New Records of Tardigrades from the Republic of South Africa with Integrative Description of a New Mesobiotus Species (Tardigrada: Eutardigrada: Macrobiotidae) †
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Specimen Extraction
2.2. Microscopy and Imaging
2.3. Morphometry and Morphological Nomenclature
2.4. DNA Sequencing
2.5. Species Identification
3. Results
3.1. New Records
- 1.
- Echiniscus africanus Murray, 1907 [48]
- 2.
- Minibiotus pentannulatus Londoño, Daza, Lisi & Quiroga, 2017 [34]
3.2. Taxonomic Account of the New Species
3.3. Description of the New Species
3.3.1. Type Locality
3.3.2. Etymology
3.3.3. Type Depositories
3.3.4. Description of Animals (Measurements and Statistics in Table 1)
| Character | N | Range | Mean | SD | Holotype | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µm | pt | µm | pt | µm | pt | µm | pt | ||||||
| Body length | 19 | 301 | – | 591 | – | 448 | – | 71 | – | 591 | – | ||
| Buccal tube | |||||||||||||
| Buccal tube length | 20 | 37.0 | – | 61.6 | – | 51.6 | – | 6.5 | – | 60.0 | – | ||
| Stylet support insertion point | 20 | 28.4 | – | 48.2 | 75.5 | – | 78.8 | 39.9 | 77.3 | 5.1 | 0.8 | 46.5 | 77.5 |
| Buccal tube external width | 20 | 5.0 | – | 10.2 | 13.5 | – | 18.3 | 8.0 | 15.4 | 1.3 | 1.0 | 9.1 | 15.1 |
| Buccal tube internal width | 20 | 3.9 | – | 7.8 | 10.3 | – | 14.8 | 6.1 | 11.7 | 1.1 | 1.1 | 7.2 | 12.0 |
| Ventral lamina length | 15 | 26.8 | – | 41.0 | 60.2 | – | 68.0 | 34.4 | 64.2 | 4.1 | 1.9 | 37.8 | 62.9 |
| Placoid lengths | |||||||||||||
| Macroplacoid 1 | 20 | 5.0 | – | 11.4 | 13.5 | – | 19.2 | 8.6 | 16.5 | 1.8 | 1.8 | 10.3 | 17.2 |
| Macroplacoid 2 | 20 | 3.9 | – | 9.1 | 10.0 | – | 16.4 | 6.4 | 12.4 | 1.4 | 1.6 | 7.2 | 11.9 |
| Macroplacoid 3 | 20 | 4.8 | – | 10.6 | 13.0 | – | 18.4 | 8.0 | 15.3 | 1.6 | 1.5 | 9.4 | 15.7 |
| Microplacoid | 20 | 2.6 | – | 7.8 | 7.0 | – | 12.6 | 5.3 | 10.1 | 1.2 | 1.6 | 4.7 | 7.9 |
| Macroplacoid row | 19 | 19.0 | – | 32.9 | 42.0 | – | 54.9 | 25.4 | 48.3 | 4.3 | 3.9 | 29.6 | 49.3 |
| Placoid row | 20 | 18.7 | – | 39.6 | 50.5 | – | 67.6 | 30.9 | 59.6 | 5.6 | 4.5 | 36.3 | 60.5 |
| Claw I heights | |||||||||||||
| External primary branch | 12 | 9.2 | – | 13.6 | 19.9 | – | 26.5 | 12.3 | 23.8 | 1.3 | 2.3 | 13.6 | 22.7 |
| External secondary branch | 12 | 7.9 | – | 13.1 | 17.1 | – | 26.3 | 10.7 | 21.2 | 1.3 | 3.0 | 10.4 | 17.3 |
| Internal primary branch | 11 | 8.0 | – | 13.0 | 19.1 | – | 27.5 | 11.4 | 23.2 | 1.5 | 2.1 | 13.0 | 21.7 |
| Internal secondary branch | 9 | 6.8 | – | 10.8 | 17.1 | – | 22.4 | 9.6 | 19.6 | 1.2 | 1.6 | 10.3 | 17.1 |
| Claw II heights | |||||||||||||
| External primary branch | 16 | 10.8 | – | 14.8 | 20.8 | – | 28.6 | 13.2 | 25.6 | 1.2 | 2.0 | 14.5 | 24.1 |
