Precision Lost with Complexity: On an Extraordinary New Species of Pholcidae (Araneae, Smeringopinae) from Western DR Congo †
Abstract
1. Introduction
2. Materials and Methods
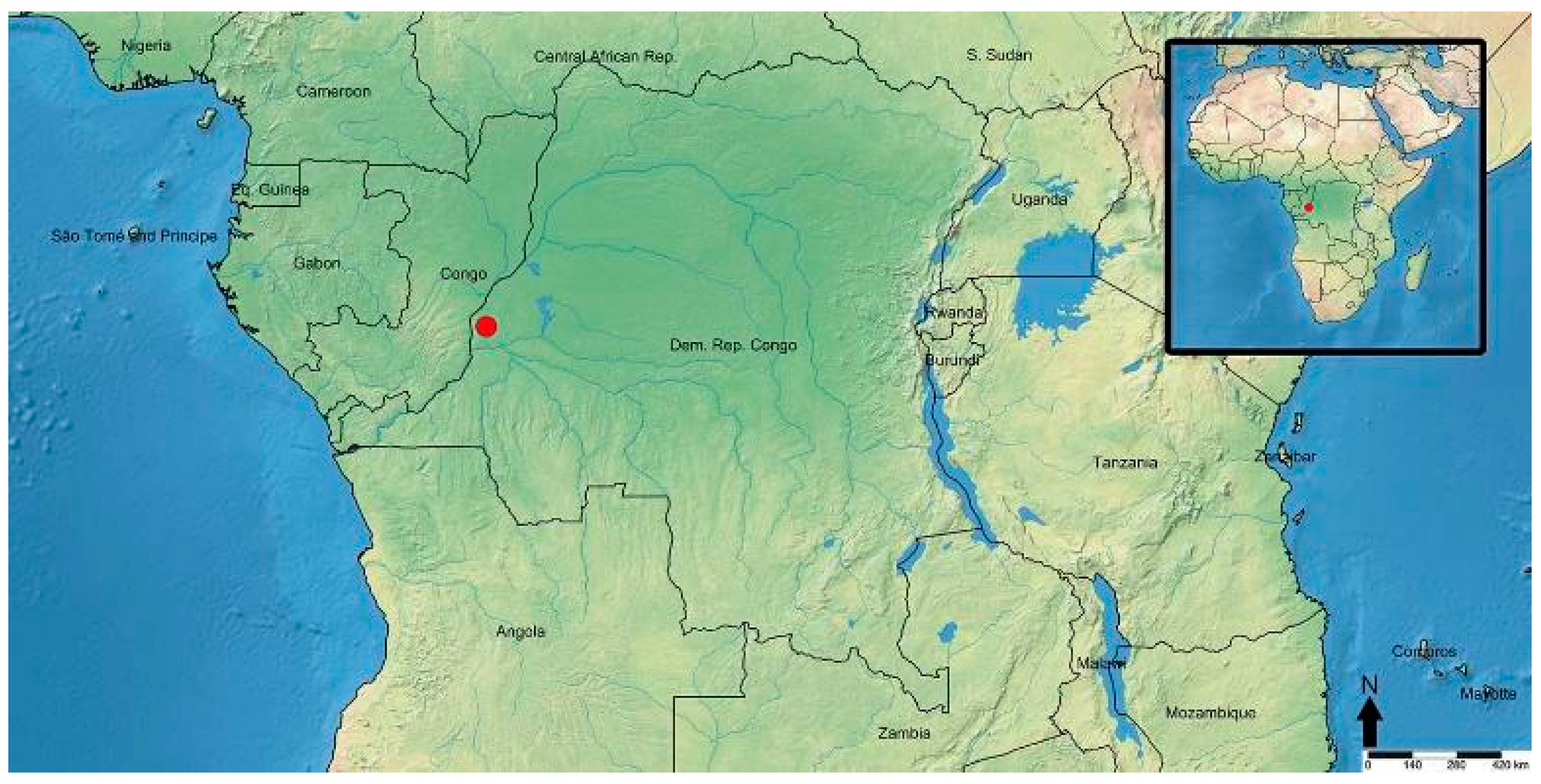
2.1. Collection and Depository
2.2. Description, Imaging and Illustrations
2.3. Abbreviations
2.4. Molecular Analyses
2.4.1. DNA Extraction, Amplification, Sequencing and Curation
2.4.2. Phylogenetic Analyses
3. Results
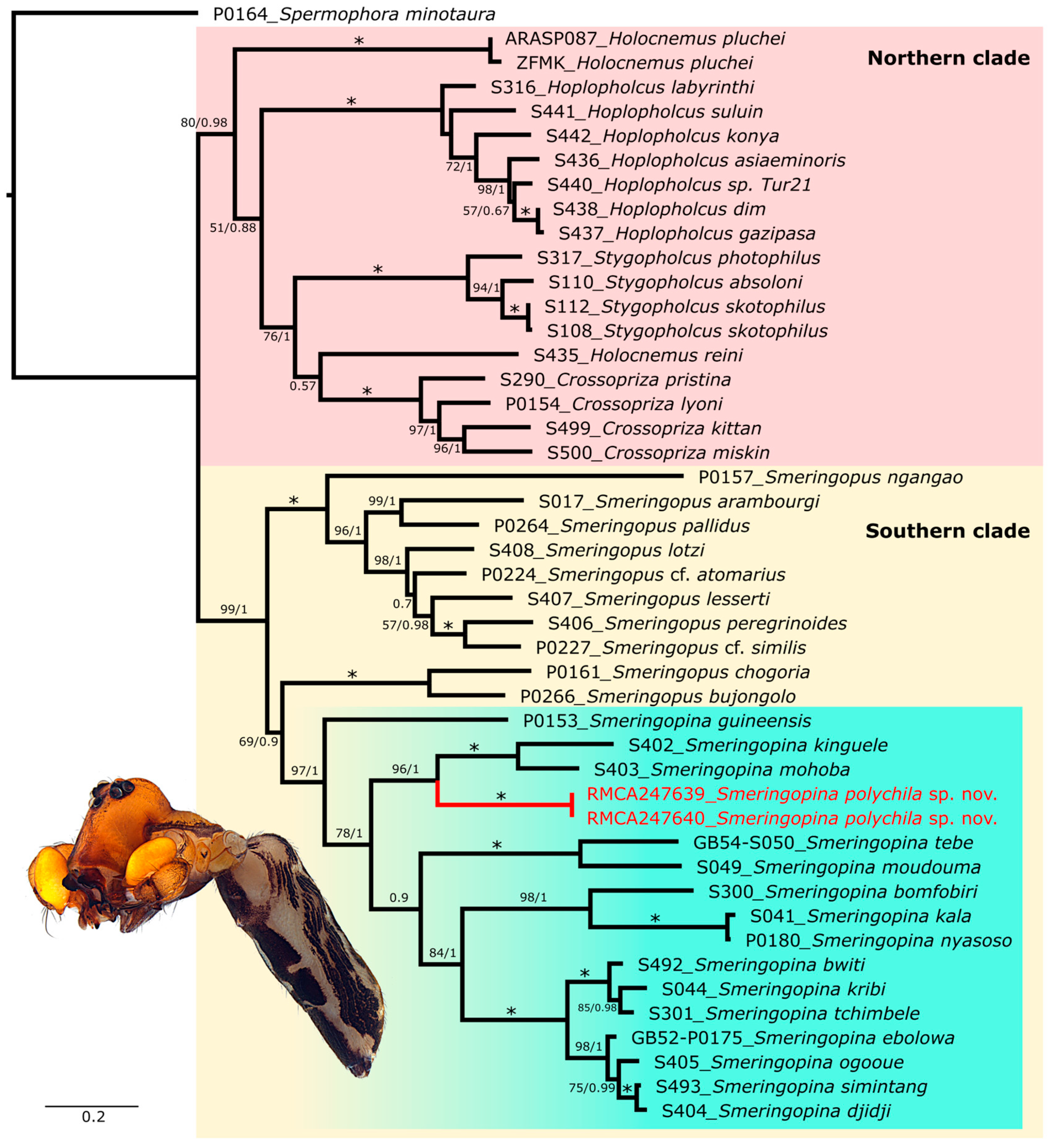
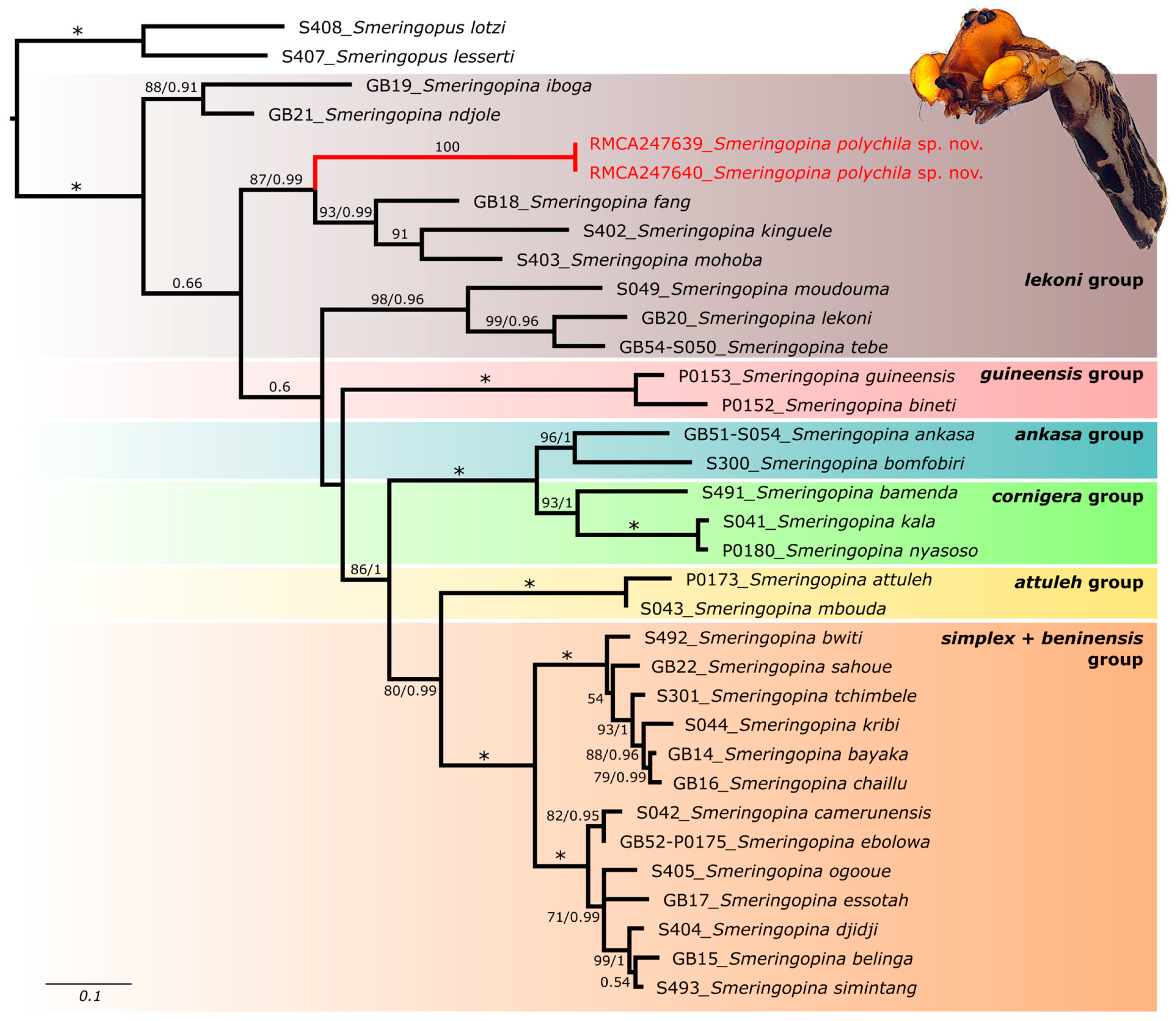
3.1. Molecular Identification and Phylogeny
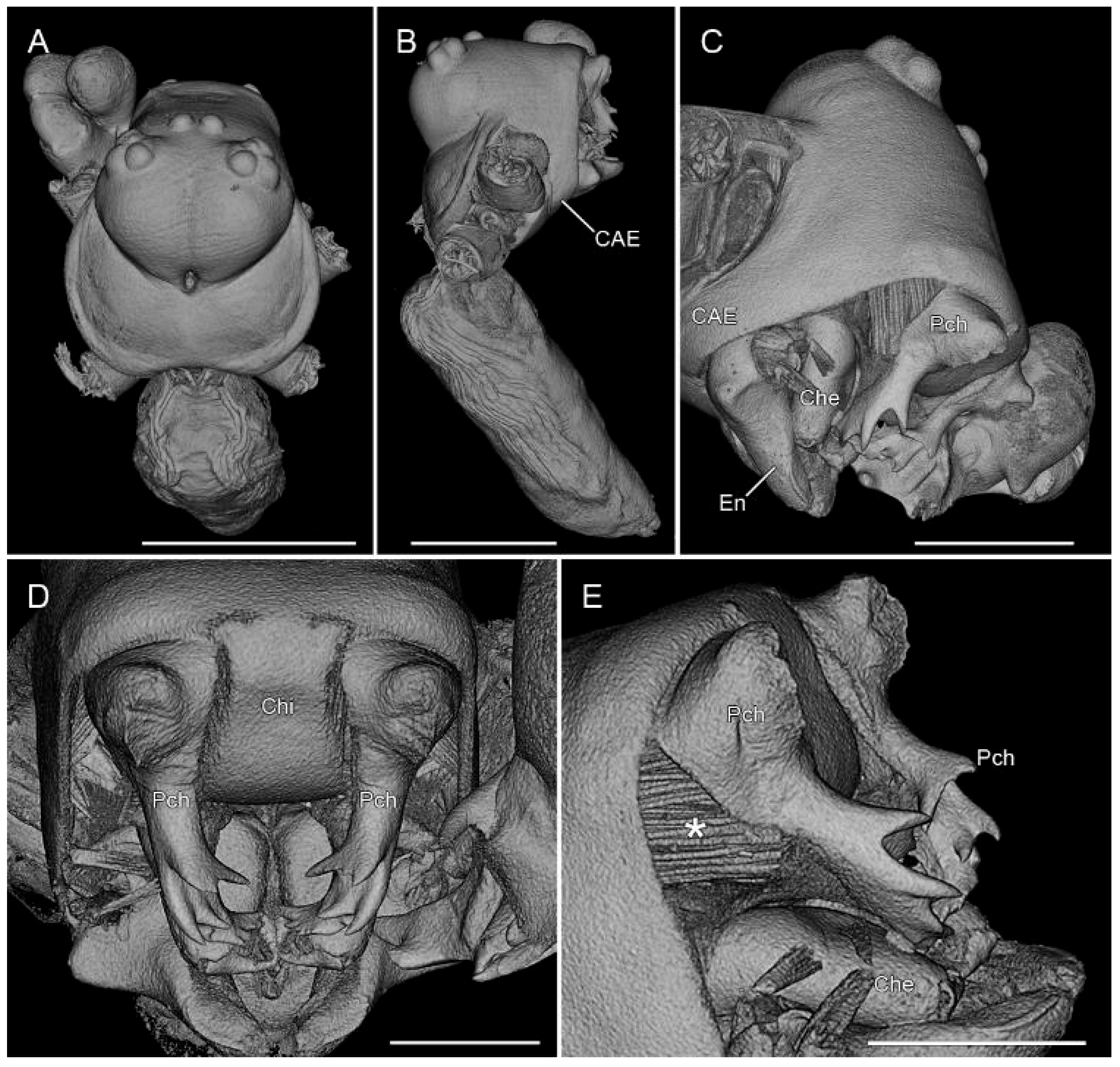
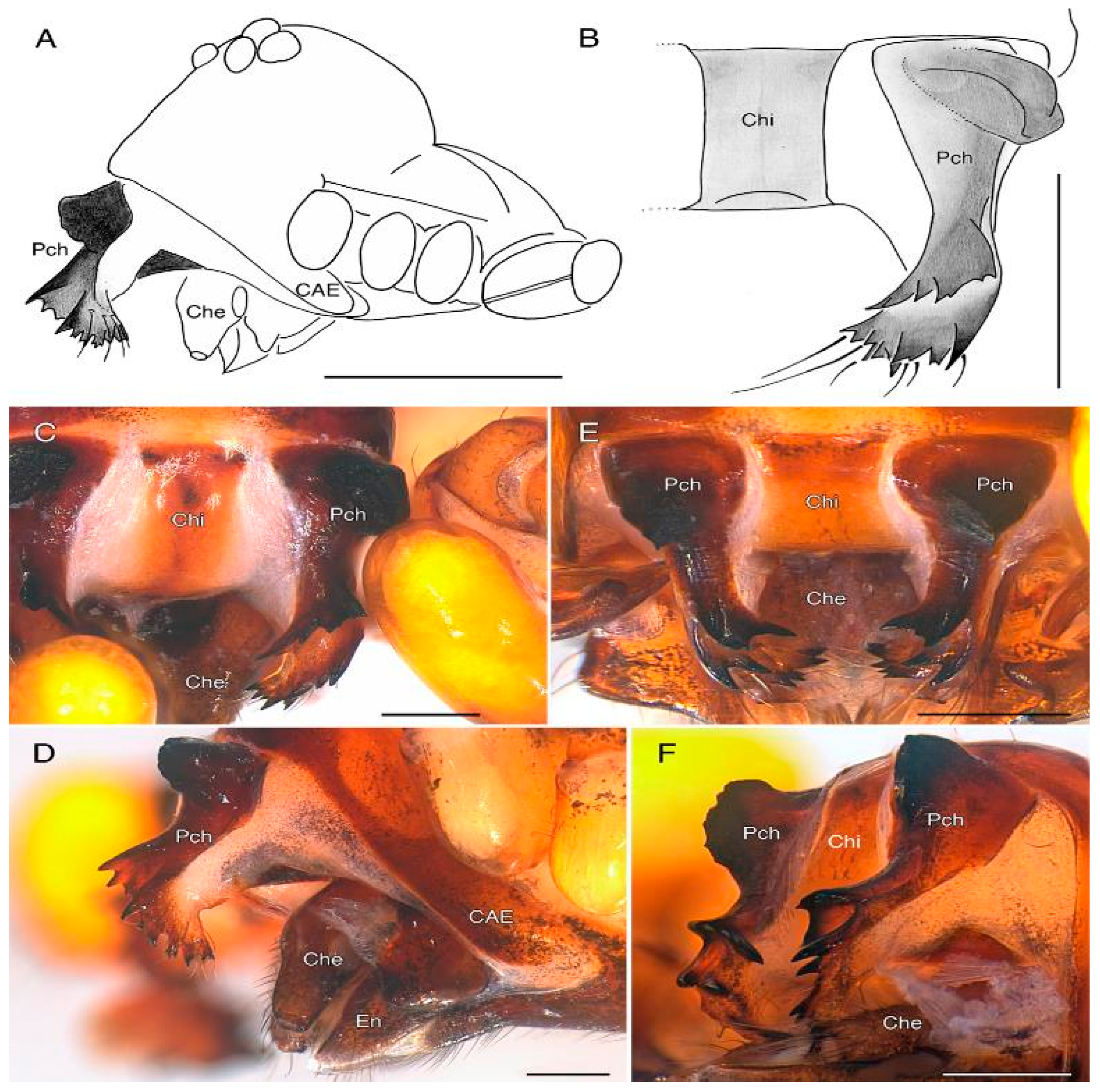
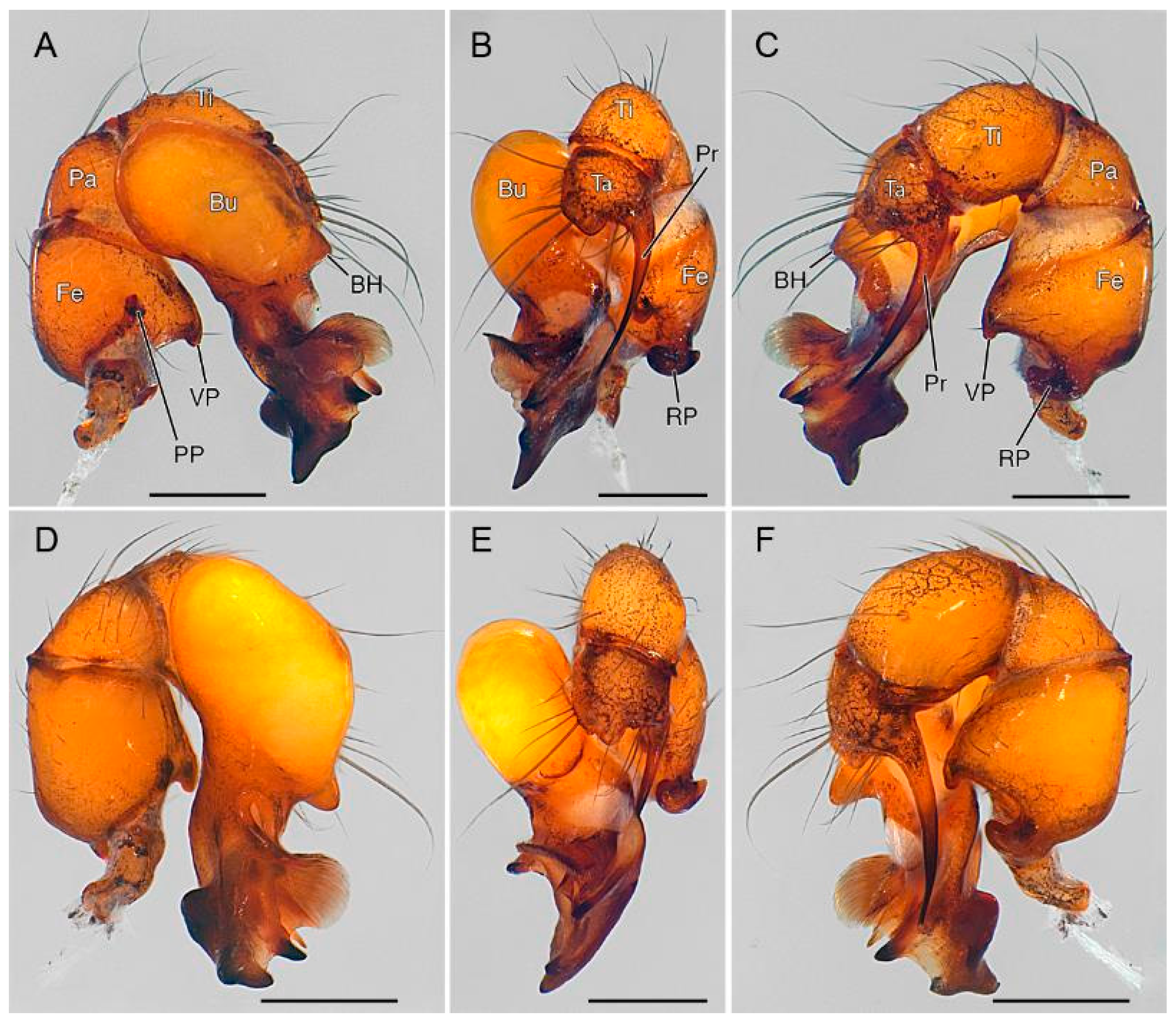
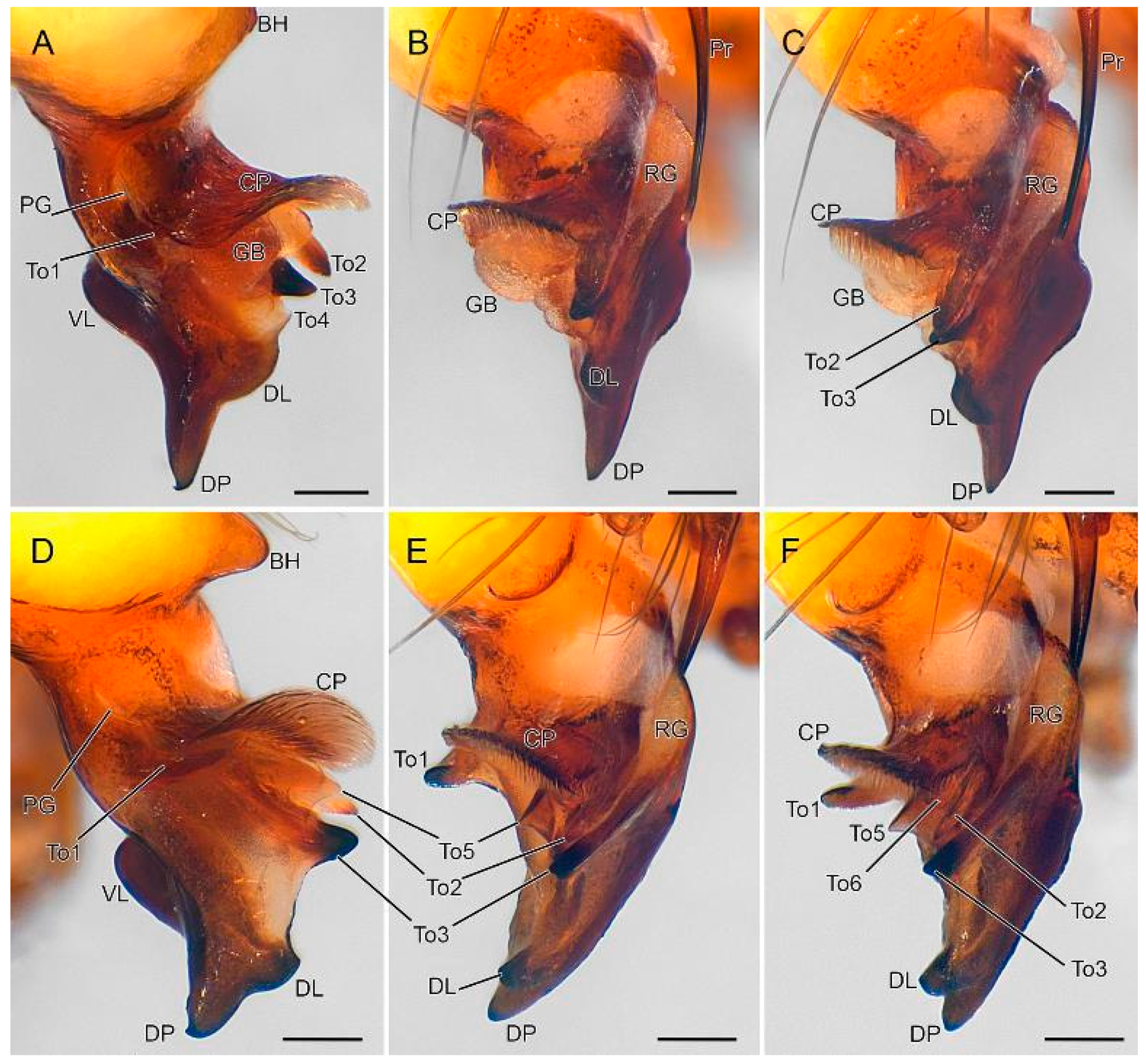
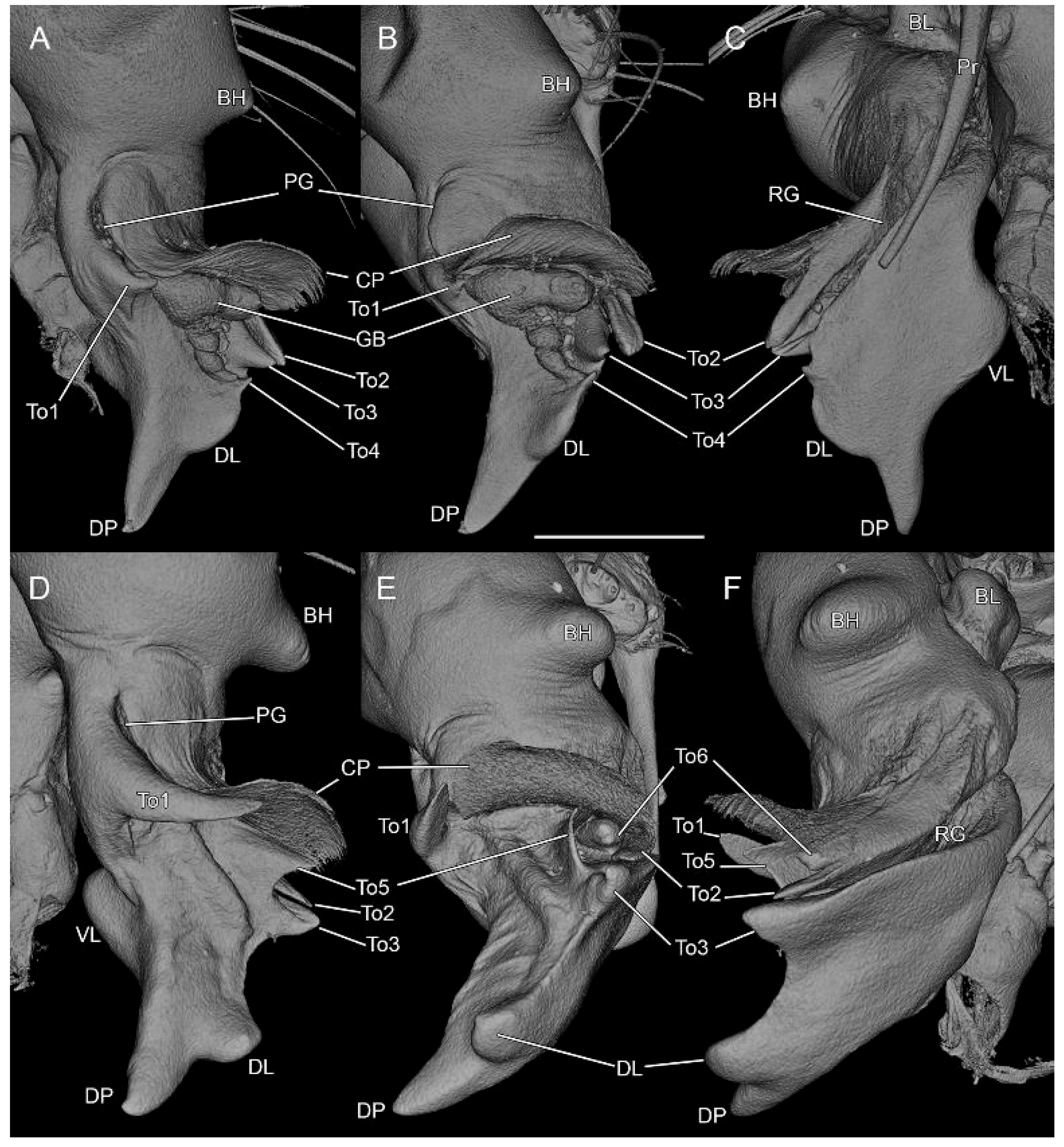
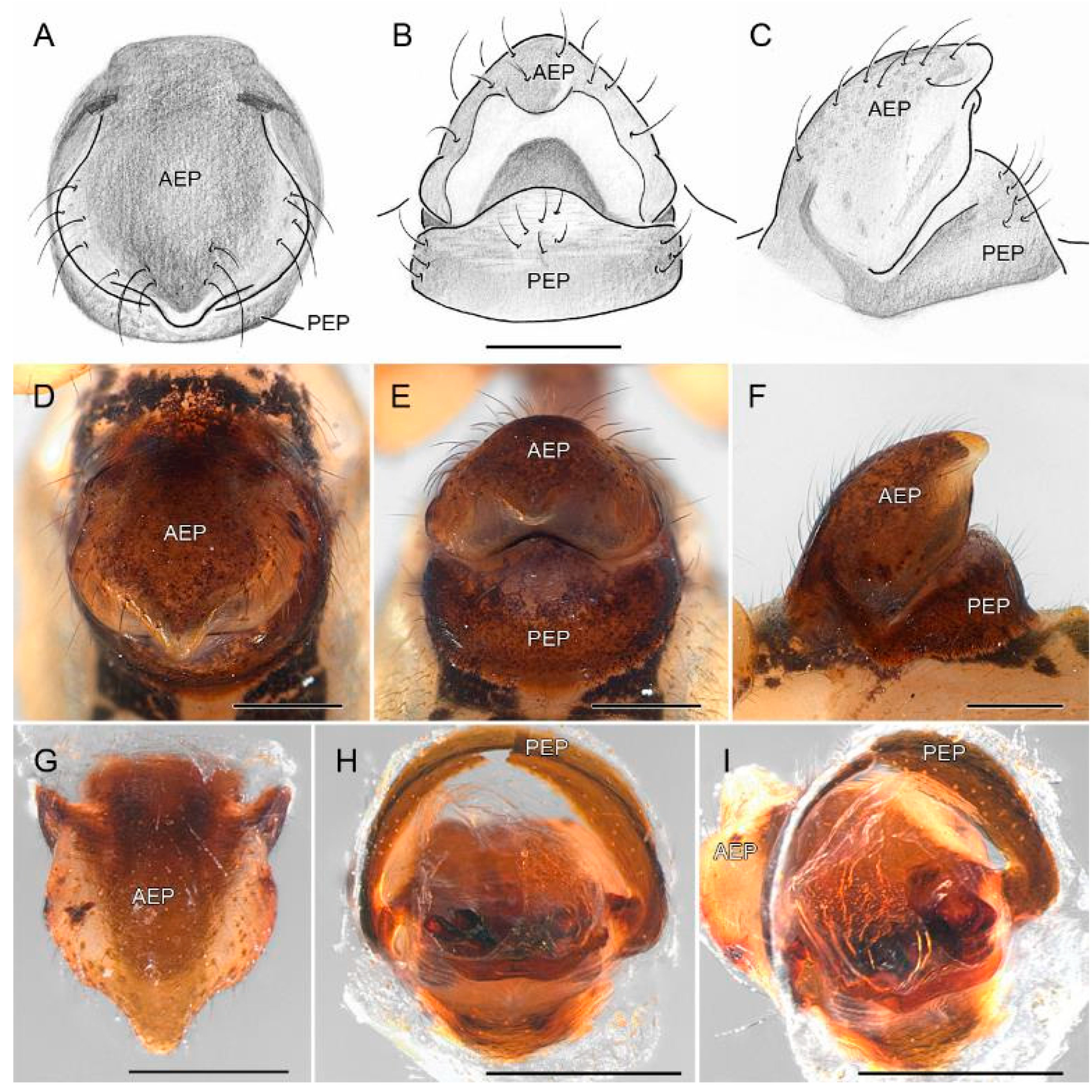
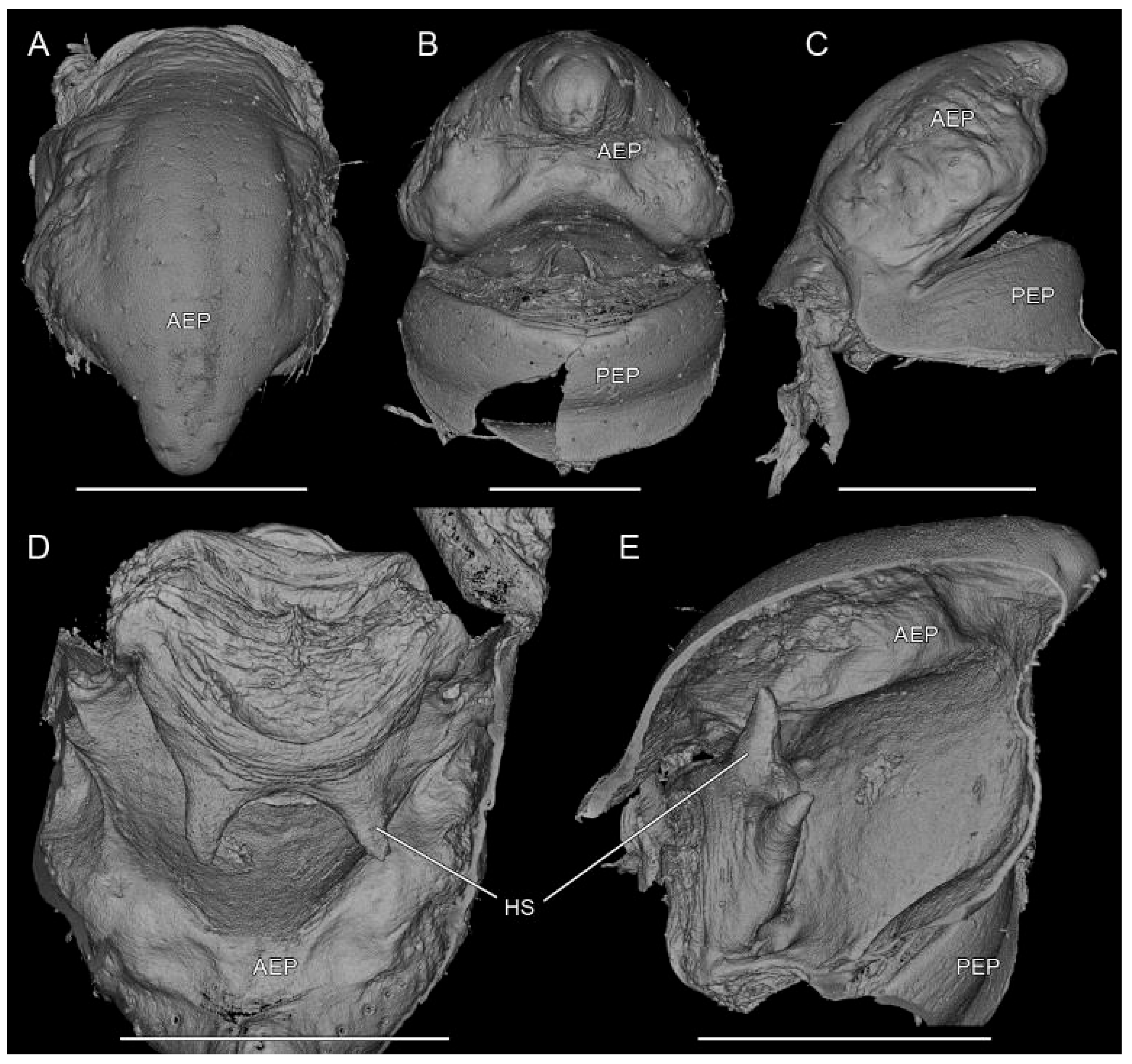
3.2. Taxonomy
4. Discussion
4.1. Phylogenetic Position of Smeringopina polychila sp. nov.
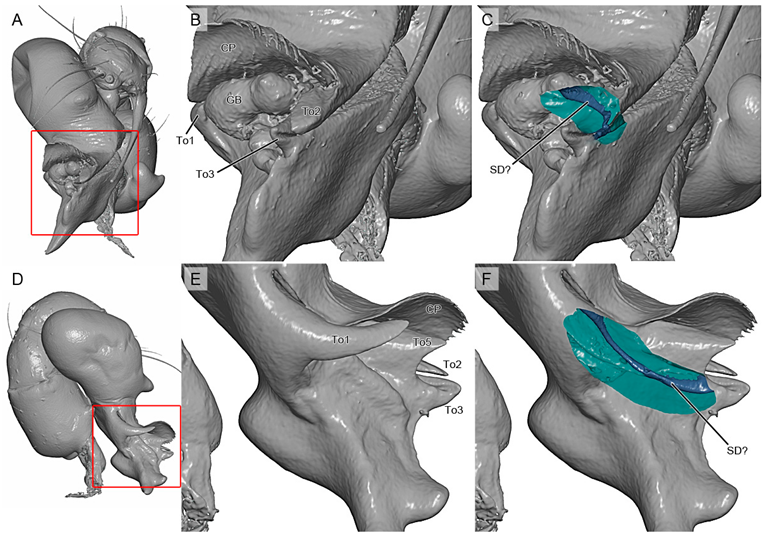
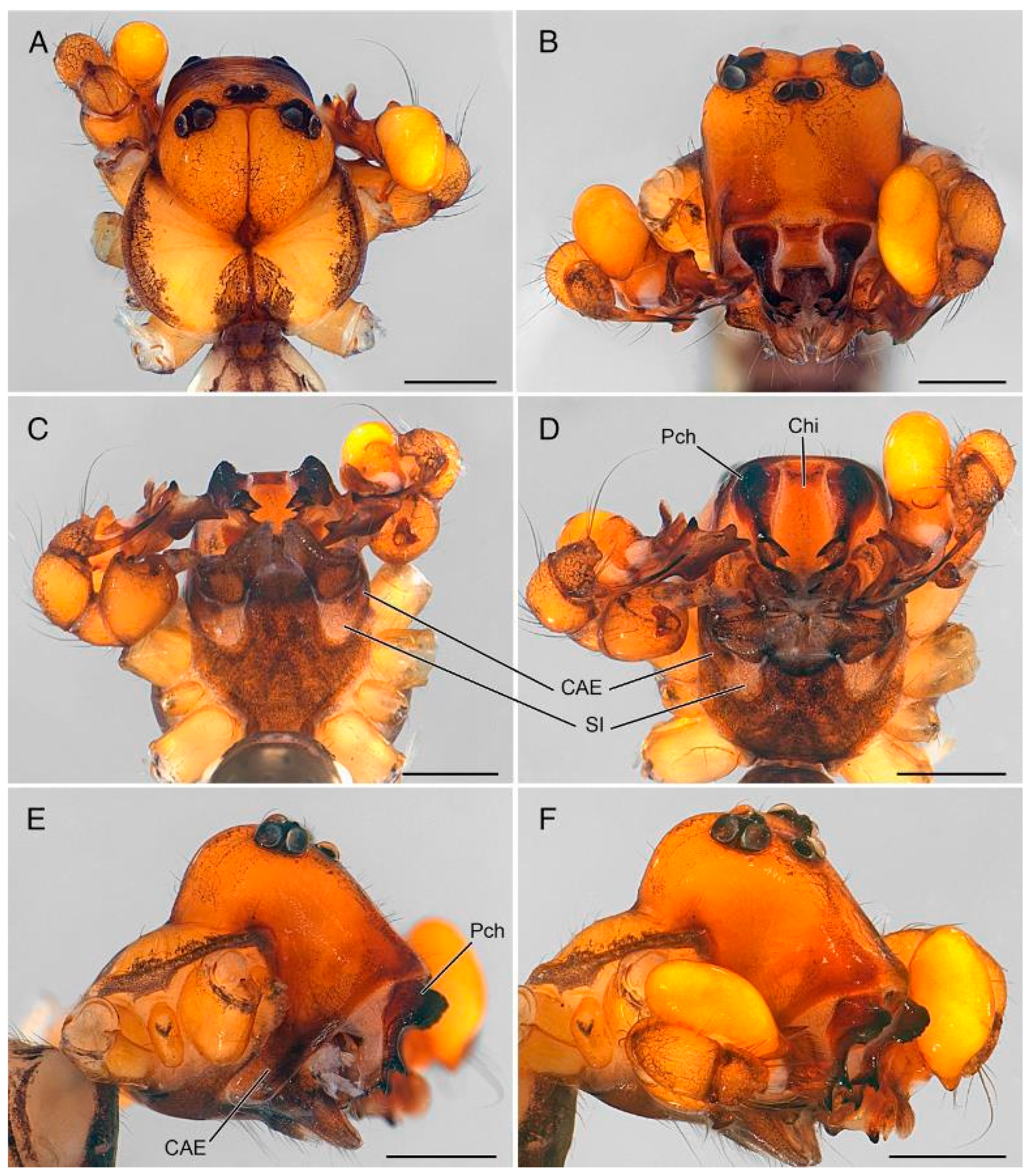
4.2. Sexual Characters and Variations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1
| Primer Name | Oligonucleotides | Reference | Target Region |
|---|---|---|---|
| C1-J-1718-spider | 5′-GGNGGATTTGGAAATTGRTTRGTTCC-3′ | [22] Vink et al. (2005) | Mitochondrial COI |
| C1-N-2776-spider | 5′-GGATAATCAGAATANCGNCGAGG-3′ | ||
| LR-N-13398 (16Sar) | 5′-CGCCTGTTTAACAAAAACAT-3′ | [23] Simon et al. (1994) | Mitochondrial 16S rRNA |
| LR-J-12887 (16Sbr) | 5′-CCGGTCTGAACTCAGATCACGT-3′ | ||
| H3aF | 5′-ATGGCTCGTACCAAGCAGACVGC-3′ | [24] Colgan et al. (1998) | Nuclear histone H3 |
| H3aR | 5′-ATATCCTTRGGCATRATRGTGAC-3′ |
Appendix A.2
| Target Region | Initial Denaturation | Denaturation | Annealing | Elongation | Final Elongation | # of Cycles |
|---|---|---|---|---|---|---|
| COI | 95 °C—15 min | 94 °C—30 s | 57 °C—90 s | 72 °C—90 s | 72 °C—10 min | 45 |
| 16S | 95 °C—15 min | 94 °C—45 s | 45 °C—45 s | 72 °C—60 s | 5 | |
| 94 °C—45 s | 48 °C—45 s | 72 °C—60 s | 72 °C—10 min | 30 | ||
| H3 | 95 °C—15 min | 94 °C—40 s | 54 °C—50 s | 72 °C—60 s | 72 °C—10 min | 40 |
Appendix A.3
| Code | Species | Genetic Markers | Reference(s) | |||||
|---|---|---|---|---|---|---|---|---|
| 12S | 16S | 18S | 28S | COI | H3 | |||
| RMCA_247639 | Smeringopina polychila sp. nov. | PQ350127 | PQ349817 | PQ356605 | Present study | |||
