Abstract
Mastogloia is a large, morphologically diverse genus of primarily benthic marine species defined by the presence of partecta (chambers) on the valvocopula (girdle band next to the valve). Several genera have been found with valves that resemble Mastogloia but lack the ring of partecta; the most diverse group is in freshwaters, especially Aneumastus, but there are two marine genera, both monotypic. A third such species has been found in Cuba and is described here as Paramastogloia cubana gen. nov., sp. nov. Paramastogloia cubana, Mastoneis biformis, and Mastogloiopsis biseriata each resemble the valve structure of some species of Mastogloia but do not resemble one another. Paramastogloia is indistinguishable in light microscopy (LM) from naviculoid diatoms and had been identified as Navicula cf. sovereigniae. The resemblance of P. cubana to Mastogloia is in the areolae, particularly to those of M. umbra, M. dicephala, and M. mammosa, three species not likely to be in one monophyletic group. Mastoneis has been observed in LM from widespread warm-water localities and resembles some Mastogloia in having costae extending partway across the virgae (interstriae); new ultrastructural details are presented showing the girdle bands and absence of partecta, and a clearer genus diagnosis is proposed. The third genus, Mastogloiopsis, was established with ultrastructure and resembles species of Mastogloia sec. Marginulatae. The range of areolar characters that might be admissible to Paramastogloia is unknown, so the generic diagnosis has been left imprecise to allow for the possibility of other species. The relationships among these genera await genetic studies, which are still scarce in Mastogloiaceae.
1. Introduction
Diatoms are photosynthetic, eukaryotic microbes that are important primary producers in aquatic ecosystems; their fossilized remains (diatomaceous earth) have various industrial uses ranging from filtration to dynamite [1]. They are enclosed in silica shells that consist of two valves (analogous to bivalves) separated by girdle bands. Among the marine benthic genera is Mastogloia Thwaites, with over 400 species and very wide variety of valve structure [2,3,4,5] but united by the shared character of a ring of chambers (partecta) on the girdle band adjacent to each valve (valvocopula). Wall structure, revealed by scanning electron microscopy (SEM) over the last 50 years [5], comprises the basal silica layer and the character of the perforations through it (areolae). Ross et al. [6] defined several kinds of areolae, but Paddock & Kemp [3], in dealing with the complexity of Mastogloia, followed those recommendations ”rather loosely” [3] (p. 102), categorizing walls as laminar when they appear to have one layer, or cavitate, i.e., thicker walls that “can be thought of” [3] (p. 81) as having an inner and outer layer. The former structure occurs in several of the most common species of Mastogloia, e.g., M. ovalis A.Schmidt, M. binotata (Grunow) Cleve, M. crucicula (Grunow) Cleve, and M. fimbriata (Brightwell) Cleve [3]. The latter situation is distinct from that in Pleurosigmataceae and Haslea, where there are basal and tegumental layers joined by partitions called saepes [7,8,9]. Cavitate walls were classified [3] as loculate, externally or internally pseudoloculate, and alveolate. The structure and taxonomy of >40 Mastogloia species claimed for Yap were recently reviewed [5]; suffice it to say here that wall complexity has been important in recognizing related genera, particularly because there are only five Mastogloia gene sequences—only one identified to species [10].
There are some genera with valve wall structures resembling one of the distinctive types in Mastogloia yet lacking the definitive chambers (i.e., apartectal mastogloids). The other genera are now placed close to Mastogloia in Mastogloiaceae [11,12], the morphology supported by molecular data only for Aneumastus D.G.Mann & Stickle and Decussiphycus Guiry & K.Ghandi. The best-known and most diverse of these apartectal genera is Aneumastus, separated from Navicula Bory in 1990 [4] (p. 663) because of a series of small chambers formed between the valve mantle and pars interior of the valvocopula. It is a freshwater genus that now includes species flocks formed by adaptive radiation in ancient lakes [13,14,15]. Decussiphycus is also a freshwater genus with a few species also formerly in Navicula [10,16,17]. It was recently shown molecularly to belong in the Mastogloiaceae [10] (the authors placed it in Mastogloiales but proposed reducing that taxon to coextensive with the family). The marine apartectal mastogloids Mastoneis Cleve [18] and Mastogloiopsis Lobban & J.N. Navarro [19] are so far both monotypic. The former, originally described as Stauroneis biformis Grunow [20], has been reported occasionally from widespread warm-water locations but is still poorly known ultrastructurally. It was not mentioned in the monographic SEM survey of diatom genera [4], nor in Cox’s [11] recent classification of diatoms and the position of Mastoneis is still listed as Naviculaceae in AlgaeBase [12], although Lobban & Navarro [19] proposed classifying it in Mastogloiales, without a family designation. However, it is common in biofilm on shell sand in Guam and I have studied it in SEM. Mastogloiopsis was described from Puerto Rico, Easter Island, and Guam [19] with a strong resemblance to Mastogloia sec. Marginulatae Simonsen ex Lobban [5].
The apartectal mastogloid genera known to date show some Mastogloia-like character (s) but there is no reason to assume that these are the only genera, either marine or freshwater, nor that others would be recognizable in LM. Ultrastructure is often needed to confirm Mastogloia-like characters. A species from Cuba, identified by Siqueiros Beltrones et al. [21] (figs A20 l–p) with LM as Navicula cf. sovereigniae (recently transferred to Placoneis sovereigniae (Hustedt) Torgan & Donadel [22]), was revealed by SEM to have wall characters like some Mastogloia spp. and to have valvocopulae without partecta. The objective of this paper is to describe this new species in comparison to Mastoneis and Mastogloiopsis, incorporating new observations, and to make the case for its placement in a new genus.
2. Materials and Methods
Cuban samples were provided by Dr. D.A. Siqueiros Beltrones; collection and cleaning protocol were described in Siqueiros Beltrones et al. [21,23]. Materials for the comparable taxa were taken from the Guam Diatom Collection in GUAM Herbarium; Mastoneis has been collected frequently in subtidal biofilm in Apra Harbor, Guam (Outhouse Beach samples GU52N-7, GU52O-2, and -4, GU52X-5). Preparation of Guam samples and microscopy were described in detail by Lobban [5]. Briefly, acid cleaning with nitric acid was followed by rinsing and mounting on coverslips or SEM stubs, the latter sputter-coated with gold. Light microscopy of all materials was carried out with Nikon 80i differential interference contrast, SEM with a Thermo-Fisher desktop Phenom XL-G2 at 10 kV, and low emission settings. Of the abundant specimens of P. cubana, 27 were recorded between LM and SEM; observations of Mastoneis are based on 23 recorded specimens.
3. Results
- Mastogloiales D.G.Mann & Round
- Mastogloiaceae Mereschkowsky
- Paramastogloia gen. nov.
Diagnosis: Valve wall resembling certain Mastogloia spp. in the pseudolocular wall construction but with the valvocopula (1st pleura) bearing an apparently solid ridge in lieu of partecta; also differing from marginal spaces in Aneumastus and Mastogloiopsis, and from those genera and Mastoneis in areolar characters.
Type species: Paramastogloia cubana sp. nov.
Etymology: Gr. παρά, beside/near + μαστογλοια, Mastogloia.
Phycobank registration: http://phycobank.org/105485 (accessed on 26 April 2025).
Comment: Given that this genus is presently monotypic, the diagnosis has been written to permit similar areolar structures to be admissible, while giving some boundaries to distinguish it from Aneumastus and Mastogloiopsis.
Diagnosis: Valves elliptico-lanceolate, rostrate, 13–28 µm long, 8.0–9.5 µm wide, striae radiate, 22–23 in 10 µm; areolae transapically elongated, cavitate with the external corners of the cavity usually covered, leaving single large, irregular exterior foramen; internal foramina in clusters of four.