| External secondary branch | 15 | 8.7 | – | 13.1 | 17.2 | – | 26.3 | 11.0 | 21.3 | 1.3 | 2.2 | 11.3 | 18.8 |
| Internal primary branch | 15 | 10.3 | – | 15.0 | 21.8 | – | 27.0 | 12.7 | 24.3 | 1.1 | 1.6 | 13.8 | 22.9 |
| Internal secondary branch | 13 | 7.9 | – | 14.2 | 17.2 | – | 26.0 | 10.9 | 21.0 | 1.7 | 2.4 | 10.4 | 17.3 |
| Claw III heights | |||||||||||||
| External primary branch | 12 | 11.5 | – | 15.9 | 22.8 | – | 29.7 | 13.5 | 25.8 | 1.4 | 1.9 | 13.7 | 22.8 |
| External secondary branch | 10 | 9.5 | – | 13.6 | 18.6 | – | 26.2 | 11.9 | 22.4 | 1.3 | 2.2 | 11.2 | 18.6 |
| Internal primary branch | 15 | 9.4 | – | 15.3 | 22.3 | – | 27.6 | 12.4 | 24.4 | 1.5 | 1.5 | 13.4 | 22.3 |
| Internal secondary branch | 10 | 8.0 | – | 13.5 | 17.6 | – | 25.2 | 11.2 | 21.6 | 2.0 | 2.1 | 11.7 | 19.5 |
| Claw IV heights | |||||||||||||
| Anterior primary branch | 16 | 7.7 | – | 17.6 | 20.8 | – | 30.9 | 14.2 | 27.2 | 2.4 | 2.9 | 15.0 | 25.0 |
| Anterior secondary branch | 14 | 6.1 | – | 13.8 | 16.5 | – | 28.2 | 11.4 | 22.0 | 2.1 | 3.1 | 11.1 | 18.4 |
| Posterior primary branch | 16 | 7.5 | – | 18.2 | 20.3 | – | 32.1 | 14.9 | 28.5 | 2.5 | 3.1 | 14.8 | 24.7 |
| Posterior secondary branch | 15 | 5.8 | – | 13.8 | 15.7 | – | 26.6 | 11.7 | 22.4 | 2.0 | 2.7 | 12.4 | 20.7 |



3.3.5. Description of Eggs (Measurements and Statistics in Table 2)
| Character | N | Range | Mean | SD | ||
|---|---|---|---|---|---|---|
| Egg bare diameter | 10 | 60.2 | – | 78.6 | 68.9 | 6.0 |
| Egg full diameter | 10 | 127.4 | – | 155.0 | 139.0 | 8.9 |
| Process height | 31 | 27.8 | – | 44.2 | 35.6 | 4.1 |
| Process base width | 31 | 20.2 | – | 26.6 | 22.7 | 1.8 |
| Process base/height ratio | 31 | 52% | – | 82% | 64% | 8% |
| Inter-process distance | 22 | 2.3 | – | 6.0 | 4.3 | 1.1 |
| Number of processes on the egg circumference | 11 | 8 | – | 9 | 8.9 | 0.3 |
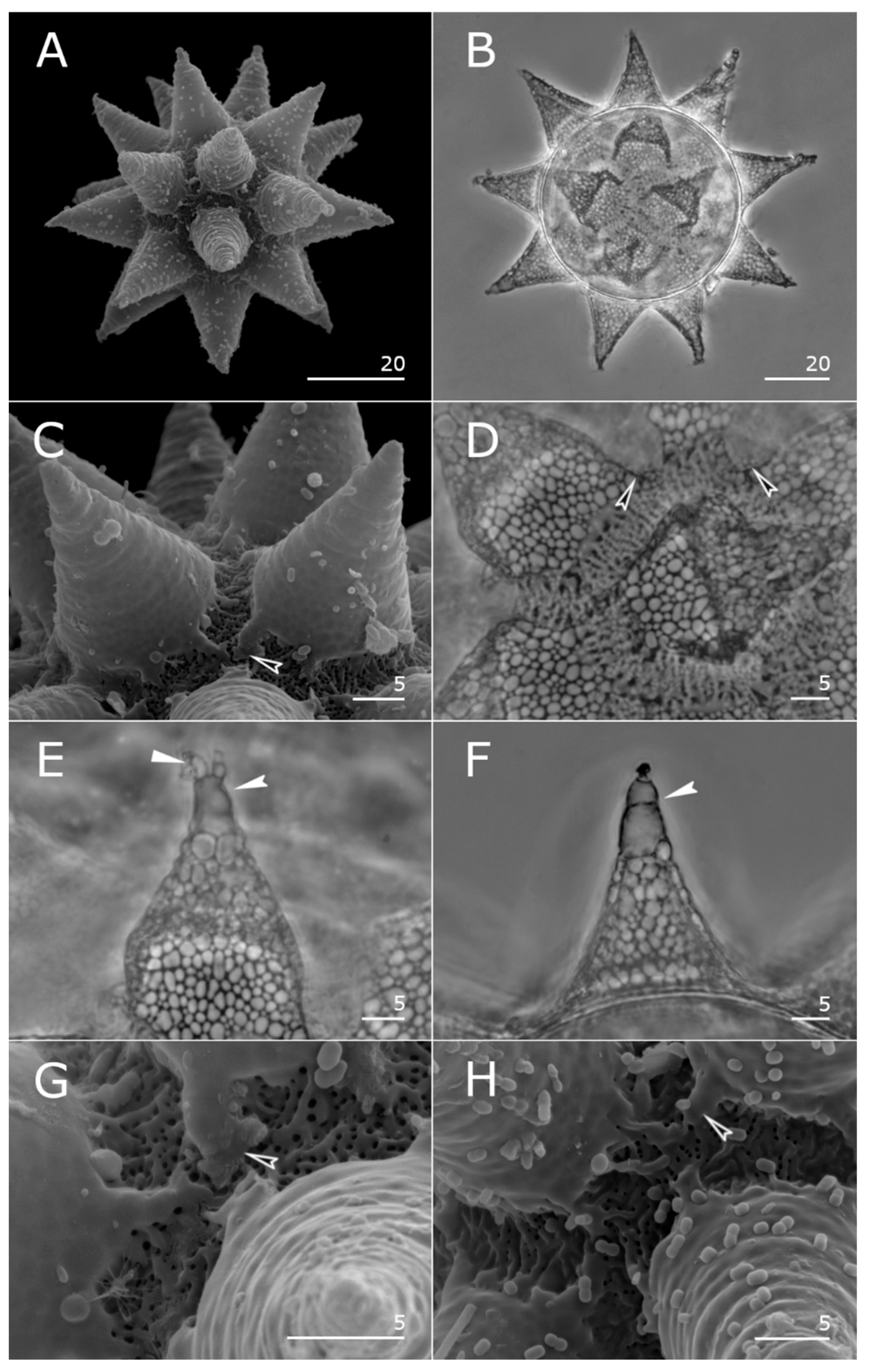
3.3.6. DNA Sequences
3.3.7. Genetic Distances
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, D.R.; Guidetti, R.; Rebecchi, L. Phylum Tardigrada. In Thorp and Covich’s Freshwater Invertebrates; Academic Press: Cambridge, MA, USA, 2015; pp. 347–380. [Google Scholar] [CrossRef]
- Degma, P.; Guidetti, R. Actual Checklist of Tardigrada Species, 43rd ed.; Checklist Database: Modena, Italy, 2009–2024. [Google Scholar] [CrossRef]
- McInnes, S.; Michalczyk, Ł.; Kaczmarek, Ł. Annotated zoogeography of non-marine Tardigrada Part IV: Africa. Zootaxa 2017, 4284, 1–74. [Google Scholar] [CrossRef]
- Stec, D.; Kristensen, R.M.; Michalczyk, Ł. An integrative description of Minibiotus ioculator sp. nov. from the Republic of South Africa with notes on Minibiotus pentannulatus Londoño et al., 2017 (Tardigrada: Macrobiotidae). Zool. Anz. 2020, 286, 117–134. [Google Scholar] [CrossRef]