| RMCA_247640 | Smeringopina polychila sp. nov. | PQ350128 | PQ349818 | PQ356606 | Present study | |||
| M089 | Cenemus culiculus | ON509570 | ON504299 | ON497107 | [29] Huber & Meng 2023 | |||
| BB05 | Crossopriza lyoni | AY560689 | AY560774 | [26] Bruvo-Mađarić et al., 2005 | ||||
| Is2 | Crossopriza lyoni | AY560690 | AY560775 | [26] Bruvo-Mađarić et al., 2005 | ||||
| P0154 | Crossopriza lyoni | JX023767 | JX023860 | JX023957 | JX024070 | JX023551 | JX023619 | [2] Eberle et al., 2018; [14] Huber et al., 2018 |
| SB026 | Crossopriza lyoni | MG267734 | MG268895 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||||
| S499 | Crossopriza kittan | MG267718 | MG268017 | MG268336 | MG268599 | MG268821 | MG269116 | [2] Eberle et al., 2018; [14] Huber et al., 2018; [29] Huber & Meng 2023 |
| S500 | Crossopriza miskin | MG267719 | MG268007 | MG268337 | MG268600 | MG268820 | MG269115 | [2] Eberle et al., 2018; [14] Huber et al., 2018; [29] Huber & Meng 2023 |
| S290 | Crossopriza pristina | MG267546 | MG267826 | MG268155 | MG268469 | MG268894 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |
| S435 | Holocnemus reini | MG267665 | MG267951 | MG268277 | MG268590 | MG268890 | MG269111 | [2] Eberle et al., 2018; [14] Huber et al., 2018; [29] Huber & Meng 2023 |
| P0233 | Holocnemus hispanicus | JX024020 | JX023600 | JX023680 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||
| BB06 | Holocnemus pluchei | AY560691 | AY560776 | [26] Bruvo-Mađarić et al., 2005 | ||||
| HpIs2 | Holocnemus pluchei | AY560692 | AY560777 | [26] Bruvo-Mađarić et al., 2005 | ||||
| ZFMK | Holocnemus pluchei | JX023832 | JX024036 | JX024132 | JX023687 | [27] Dimitrov et al., 2013 | ||
| ARASP087 | Holocnemus pluchei | KY015507 | KY016611 | KY017265 | KY017849 | [28] Wheeler et al., 2017 | ||
| S436 | Hoplopholcus asiaeminoris | MG267666 | MG267952 | MG268278 | MG268813 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||
| S009 | Hoplopholcus cecconii | MG267447 | MG268084 | MG268811 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||
| S438 | Hoplopholcus dim | MG267668 | MG267954 | MG268280 | MG268815 | [2] Eberle et al., 2018; [14] Huber et al., 2018; [16] Huber 2020 | ||
| S057 | Hoplopholcus forskali | MG267446 | MG267758 | MG268083 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||
| S437 | Hoplopholcus gazipasa | MG267667 | MG267953 | MG268279 | MG268814 | [2] Eberle et al., 2018; [14] Huber et al., 2018; [16] Huber 2020 | ||
