Abstract
Three new species of Trichorhina are described from iron ore caves in the Serra dos Carajás Mountain Range, located in the Amazon Forest, Brazil. Trichorhina tucupi n. sp. occurs in Serra Leste, Serra da Bocaina and Serra do Tarzan Mountain Ranges. Trichorhina tacaca n. sp. occurs in caves in the Serra Norte Mountain Range. Trichorhina piloi n. sp. occurs in the Serra Sul and Serra Norte Mountain Ranges. Statistical methods were applied to investigate the putative morphological patterns of these species and to investigate their potential use in distinguishing Trichorhina species from epigean and hypogean habitats.
1. Introduction
Historically, most cave-dwelling oniscidean isopod species were described in northern regions of the globe. However, over the past two decades, extensive surveys in Brazilian karst systems have revealed a significant diversity of Oniscidea in this region. Nearly 250 species of terrestrial isopods have already been identified in Brazil [1,2,3,4,5,6,7,8], with approximately one hundred species inhabiting subterranean environments [8,9,10]. Among them, Trichorhina Budde-Lund, 1908 species are frequently encountered in Brazilian caves and surrounding areas.
Overall, the genus Trichorhina comprises 86 species, with 37 occurring in Brazil, 20 of which have been recorded in caves [10,11]. Like other members of the Platyarthridae family, the Trichorhina species possess lateral lobes on the cephalon, biarticulate antennal flagellum, dorsal surface covered with scale-setae, and a lack of pleopodal lungs, rendering them incapable of volvation [12]. The genus was redefined by Carpio-Diaz et al. [13]. Despite significant advancements in the taxonomy of this genus in recent years, which have greatly enhanced our understanding of its diversity, distribution, and morphological variation [10,13,14], the molecular data indicate that Platyartridae is paraphyletic [15,16]. This underscores the urgent need for a comprehensive review of this taxon.
In general, Trichorhina species are highly endemic, with a few exceptions, such as Trichorhina tomentosa (Budde-Lund, 1893) and Trichorhina heterophthalma Lemos de Castro, 1964, whose distribution may have been facilitated by human activity [17]. Most Trichorhina species found in Brazilian caves are restricted to one or a few locations and are considered troglophilic for two main reasons: limited knowledge regarding their true distribution (including their potential presence in epigean habitats, as observed in Trichorhina quadriocellata Campos-Filho, Borja-Arrieta & Bichuette, 2023) and a limited understanding of traits associated with their adaptation to subterranean environments [10].
The regression or absence of eyes is one of the most characteristic features associated with subterranean life. When present, the number of ommatidia serves as the primary distinguishing trait among Trichorhina species [10]. Ocular structures exhibit considerable variability. However, this variability is not consistently correlated with either epigean or hypogean habits, as observed across various taxonomic groups. Some Trichorhina species found in caves are completely eyeless, including Trichorhina anhanguera Campos-Filho, Araujo & Taiti, 2014, Trichorhina cipoensis Campos-Filho, Bichuette & Taiti, 2016, Trichorhina pataxosi Campos-Filho, Bichuette & Taiti, 2016, and Trichorhina. kaingangi Campos-Filho, 2015 [18,19,20,21]. In contrast, Trichorhina curupira Campos-Filho, Araujo & Taiti, 2014, has three ommatidia, while Trichorhina yiara Campos-Filho, Araujo & Taiti, 2014, has ten [18]. Among epigean species, at least ten are anophthalmic, while others possess varying numbers of ommatidia: one in Trichorhina tomentosa and Trichorhina macrops Souza-Kury, 1993, two in Trichorhina heterophthalma, and up to fifteen in Trichorhina tropidocerata Souza, Araujo & Campos-Filho, 2011 [17,20,22,23]. Another trait often considered troglomorphic in many taxa is the reduction in or absence of body pigmentation. This trait is highly variable in Trichorhina, as it is in many endogean species, such as Trichorhina brasilensis Andersson, 1960, which is depigmented and also anophthalmic [24].
In this context, classical troglomorphisms do not apply to Trichorhina. No studies have identified specific characteristics that would indicate a possible restriction to subterranean life for species within this genus, making it challenging to ascertain their ecological and evolutionary status. Campos-Filho et al. [18,25] emphasize the importance of conducting systematic collections in the epigean environments surrounding caves before defining their troglomorphic condition, a process that is not always feasible or successful. Nonetheless, Trichorhina baiana Campos-Filho, Gallão & Bichuette, 2023, was classified as troglobitic by the authors based on the observation that this anophthalmic and unpigmented species inhabits a xeric region where populations appear to thrive exclusively in the subterranean realm [10].
Trichorhina species documented in Brazilian caves are associated with a wide variety of lithologies and biomes. In the Amazon region, three species have been collected in caves and are considered troglophiles: Trichorhina yiara and Trichorhina curupira, from the Altamira region, and Trichorhina araguaia Campos-Filho, López-Orozco and Taiti, 2023, from the São Geraldo do Araguaia region [10,18]. Additionally, four species have been recorded in epigean habitats: Trichorhina amazonica Souza-Kury, 1997; Trichorhina paraensis Souza-Kury, 1997; Trichorhina pittieri (Pearse, 1921), and Trichorhina tomentosa [17,18,26,27].
Recent surveys conducted in iron ore caves of Serra dos Carajás, as part of the Brazilian licensing procedures, have revealed numerous Trichorhina specimens. This study investigates their morphological variations and distribution, leading to the identification and description of three new species for the genus. Additionally, we provide insights into Trichorhina’s ecomorphology, analyzing body and habitat traits through morphometric comparisons between epigean and hypogean taxa.
2. Materials and Methods
2.1. Collection
The material analyzed is deposited in the Subterranean Invertebrate Collection of Lavras (ISLA) at the Federal University of Lavras (UFLA) in Lavras, Minas Gerais, Brazil. Most specimens were collected from hypogean habitats (caves), with additional collections made in the epigean environments of the Serra Leste Mountain Range (Figure 1). Cave specimens were sampled throughout the entire extent of each cave by independent consulting companies, with reports on cave sampling available as Supporting Information in the work of Jaffé et al. [19]. Specimens from forest soil were collected using various methods, as detailed in the work of Oliveira et al. [28], including hay-baited traps (HBT), hand collection (HC, or CM in Portuguese, referring to the collection vouchers), and soil samples (SS, or AS in Portuguese, referring to the collection vouchers).
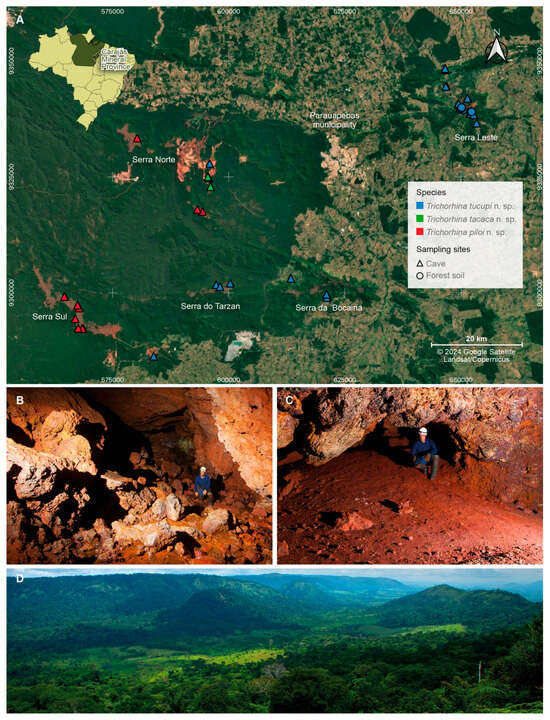
Figure 1.
Occurrence locations and habitat types where the three new Trichorhina species were sampled. (A) Species distribution in the Carajás Mineral Province, Pará state, Brazil; (B) cave ST_0041, one of the sampling sites of Trichorhina tucupi n. sp.; (C) cave S11C_0082, habitat of Trichorhina piloi n. sp.; (D) landscape in the Serra Leste Mountain range, epigean region where T. tucupi n. sp. specimens were collected. The map was created by Marcus Paulo Alves de Oliveira. Satellite image source: Google Earth 2024. Photographs (B–D) were provided by Ataliba Coelho.
The specimens were photographed under a stereomicroscope ZEISS Stemi 2000 with a coupled camera and measured in the software ZEN Blue edition. Individuals were previously sexed and identified as juveniles or adults, posteriorly dissected, and mounted on semi-permanent slides using Hoyer as medium [29]. Moreover, the topology of noduli laterales was assessed to identify and describe the species [20,30]. The position of noduli laterales was measured in the microscope Zeiss Axio Scope A1 (Jena, Germany; Made in China), equipped with an ocular lens with a millimetric scale. Illustrations were made with the aid of a camera lucida coupled with the optical microscope Leica DM750 (Heerbrugg, Switzerland; Made in China).