Holotype: Figure 1d, from specimen on stub 2067, according to Article 40.5 of the International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code, Turland et al. [24]; this Art. not modified at the Madrid Botanical Congress in 2024).
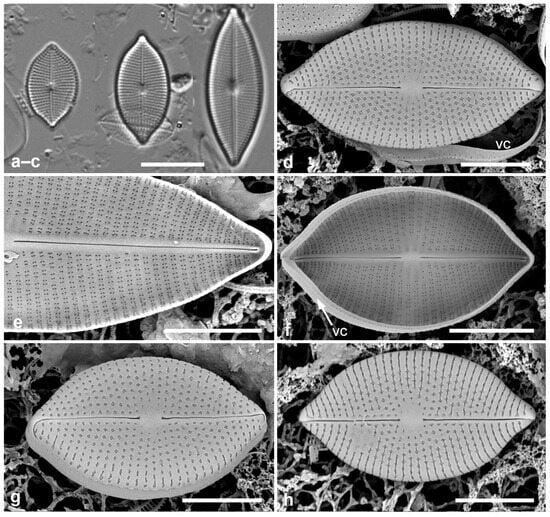
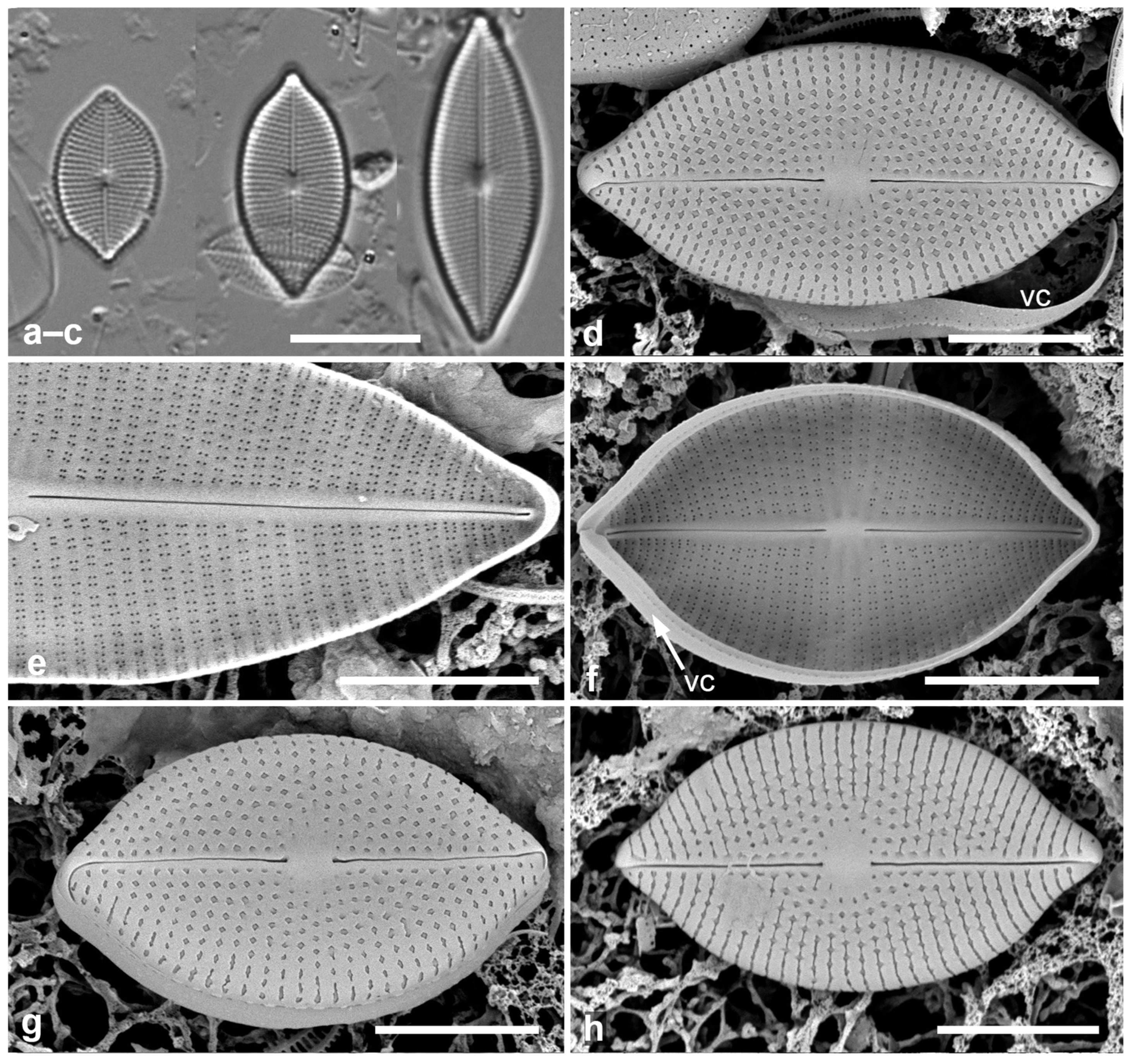
Figure 1.
Paramastogloia cubana sp. nov. (a–c) Valves from isotype slide in LM showing length variation with little width variation. (d) Holotype valve exterior view in SEM with partial view of valvocopula (vc). (e) Interior of a large specimen, valve without valvocopula. (f) Typical specimen, valve interior with valvocopula (vc) lacking partecta. (g) Exterior of valve with valvocopula in slightly oblique view, showing shallow mantle. (h) Exterior of specimen with variant foramina. Scale bars: (a–c) = 10 µm, (d–h) = 5 µm.
Figure 1.
Paramastogloia cubana sp. nov. (a–c) Valves from isotype slide in LM showing length variation with little width variation. (d) Holotype valve exterior view in SEM with partial view of valvocopula (vc). (e) Interior of a large specimen, valve without valvocopula. (f) Typical specimen, valve interior with valvocopula (vc) lacking partecta. (g) Exterior of valve with valvocopula in slightly oblique view, showing shallow mantle. (h) Exterior of specimen with variant foramina. Scale bars: (a–c) = 10 µm, (d–h) = 5 µm.
Isotype: Specimen at 14.6 mm E and 5.7 mm S of the mark on slide 4133, deposited at Academy of Natural Sciences of Drexel University, Philadelphia (ANSP), accession # ANSP-GC 16017. There are many specimens on the slide (Figure 1a–c).
Type locality: Cuba, Sabana-Camagüey archipelago, Playa Las Gaviotas, 79°11′ to 78°56′ W, and 22°39′ to 22°29′ N, within the biosphere reserve Buenavista, itself within the marine protected area Refugio de Vida Silvestre Cayo Santa María. Sample P5-0, coll. Erisbel Echevarría-Herrera, Instituto Politécnico Nacional, La Paz, Mexico, 8 July 2022 (rainy season).
Description: Valves elliptico-lanceolate, rostrate, with shallow mantle; 13–28 µm long, 8.0–9.5 µm wide, striae radiate, 22–23 in 10 µm, central area oval (Figure 1a–d,g). Raphe straight, central endings distant. Areolae transapically rectangular, with irregular external foramina (Figure 1d,g,h), elongated near and on margin (Figure 1d,g); internally, clusters of four foramina (Figure 1e,f). Valve structure thus “cavitate, pseudoloculate (externally)”, according to classification of Mastogloia valves in Paddock & Kemp [3] [2.2.1.3]; the classification within that appears to be 2.2.1.3.9, where the clusters of four internal pores comprise one pair from each of two transapically elongate areolae (Figure 2b,c vs. Figure 2d), as shown in the eroded valve in Figure 2a.