- Tumanov, D.V. Integrative description of Mesobiotus anastasiae sp. nov. (Eutardigrada, Macrobiotoidea) and first record of Lobohalacarus (Chelicerata, Trombidiformes) from the Republic of South Africa. Eur. J. Taxon. 2020, 726, 102–131. [Google Scholar] [CrossRef]
- Morek, W.; Surmacz, B.; López-López, A.; Michalczyk, Ł. Everything is not everywhere: Time-calibrated phylogeography of the genus Milnesium (Tardigrada). Mol. Ecol. 2021, 30, 3590–3609. [Google Scholar] [CrossRef] [PubMed]
- Gąsiorek, P.; Vončina, K.; Bochnak, M.; Surmacz, B.; Morek, W.; Michalczyk, Ł. Echiniscidae (Heterotardigrada) of South Africa. Zootaxa 2022, 5156, 1–238. [Google Scholar] [CrossRef]
- Vecchi, M.; Cesari, M.; Bertolani, R.; Jönsson, K.I.; Rebecchi, L.; Guidetti, R. Integrative systematic studies on tardigrades from Antarctica identify new genera and new species within Macrobiotoidea and Echiniscoidea. Invertebr. Syst. 2016, 30, 303–322. [Google Scholar] [CrossRef]
- Stec, D.; Smolak, R.; Kaczmarek, Ł.; Michalczyk, Ł. An integrative description of Macrobiotus paulinae sp. nov. (Tardigrada: Eutardigrada: Macrobiotidae: hufelandi group) from Kenya. Zootaxa 2015, 4052, 501–526. [Google Scholar]
- Roszkowska, M.; Stec, D.; Gawlak, M.; Kaczmarek, Ł. An integrative description of a new tardigrade species Mesobiotus romani sp. nov. (Macrobiotidae: harmsworthi group) from Ecuadorian Pacific coast. Zootaxa 2018, 4450, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Pilato, G. Analisi di nuovi caratteri nello studio degli Eutardigradi. Animalia 1981, 8, 51–57. [Google Scholar]
- Pilato, G.; Binda, M.G. Definition of families, subfamilies, genera and subgenera of the Eutardigrada, and keys to their identification. Zootaxa 2010, 2404, 1–52. [Google Scholar] [CrossRef]
- Pilato, G. Structure, intraspecific variability and systematic value of the buccal armature of eutardigrades. Zool. Anz. 1972, 10, 65–78. [Google Scholar] [CrossRef]
- Michalczyk, Ł.; Kaczmarek, Ł. A description of the new tardigrade Macrobiotus reinhardti (Eutardigrada: Macrobiotidae, harmsworthi group) with some remarks on the oral cavity armature within the genus Macrobiotus Schultze. Zootaxa 2003, 331, 1–24. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Michalczyk, Ł. The Macrobiotus hufelandi group (Tardigrada) revisited. Zootaxa 2017, 4363, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, Ł.; Cytan, J.; Zawierucha, K.; Diduszko, D.; Michalczyk, Ł. Tardigrades from Peru (South America), with descriptions of three new species of Parachela. Zootaxa 2014, 3790, 357–379. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Bartylak, T.; Stec, D.; Kulpa, A.; Kepel, M.; Kepel, A.; Roszkowska, M. Revisiting the genus Mesobiotus Vecchi et al., 2016 (Eutardigrada, Macrobiotidae)—Remarks, updated dichotomous key and an integrative description of new species from Madagascar. Zool. Anz. 2020, 287, 121–146. [Google Scholar] [CrossRef]