| S442 | Hoplopholcus konya | MG267671 | MG267958 | MG268284 | MG268558 | MG268810 | [2] Eberle et al., 2018; [14] Huber et al., 2018; [16] Huber 2020 | |
| S316 | Hoplopholcus labyrinthi | MG267560 | MG267849 | MG268172 | MG268808 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||
| S315 | Hoplopholcus minotaurinus | MG267559 | MG267848 | MG268171 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||
| S314 | Hoplopholcus minous | MG267558 | MG267847 | MG268170 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||
| S439 | Hoplopholcus patrizii | MG267955 | MG268281 | MG268807 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||
| S440 | Hoplopholcus sp. Tur21 | MG267669 | MG267956 | MG268282 | MG268812 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||
| S441 | Hoplopholcus suluin | MG267670 | MG267957 | MG268283 | MG268809 | MG269183 | [2] Eberle et al., 2018; [14] Huber et al., 2018; [16] Huber 2020 | |
| GB51—S054 | Smeringopina ankasa—GB51 | MG268634 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||||
| Smeringopina ankasa—S054 | MG267772 | MG268081 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||||
| P0173 | Smeringopina attuleh | JX023874 | JX023633 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||||
| S491 | Smeringopina bamenda | MG267710 | MG267997 | MG269120 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||
| GB14 | Smeringopina bayaka | MG268662 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||||
| GB15 | Smeringopina belinga | MG268665 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||||
| P0152 | Smeringopina bineti | JX023765 | JX023858 | JX023617 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||
| S300 | Smeringopina bomfobiri | MG267554 | MG267834 | MG268163 | MG268633 | MG269118 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |
| S492 | Smeringopina bwiti | MG267711 | MG267998 | MG268659 | MG269068 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||
| S042 | Smeringopina camerunensis | MG267773 | MG268079 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||||
| GB16 | Smeringopina chaillu | MG268663 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||||
| S404 | Smeringopina djidji | MG267602 | MG267930 | MG268220 | MG268666 | MG269069 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |