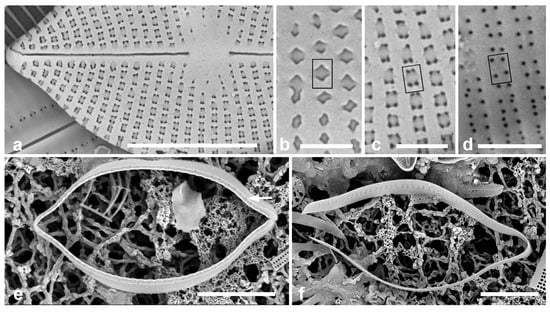
Figure 2.
Paramastogloia cubana sp. nov. (cont.). (a) Eroded (or incompletely developed?) exterior without external foramina showing pores through the transapically rectangular areolae at each corner. (b–d) Details of foramina showing position of one areola (rectangles on each figure) in normal exterior (b, from Figure 1d); eroded exterior (c from a); and interior (d). Note that the closest four foramina in the interior view derive from two areolae. (e) Isolated valvocopula (1st pleura) in advalvar view, showing row of pores on edge of pars interior (arrow). (f) Isolated 2nd pleura showing two rows of pores on pars exterior. Scale bars: (a,e,f) = 5 µm, (b–d) = 1 µm.
Figure 2.
Paramastogloia cubana sp. nov. (cont.). (a) Eroded (or incompletely developed?) exterior without external foramina showing pores through the transapically rectangular areolae at each corner. (b–d) Details of foramina showing position of one areola (rectangles on each figure) in normal exterior (b, from Figure 1d); eroded exterior (c from a); and interior (d). Note that the closest four foramina in the interior view derive from two areolae. (e) Isolated valvocopula (1st pleura) in advalvar view, showing row of pores on edge of pars interior (arrow). (f) Isolated 2nd pleura showing two rows of pores on pars exterior. Scale bars: (a,e,f) = 5 µm, (b–d) = 1 µm.
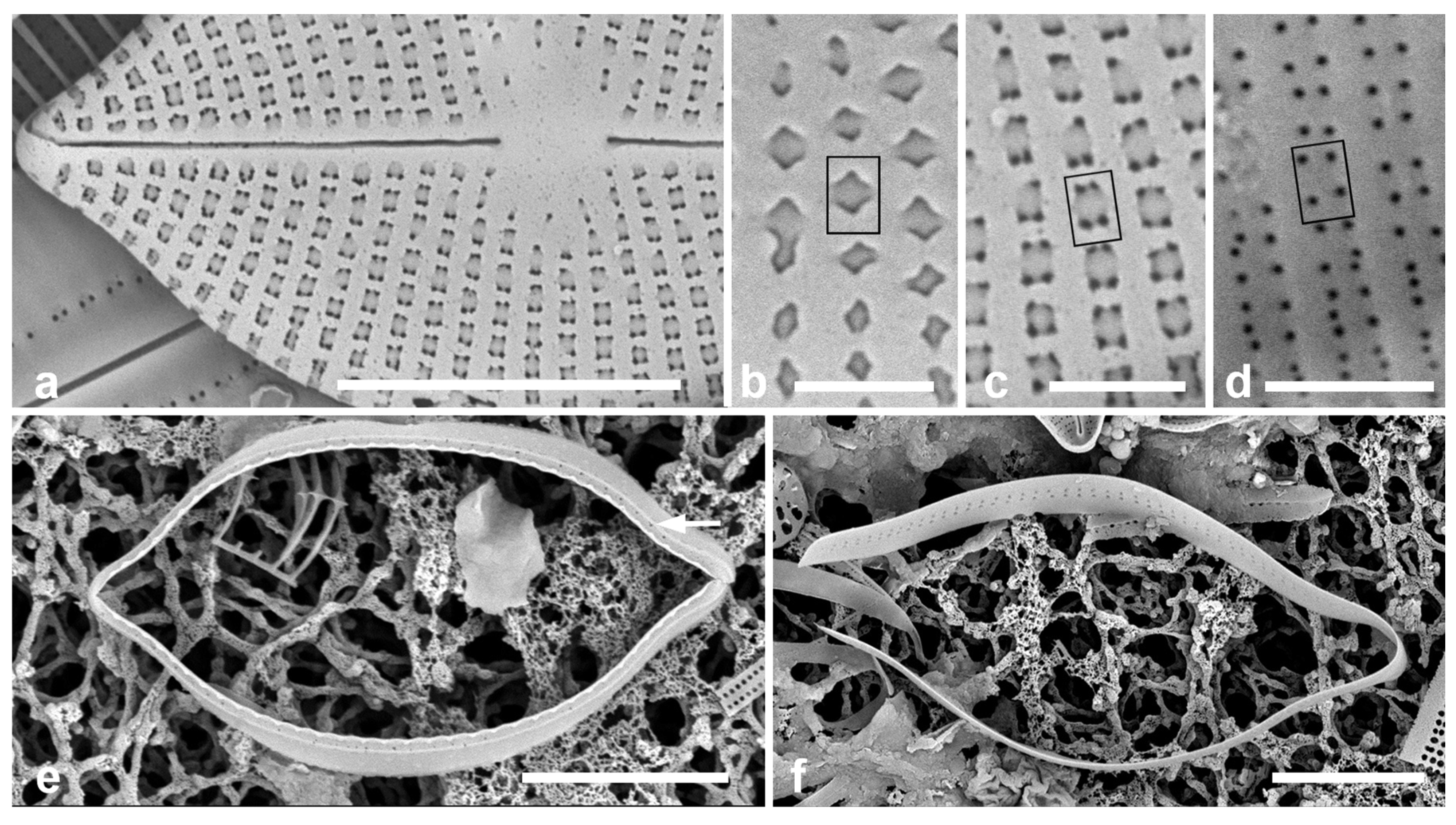
Girdle bands: Valvocopula (1st pleura) (Figure 1d,f and Figure 2e) with a narrow ledge and a line of pores on the edge of the pars interior; second band with two rows of pores on the pars exterior. In contrast to Mastogloia, there is no great distinction between the bands, so they should both be referred to as pleurae [25]. There might also be a narrow third pleura, but it was not detected.
Etymology: (L.) cubana, from the collection site in Cuba.
Distribution: So far known only from two samples at the type locality, P5-0 and P5-1.
Phycobank registration: http://phycobank.org/105486 (accessed on 26 April 2025).
References: LM: Grunow [20], p. 154, pl. 13, fig 7 (basionym: Stauroneis biformis); Mann [26], p. 95, pl. 20, fig 7 (as Navicula biformis (Grunow) A.Mann); Hendey [27], p. 139, pl. 6, fig 71; Witkowski et al. [28], p. 264, pl. 101, fig 5; Hein et al. [29], p. 74, pl. 32, fig 3, pl. 49, figs 1, 2. SEM: Güttinger [30] 2.05.30-1; Lobban & Navarro [19], p. 261, figs 30, 31.
Description: Cells motile in biofilm over shell sand, not stationary on mucilage stalks or in bubbles. Two plastids fore-and-aft with four lobes at right angles and central pyrenoids (Figure 3a). Valves broadly lanceolate with strong differentiation between inner and outer zones and a thickened partial stauros extending from the central area (Figure 3d,e and Figure 4b). Striae 16 in 10 µm, costae 8–9 in 10 µm, 2–3 striae between costae. In SEM, the outer zone subtended by transapical costae. Walls cavitate, pseudoloculate; areolae throughout with small single outer foramen and four internal foramina (Figure 3d,e and Figure 4b); external foramina in the outer zone generally circular, those in inner zone slightly apically elongate (oval) (Figure 4a). Sternum lanceolate in each branch (Figure 3d), raphe strongly sinuous externally, straight internally with thickened sternum (Figure 3a,b,d,e).