- Kiosya, Y.; Pogwizd, J.; Matsko, Y.; Vecchi, M.; Stec, D. Phylogenetic position of two Macrobiotus species with a revisional note on Macrobiotus sottilei Pilato, Kiosya, Lisi & Sabella, 2012 (Tardigrada: Eutardigrada: Macrobiotidae). Zootaxa 2021, 4933, 113–135. [Google Scholar] [CrossRef]
- Michalczyk, Ł.; Kaczmarek, Ł. The Tardigrada Register: A comprehensive online data repository for tardigrade taxonomy. J. Limnol. 2013, 72, 175–181. [Google Scholar] [CrossRef]
- Bertolani, R.; Guidetti, R.; Marchioro, T.; Altiero, T.; Rebechi, L.; Cesari, M. Phylogeny of Eutardigrada: New molecular data and their morphological support lead to the identification of new evolutionary lineages. Mol. Phylogenetics Evol. 2014, 76, 110–126. [Google Scholar] [CrossRef]
- Stec, D.; Vecchi, M.; Calhim, S.; Michalczyk, Ł. New multilocus phylogeny reorganises the family Macrobiotidae (Eutardigrada) and unveils complex morphological evolution of the Macrobiotus hufelandi group. Mol. Phylogenetics Evol. 2021, 160, 106987. [Google Scholar] [CrossRef]
- Perry, E.; Miller, W.R.; Kaczmarek, Ł. Recommended abbreviations for the names of genera of the phylum Tardigrada. Zootaxa 2019, 4608, 145–154. [Google Scholar] [CrossRef]
- Casquet, J.; Thebaud, C.; Gillespie, R.G. Chelex without boiling, a rapid and easy technique to obtain stable amplifiable DNA from small amounts of ethanol-stored spiders. Mol. Ecol. Resour. 2012, 12, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Sands, C.J.; McInnes, S.J.; Marley, N.J.; Goodall-Copestake, W.P.; Convey, P.; Linse, K. Phylum Tardigrada: An “individual” approach. Cladistics 2008, 24, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Mironov, S.V.; Dabert, J.; Dabert, M. A new feather mite species of the genus Proctophyllodes Robin, 1877 (Astigmata: Proctophyllodidae) from the Long-tailed Tit Aegithalos caudatus (Passeriformes: Aegithalidae)—Morphological description with DNA barcode data. Zootaxa 2012, 3253, 54. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Katoh, K.; Toh, H. Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 2008, 9, 286–298. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Dastych, H. The Tardigrada from Antarctic with descriptions of several new species. Acta Zool. Cracoviensia 1984, 27, 377–436. [Google Scholar]
- Tumanov, D.V. Two new species of Macrobiotus (Eutardigrada, Macrobiotidae) from Tien Shan (Kirghizia), with notes on Macrobiotus tenuis group. Zootaxa 2005, 1043, 33–46. [Google Scholar] [CrossRef]