| GB52—P0175 | Smeringopina ebolowa—GB52 | MG268668 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||||
| Smeringopina ebolowa—P0175 | JX023786 | JX023876 | JX023635 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||||
| GB17 | Smeringopina essotah | MG268664 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||||
| GB18 | Smeringopina fang | MG268654 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||||
| P0153 | Smeringopina guineensis | JX023766 | JX023859 | JX023550 | JX023618 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||
| GB19 | Smeringopina iboga | MG268645 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||||
| S041 | Smeringopina kala | MG267459 | MG267774 | MG268078 | MG268387 | MG268622 | MG269119 | [2] Eberle et al., 2018; [14] Huber et al., 2018 |
| S402 | Smeringopina kinguele | MG267600 | MG267928 | MG268219 | MG268527 | MG269181 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |
| S044 | Smeringopina kribi | MG267460 | MG267775 | MG268080 | MG268660 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||
| GB20 | Smeringopina lekoni | MG268652 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||||
| S043 | Smeringopina mbouda | MG267776 | MG268082 | MG269066 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||
| S403 | Smeringopina mohoba | MG267601 | MG267929 | MG268655 | MG269182 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||
| S049 | Smeringopina moudouma | MG267462 | MG267777 | MG268077 | MG268386 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||
| GB21 | Smeringopina ndjole | MG268644 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||||
| P0180 | Smeringopina nyasoso | JX023790 | JX023880 | JX023565 | JX023639 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||
| S405 | Smeringopina ogooue | MG267603 | MG267931 | MG268669 | MG269070 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||
| GB22 | Smeringopina sahoue | MG268658 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||||
| S493 | Smeringopina simintang | MG267716 | MG267999 | MG268329 | MG268667 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||
| S301 | Smeringopina tchimbele | MG267835 | MG268164 | MG268661 | MG269067 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||
| GB54—S050 | Smeringopina tebe—GB54 | MG268653 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||||