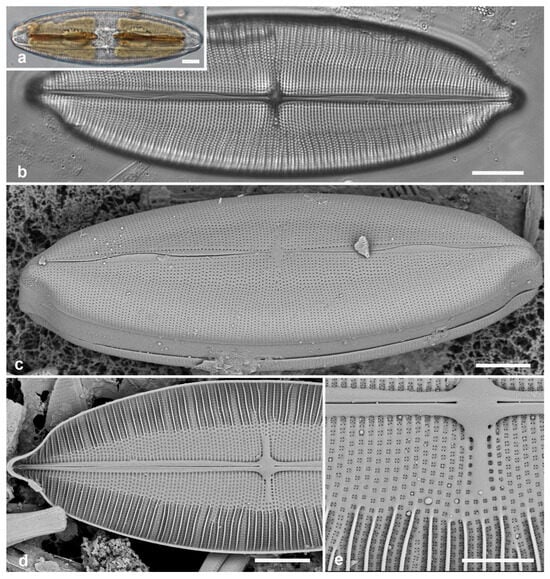
Figure 3.
Mastoneis biformis. (a) Living cell, slightly oblique of valve view, showing two lobed plastids “fore-&-aft” with pyrenoids. (b) Valve in LM. (c) Valve exterior in SEM, valve view. (d,e) Valve interior, general view and detail of central area, showing foramina, costae, stauros, and thickened sternum. Scale bars: (a–e) = 10 µm, (e) = 5 µm.
Figure 3.
Mastoneis biformis. (a) Living cell, slightly oblique of valve view, showing two lobed plastids “fore-&-aft” with pyrenoids. (b) Valve in LM. (c) Valve exterior in SEM, valve view. (d,e) Valve interior, general view and detail of central area, showing foramina, costae, stauros, and thickened sternum. Scale bars: (a–e) = 10 µm, (e) = 5 µm.
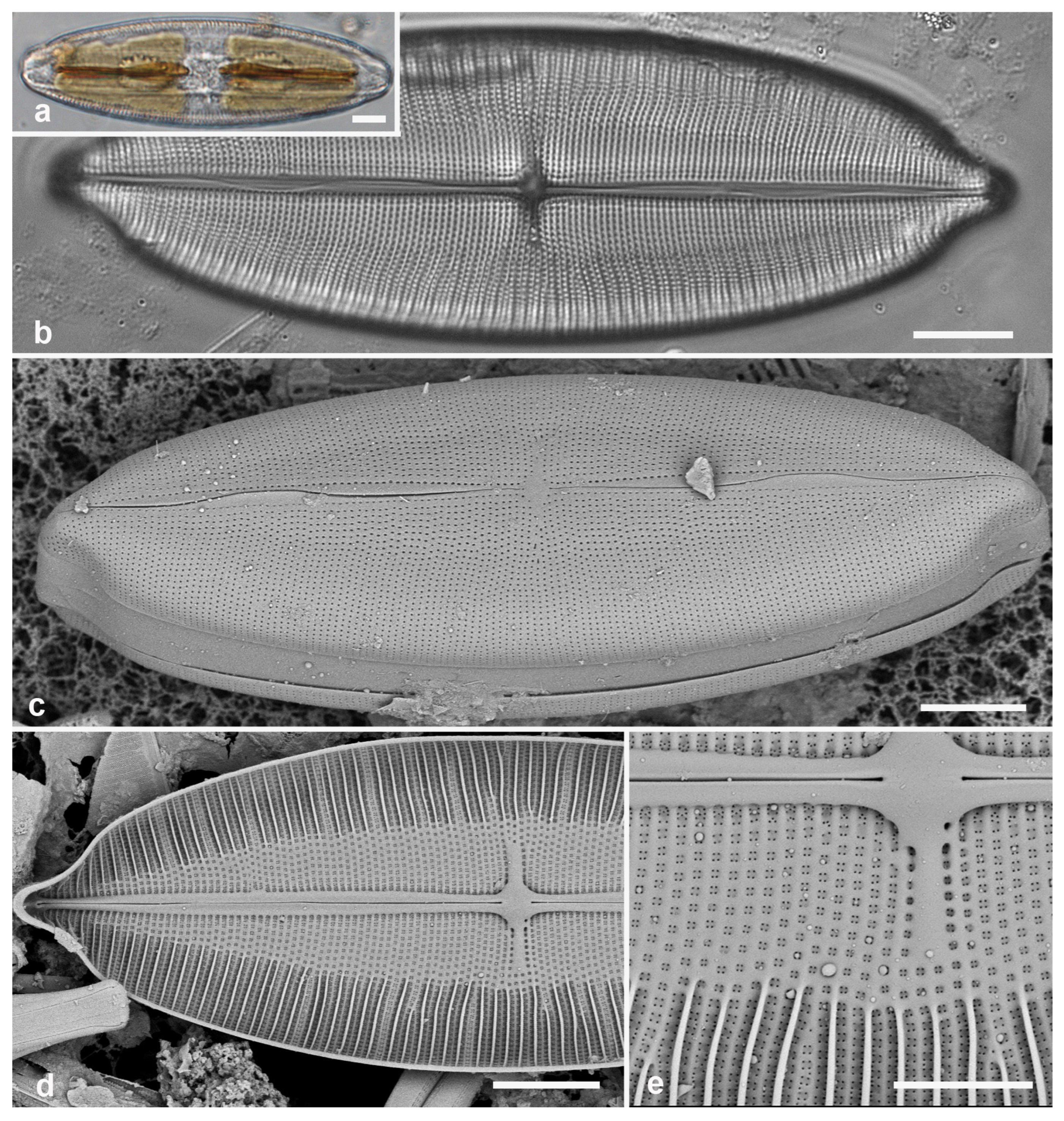
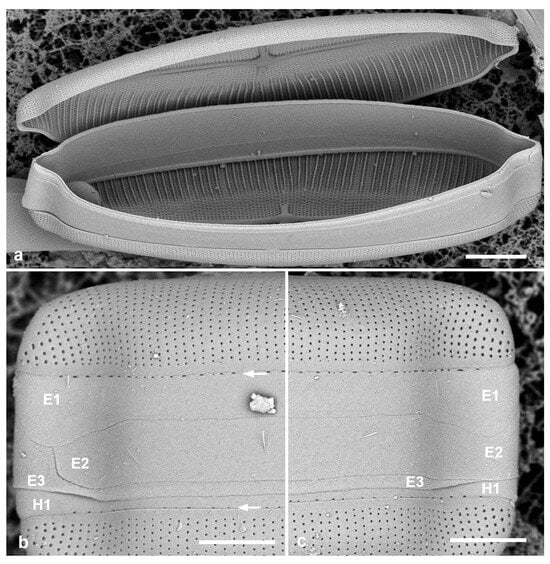
Figure 4.
Mastoneis biformis girdle bands, observed in SEM of whole mounts made from living material. (a) Frustule broken open to show lack of partecta. (b,c) Apices of one frustule, showing the three pleurae (E1, E2, E3) of the epicingulum, the 1st pleura of the hypocingulum (H1) just visible. The 1st pleurae (E1, H1) have a line of small pores along the edge of the pars interior (arrows, Figure 1b). Scale bars: (a) = 10 µm, (b,c) = 5 µm.
Figure 4.
Mastoneis biformis girdle bands, observed in SEM of whole mounts made from living material. (a) Frustule broken open to show lack of partecta. (b,c) Apices of one frustule, showing the three pleurae (E1, E2, E3) of the epicingulum, the 1st pleura of the hypocingulum (H1) just visible. The 1st pleurae (E1, H1) have a line of small pores along the edge of the pars interior (arrows, Figure 1b). Scale bars: (a) = 10 µm, (b,c) = 5 µm.
