenfil, J.C.; Barrera-Pérez, M.; Reyes-Novelo, E.; Chablé-Santos, J.; Selem-Salas, C.; Vazquez-Prokopec, G.; Manrique-Saide, P.; et al. Chicken coops, Triatoma dimidiata infestation and its infection with Trypanosoma cruzi in a rural village of Yucatan, Mexico. Rev. Inst. Med. Trop. Sao Paulo. 2015, 57, 269. [Google Scholar] [CrossRef]
- Dumonteil, E.; Tripet, F.; Ramirez-Sierra, M.J.; Payet, V.; Lanzaro, G.; Menu, F. Assessment of Triatoma dimidiata dispersal in the Yucatan Peninsula of Mexico by morphometry and microsatellite markers. Am. J. Trop. Med. Hyg. 2007, 76, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rejon, J.E.; Tzuc-Dzul, J.C.; Cetina-Trejo, R.; Madera-Navarrete, M.I.; Cigarroa-Toledo, N.; Chan-Perez, J.I.; Ortega-Pacheco, A.; Torres-Chable, O.; Pietri, J.E.; Baak-Baak, C.M. Identification of parasitic arthropods collected from domestic and wild animals in Yucatan, Mexico. Ann. Parasitol. 2021, 67, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Marín, E.; Barrera-Pérez, M.A.; Rodríguez-Félix, M.E.; Escobedo, F.; Zavala, J.E. Indices entomológicos de Triatoma dimidiata en el estado de Yucatán. Rev. Biomédica. 1991, 2, 20–29. [Google Scholar]
- Dumonteil, E.; Ramirez-Sierra, M.J.; Ferral, J.; Euan-Garcia, M.; Chavez-Nuñez, L. Usefulness of community participation for the fine temporal monitoring of house infestation by non-domiciliated triatomines. J. Parasitol. 2009, 95, 469–471. [Google Scholar] [CrossRef]
- May-Concha, I.; Guerenstein, P.G.; Ramsey, J.M.; Rojas, J.C.; Catalá, S. Antennal phenotype of Mexican haplogroups of the Triatoma dimidiata complex, vectors of Chagas disease. Infect. Genet. Evol. 2016, 40, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Cruz, D.D.; Arellano, E.; Denis-Ávila, D.; Ibarra-Cerdeña, C.N. Identifying Chagas disease vectors using elliptic Fourier descriptors of body contour: A case for the cryptic dimidiata complex. Parasit. Vectors. 2020, 13, 332. [Google Scholar] [CrossRef]
- Harry, M. Use of the median process of the Pygophore in the identification of Rhodnius nasutus, R. Neglectus, R. Prolixus and R. robustus (Hemiptera: Reduviidae). Ann. Trop. Med. Parasitol. 1993, 87, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Gil-Santana, H.R.; Galvão, C. Description of the male genitalia of Belminus rugulosus Stål and Belminus corredori Galvão & Angulo, and comments on the holotype of Parabelminus yurupucu Lent & Wygodzinsky (Hemiptera: Heteroptera: Reduviidae: Triatominae: Bolboderini). Zootaxa 2013, 46, 587–596. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).