| Smeringopina tebe—S050 | MG267461 | MG267778 | MG268076 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||||
| S017 | Smeringopus arambourgi | MG267458 | MG267779 | MG268039 | MG268382 | MG269061 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |
| P0266 | Smeringopus bujongolo | JX023837 | JX023933 | JX024045 | JX023697 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||
| P0224 | Smeringopus cf. atomarius | JX023910 | JX024012 | JX024117 | JX023673 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||
| P0227 | Smeringopus cf. similis | JX023820 | JX023912 | JX024015 | JX024119 | JX023675 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |
| P0161 | Smeringopus chogoria | JX023774 | JX023866 | JX023963 | JX023555 | [27] Dimitrov et al., 2013; [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||
| P0143 | Smeringopus cylindrogaster | JX023850 | JX023951 | JX024064 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||
| P0178 | Smeringopus cylindrogaster | JX023878 | JX023975 | JX024084 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||
| S407 | Smeringopus lesserti | MG267605 | MG267933 | MG268222 | MG268530 | MG268871 | MG269062 | [2] Eberle et al., 2018; [14] Huber et al., 2018 |
| S408 | Smeringopus lotzi | MG267606 | MG267934 | MG268223 | MG268529 | MG268872 | MG269063 | [2] Eberle et al., 2018; [14] Huber et al., 2018 |
| P0257 | Smeringopus mgahinga | JX023927 | JX024038 | JX023688 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |||
| P0265 | Smeringopus mpanga | JX023932 | JX023696 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||||
| BB28 | Smeringopus natalensis | AY560717 | AY560755 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||||
| P0157 | Smeringopus ngangao | JX023770 | JX023863 | JX023960 | JX024073 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | ||
| P0264 | Smeringopus pallidus | JX023836 | JX023931 | JX024044 | JX024136 | JX023695 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |
| S406 | Smeringopus peregrinoides | MG267604 | MG267932 | MG268221 | MG268528 | MG269060 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |
| S108 | Stygopholcus skotophilus | MG267487 | MG268112 | MG268428 | MG268938 | [2] Eberle et al., 2018; [14] Huber et al., 2018; [29] Huber & Meng 2023 | ||
| S109 | Stygopholcus montenegrinus | MG267488 | MG268936 | [2] Eberle et al., 2018; [14] Huber et al., 2018; [29] Huber & Meng 2023 | ||||