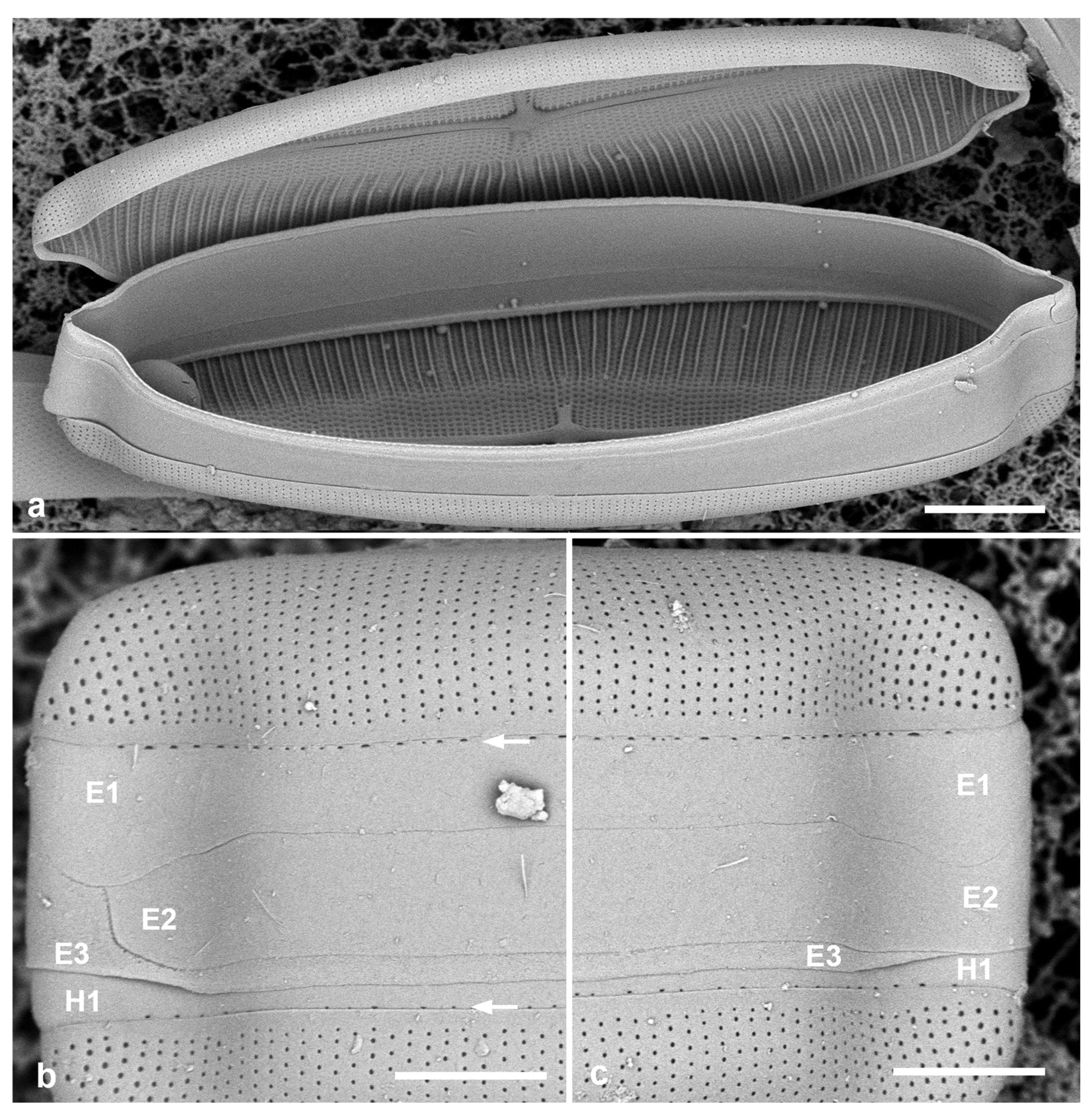
Girdle bands: The opened frustule (Figure 4a) proves the absence of partectal rings. All pleurae hyaline except for a line of small pores along the edge of the pars interior of the 1st pleura (valvocopula) (Figure 3c and Figure 4b,c). The 1st and 2nd pleurae are of approximately equal depth, and—curiously—open at the same pole, the ligule of the 3rd pleura filling the gap for both (Figure 4b,c).
Distribution: Described from the Red Sea; pantropical (Philippines, Guam, Bahamas, Kuwait) but rarely reported.
Comments: The arrangement of the plastids is like the usual form in Mastogloia (other forms are known from a few Mastogloia species [5]). The areolae can be categorized, parallel to Mastogloia [3], as 2.2.2: cavitate with internal costae, a pattern that is common in that genus but accompanies many other external patterns and was not subcategorized by Paddock & Kemp [3].
- Additional Observation of Mastogloiopsis biseriata. Figure 5a,b.
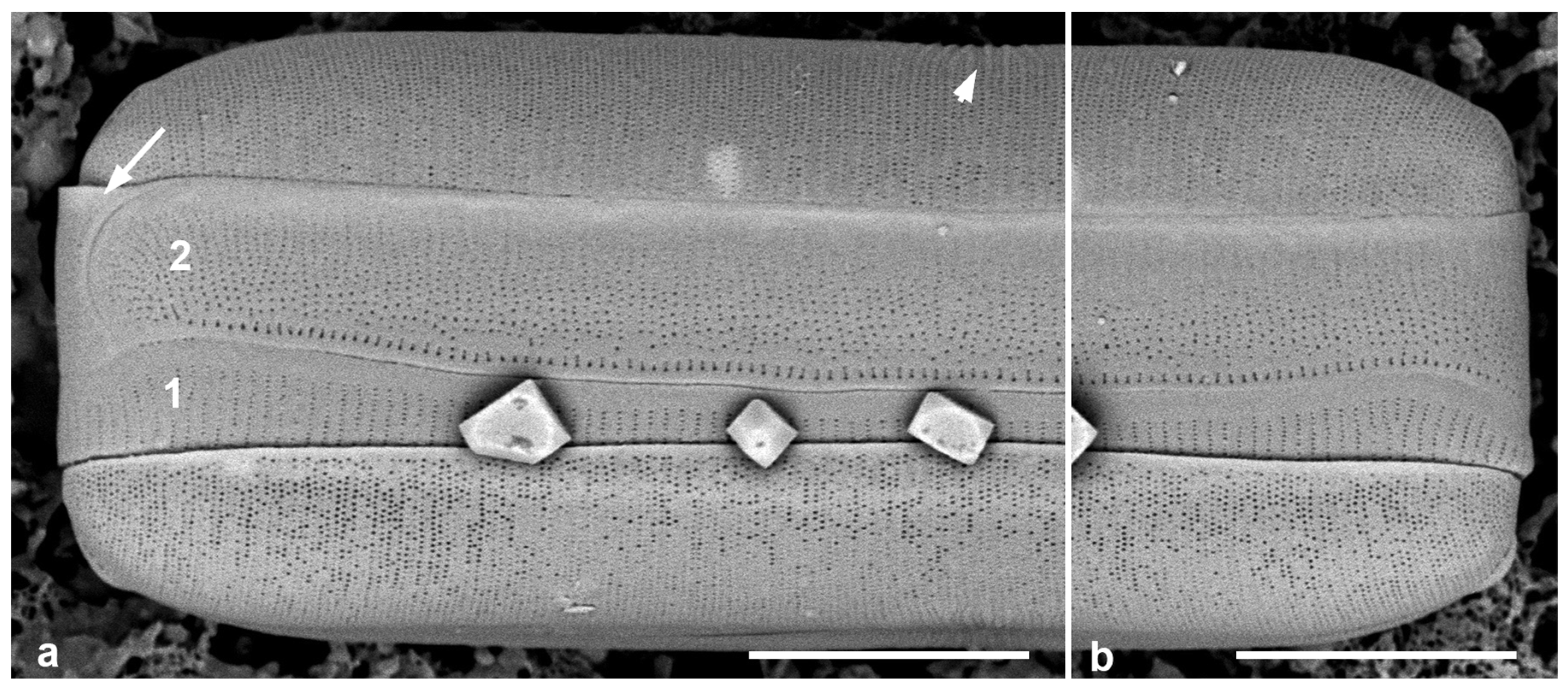
This species was described with LM and SEM from Guam and Puerto Rico, including plastids and mucilage stalks [19]. More recent whole-mount material from Guam sample # GU52AQ-2) was examined for the girdle bands. While Lobban & Navarro [19] observed three bands, two were valvocopulae; thus, there are two bands in each of the epicingulum and the mature hypocingulum. New images (Figure 5a,b) confirm the presence of two different bands: the 1st pleura (valvocopula) with less densely spaced striae (40 in 10 µm) than the 2nd pleura (55 in 10 µm), but the areolae are more widely spaced along the striae on the 2nd pleura, 7 in 1 µm in the 1st pleura vs. 12 in 1 µm in the 2nd pleura. There is a row of larger areolae along the advalvar edge of the 2nd pleura. There is an apparent cap at one end of the 2nd pleura; this shows no hint of a narrow band comprising a 3rd pleura with a large ligule, but it could be merely a cap piece, symmetrical on both sides of the frustule; or it may be that the open end of the 2nd pleura comes around the apex to fit. The first two possibilities occur in other diatoms (see Mastoneis, above), the other perhaps not.