2.2. Morphometry
Attempting to elucidate putative ecomorphological patterns, we conducted morphometric analyses in 68 vouchers of Trichorhina (Table S1). Multivariate statistical analyses based on morphometric data have been used in taxonomic studies to corroborate the species distinction [31,32,33]. These analyses cannot include species with fewer than three adult individuals for statistical reasons. Characters unrelated to sexual dimorphisms were measured to allow for both males and females to be used in the analysis and thereby optimize sample sizes. All structures of interest were photographed with the camera Axiocam 105 Colour, coupled with the microscope Zeiss Axio Scope A1, and measurements were obtained through the software ZEN Blue edition (v. 2012).
Overall, 16 morphometric variables were obtained: (1) cep_ep1: reason between width of cephalon and epimeron 1; (2) ep1_ep7: reason between width of epimera 1 and 7; (3) ep1_ep4: reason between width of epimera 1 and 4; (4) a2_tl: reason between total length of antenna and body; (5) p1_p7: reason between length of pereopods 1 (P1) and 7 (P7); (6) u_exo_end: reason between length of uropod exopod and endopod; (7) p1_bas: reason between length and width of P1 basis; (8) p1_isc: reason between length and width of P1 ischium; (9) p1_mer: reason between length and width of P1 merus; (10) p1_car: reason between length and width of P1 carpus; (11) p1_pro: reason between length and width of P1 propodus; (12) p7_bas: reason between length and width of P7 basis; (13) p7_isc: reason between length and width of P7 ischium; (14) p7_mer: reason between length and width of P7 merus; (15) p7_car: reason between length and width of P7 carpus; (16) p7_pro: reason between length and width of P7 propodus.
All statistical analyses were performed in R v. 4.0.2 [34]. To reduce multicollinearity among morphometric variables, we first performed Spearman correlation tests and retained only those variables with rho < 0.700 and p < 0.050, or those with no significant correlation (p > 0.050). This step was conducted using the ‘rcorr’ function from the ‘Hmisc’ package. Species differentiation was then assessed through Canonical Variates Analysis (CVA), based on Linear Discriminant Analysis (LDA), implemented via the ‘MASS’ package. The resulting canonical scores were subjected to permutational multivariate analysis of variance using the ‘adonis2’ function from the ‘vegan’ package to evaluate the overall morphometric separation among species. When significant differences were detected, we examined the LDA loadings to determine which variables contributed most to the observed variation along the canonical axes (CV1 and CV2). Post hoc pairwise comparisons between species were conducted using the ‘pairwise.perm.manova’ function from the ‘RVAideMemoire’ package.
Subsequently, we selected specimens of the same species (Trichorhina tucupi n. sp.) and location (Serra Leste) to evaluate potential morphometric differences between habitats (caves and forest soils). We chose T. tucupi n. sp. due to the higher availability of individuals, wide distribution, and their occurrence in both epigean (soil) and hypogean habitats. Only T. tucupi n. sp. specimens sampled in Serra Leste were included in this analysis to avoid potential biases related to sampling location. We used a permutational multivariate analysis of variance (‘adonis2’ with habitats as categories—caves and forest soils). If differences were detected, we used LDA loadings to determine which metrics contributed the most to the observed variance on the canonical axis from CVA. Finally, we conducted mean tests using generalized linear models (function ‘glm’, package ‘stats’, gaussian distribution) on the selected morphometric variables (those with the highest contribution to CV1) to assess the statistical differences between the categories.
3. Results
3.1. Taxonomy
SuborderOniscidea Latreille, 1802
FamilyPlatyarthridae Verhoeff, 1949
GenusTrichorhina Budde-Lund, 1908
Type species: Bathytropa thermophila Dollfus, 1896 [=Trichorhina tomentosa (Budde-Lund, 1893)] by original designation [35].
Diagnosis. See Carpio-Díaz et al. [13].
Trichorhina tucupi Cardoso and Bastos-Pereira n. sp.
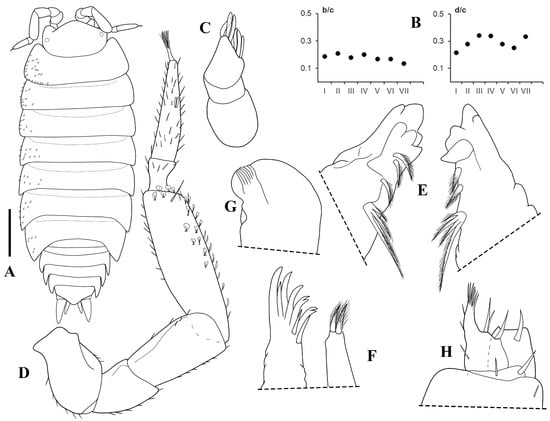
Figure 2.
Trichorhina tucupi n. sp. male paratype (ISLA15129): (A) habitus, dorsal view; (B) b/c and d/c coordinates of noduli laterales; (C) antennula; (D) antenna; (E) left and right mandible; (F) maxillula; (G) maxilla; (H) maxilliped. Scale-bar: 0.5 mm.
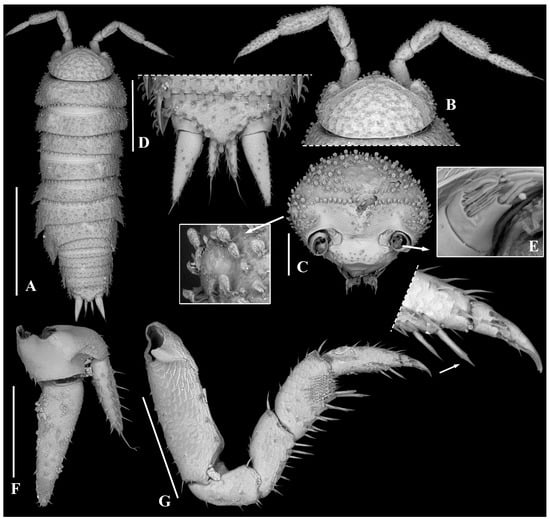
Figure 3.
Trichorhina tucupi n. sp. Female paratype (ISLA15129): (A) habitus, dorsal view; (B) cephalon, dorsal view; (C) cephalon, frontal view; (D) pleonite 5, pleotelson and uropods, dorsal view; (E) antennula; (F) uropod; (G) pereopod 1. Scale-bars: (A), 1 mm; (B–G), 200 µm.
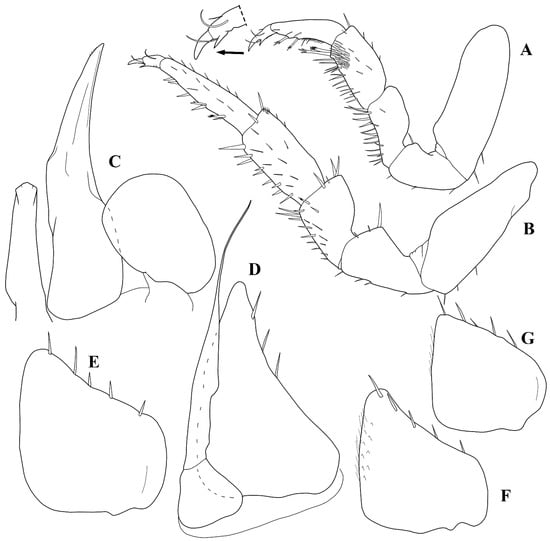
Figure 4.
Trichorhina tucupi n. sp. Male paratype (ISLA15129): (A) pereopod 1; (B) pereopod 7; (C) genital papilla and pleopod 1; (D) pleopod 2; (E) pleopod 3 exopod; (F) pleopod 4 exopod; (G) pleopod 5 exopod.