| S112 | Stygopholcus skotophilus | MG267490 | MG268114 | MG268430 | MG268937 | [2] Eberle et al., 2018; [14] Huber et al., 2018; [29] Huber & Meng 2023 | ||
| S317 | Stygopholcus photophilus | MG267561 | MG267850 | MG268173 | MG268934 | MG269114 | [2] Eberle et al., 2018; [14] Huber et al., 2018 | |
| S110 | Stygopholcus absoloni | MG267489 | MG268113 | MG268429 | MG268935 | [2] Eberle et al., 2018; [14] Huber et al., 2018; [29] Huber & Meng 2023 | ||
| P0164 | Spermophora minotaura | JX023777 | JX023869 | JX023965 | JX024077 | JX023557 | JX023624 | [2] Eberle et al., 2018; [14] Huber et al., 2018 |
Appendix A.4
References
- World Spider Catalog Version 26 (2025). Natural History Museum Bern. Available online: http://wsc.nmbe.ch (accessed on 1 June 2025). [CrossRef]
- Eberle, J.; Dimitrov, D.; Valdez-Mondragón, A.; Huber, B.A. Microhabitat change drives diversification in pholcid spiders. BMC Evol. Biol. 2018, 18, 141. [Google Scholar] [CrossRef]
- Huber, B.A. Sexual selection in pholcid spiders (Araneae, Pholcidae): Artful chelicerae and forceful genitalia. J. Arachnol. 1999, 27, 135–141. [Google Scholar]
- Huber, B.A. High species diversity, male-female coevolution, and metaphyly in Southeast Asian pholcid spiders: The case of Belisana Thorell 1898 (Araneae, Pholcidae). Zoologica 2005, 155, 1–126. [Google Scholar]
- Huber, B.A. Two new genera of small, six-eyed pholcid spiders from West Africa, and first record of Spermophorides for mainland Africa (Araneae: Pholcidae). Zootaxa 2007, 1635, 23–43. [Google Scholar] [CrossRef]
- Huber, B.A. Revision and cladistic analysis of Pholcus and closely related taxa (Araneae, Pholcidae). Bonn. Zool. Monogr. 2011, 58, 1–509. [Google Scholar]
- Huber, B.A. Revision and cladistic analysis of the Afrotropical endemic genus Smeringopus Simon, 1890 (Araneae: Pholcidae). Zootaxa 2012, 3461, 1–138. [Google Scholar] [CrossRef]
- Huber, B.A. Revision and cladistic analysis of the Guineo-Congolian spider genus Smeringopina Kraus (Araneae, Pholcidae). Zootaxa 2013, 3713, 1–160. [Google Scholar] [CrossRef] [PubMed]
- Huber, B.A. A new genus of ground and litter-dwelling pholcine spiders from Sarawak (Araneae, Pholcidae). Eur. J. Taxon. 2016, 186, 1–15. [Google Scholar] [CrossRef]