- Londoño, R.; Daza, A.; Lisi, O.; Quiroga, S. New species of water bear Minibiotus pentannulatus (Tardigrada: Macrobiotidae) from Colombia. Rev. Mex. De Biodivers. 2017, 88, 807–814. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Zawierucha, K.; Buda, J.; Stec, D.; Gawlak, M.; Michalczyk, Ł.; Roszkowska, M. An integrative redescription of the nominal taxon for the Mesobiotus harmsworthi group (Tardigrada: Macrobiotidae) leads to descriptions of two new Mesobiotus species from Arctic. PLoS ONE 2018, 13, e0204756. [Google Scholar] [CrossRef] [PubMed]
- Stec, D. Mesobiotus datalanicus sp. nov. a new tardigrade species (Macrobiotidae: Mesobiotus harmsworthi group) from Lâm Ðông province in Vietnam. Zootaxa 2019, 4679, 164–180. [Google Scholar] [CrossRef]
- Tumanov, D.V.; Androsova, E.D.; Gavrilenko, M.D.; Kalimullin, A.A. Integrative description of two new species of the genus Mesobiotus (Eutardigrada, Macrobiotoidea) from Russia, with an updated phylogeny of the genus. Eur. J. Taxon. 2024, 947, 20–52. [Google Scholar] [CrossRef]
- Stec, D. An integrative description of two new Mesobiotus species (Tardigrada: Eutardigrada: Macrobiotidae) with updated genus phylogeny. Zool. Stud. 2022, 61, e85. [Google Scholar] [CrossRef]
- Itang, L.A.; Stec, D.; Mapalo, M.A.; Mirano-Bascos, D.; Michalczyk, Ł. An integrative description of Mesobiotus dilimanensis, a new tardigrade species from the Philippines (Eutardigrada: Macrobiotidae: furciger group). Raffles Bull. Zool. 2020, 68, 19–31. [Google Scholar] [CrossRef]
- Massa, E.; Guidetti, R.; Cesari, M.; Rebecchi, L.; Jönsson, K.I. Tardigrades of Kristianstads Vattenrike Biosphere Reserve with description of four new species from Sweden. Sci. Rep. 2021, 11, 4861. [Google Scholar] [CrossRef]
- Erdmann, W.; Kosicki, J.Z.; Kayastha, P.; Mioduchowska, M.; Kaczmarek, Ł. An integrative description of Mesobiotus mandalori sp. nov. (Eutardigrada, Macrobiotoidea) from Poland. Eur. Zool. J. 2024, 91, 378–394. [Google Scholar] [CrossRef]
- Atherton, S.; Hulterström, J.; Guidetti, R.; Jönsson, K. Three new species of Mesobiotus (Eutardigrada: Macrobiotidae) from Sweden with an updated phylogeny of the genus. Sci. Rep. 2025, 15, 4535. [Google Scholar] [CrossRef]
- Vecchi, M.; McDaniel, J.L.; Chartrain, J.; Vuori, T.; Walsh, E.J.; Calhim, S. Morphology, phylogenetic position, and mating behaviour of a new Mesobiotus (Tardigrada) species from a rock pool in the Socorro Box Canyon (New Mexico, USA). Eur. Zool. J. 2023, 90, 708–725. [Google Scholar] [CrossRef]
- Tumanov, D.V.; Pilato, G. A new species of Eutardigrade (Macrobiotidae) from New Zealand. Zootaxa 2019, 4603, 431–447. [Google Scholar] [CrossRef] [PubMed]
- Stec, D. Integrative descriptions of two new Mesobiotus species (Tardigrada, Eutardigrada, Macrobiotidae) from Vietnam. Diversity 2021, 13, 605. [Google Scholar] [CrossRef]