Zoobank: urn:lsid:zoobank.org:act:30F73932-34E0-41A5-98B2-20FE4CE65DAF
Type material. Holotype. 1M; Brazil, Pará state, Curionópolis municipality, Serra Leste plateau, SL_0004 cave, −5.9637, −49.6497, leg. Bioespeleo; ISLA96998. Paratypes. 8M12F; same data as for holotype, ISLA15129; 12M18F, same data as for holotype, ISLA15963. Additional material. 1M1F, SL_0005 cave, −5.9636, −49.6497, leg. Bioespeleo, ISLA15978; 1F, SL_0005 cave, leg. Bioespeleo, ISLA15960; 1F, SL_0015 cave, −5.96230, −49.65006, leg. Bioespeleo, ISLA15120; 3F, SL_0016 cave, −5.9702, −49.6476, leg. Bioespeleo, ISLA15127; 3F, SL_0016 cave, leg. Bioespeleo, ISLA15967; 8M4F, SL_0031 cave, −5.9723, −49.6435, leg. Bioespeleo, ISLA15106; 2M1F, SL_0031 cave, leg. Bioespeleo, ISLA15958; 3M3F, SL_0033 cave, leg. Bioespeleo, ISLA15868; 1J, SL_0033 cave, 27.VII.2010, leg. Carste, ISLA7033; 1F, SL_0052 cave, leg. Bioespeleo, ISLA15959; 2M3F, SL_0054 cave, −5.98206, −49.62819, leg. Bioespeleo, ISLA15962; 1F, SL_0055 cave, −5.9819, −49.6272, leg. Bioespeleo, ISLA15996; 1F, SL_0069 cave, −5.9840, −49.6206, leg. Bioespeleo, ISLA15126; 9M12F, SL_0093 cave, leg. Bioespeleo, ISLA15125; 7M12F, SL_0093 cave, leg. Bioespeleo, ISLA15130; 10M10F, SL_0093 cave, leg. Bioespeleo, ISLA7432; 1F, SL_0096 cave, −5.9531, −49.6318, 06.VII.2010, leg. Carste, ISLA7035; 5M4F, SL_0097 cave, leg. Bioespeleo, ISLA15875; 10M10F, SL_0097 cave, 28.VII.2010, leg. Carste, ISLA7034; 1M2F, SL_0102 cave, −5.9280, −49.6738, leg. Bioespeleo, ISLA15852; 1M, SL_0103 cave, leg. Bioespeleo, ISLA6191; 1M2F, SL_0104 cave, −5.9289, −49.6750, 11.I.2012, leg. Carste, ISLA6177; 3M19F, SL_0103 cave, −5.8962, −49.6762, 11.I.2012, leg. Carste, ISLA6200; 1M1F, SL_0106 cave, −5.8960, −49.6761, 16.I.2012, leg. Carste, ISLA6198; 8M5F, SL_0108 cave, −5.8938, −49.6764, 12.I.2012, leg. Carste, ISLA6201; 5M5F, SL_0108 cave, leg. Bioespeleo, ISLA6187; 2M1F, SL_0109 cave, −5.8930, −49.6763, leg. Bioespeleo, ISLA6196; 2M, SL_0109 cave, 28.VI.2012, leg. Carste, ISLA6199; 4M5F, SL_0109 cave, leg. Bioespeleo, ISLA15854; 2M1F, SL_0110 cave, leg. Bioespeleo, ISLA15845; 1M3F, SL_0112 cave, −5.8941, −49.6763, leg. Bioespeleo, ISLA6190; 1M, SL_0166 cave, 01-19.III.2016, leg. Spelayon, ISLA39612; 1F, AS-T1C, 29.IV.2017, leg. MP Oliveira, ISLA96084; 1F, AS-T1D, 25.IV.2017, leg. MP Oliveira, ISLA96121; 1M1F, AS-T1E, 4.V.2017, leg. MP Oliveira, ISLA96092; 1F, AS-T2D, 27.IV.2017, leg. MP Oliveira, ISLA96107; 2F, AS-T3A, 3.V.2017, leg. MP Oliveira, ISLA96105; 2F, AS-T3B 1C, −5.9776, −49.6288, 3.V.2017, leg. MP Oliveira, ISLA76577; 1, CM-T1A, −5.9727, −49.6429, 15.III.2017, leg. MP Oliveira, ISLA76574; 1, CM-T1B, −5.9732, −49.6425, 15.III.2017, leg. MP Oliveira, ISLA76575; 1M, CM-T1C, 15.III.2017, leg. MP Oliveira, ISLA96089; 1M, CM-T3A, 16.III.2017, leg. MP Oliveira, ISLA96090; 2F, HBT-T1Bb, 25.IV.2017, leg. MP Oliveira, ISLA96100; 4F, HBT-T1Ca, 25.IV.2017, leg. MP Oliveira, ISLA96094; 3M10F, HBT-T1Db, 25.IV.2017, leg. MP Oliveira, ISLA96098; 2F, HBT-T1E, 25.IV.2017, leg. MP Oliveira, ISLA96079; 1F, HBT-T1Ee, 25.IV.2017, leg. MP Oliveira, ISLA96099; 1F, HBT-T2Ac, 25.IV.2017, leg. MP.
Oliveira, ISLA96106; 2F, HBT-T2Bc, 25.IV.2017, leg. MP Oliveira, ISLA96104; 2M4F, HBT-T2Bd, 25.IV.2017, leg. MP Oliveira, ISLA96091; 4F, HBT-T2Da, 25.IV.2017, leg. MP Oliveira, ISLA96097; 1M1F, HBT-T2Ed, 25.IV.2017, leg. MP Oliveira, ISLA96096; 1F, HBT-T3Aa, 26.IV.2017, leg. MP Oliveira, ISLA96095; 5M15F, HBT-T3Be, 26.IV.2017, leg. MP Oliveira, ISLA96109; 1M1F, HBT-T3Dd, 26.IV.2017, leg. MP Oliveira, ISLA96102; 1M, HBT-T3Eb, 25.IV.2017, leg. MP Oliveira, ISLA96086. Pará state, Canaã dos Carajás municipality, Serra da Bocaina plateau: 2F, SB_0214 cave, 08-22.V.2013, leg. Carste(ISO)0100, ISLA96110; 4F, SB_0219 cave, 10-20.IX.2013, leg. Carste0106, ISLA96115; 1F, SB_0219 cave, 08-22.V.2013, leg. Carste083, ISLA96114; 1F, SB_0219 cave, 10-20.IX.2013, leg. Carste086, ISLA96112; 1F, SB_0219 cave, 08-22.V.2013, leg. Carste0110, ISLA96111; 1M2F, SB_0219 cave, 10-20.IX.2013, leg. Carste098, ISLA96113; 1M1F, SB_0233 cave, −6.34086, −49.90487, 12-22.X.2013, leg. Carste107, ISLA96117; 3F, SB_0242 cave, −6.33450, −49.90553, 12-22.X.2013, leg. Carste102, ISLA96116. Pará state, Canaã dos Carajás municipality, Serra do Tarzan plateau: 1F, ST_0034 cave, −6.3201, −50.1128, 18.VII.2016, leg. Bioespeleo; ISLA43723. Pará state, Parauapebas municipality, Serra Norte plateau: 1F, N5W_0001 cave, −6.07974, −50.13333, leg. Carste 123, ISLA96067.
Diagnosis. Body colorless and robust with continuous outline; cephalon with well-developed lateral lobes; eyes composed of one ommatidium; pereonite 1 epimeron slightly directed frontward, reaching eye; pleotelson triangular, lateral sides concave with pointed apex; antennula distal article with nine apical and sub-apical aesthetascs; pleopod 1 exopod sub-ovoid, outer margin straight.
Description. Maximum body length: 2.5 mm. Colorless body. Body (Figure 2A and Figure 3A) robust, pereonite 1 epimeron slightly directed frontward, reaching the eye, pereonites 2–7 gradually directed backward, pleon outline continuous with that of pereonite 7, pleonites posterior point developed. Dorsum covered with fan-shaped scale-setae (Figure 3A–D). One line of noduli laterales near posterior margins, and far from lateral margins on pereonites 3, 4, and 7 (Figure 2B). Cephalon (Figure 3B,C) with well-developed lateral lobes, suprantennal line bent downwards in middle, frontal line as row of scales, eyes composed of one ommatidium. Pleotelson (Figure 3D) triangular, lateral sides concave with pointed apex. Antennula (Figure 2C and Figure 3E) of three articles, proximal and distal articles subequal in length, distal article with nine apical and sub-apical aesthetascs. Antenna (Figure 2D) reaching back posterior margin of pereonite 1, flagellum shorter than fifth article of peduncle, second article about three times as long as first, with one set of aesthetascs, apical organ short with long free sensilla. Mandibles (Figure 2E) with molar penicil dichotomized, with four branches, left mandible with 2 + 1 penicils, right mandible with 1 + 1 penicils. Maxillula (Figure 2F) inner endite with two setose penicils, outer endite with 4 + 4 teeth, two of them cleft. Maxilla (Figure 3G) with setose and bilobate apex, outer lobe about three times wider than inner lobe. Maxilliped (Figure 2H) basis rectangular bearing sparse scale-setae, palp with two distinct setae on basal article, endite subrectangular, medial seta surpassing distal margin. Pereopod 1 (Figure 3G) carpus bearing transverse antennal grooming brush, and distal seta with double-fringed apex, pereopod 7 (Figure 4B) merus and carpus bearing sparse setae on sternal margin. Uropod (Figure 3F) exopod twice as long as endopod, endopod inserted at same level as exopod.