- Huber, B.A.; Nuñeza, O.M. Evolution of genital asymmetry, exaggerated eye stalks, and extreme palpal elongation in Panjange spiders (Araneae: Pholcidae). Eur. J. Taxon. 2015, 169, 1–46. [Google Scholar] [CrossRef]
- Aharon, S.; Huber, B.A.; Gavish-Regev, E. Daddy-long-leg giants: Revision of the spider genus Artema Walckenaer, 1837 (Araneae, Pholcidae). Eur. J. Taxon. 2017, 376, 1–57. [Google Scholar] [CrossRef]
- Huber, B.A.; Kwapong, P. West African pholcid spiders: An overview, with descriptions of five new species (Araneae, Pholcidae). Eur. J. Taxon. 2013, 59, 1–44. [Google Scholar] [CrossRef]
- Huber, B.A.; Meng, G.L.; Král, J.; Ávila Herrera, I.M.; Carvalho, L.S. Diamonds in the rough: Ibotyporanga (Araneae, Pholcidae) spiders in semi-arid Neotropical environments. Eur. J. Taxon. 2024, 963, 1–169. [Google Scholar] [CrossRef]
- Huber, B.A.; Eberle, J.; Dimitrov, D. The phylogeny of pholcid spiders: A critical evaluation of relationships suggested by molecular data (Araneae, Pholcidae). ZooKeys 2018, 789, 51–101. [Google Scholar] [CrossRef] [PubMed]
- Huber, B.A.; Carvalho, L.S. Filling the gaps: Descriptions of unnamed species included in the latest molecular phylogeny of Pholcidae (Araneae). Zootaxa 2019, 4546, 1–96. [Google Scholar] [CrossRef] [PubMed]
- Huber, B.A. Revision of the spider genus Hoplopholcus Kulczyński (Araneae, Pholcidae). Zootaxa 2020, 4726, 1–94. [Google Scholar] [CrossRef]
- Huber, B.A. Revisions of Holocnemus and Crossopriza: The spotted-leg clade of Smeringopinae (Araneae, Pholcidae). Eur. J. Taxon. 2022, 795, 1–241. [Google Scholar] [CrossRef]
- Huber, B.A. The South American spider genera Mesabolivar and Carapoia (Araneae, Pholcidae): New species and a framework for redrawing generic limits. Zootaxa 2018, 4395, 1–178. [Google Scholar] [CrossRef]
- Jocqué, R.; Jocque, M.; Mbende, M. A new Cangoderces (Araneae, Telemidae) from DR Congo, the first telemid from Central Africa. Zootaxa 2022, 5162, 430–438. [Google Scholar] [CrossRef]
- Jocqué, R. How to rehydrate dried spiders. Newsl. Br. Arachnol. Soc. 2008, 112, 5. [Google Scholar]
- Beccaloni, J. A preliminary comparison of Trisodium Phosphate with Agepon and Decon90 as wetting agents to hydrate dried arachnida and myriapoda specimens. NatSCA News 2012, 22, 71–79. [Google Scholar]
- SimpleMappr, an Online Tool to Produce Publication-Quality Point Maps. Shorthouse D.P. 2010. Available online: https://www.simplemappr.net (accessed on 1 January 2023).