- Guidetti, R.; Gneuß, E.; Cesari, M.; Altiero, T.; Schill, R.O. Life-history traits and description of the new gonochoric amphimictic Mesobiotus joenssoni (Eutardigrada: Macrobiotidae) from the island of Elba, Italy. Zool. J. Linn. Soc. 2020, 188, 848–859. [Google Scholar] [CrossRef]
- Tumanov, D.V. Mesobiotus nikolaevae sp. n. (Eutardigrada: Macrobiotidae), a new species of Tardigrada from Croatia. Invertebr. Zool. 2018, 15, 402–419. [Google Scholar] [CrossRef]
- Murray, J. Some South African Tardigrada. J. R. Microsc. Soc. 1907, 5, 515–524. [Google Scholar] [CrossRef]
- Węglarska, B. Die Tardigraden Vietnams. Acta Soc. Zool. Bohemoslov. 1962, 26, 300–307. [Google Scholar]
- Gąsiorek, P.; Kristensen, R.M. Echiniscidae (Heterotardigrada) of Tanzania and Uganda. Trop. Zool. 2018, 31, 131–160. [Google Scholar] [CrossRef]
- Gąsiorek, P.; Vončina, K. New Echiniscidae (Heterotardigrada) from Amber Mountain (Northern Madagascar). Evol. Syst. 2019, 3, 29–39. [Google Scholar] [CrossRef]
- Spallanzani, L. Opuscoli di fisica animale e vegetabile. Soc. Tipogr. De Class. Ital. 1777, 2, 181–253. [Google Scholar]
- Richters, F. Tardigrada. Handb. Der Zool. 1926, 3, 1–68. [Google Scholar]
- Schuster, R.O.; Nelson, D.R.; Grigarick, A.A.; Christenberry, D. Systematic criteria of the Eutardigrada. Trans. Am. Microsc. Soc. 1980, 99, 284–303. [Google Scholar] [CrossRef]
- Thulin, G. Über die Phylogenie und das System der Tardigraden. Hered. Lund 1928, 11, 207–266. [Google Scholar] [CrossRef]
- Marley, N.J.; McInnes, S.J.; Sands, C.J. Phylum Tardigrada: A re-evaluation of the Parachela. Zootaxa 2011, 2819, 51–64. [Google Scholar] [CrossRef]
- Binda, M.G.; Pilato, G.; Lisi, O. Remarks on Macrobiotus furciger Murray, 1906 and description of three new species of the furciger group (Eutardigrada, Macrobiotidae). Zootaxa 2005, 1075, 55–68. [Google Scholar] [CrossRef]
- Vecchi, M.; Dykyy, I.; Khoyetskyy, P.; Vuori, T.; Calhim, S.; Trokhymets, V. The tardigrade Mesobiotus aradasi (Binda, Pilato & Lisi, 2005) is widely distributed along the Antarctic Peninsula. Polar Biol. 2024, 47, 227–238. [Google Scholar] [CrossRef]
- Short, K.A.; Sands, C.J.; McInnes, S.J.; Pisani, D.; Stevens, M.I.; Convey, P. An ancient-specific species complex: Large divergences between multiple Antarctic lineages of the tardigrade genus Mesobiotus. Mol. Phylogenetics Evol. 2022, 170, 107429. [Google Scholar] [CrossRef]
- Stec, D.; Kristensen, R.M. An integrative description of Mesobiotus ethiopicus sp. nov. (Tardigrada: Eutardigrada: Parachela: Macrobiotidae: harmsworthi group) from the northern Afrotropical region. Turk. J. Zool. 2017, 41, 800–811. [Google Scholar] [CrossRef]
- Murray, J. XXV-Arctic Tardigrada, collected by Wm. S. Bruce. Earth Environ. Sci. Trans. R. Soc. Edinb. 1907, 45, 669–681. [Google Scholar] [CrossRef]