Male: Pereopod 1 (Figure 4A) merus and carpus bearing dense number of setae on sternal margin. Genital papilla (Figure 4C) with triangular ventral shield and subapical orifices. Pleopod 1 (Figure 4C) exopod sub-ovoid, outer margin straight, endopod about three times longer than exopod, distal portion tapering with small setae on apex. Pleopod 2 (Figure 4D) exopod triangular, outer margin concave with three setae, endopod longer than exopod. Pleopod 3 and 4 exopods (Figure 4E,F) trapezoidal with five setae on distal margin. Pleopod 5 exopod (Figure 4G) triangular with five setae on distal margin.
Etymology. The epithet refers to “tucupi”, as it is called in Portuguese, from the tupi-guarani language, ticu = juice + pi = raw thing: consists of a yellow sauce extracted from wild manioc root, widely used for cooking in Northern Brazil, including in the state of Pará, where the species occurs.
Taxonomic remarks. In Brazil, two known species of Trichorhina present one ommatidia, as Trichorhina tucupi n. sp., Trichorhina macrops, and Trichorhina tomentosa. Trichorhina tomentosa occurs in Tropical America, with records in greenhouses worldwide [36]. Trichorhina tucupi n. sp. differs from T. tomentosa by the number of noduli laterales on pereonite VII (one pair in the new species versus two in T. tomentosa), the number of teeth on maxillula endite (4 + 4 in the new species versus 4 + 3 in T. tomentosa), the absence of a modification on the ischium of pereopod 7, which is present in T. tomentosa. Trichorhina macrops is an epigean species collected in Northeastern Brazil [20]. Despite sharing the condition of one ommatidium, it is expressively larger and darker in T. macrops, which indeed is the reason for the species’ name. Also, T. tucupi n. sp. is colorless, while T. macrops have small light brown spots along the body. Differences are also observed in the pleon outline in relation to pereonite 7, which is continuous in T. tucupi n. sp. and not continuous in T. macrops. The lateral lobes on cephalon are well-developed in T. tucupi n. sp. versus small in T. macrops. Moreover, pleopod 1 exopod is sub-ovoid in T. tucupi n. sp. and heart-shaped in T. macrops.
Trichorhina tacaca Cardoso and Bastos-Pereira n. sp.
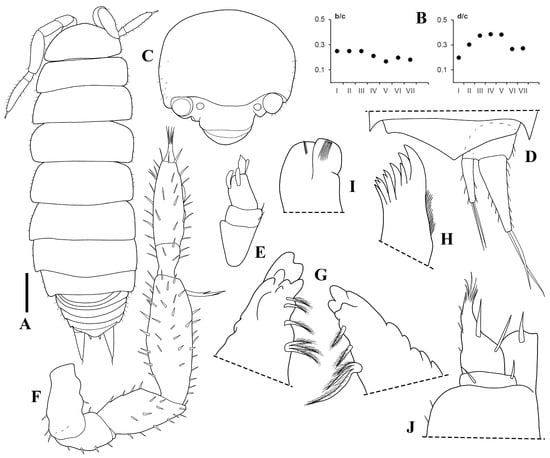
Figure 5.
Trichorhina tacaca n. sp. Male paratype (ISLA6296): (A) habitus, dorsal view; (B) b/c and d/c coordinates of noduli laterales; (C) cephalon, frontal view; (D) pleonite 5, pleotelson and uropod, dorsal view; (E) antennula; (F) antenna; (G) left and right mandible; (H) maxillula; (I) maxilla; (J) maxilliped. Scale-bar: 0.2 mm.
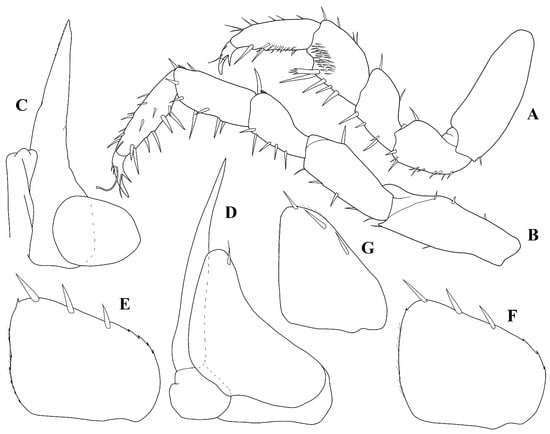
Figure 6.
Trichorhina tacaca n. sp. Male paratype (ISLA6296): (A) pereopod 1; (B) pereopod 7; (C) pleopod 1; (D) pleopod 2; (E) pleopod 3 exopod; (F) pleopod 4 exopod; (G) pleopod 5 exopod.
Zoobank: urn:lsid:zoobank.org:act:52E10169-1171-4870-911A-B3AAA2FF8DD8
Type material. Holotype. 1M; Brazil, Pará state, Parauapebas municipality, Serra Norte plateau, N5SM1_0028 (GEM-1782) cave, −6.1089, −50.1351, leg. Carste, ISLA15806. Paratypes. 2M, N5SM1_0028 (GEM-1782) cave, same data as holotype, ISLA6296. Additional material. Brazil, Pará state, Parauapebas municipality, Serra Norte plateau: 1F in slide, N5SM1_00036 (GEM 1790) cave, −6.1063, −50.1371, 28.VIII.2010, leg. Carste, ISLA7027; 1M in slide, N5SM2_0042 (GEM 1770) cave, −6.1323, −50.1364, 30.X.2010, leg. Carste, ISLA15808; 1F in slide, N5SM2_0036 (GEM 1713) cave, −6.1332, −50.1345, 30.X.2010, leg. Carste, ISLA6302; 1F, N5SM2_0023 (GEM-1722) cave, −6.1351, −50.1349, 30.X.2010, leg. Carste, ISLA15806 1F, N5SM2_0016 (GEM-1734) cave, −6.1381, −50.1331, 30.X.2010, leg. Carste, ISLA9700.
Diagnosis. Body colorless and slender; cephalon with lateral lobes not developed, suprantennal line bent downwards in the middle, eyes absent; pleotelson triangular with straight lateral margins and pointed apex, antennula distal articles with two apical and two sub-apical aesthetascs, antenna fifth article of peduncle with medial part wider than base; pleopod 1 exopod sub-ovoid, outer margin straight, endopod about three times longer than exopod with distal portion tapering.
Description.
Maximum body length: 1.5 mm. Colorless body. Body (Figure 5A) slender, pereonites gradually directed backward. Dorsum covered with fan-shaped scale-setae. One line of noduli laterales inserted near posterior margin, and far from lateral margin on pereonites 3–5 (Figure 5B). Cephalon (Figure 5C) lateral lobes not developed, suprantennal line bent downwards in middle, eyes absent. Pleotelson (Figure 5D) triangular, lateral margins straight with pointed apex. Antennula (Figure 5E) of three articles, proximal and distal articles subequal in length, distal article with two apical and two sub-apical aesthetascs. Antenna (Figure 5F) reaching back posterior margin of pereonite 2, fifth article of peduncle medial part wider than base, flagellum as long as fifth article of peduncle, second article about three times as long as first, with two sets of aesthetascs, apical organ short with long free sensilla. Mandibles (Figure 5G) with molar penicil dichotomized, with three branches, left mandible with 2 + 1 penicils, right mandible with 1 + 1 penicils. Maxillula (Figure 5H) inner endite with two setose penicils, outer endite with 4 + 3 teeth, two of them cleft at apex. Maxilla (Figure 5G) with setose and bilobate apex, outer lobe wider than inner lobe. Maxilliped (Figure 5J) basis rectangular bearing sparse scale-setae, palp with two distinct setae on basal article, endite subrectangular, medial seta surpassing distal margin. Pereopod 1 (Figure 6A) carpus bearing transverse antennal grooming brush, dactylus with short inner claw, ungual seta simple shorter than outer claw, dactylar seta simple longer than outer claw; pereopod 7 (Figure 5B) merus and carpus bearing sparse setae on sternal margin. Uropod (Figure 5D) exopod twice as long as endopod, endopod inserted at same level as exopod. Pereopods with no modifications.
Male: Genital papilla (Figure 6C) with triangular ventral shield and subapical orifices. Pleopod 1 (Figure 6C) exopod sub-ovoid, outer margin straight, endopod about three times longer than exopod, distal portion tapering with small setae on apex. Pleopod 2 (Figure 6D) exopod triangular, outer margin concave with one seta, endopod longer than exopod. Pleopod 3 and 4 exopods (Figure 6E,F) trapezoidal with three setae on distal margin. Pleopod 5 exopod (Figure 6G) triangular with three setae on distal margin.