The characters of Mastogloiopsis include the pars interior of valvocopula interlocking with the valve and bearing a porate, apparently hollow flange with no evidence that this is divided into chambers. Mastogloiopsis (Figure 5) has morphology very much like Mastogloia sec. Marginulatae Simonsen ex Lobban [5], to which it may be related because of the synapomorphic curve in the raphe sternum. However, areolae in Mastogloia sec. Marginulatae are cavitate [3] (category 2.2.2.1), whereas in Mastogloiopsis, the wall is laminar with simple areolae.
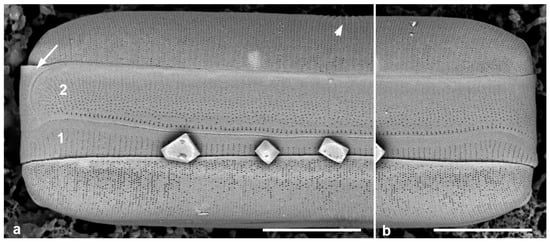
Figure 5.
Mastogloiopsis biseriata girdle bands. (a,b) Apices of one frustule in whole mount, SEM, showing the two pleurae (1, 2), i.e., the valvocopula with less dense striae than the 2nd pleura. Arrow in (a) indicates the enigmatic piece of band coming around the apex; arrowhead indicates the slightly raised virgae between the more widely spaced central striae. Scale bars = 5 µm.
Figure 5.
Mastogloiopsis biseriata girdle bands. (a,b) Apices of one frustule in whole mount, SEM, showing the two pleurae (1, 2), i.e., the valvocopula with less dense striae than the 2nd pleura. Arrow in (a) indicates the enigmatic piece of band coming around the apex; arrowhead indicates the slightly raised virgae between the more widely spaced central striae. Scale bars = 5 µm.
4. Discussion
4.1. Paramastogloia
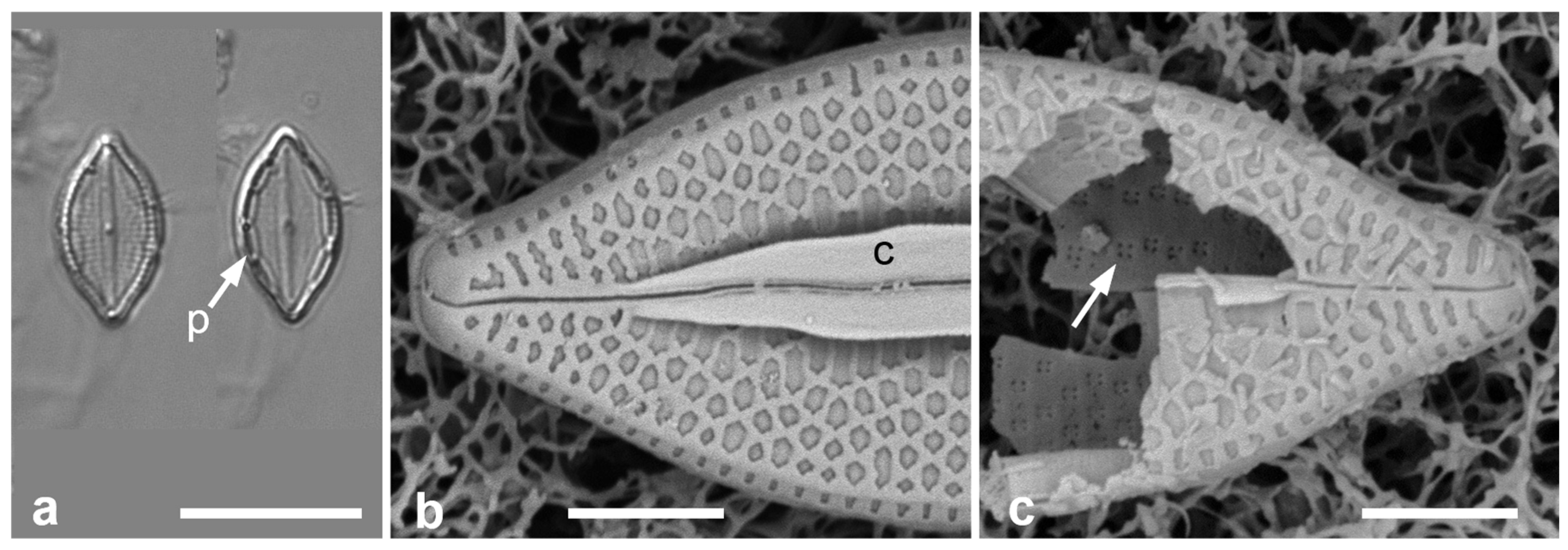
Given the absence of partecta, what characters of Paramastogloia suggest a relationship to Mastogloia? Among the huge diversity of wall structure in Mastogloia, there are at least three species in which both inner and outer areolar form are the same as in Paramastogloia: M. umbra Paddock & Kemp [31] was described with SEM, M. dicephala Voigt and M. mammosa Voigt [32] were described from LM but have been observed in Guam (unpublished data). I have observed M. umbra [5] and show additional images in Figure 6 to contrast the appearance in LM and to show the similarity of the areolae. SEM images of the other two will be included in a paper (in prep.) on Voigt’s Asian Mastogloia spp. These three have never been associated with any of Hustedt’s [2] artificial groups and no relationship among them has been proposed; other features of the valve structure, especially the conopea of M. umbra, suggest any subgeneric relationship among these three Mastogloia spp. is unlikely.
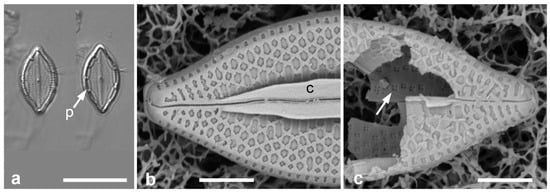
Figure 6.
Comparable Mastogloia valves: M. umbra from Guam (samples GU52P-1 [LM] and GU52O-4 [SEM]). (a) Valves with partecta (p) in LM. (b,c) SEM showing, respectively, exterior with conopeum (c) and interior foramina (arrow). Scale bars: (a) = 10 µm, (b,c) = 2 µm.