- Vink, C.J.; Thomas, S.M.; Paquin, P.; Hayashi, C.Y.; Hedin, M. The effects of preservatives and temperatures on arachnid DNA. Invertebr. Syst. 2005, 19, 99–104. [Google Scholar] [CrossRef]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, Weighting, and Phylogenetic Utility of Mitochondrial Gene Sequences and a Compilation of Conserved Polymerase Chain Reaction Primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Colgan, D.J.; McLauchlan, A.; Wilson, G.D.F.; Livingston, S.P.; Edgecombe, G.D.; Macaranas, J.; Cassis, G.; Gray, M.R. Histone H3 and U2 snRNA DNA sequences and arthropod molecular evolution. Aust. J. Zool. 1998, 46, 419–437. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data System. Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Bruvo-Madarić, B.; Huber, B.A.; Steinacher, A.; Pass, G. Phylogeny of pholcid spiders (Araneae: Pholcidae): Combined analysis using morphology and molecules. Mol. Phylogenetics Evol. 2005, 37, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.; Astrin, J.J.; Huber, B.A. Pholcid spider molecular systematics revisited, with new insights into the biogeography and the evolution of the group. Cladistics 2013, 29, 132–146. [Google Scholar] [CrossRef]
- Wheeler, W.C.; Coddington, J.A.; Crowley, L.M.; Dimitrov, D.; Goloboff, P.A.; Griswold, C.E.; Hormiga, G.; Prendini, L.; Ramírez, M.J.; Sierwald, P.; et al. The spider tree of life: Phylogeny of Araneae based on target-gene analyses from an extensive taxon sampling. Cladistics 2017, 33, 576–616. [Google Scholar] [CrossRef]
- Huber, B.A.; Meng, G.L. On the mysterious Seychellois endemic spider genus Cenemus (Araneae, Pholcidae). Arthropod Syst. Phylogeny 2023, 81, 179–200. [Google Scholar] [CrossRef]
- Astrin, J.J.; Misof, B.; Huber, B.A. The pitfalls of exaggeration: Molecular and morphological evidence suggests Kaliana is a synonym of Mesabolivar (Araneae: Pholcidae). Zootaxa 2007, 1646, 17–30. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36 (Suppl. 2), W465–W469. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, F.; Correia, D.; Lefort, V.; Doppelt-Azeroual, O.; Mareuil, F.; Cohen-Boulakia, S.; Gascuel, O. NGPhylogeny.fr: New generation phylogenetic services for non-specialists. Nucleic Acids Res. 2019, 47, W260–W265. [Google Scholar] [CrossRef]
- Mesquite: A Modular System for Evolutionary Analysis, Version 3.8, Maddison, W.P. and D.R. Maddison. 2023. Available online: http://www.mesquiteproject.org (accessed on 10 January 2024).
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2016, 34, 772–773. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Lanfear, R.; Calcott, B.; Ho, S.Y.; Guindon, S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef]
- Zwickl, D.J. Genetic Algorithm Approaches for the Phylogenetic Analysis of Large Biological Sequence Datasets Under the Maximum Likelihood Criterion. Ph.D. Dissertation, The University of Texas, Austin, TX, USA, May 2006. [Google Scholar]
- Sukumaran, J.; Holder, M.T. DendroPy: A Python library for phylogenetic computing. Bioinformatics 2010, 26, 1569–1571. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogeny. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Figtree Version 1.4.4, Rambaut R. 2006–2018. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 9 January 2024).
- Kraus, O. Araneenstudien 1. Pholcidae (Smeringopodinae, Ninetinae). Senckenberg. Biol. 1957, 38, 217–243. [Google Scholar]
- Huber, B.A. Beyond size: Sexual dimorphisms in pholcid spiders. Arachnology 2021, 18, 656–677. [Google Scholar] [CrossRef]
- Huber, B.A. Genital morphology, copulatory mechanism and reproductive biology in Psilochorus simoni (Berland, 1911) (Pholcidae; Araneae). Neth. J. Zool. 1994, 44, 85–99. [Google Scholar] [CrossRef]
- Huber, B.A. Copulatory mechanism in Holocnemus pluchei and Pholcus opilionoides, with notes on male cheliceral apophyses and stridulatory organs in Pholcidae (Araneae). Acta Zool. 1995, 76, 291–300. [Google Scholar] [CrossRef]
- Huber, B.A. On American ‘Micromerys’ and Metagonia (Araneae, Pholcidae), with notes on natural history and genital mechanics. Zool. Scr. 1997, 25, 341–363. [Google Scholar] [CrossRef]
- Huber, B. Genital mechanics in some neotropical pholcid spiders (Araneae: Pholcidae), with implications for systematics. J. Zool. 1998, 244, 587–599. [Google Scholar] [CrossRef]
- Huber, B.A.; Eberhard, W.G. Courtship, copulation, and genital mechanics in Physocyclus globosus (Araneae, Pholcidae). Can. J. Zool. 1997, 74, 905–918. [Google Scholar] [CrossRef]
- Huber, B.A.; Pérez-González, A.; Astrin, J.J.; Blume, C.; Baptista, R. Litoporus iguassuensis Mello-Leitão, 1918 (Araneae, Pholcidae): Camouflaged retreat, sexual dimorphism, female color polymorphism, intra-specific genital variation, and description of the male. Zool. Anz. 2013, 252, 511–521. [Google Scholar] [CrossRef]
- Huber, B.A.; Dimitrov, D. Slow genital and genetic but rapid non-genital and ecological differentiation in a pair of spider species (Araneae, Pholcidae). Zool. Anz. 2014, 253, 394–403. [Google Scholar] [CrossRef]
- Wheeler, Q.D.; Platnick, N. The phylogenetic species concept. In Species Concepts and Phylogenetic Theory. A Debate; Wheeler, Q.D., Meier, R., Eds.; Columbia University Press: New York, NY, USA, 2000; pp. 55–69. [Google Scholar]
- Eberhard, W.G.; Huber, B.A.; Rodriguez, R.L.S.; Briceño, R.D.; Salas, I.; Rodriguez, V. One size fits all? Relationships between the size and degree of variation in genitalia and other body parts in twenty species of insects and spiders. Evolution 1998, 52, 415–431. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henrard, A.; Jocqué, R.; Smitz, N.; Grignet, V. Precision Lost with Complexity: On an Extraordinary New Species of Pholcidae (Araneae, Smeringopinae) from Western DR Congo. Taxonomy 2025, 5, 57. https://doi.org/10.3390/taxonomy5040057
Henrard A, Jocqué R, Smitz N, Grignet V. Precision Lost with Complexity: On an Extraordinary New Species of Pholcidae (Araneae, Smeringopinae) from Western DR Congo. Taxonomy. 2025; 5(4):57. https://doi.org/10.3390/taxonomy5040057
Chicago/Turabian StyleHenrard, Arnaud, Rudy Jocqué, Nathalie Smitz, and Virginie Grignet. 2025. "Precision Lost with Complexity: On an Extraordinary New Species of Pholcidae (Araneae, Smeringopinae) from Western DR Congo" Taxonomy 5, no. 4: 57. https://doi.org/10.3390/taxonomy5040057
APA StyleHenrard, A., Jocqué, R., Smitz, N., & Grignet, V. (2025). Precision Lost with Complexity: On an Extraordinary New Species of Pholcidae (Araneae, Smeringopinae) from Western DR Congo. Taxonomy, 5(4), 57. https://doi.org/10.3390/taxonomy5040057