Etymology. The epithet refers to “tacacá”, from the tupi-guarani language, meaning gum, mucilage: consists of a traditional soup from the Brazilian state of Pará, where the species occurs.
Taxonomic remarks. Currently, 28 anophthalmic species are known for Trichorhina worldwide, 14 of them occurring in Brazil [1,10]. Two of these species have no records in caves and are widespread in South America. However, some material deserves more detailed analysis to confirm this distribution. One species, Trichorhina pittieri (Pearse, 1921), was first collected in Venezuela but then recorded in British Guyana and in Brazil (Pará state). However, as mentioned by Campos-Filho et al. [10], there are some incongruences in the species identified by Lemos de Castro [37]. Future examination of these species is needed to ensure their taxonomy. The second species is Trichorhina brasilensis, with records in Paraguay and Southern Brazil, in the state of Santa Catarina [1].
Considering the 12 anophthalmic species of Trichorhina recorded for caves in Brazil, two of them described in the present work, differences are observed in body shape, cephalon lateral lobes, suprantennal line, number of aesthetascs in antennula, number of penicils in mandibular molar penicil, number of teeth on maxillula outer endite, male pleopod 1 exopod shape and setae on male pleopod 2 exopod outer margin. All of them are colorless, except for Trichorhina marianae Campos-Filho, Gallão and Bichuette, 2023, which is pale yellowish [10].
Trichorhina tacaca n. sp. is similar to Trichorhina baiana Campos-Filho, Gallão and Bichuette, 2023, and Trichorhina bessiae Campos-Filho, Carpio-Díaz and Bichuette, 2023, by the slender body, pleon outline continuous with pereon and not-developed cephalon lateral lobes [10]. These species differ by the number of aesthetascs of antennula (T. tacaca n. sp.: 2 apical and 2 subapical; T. baiana: 6 apical; T. bessiae: at least 16), by the number of branches on mandibular molar penicil (T. tacaca n. sp.: dicothomized with 3 branches; T. baiana: dicothomized with 6 branches; T. bessiae: simple); by the number and type of teeth on maxillula outer endite (T. tacaca n. sp.: 4 + 3, 2 of them cleft; T. baiana: 4 + 4, 3 of them cleft; T. bessiae: 3 + 4); by the shape of male pleopod I exopod (T. tacaca n. sp.: subovoid; T. baiana: ovoid; T. bessiae: sub-rectangular), and by the number of setae on the outer margin of male pleopod 2 exopod (1 in T. tacaca n. sp. and T. baiana versus 3 in T. bessiae).
Trichorhina piloi Cardoso, Bastos-Pereira and Oliveira n. sp.
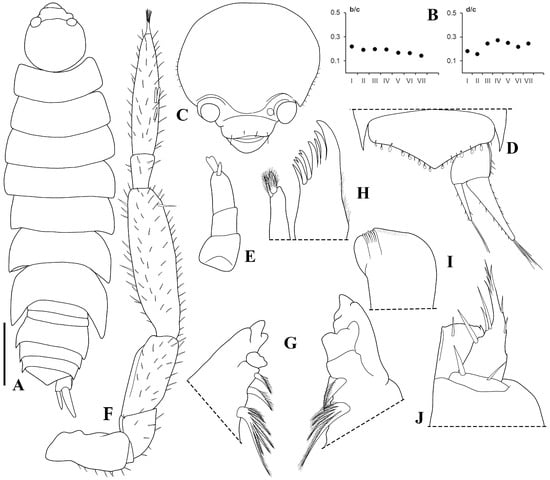
Figure 7.
Trichorhina piloi n. sp. Male paratype (ISLA44679). (A) habitus, dorsal view; (B) b/c and d/c coordinates of noduli laterales; (C) cephalon, frontal view; (D) pleonite 5, pleotelson and uropod, dorsal view; (E) antennula; (F) antenna; (G) left and right mandible; (H) maxillula; (I) maxilla; (J) maxilliped. Scale-bar: 0.2 mm.
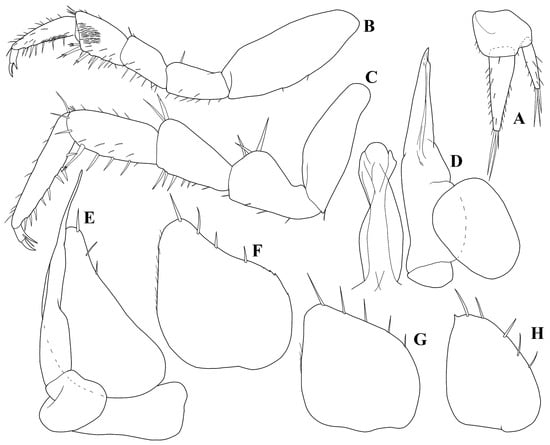
Figure 8.
Trichorhina piloi n. sp. male paratype (ISLA44679): (A) uropod; (B) pereopod 1; (C) pereopod 7; (D) pleopod 1; (E) pleopod 2; (F) pleopod 3 exopod; (G) pleopod 4 exopod; (H) pleopod 5 exopod.
Zoobank: urn:lsid:zoobank.org:act:32CD873D-F6D9-47E8-B71F-3A219D9C8907
Type material. Holotype. 1M (in slide); Brazil, Pará state, Canaã dos Carajás municipality, Serra Sul plateau, S11C_0068 cave, −6.4014, −50.3795, 15.IV.2016, leg. Bioespeleo, ISLA44679. Paratypes. 1F, S11C_0068 cave, 02.IX.2015, leg. Bioespeleo, ISLA44680; 1F, S11C_0068 cave, 19.IV.2016, leg. Bioespeleo, ISLA96070; 1M, S11C_0068 cave, 15.IV.2016, leg. Bioespeleo, ISLA44679. Additional material. 1M in slide, S11C_0070 cave, −6.4011, −50.3792, 19.IV.2016, leg. Bioespeleo, ISLA96073; 1M1F, S11C_0082 cave, −6.4029, −50.3812, 04.IX.2015, leg. Bioespeleo, ISLA44681; 1M, S11C_0091 cave, 12.III.2016, leg. Bioespele, ISLA96069; 1F in slide, S11C_0120 cave, 11.IV.2016, leg. Bioespeleo, ISLA44682; 1F in slide, S11C_0120 cave, 10.VIII.2015, leg. Bioespeleo, ISLA96071; 1F in slide, S11C_0205 cave, 24.VII.2015, leg. Bioespeleo, ISLA96072; 1M in slide, S11B_0186 cave, −6.3397, −50.4155, 21.III.2019, leg. Ativo, ISLA96064; 1F, S11B_0006 cave, −6.3565, −50.3901, 19.I.2019, leg. Ativo, ISLA96065. Brazil, Pará state, Parauapebas municipality, Serra Norte plateau: 1F, N8_0038 cave, −6.1737, −50.1474, 17.VII-4.VIII.2014, leg. Carste (iso)1187, ISLA37884; 1F, N8_0038 cave, 02.-29.IV.2015, leg. Carste (iso)0671, ISLA 30510; 1M1F, N8_0038 cave, 02.-29.IV.2015, leg. Carste (iso)0671, ISLA 30511; 1M, N8_0038 cave, 02.-29.IV.2015, leg. Carste (iso)0659, ISLA 30498; 1M, N8_0019 cave, −6.1697, −50.1574, 02.-29.IV.2015, leg. Carste (iso)0671, ISLA 30504; 1F, N1_0037 cave, −6.030922, −50.27478, 04.IX-06.X.2014, leg. CARSTE(iso)1197, ISLA37894.
Diagnosis. Body colorless and slender; cephalon with lateral lobes not developed, suprantennal line bent downwards in middle; eyes absent; pleotelson triangular with lateral margins slightly sinuous and right-angle apex; antennula distal articles with two aesthetascs; pleopod 1 exopod sub-rectangular, outer margin straight, endopod about three times longer than exopod with distal portion tapering.