Placement in a genus. Although in LM, valves of Paramastogloia resemble Placoneis, the wall structure in that genus is laminate with small poroids closed internally by volate occlusions [4] (p. 484). The pseudoloculate walls of Paramastogloia strongly suggest a relationship with Mastogloia. Several genera have been established for species resembling Mastogloia but lacking partecta. I consider these and, firstly, the alternative possibility of placing the new species in Mastogloia until further evidence (e.g., molecular) could be found, and argue that a new genus is necessary.
Why not Mastogloia? This genus has been defined, beginning with Hustedt [2], by the presence of partecta on the valvocopula, to the extent that he included Stigmaphora, with two partectate species of open ocean plankton recovered from the digestive tracts of salps [33]. Kemp & Paddock [34] defended separation of the two genera but this remains unresolved [35]. Anything without partecta has been excluded: species that are now included in Aneumastus, etc., were transferred from Navicula if not newly discovered. Thus, there is presently no justification for assigning the new species to Mastogloia.
Why not Aneumastus? The diagnosis of this genus [4] (p. 663) unfortunately relies on an absent character, “Genus novum Mastogloiae Thwaites affine, a qua absentia partectorum genuinorum praecipua differt” [differs chiefly in the absence of genuine partecta]. By this definition, all the other apartectal mastogloids could be included here, but that would likely create another polyphyletic genus. The definition of the genus therefore cannot be interpreted to include every species that fits the two criteria. However, the defining feature of Aneumastus is the series of tiny chambers formed between the valvocopula pars interior and thickened valve margin, opening through a single row of pores in the pars exterior of the valvocopula [4]. While there is a row of pores on the pars exterior of Paramastogloia (Figure 2e), there is no indication of a thickening at the valve margin (Figure 1e) that could combine to form even a channel.
4.2. Diagnosis of Mastoneis
Mastoneis has been monotypic since its establishment by Cleve in 1894 [18]. Grunow [20] recognized an affinity to Mastogloia valves but saw no partecta attached to any of several specimens and put them in Stauroneis because of the partial stauros. He noted a “double structure” in the species and interpreted the striae in the outer zone as being 20 in 0.001” (‘Paris inch’ converts to 7.4 in 10 µm; see [36], table 2), and in the inner zone 40 in 0.001” (=15 in 10 µm), “structura valvarum duplici: striis validioribus abbreviatis marginalibus 20 in 0.001” et tenuioribus lineam mediam attingentibus sub lucem obliquam tantum conspicuis” (valve structure double: stronger, shortened marginal striae 20 in 0.001” and thinner lines reaching the middle visible only in oblique light).
These numbers must refer to the costa density in the outer zone and the stria density (the same in both zones), respectively, but his comment on the need for oblique light is puzzling, considering that the stria density is well within the resolving power of the microscopes of his day. Witkowski et al. [28] and Hein et al. [29] gave the stria density ranging from 15 in 10 µm near the middle to 18 in 10 µm near the apices and those metrics are close to the Guam specimens shown here. Cleve’s [18] (p. 194) diagnosis of Mastoneis was, “Valve with double structure. The exterior stratum with transverse striae, composed of puncta; the interior with transverse costae, directed from the margin, where they are thicker, towards the median line”. It seems that the “double structure” described by Grunow is a transapical feature, but Cleve described the valve structure from outside to inside, i.e., in the pervalvar axis. Already a century ago, Mann [26] saw no reason to distinguish this species from Navicula on the grounds of the “double markings”, but this view has not been accepted in the intervening century and no clearer diagnosis of Mastoneis has been offered. I therefore propose the following emended diagnosis to distinguish it from other putative members of Mastogloiales/Mastogloiaceae, i.e., Mastogloia, Aneumastus, and Decussiphycus (molecularly related [10]), plus Mastogloiopsis and Paramastogloia (Table 1).

Table 1.
Comparison of Mastogloiaceae genera discussed here.
Mastoneis Cleve 1894, emend. Lobban
Diagnosis: Valves with the combination of pseudoloculate walls, external foramina in a single zone of nearly uniform single poroids vs. internal with four foramina per areola, and every 2nd or 3rd interstria (virga) thickened internally by costae in a distinctive outer zone, evident in LM. Differing from Mastogloia in the absence of partecta on the valvocopulae, from Aneumastus and Mastogloiopsis in lack of any small chambering or channel between valvocopula and valve, and from Decussiphycus in plastid shape, wall structure, and transapical striae.
5. Conclusions
The new species cannot be assigned to any of the known Mastogloia-like genera. The three marine genera each share valve characters with certain species of Mastogloia but not with each other. The range of areolar shape admissible to the new genus can only be determined when another potential candidate is found. Similarly, the absence of costae has not yet been defined as a character state for Paramastogloia. Monotypic genera are always a challenge to define and often cannot be clearly understood until a second species is discovered [37]. Thus, the exact details of the wall structure have been omitted from the generic description because one cannot predict how variable these might be before needing a further new species. Evolutionary connections of the marine apartectate genera to Mastogloia are suggested by morphology but molecular data are needed to establish them and to confirm the systematic placement of these genera in Mastogloiaceae.
Funding
SEM and write-up were supported by the National Science Foundation under EPSCoR Grant Number OIA-1946352 for the University of Guam Ecosystems Collaboration for Corals and Oceans (GECCO) program, Biorepository component.
Data Availability Statement
All images have been trimmed to fit into plates. The original image files and related images are available from the author on reasonable request.
Acknowledgments
I thank David A. Siqueiros Beltrones for sharing the samples from Cuba, collected and processed by Erisbel Echevarría-Herrera, and the three reviewers for thoughtful suggestions.
Conflicts of Interest
The author declares no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Smol, J.P.; Stoermer, E.F. (Eds.) The Diatoms: Applications for the Environmental and Earth Sciences, 2nd ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Hustedt, F. Die Kieselalgen Deutschlands, Österreichs und der Schweiz. In Rabenhorst’s Kryptogamenflora; Band 7, Teil 2, Lief. 4; Akademischen Verlagsgesellschaft: Frankfurt am Main, Germany, 1933; Johnson Reprint, New York, NY, USA, 1962. [Google Scholar]
- Paddock, T.B.B.; Kemp, K.D. An illustrated survey of the morphological features of the diatom genus Mastogloia. Diatom Res. 1990, 5, 73–103. [Google Scholar] [CrossRef]
- Round, F.E.; Crawford, R.M.; Mann, D.G. The Diatoms: Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Lobban, C.S. Towards a biogeography of Mastogloia (Diatomeae: Bacillariophyceae): A preliminary checklist for Yap, Micronesia, with new ultrastructure images and a review of problems. Nova Hedw. 2025, 120, 313–417. [Google Scholar] [CrossRef]
- Ross, R.; Cox, E.J.; Karayeva, N.I.; Mann, D.G.; Paddock, T.B.B.; Simonsen, R.; Sims, P.A. An amended terminology for the siliceous components of the diatom cell. Nova Hedw. Beih. 1979, 64, 513–533. [Google Scholar]
- Sterrenburg, F.A.S.; Tiffany, M.A.; Meave del Castillo, M.E. Valve morphogenesis in the diatom genus Pleurosigma W. Smith (Bacillariophyceae): Nature’s alternative sandwich. J. Nanosci. Nanotech. 2005, 5, 140–145. [Google Scholar] [CrossRef]
- Sterrenburg, F.A.S.; Tiffany, M.A.; Hinz, F.; Herwig, W.E.; Hargraves, P.E. Seven new species expand the morphological spectrum of Haslea. A comparison with Gyrosigma and Pleurosigma (Bacillariophyta). Phytotaxa 2015, 207, 143–162. [Google Scholar] [CrossRef]
- Lobban, C.S.; Perez, C.O.; Ashworth, M.P. Non-blue Haslea species (Bacillariophyceae: Naviculaceae) in the benthic marine flora of Guam (Mariana Islands, Western Pacific Ocean). Diatom Res. 2020, 35, 163–183. [Google Scholar] [CrossRef]
- Mironov, A.; Glushchenko, A.; Kezlya, E.; Maltsev, Y.; Iurmanov, A.; Liu, Y.; Kulikovskiy, M. Decussiphycus sinensis sp. nov. (Bacillariophyceae, Mastogloiales)—A new species described from China, with comments on phylogenetic position of the genus. PhytoKeys 2025, 254, 1–19. [Google Scholar] [CrossRef]
- Cox, E.J. Diatoms, Diatomeae (Bacillariophyceae s.l., Bacillariophyta). In Engler’s Syllabus of Plant Families, 13th ed.; Frey, W., Ed.; Borntraeger Science Publishers: Stuttgart, Germany, 2015; Part 2/1; pp. 64–103. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-wide Electronic Publication, National University of Ireland: Galway, Ireland. Available online: https://www.algaebase.org (accessed on 16 February 2025).