Description. Maximum body length: 2.5 mm. Colorless body. Body (Figure 7A) slender, pereonites gradually directed backward. Dorsum covered with fan-shaped scale-setae. One line of noduli laterales inserted progressively near posterior margin, and near lateral margin on pereonites 1 and 2, far from lateral margin on pereonite 4 and 7 (Figure 7B). Cephalon (Figure 7C) lateral lobes not developed, suprantennal line bent downwards in middle, eyes absent. Pleotelson (Figure 7D) triangular, lateral margins sinuous with pointed apex. Antennula (Figure 7E) of three articles, proximal and distal articles subequal in length, distal article with two apical aesthetascs. Antenna (Figure 7F) reaching back posterior margin of pereonite 1, flagellum as long as fifth article of peduncle, second article about three times as long as first, with two sets of aesthetascs, apical organ short with long free sensilla. Mandibles (Figure 7G) with molar penicil dichotomized, with five to seven branches, left mandible with 2 penicils, right mandible with 1 + 1 penicils. Maxillula (Figure 7H) inner endite with two setose penicils, outer endite with 4 + 4 teeth, two of them cleft at apex. Maxilla (Figure 7I) with setose and bilobate apex, outer lobe wider than inner lobe. Maxilliped (Figure 7J) basis rectangular, palp with two distinct seta with double-fringed apex, dactylus with short inner claw, ungual seta and dactylar seta simple as long as outer claw; pereopod 7 (Figure 8C) merus and carpus bearing sparse setae on sternal margin. Male: Genital papilla (Figure 8D) with triangular ventral shield and subapical orifices. Pleopod 1 (Figure 8D) exopod sub-rectangular, outer margin straight, endopod about three times longer than exopod, distal portion tapering with small setae on apex. Pleopod 2 (Figure 8E) exopod triangular, outer margin concave with three setae, endopod longer than exopod. Pleopod 3–5 exopods (Figure 8F–H) trapezoidal with four to five setae on distal margin.
Etymology. The epithet honors Luis Beethoven Piló, a brilliant geographer and person who made important contributions to Brazilian speleology, especially studying Amazon iron ore caves, who unfortunately left us in 2022.
Taxonomic remarks. Trichorhina piloi n. sp. is similar to Trichorhina tacaca n. sp. due to the absence of eyes, slender body, and not-developed cephalon lateral lobes, as well as to Trichorhina baiana and Trichorhina bessiae Campos-Filho, Carpio-Díaz & Bichuette, 2023 [10]. These species differ by the number of aesthetascs of antennula (T. piloi n. sp.: 2 apical; T. tacaca n. sp.: 2 apical and 2 subapical; T. baiana: 6 apical; T. bessiae: at least 16), by the branches on mandible molar penicil (T. piloi n. sp.: dicothomized with 5 branches, T. tacaca n. sp.: dicothomized with 3 branches; T. baiana: dicothomized with 6 branches; T. bessiae: simple); by the number and type of teeth on maxillula outer endite (T. piloi n. sp.: 4 + 3, 2 of them cleft; T. tacaca n. sp.: 4 + 3, 2 of them cleft; T. baiana: 4 + 4, 3 of them cleft; T. bessiae: 3 + 4); and by the shape of male pleopod I exopod (T. tacaca n. sp.: subovoid; T. baiana: ovoid).
3.2. Morphometry
The morphometric variables were independent of each other (rho < 0.70 for all cases, Figure S1), indicating that they present distinct conditions in the species. Morphometric data distinguishes the three new Trichorhina species (Figure 9, Table 1). We found that ep1_ep4 (reason between width of epimera 1 and 4), ep1_ep7 (reason between width of epimera 1 and 7), cep_ep1 (reason between width of cephalon and epimeron 1), and p1_mer (reason between length and width of P1 merus) were the most informative morphometric variables for distinguishing among the evaluated species (Table S2). The variables ep1_ep4, ep1_ep7, and cep_ep1 accounted for 65.66% of the species differentiation along CV1, whereas p1_mer primarily contributed to the separation along CV2 (Table S2). When considering only specimens of T. tucupi n. sp., we did not find morphometric differences between specimens inhabiting caves and those from forest soils (permutational multivariate analysis with p > 0.005), meaning that cave-dwelling and epigean T. tucupi n. sp. exhibit similar morphological patterns.
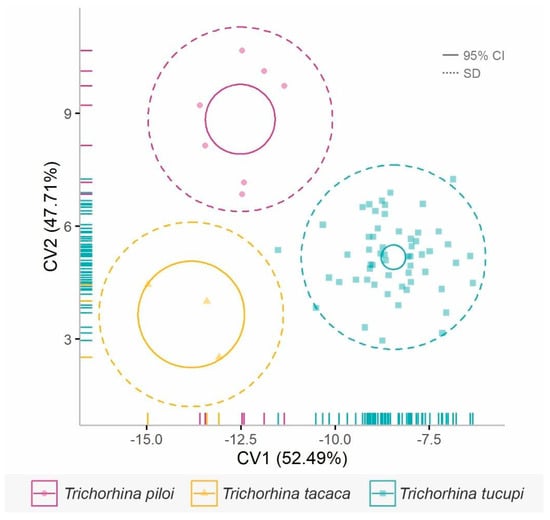
Figure 9.
Canonical Variates Analysis (CVA) of morphometric ratios obtained from the new Trichorhina specimens. The solid circle line represents the 95% confidence interval (95% CI) for the group mean, while the dashed line indicates the standard deviation for the entire population (SD).

Table 1.
Morphometric distinction among the three newly identified Trichorhina species. We present the results of the permutational multivariate analysis of variance (F and p values), conducted using the CVA scores.
4. Discussion
Our study reveals three newly depigmented species of Trichorhina, two of which are anophthalmic (Trichorhina tacaca n. sp. and Trichorhina piloi n. sp.), while one has one single ommatidium (Trichorhina tucupi n. sp.). Among these species, T. tucupi n. sp. is found in both caves and forest soils within ferruginous Amazon geosystems, whereas T. piloi n. sp. and T. tacaca n. sp. has only been sampled in caves. This region is notable for its rich biodiversity and strong potential for further discoveries, given the concentration of environmental studies [38,39,40,41]. We observed that, for this group, some classical troglomorphisms, such as depigmentation and the reduction/absence of ocular structures, do not necessarily indicate restriction to the subterranean realm. Complementarily, our morphometric approach supported the distinction among these species. Furthermore, no morphometric differences were detected between cave-dwelling and forest soil specimens of T. tucupi n. sp., highlighting the ability of these populations to disperse across distinct landscape compartments.
The absence of pigmentation and anophthalmia are commonly associated with troglobitic species; however, a significant knowledge gap remains regarding what constitutes troglomorphism in isopods. Alpioniscus fragilis (Budde-Lund, 1909) [42] (Trichoniscidae), for instance, exhibits these traits but inhabits both endogean and aquatic environments [43,44]. Likewise, Alpioniscus thanit Taiti & Argano, 2009 [45], is also depigmented and anophthalmic, occurring in both cave and endogean habitats, yet it is not classified as troglobitic like its congeners [44,45]. These examples underscore the remarkable adaptive plasticity of such species, enabling them to occupy discontinuous habitats provided that suitable ecological conditions are met [44]. In the case of the newly described Trichorhina species, depigmentation and anophthalmia may reflect adaptations to specific environmental features, such as the absence of or reduction in light, which are not exclusive to caves but also occur in soil layers, rocky substrates, and the forest leaf litter of the Amazon. Therefore, their typical subterranean morphology does not appear to pose a disadvantage in terms of their ability to inhabit endogean ecosystems.
Although discussions on this issue are still incipient for isopods, the convergent or parallel evolution among animals living in deep soil and subterranean environments is being widely explored for several taxonomic groups [46]. Luo et al. [47] investigated the morphology of Bergrothia saulcyi beetles (Reitter, 1877) (Coleoptera) through multiple approaches. The authors elucidated a morphological pattern consistent with the endogean or cave habitat, although the beetles were found to be associated with leaf litter not only in this work, but also in that of Hlavác [48]. Therefore, Luo et al. [47] indicated that it is plausible to assume that this species is adapted to occasionally live in deeper soil layers, or rock cracks. From this perspective, it is plausible to assume that the species of Trichorhina described here, like other congenerics, transit from leaf litter and soil layers to the cave environment. Since they are subject to similar environmental conditions and, therefore, similar selective pressures to the subterranean ones, this is possibly why they present with a convergent morphology. The absence of significant differences in the morphology of individuals from epigean and hypogean samples of Trichorhina tucupi n. sp. corroborates such hypotheses of adaptations that allow them to deal with a gradient of environmental conditions.
The specimens of Trichorhina tacaca n. sp. and Trichorhina piloi n. sp. analyzed in this study were exclusively collected from caves, and our results suggest their potential to use and occupy different landscape compartments. These species occur in regions of Carajás with extensive cave inventories. However, no studies specifically sampling Isopoda in forests or other surface ecosystems have been conducted. In contrast, T. tucupi n. sp. originates from the Serra Leste region, where a targeted study applied four distinct methods to sample Isopoda in forests and savannas, resulting in the collection of specimens from five different families [28]. In this sense, systematic collections in epigean habitats surrounding caves in other parts of Carajás are likely to yield additional specimens of T. tacaca n. sp. and T. piloi n. sp. For this purpose, we strongly recommend using hay-baited traps, as 80% of Trichorhina specimens were collected with this method [28]. Despite the extensive inventories in caves in Carajás, additional collections may expand the distribution of T. tacaca n. sp. and T. piloi n. sp., in addition to providing new specimens to strengthen the distinct morphometric patterns revealed in the present study.
5. Conclusions
This study not only describes three new taxa but also introduces statistical methods to distinguish Trichorhina species and explore potential morphological patterns within the genus. These findings are particularly significant, as accurate species identification and evidence of its potential restriction to subterranean habitats have critical implications for the conservation of species, caves, and surrounding landscapes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/taxonomy5020030/s1, Figure S1: Spearman correlation between morphometric variables measured in the specimens. Variables were considered autocorrelated when rho > |0.700|; Table S1: Morphometric variables measured in 68 individuals of the three new Amazonian species of Trichorhina, according to mountain range, location, and habitat type where they were sampled; Table S2. Coefficients from Canonical Variates Analysis (CVA) based on morphometric ratios obtained from Trichorhina specimens (Oniscidea: Platyarthridae). CV: canonical variate axis (linear discriminants). The highest absolute values for each axis, highlighted in bold, indicate the morphometric variables that most strongly contribute to species differentiation along that axis.
Author Contributions
Conceptualization, G.M.C., R.B.-P. and M.P.A.d.O.; methodology, G.M.C. and R.B.-P.; software, R.B.-P. and M.P.A.d.O.; validation, G.M.C., R.B.-P. and M.P.A.d.O.; formal analysis, R.B.-P. and M.P.A.d.O.; investigation, G.M.C. and R.B.-P.; resources, M.P.A.d.O.; data curation, R.L.F.; writing—original draft preparation, G.M.C. and R.B.-P.; writing—review and editing, G.M.C., R.B.-P., M.P.A.d.O. and R.L.F.; visualization, M.P.A.d.O.; supervision, R.L.F.; project administration, M.P.A.d.O.; funding acquisition, M.P.A.d.O. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank Vale’s Environmental Licensing and Speleology Department and BioEspeleo Consultoria Ambiental for the financial support to carry out this project. Also, the authors thank the CNPq (National Council for Scientific and Technological Development) and the productive scholarship of RLF (CNPq n. 302925/2022-8).
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the team from BioEspeleo Consultoria Ambiental for administrative and logistical support during this project. Special thanks to Tatiana Marcela (Vale’s Environmental Licensing and Speleology Department) for promoting this project.
Conflicts of Interest
The author Marcus Paulo Alves Oliveira is employed by BioEspeleo Consultoria Ambiental. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Abbreviations
The following abbreviations are used in this manuscript:
| ISLA | Subterranean Invertebrate Collection of Lavras |
| UFLA | Federal University of Lavras (UFLA) in Lavras, Minas Gerais, Brazil |
| HBT | Hay-baited traps |
| HC | Hand collection |
| SS | Soil samples |
| CVA | Canonical Variates Analysis |
| LDA | Linear Discriminant Analysis |
References
- Campos-Filho, I.S.; Cardoso, G.M.; Aguiar, J.O. Catalogue of terrestrial isopods (Crustacea, Isopoda, Oniscidea) from Brazil: An update with some considerations. Nauplius 2018, 26, e2018038. [Google Scholar] [CrossRef]
- Campos-Filho, I.S.; Cardoso, G.M.; Aguiar, J.O. New species and first record of Alloniscus Dana, 1854 (Isopoda: Oniscidae: Alloniscidae) from Brazil. Nauplius 2018, 26, e2018014. [Google Scholar] [CrossRef]
- Campos-Filho, I.S.; Fernandes, C.M.; Cardoso, G.M.; Bichuette, M.E.; Aguiar, J.O.; Taiti, S. Two new species and new records of terrestrial isopods (Crustacea, Isopoda, Oniscidea) from Brazilian caves. Zootaxa 2019, 4564, 422–448. [Google Scholar] [CrossRef] [PubMed]
- Campos-Filho, I.S.; Fernandes, C.M.; Cardoso, G.M.; Bichuette, M.E.; Aguiar, J.O.; Taiti, S. New species and new records of terrestrial isopods (Crustacea, Isopoda, Oniscidea) of the families Philosciidae and Scleropactidae from Brazilian caves. Eur. J. Taxon. 2020, 606, 1–38. [Google Scholar] [CrossRef]
- Cardoso, G.M.; Bastos-Pereira, R.; Souza, L.A.; Ferreira, R.L. New troglobitic species of Xangoniscus (Isopoda: Styloniscidae) from Brazil, with notes on their habitats and threats. Zootaxa 2020, 4819, 84–108. [Google Scholar] [CrossRef]
- Cardoso, G.M.; Bastos-Pereira, R.; Souza, L.A.; Ferreira, R.L. New cave species of Pectenoniscus Andersson, 1960 (Isopoda: Oniscidea: Styloniscidae) and an identification key for the genus. Nauplius 2020, 28, e2020039. [Google Scholar] [CrossRef]
- Cardoso, G.M.; Bastos-Pereira, R.; Souza, L.A.; Ferreira, R.L. Chaimowiczia: A new Iuiuniscinae genus from Brazil (Oniscidea, Synocheta, Styloniscidae) with the description of two new troglobitic species. Subterr. Biol. 2021, 39, 45–62. [Google Scholar] [CrossRef]
- Cardoso, G.M.; Ferreira, R.L. New troglobitic species of Pectenoniscus Andersson, 1960 (Isopoda: Oniscidea: Styloniscidae) from Bahia state, Brazil. Stud. Neotrop. Fauna Environ. 2024, 59, 202–223. [Google Scholar] [CrossRef]
- Campos-Filho, I.S.; Gallo, J.S.; Gallão, J.E.; Torres, D.F.; Carpio-Díaz, Y.M.; López-Orozco, C.M.; Borja-Arrieta, R.; Taiti, S.; Bichuette, M.E. Expanding the knowledge on the diversity of the cavernicolous Styloniscidae Vandel, 1952 (Oniscidea, Synocheta) from Brazil, with descriptions of two new species from the semiarid karst regions. ZooKeys 2022, 1101, 35–55. [Google Scholar] [CrossRef]
- Campos-Filho, I.S.; López-Orozco, C.M.; Carpio-Díaz, Y.M.; Borja-Arrieta, R.L.; Gallão, J.E.; Taiti, S.; Sfenthourakis, S.; Bichuette, M.E. Everything is similar, everything is different! Trichorhina (Oniscidea, Platyarthridae) from Brazilian caves, with descriptions of 11 new species. Biota Neotrop. 2024, 23, e20231545. [Google Scholar] [CrossRef]
- Boyko, C.B.; Campos-Filho, I.S.; Hadfield, K.A.; Hughes, T.; Merrin, K.L.; Ota, Y.; Poore, G.C.B. (Eds) (2025). World Marine, Freshwater and Terrestrial Isopod Crustaceans database. Trichorhina Budde-Lund. 1908. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=249422 (accessed on 31 May 2025).