- Gluschenko, A.; Kulikovskiy, M.; Okhapkin, A.; Kociolek, J.P. Aneumastus laosica sp. nov. and A. genkalii sp. nov.—Two new diatom species from Laos (Southeast Asia) with comments on the biogeography of the genus. Cryptogam. Algol. 2017, 38, 183–199. [Google Scholar] [CrossRef]
- Spaulding, S.; Potapova, M.; Bishop, I.W.; Lee, S.S.; Gasperak, T.S.; Jovanoska, E.; Furey, P.C.; Edlund, M.B. Diatoms.org: Supporting taxonomists, connecting communities. Diatom Res. 2021, 36, 291–304. [Google Scholar] [CrossRef]
- Stelbrink, B.; Jovanovska, E.; Levkov, Z.; Ognjanova-Rumenova, N.; Wilke, T.; Albrech, C. Diatoms do radiate: Evidence for a freshwater species flock. J. Evol. Biol. 2018, 31, 1969–1975. [Google Scholar] [CrossRef]
- Edlund, M.B.; Brant, L.A.; Levkov, Z.; Nakov, T. An emended description of Decussata (Patrick) Lange-Bertalot & Metzeltin that includes protoplast organization and detailed valve and cingulum ultrastructure. Diatom Res. 2006, 21, 269–280. [Google Scholar]
- Guiry, M.D.; Ghandi, K. Decussiphycus gen. nov.: A validation of “Decussata” (R.M.Patrick) Lange-Bertalot (Mastogloiaceae, Bacillariophyta). Not. Alg. 2019, 94, 2. [Google Scholar]
- Cleve, P.T. Synopsis of the naviculoid diatoms, Part I. Kongliga Sven.-Vetensk. Akad. Handl. 1894, 26, 1–194. [Google Scholar]
- Lobban, C.S.; Navarro, J.N. Mastogloiopsis biseriata, gen. et sp. nov., a diatom without partecta, is very similar to the Marginulatae group of Mastogloia. Nova Hedw. 2012, 94, 251–263. [Google Scholar] [CrossRef]
- Grunow, A. Über einige neue und ungenügend bekannte Arten und Gattungen von Diatomaceen. Verhandl. Kaiser. König. Zool. Bot. Ges. Wien 1863, 13, 137–162, 2 pls. [Google Scholar]
- Siqueiros Beltrones, D.A.; Echevarría Herrera, E.; López-Fuerte, F.O.; Martínez, Y.J. Species diversity of benthic marine diatoms from a Natural Protected Area in Cuba. Diversity 2025, 17, 181. [Google Scholar] [CrossRef]
- Torgan, L.C.; Donadel, L.; Gonçalves da Silva, J. A transferência de Navicula sovereignae Hustedt para o gênero Placoneis Mereschkowsky (Bacillariophyta). Iheringia Ser. Bot. 2010, 65, 107–114. [Google Scholar]
- Siqueiros Beltrones, D.A.; Echevarría Herrera, E.; López-Fuerte, F.O. Additions to the marine Mastogloia (Bacillariophyceae) from Cuban coasts; remarks on misidentified taxa. Diversity 2024, 16, 747. [Google Scholar] [CrossRef]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.-H.; Li, D.-Z.; Marhold, K.; et al. (Eds.) International Code of Nomenclature for algae, fungi, and plants. (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Veg. 2018, 159, [i]–xxxviii, 1–253. Available online: https://www.iapt-taxon.org/historic/2018.htm (accessed on 6 March 2025).
- von Stosch, H.A. An amended terminology of the diatom girdle. Nova Hedw. Beih. 1975, 53, 1–28. [Google Scholar]
- Mann, A. Marine diatoms of the Philippine Islands. Bull. United States Natl. Mus. 1925, 100 Pt 1, 1–182. [Google Scholar]
- Hendey, N.I. Some littoral diatoms of Kuwait. Nova Hedw. Beih. 1970, 31, 101–167. [Google Scholar] [CrossRef]
- Witkowski, A.; Lange-Bertalot, H.; Metzeltin, D. Diatom Flora of Marine Coasts; I.A.R.G. Gantner Verlag: Ruggell, Germany, 2000. [Google Scholar]
- Hein, M.K.; Winsborough, B.M.; Sullivan, M.J. Bacillariophyta (Diatoms) of the Bahamas; Iconographia Diatomologica; Gantner Verlag: Ruggell, Germany, 2008. [Google Scholar]
- Güttinger, W. Collection of SEM-Micrographs of Diatoms. Series 2. 50 Plates; Koeltz Scientific Books: Koenigstein, Germany, 1988. [Google Scholar]
- Paddock, T.B.B.; Kemp, K.-D. A description of some new species of the genus Mastogloia with further observations on M. elegans and M. goessii. Diatom Res. 1988, 3, 109–121. [Google Scholar] [CrossRef]
- Voigt, M. Contribution to the knowledge of the diatom genus Mastogloia. J. Roy. Microsc. Soc. Ser. 3 1942, 62, 1–20. [Google Scholar] [CrossRef]
- Wallich, G.C. On the siliceous organisms found in the digestive cavities of the Salpae, and their relation to the flint nodules of the Chalk Formation. Trans. Microsc. Soc. Lond. N. Ser. 1860, 8, 36–55, pl. II. [Google Scholar]
- Kemp, K.-D.; Paddock, T.B.B. Observations on the diatom genus Stigmaphora G. C. Wallich and two species of Mastogloia Thwaites ex Wm Smith. In Proceedings of the 9th International Diatom Symposium, Bristol, UK, 24–30 August 1986; Round, F.E., Ed.; Koenigstein & Biopress Ltd.: Bristol, UK, 1988; pp. 405–416. [Google Scholar]
- Molinari Novoa, E.A. In Guiry, M.D. & Guiry, G.M. 2024. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: https://www.algaebase.org/search/genus/detail/?genus_id=47079 (accessed on 20 February 2025).
- Schuster, T.M.; Hamilton, P.B.; Haring, V.; Edlund, M.B.; Van de Vijver, B. An introduction to the catalogue of Albert Grunow’s 19th century diatom collection at W including a palaeographic aid. Ann. Nat. Hist. Mus. Vienna B 2023, 125, 101–122. [Google Scholar]
- Lobban, C.S. Disymmetria reticulata, gen. nov., sp. nov. (Mediophyceae: Thalassiosirales), a new genus in Lauderiaceae emend., and transfer of Lauderia excentrica. Diatom 2023, 39, 25–30. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).