- Schmidt, C. Contribution to the phylogenetic system of the Crinocheta (Crustacea, Isopoda). Part 2 (Oniscoidea to Armadillidiidae). Zoosystematics Evol. 2003, 79, 3–179. [Google Scholar] [CrossRef]
- Carpio-Díaz, Y.M.; López-Orozco, C.M.; Campos-Filho, I.S.; Navas, G.R. Terrestrial isopods (Isopoda: Oniscidea) of the Botanical Garden of Cartagena “Guillermo Piñeres”, Colombia, with the description of three new species. Arthropoda Sel. 2018, 27, 301–318. [Google Scholar] [CrossRef]
- Carpio-Díaz, Y.M.; López-Orozco, C.M.; Borja-Arrieta, R.; Campos-Filho, I.S. A new species and first record of Trichorhina Budde-Lund, 1908 (Isopoda, Oniscidea, Platyarthridae) from the Department of Norte de Santander, Colombia. Nauplius 2021, 29, e2021028. [Google Scholar] [CrossRef]
- Javidkar, M.; Cooper, S.J.B.; King, R.A.; Humphreys, W.F.; Austin, A. Molecular phylogenetic analyses reveal a new southern hemisphere oniscidean family (Crustacea: Isopoda) with a unique water transport system. Invertebr. Syst. 2015, 29, 554–577. [Google Scholar] [CrossRef]
- Dimitriou, A.C.; Taiti, S.; Sfenthourakis, S. Genetic evidence against monophyly of Oniscidea implies a need to revise scenarios for the origin of terrestrial isopods. Sci. Rep. 2019, 9, 18508. [Google Scholar] [CrossRef]
- Lemos de Castro, A. Trichorhina heterophthalma nueva especie de isopodo terrestre cavernicola de Cuba. Poeyana 1964, 2a, 1–7. [Google Scholar]
- Campos-Filho, I.S.; Araujo, P.B.; Bichuette, M.E.; Trajano, E.; Taiti, S. Terrestrial isopods (Crustacea: Isopoda: Oniscidea) from Brazilian caves. Zool. J. Linn. Soc. 2014, 172, 360–425. [Google Scholar] [CrossRef]
- Campos-Filho, I.S.; Bichuette, M.E.; Taiti, S. Three new species of terrestrial isopods (Crustacea, Isopoda, Oniscidea) from Brazilian caves. Nauplius 2016, 24, e2016001. [Google Scholar] [CrossRef]
- Souza-Kury, L.A. Notes on Trichorhina I. Two new species from northeastern Brazil. Rev. Suisse De Zool. 1993, 100, 157–210. [Google Scholar] [CrossRef]
- Campos-Filho, I.S.; Mise, K.M.; Sessegolo, G.C. A new species of Trichorhina Budde-Lund, 1908 (Isopoda: Oniscidea: Platyarthridae) from Paraná caves, southern Brazil. Nauplius 2015, 23, 112–119. [Google Scholar] [CrossRef]
- Budde-Lund, G. Landisopoder fra Venezuela, insamlede af Dr. F. Meinert. Entomol. Meddelelser 1893, 4, 111–129. [Google Scholar]
- Souza, L.A.; Araújo, J.P.; Campos-Filho, I.S. The genus Trichorhina Budde-Lund in Brazil, with description of seven new species (Isopoda, Oniscidea, Platyarthridae). Iheringia Série Zool. 2011, 101, 239–261. [Google Scholar] [CrossRef]
- Andersson, Å. South American terrestrial isopods in the collection of the Swedish State Museum of Natural History. Ark. För Zool. 1960, 12, 537–570. [Google Scholar]
- Campos-Filho, I.S.; Montesanto, G.; Araujo, P.B.; Taiti, S. New species and new records of terrestrial isopods (Crustacea, Isopoda, Oniscidea) from Brazil. Iheringia Série Zool. 2017, 107, e2017034. [Google Scholar] [CrossRef]
- Souza-Kury, L.A. Two new species of Trichorhina from Brazilian Amazonia. Crustaceana 1997, 70, 180–190. [Google Scholar] [CrossRef]
- Pearse, A. Crustacea from Lake Valencia, Venezuela. Proc. United States Natl. Mus. 1921, 59, 459–462. [Google Scholar] [CrossRef]
- Oliveira, M.P.A.; Bastos-Pereira, R.; Torres, S.H.S.; Pereira, T.S.; Batista, F.M.; Alves, J.P.; Iniesta, L.F.M.; Bouzan, R.S.; Chagas-Jr, A.; Prous, X.; et al. Choosing sampling methods for Chilopoda, Diplopoda and Isopoda (Oniscidea): A case study for ferruginous landscapes in Brazilian Amazonia. Appl. Soil Ecol. 2019, 143, 181–191. [Google Scholar] [CrossRef]
- Anderson, L.E. Hoyer’s Solution as a Rapid Permanent Mounting Medium for Bryophytes. Bryologist 1954, 57, 242. [Google Scholar] [CrossRef]
- Vandel, A. Isopodes terrestres. (Deuxième Partie). In Faune de France; Fédération Française des Sociétes de Sciences Naturelles, Ed.; Fédération Française des Sociétés de Sciences Naturelles: Paris, France, 1962; Volume 66, pp. 417–931. [Google Scholar]
- Bastos-Pereira, R.; Ferreira, R.L. Spelaeogammarus uai (Bogidielloidea: Artesiidae): A new troglobitic amphipod from Brazil. Zootaxa 2017, 4231, 38–50. [Google Scholar] [CrossRef]
- Pellegrini, T.G.; Ferreira, R.L. Two new troglobitic Coarazuphium Gnaspini, Godoy & Vanin 1998 species of ground beetles from iron ore Brazilian caves (Coleoptera: Carabidae: Zuphiini). Zootaxa 2017, 4306, 551–566. [Google Scholar] [CrossRef]
- Souza, M.F.V.R.; Ferreira, R.L. Pandora is on Earth: New species of Eukoenenia (Palpigradi) emerging at risk of extinction. Invertebr. Syst. 2018, 32, 581–604. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. Available online: https://www.R-project.org/ (accessed on 31 May 2025).
- Schmidt, C.; Leistikow, A. Catalogue of genera of the terrestrial Isopoda (Crustacea: Isopoda: Oniscidea). Steenstrupia 2004, 28, 1–118. [Google Scholar]
- Schmalfuss, H. World catalog of terrestrial isopods (Isopoda: Oniscidea). Stuttg. Beiträge Zur Naturkunde Ser. A 2003, 654, 1–341. [Google Scholar]
- Lemos de Castro, A. Isópodos terrestres da Amazônia Brasileira (Isopoda, Oniscoidea). Atas Do Simpósio Sôbre A Biota Amazônica 1967, 5, 311–336. [Google Scholar]
- Fraga, R.; Tavares, V.; Simões, M.H.; Prous, X.; Girolamo-Neto, C.; Brandi, I.V.; Oliveira, G.; Trevelin, L.C. Caves as wildlife refuges in degraded landscapes in the Brazilian Amazon. Sci. Rep. 2023, 13, 6055. [Google Scholar] [CrossRef]
- Trevelin, L.C.; Gastauer, M.; Prous, X.; Nicácio, G.; Zampaulo, R.; Brandi, I.; Oliveira, G.; José, O.; Siqueira, J.O.; Jaffé, R. Biodiversity surrogates in Amazonian iron cave ecosystems. Ecol. Indic. 2019, 101, 813–820. [Google Scholar] [CrossRef]
- Jaffé, R.; Prous, X.; Calux, A.; Gastauer, M.; Nicacio, G.; Zampaulo, R.; Souza-Filho, P.W.M.; Oliveira, G.; Brandi, I.V.; Siqueira, J.O. Conserving relics from ancient underground worlds: Assessing the influence of cave and landscape features on obligate iron cave dwellers from the Eastern Amazon. PeerJ 2018, 6, e4531. [Google Scholar] [CrossRef]
- Oliveira, M.P.; Ferreira, R.L. Extending beyond individual caves: A graph theory approach broadening conservation priorities in Amazon iron ore caves. PeerJ 2024, 12, e16877. [Google Scholar] [CrossRef]
- Budde-Lund, G. Land-Isopoden. In Zoologische und Anthropologische Ergebnisse einer Forschungsreise in Westlichen und Zentralen Südafrika Ausgeführt in den Jahren 1903–1905 mit Unterstützung der Kgl; Schultze, L., Ed.; Denkschriften der Medicinisch-Naturwissenschaftlichen Gesellschaft zu Jena; Biodiversity Heritage Library: Washington, DC, USA, 1909; Volume 14, pp. 53–70. [Google Scholar]
- Taiti, S.; Argano, R. Oniscidea di Sardegna (Crustacea, Isopoda). Conserv. Habitat Invertebr. 2011, 5, 163–222. [Google Scholar]
- Taiti, S.; Argano, R.; Marcia, P.; Scarpa, F.; Sanna, D.; Casu, M. The genus Alpioniscus Racovitza, 1908 in Sardinia: Taxonomy and natural history (Isopoda, Oniscidea, Trichoniscidae). Zookeys 2018, 801, 229–263. [Google Scholar] [CrossRef]
- Taiti, S.; Argano, R. New species of terrestrial isopods from Sardinia (Isopoda: Oniscidea). Zootaxa 2009, 2318, 38–55. [Google Scholar] [CrossRef]
- Juan, C.; Guzik, M.T.; Jaume, D.; Cooper, S.B. Evolution in caves: Darwin’s ‘wrecks of ancient life’ in the molecular era. Mol. Ecol. 2010, 19, 3865–3880. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Hlavac, P.; Jaloszynski, P.; Beutel, R.G. In the twilight zone—The head morphology of Bergrothia saulcyi (Pselaphinae, Staphylinidae, Coleoptera), a beetle with adaptations to endogean life but living in leaf litter. J. Morphol. 2021, 282, 1170–1187. [Google Scholar] [CrossRef] [PubMed]
- Hlavac, P. Description of new Bergrothia from Turkey and notes on Amauropini (Coleoptera: Staphylinidae: Pselaphinae). Entomol. Probl. 1999, 30, 49–53. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).