- Murray, J. African Tardigrada. J. R. Microsc. Soc. 1913, 2, 136–144. [Google Scholar] [CrossRef]
- Dastych, H. Macrobiotus kurasi sp. nov., a new species of Tardigrada from mountains in Uganda. Bull. Del Acad. Pol. Des Sci. 1980, 28, 653–657. [Google Scholar]
- Binda, M.G. Notizie sui Tardigradi dell’Africa meridionale con descrizione di una nuova specie di Apodibius (Eutardigrada). Animalia 1984, 11, 5–15. [Google Scholar]
- Pilato, G.; Binda, M.G.; Catanzaro, R. Remarks on some tardigrades of the African fauna with the description of three new species of Macrobiotus Schultze 1834. Trop. Zool. 1991, 4, 167–178. [Google Scholar] [CrossRef][Green Version]
- Dastych, H. Paradiphascon manningi gen. n. sp. n., a new water-bear from South Africa, with the erecting of a new subfamily Diphasconinae (Tardigrada). Mitt. Hambg. Zool. Mus. Inst. 1992, 89, 125–139. [Google Scholar]
- Kaczmarek, Ł.; Beasley, C.W.; Michalczyk, Ł. The first record of the genus Haplohexapodibius Pilato & Beasley, 1987 in Africa, with notes on synonymy of Hexapodibius beasleyi Maucci, 1988, with Haplohexapodibius seductor Pilato & Beasley, 1987. Afr. Zool. 2006, 41, 290–293. [Google Scholar] [CrossRef]
- Dastych, H. Notes on the African limno-terrestrial tardigrade Ramazzottius szeptycki (Dastych, 1980)(Tardigrada). Entomol. Mitt. Zool. Mus. Hambg. 2009, 15, 87–91. [Google Scholar]
- Meyer, H.A.; Hinton, J.G. The Tardigrada of southern Africa, with the description of Minibiotus harrylewisi, a new species from KwaZulu-Natal, South Africa (Eutardigrada: Macrobiotidae). Afr. Invertebr. 2009, 50, 255–268. [Google Scholar] [CrossRef]
- Meyer, H.A.; Tsaliki, M.; Hinton, J.G. First records of water bears (Phylum Tardigrada) from Swaziland. Afr. Invertebr. 2018, 59, 47–53. [Google Scholar] [CrossRef]
- Richters, F. Moosbewohner. In Wissenschaftliche Ergebnisse der Schwedischen Südpolar-Expedition 1901–1903; Lithographisches Institut des Generalstabs: Stockholm, Sweden, 1908; Volume 6, pp. 1–16. [Google Scholar]
- Richters, F. Beiträge zur Kenntnis der Fauua der Umgebung von Frankfurt a. M. Ber. Der Senckenberg. Naturforschenden Ges. Frankf. Am Main 1902, 1902, 3–21. [Google Scholar]
- Richters, F. Neue Moosbewohner. Ber. Senckenberg. Naturforschenden Ges. Frankf. Am Main 1902, 1902.2, 23–26. [Google Scholar]
- Richters, F. Nordische Tardigraden. Zool. Anz. 1903, 27, 168–172. [Google Scholar]
- Ehrenberg, C.G. Diagnoses novarum formarum. Verhandlungen Der K. Preuss. Akad. Der Wiss. Zu Berl. 1853, 8, 526–533. [Google Scholar]
- Marcus, E. Spinnentiere oder Arachoides. IV Bärtierchen (Tardigrada). Tierwelt Dtschl. Und Der Angrenzenden Meeresteile Jena 1928, 12, 1–230. [Google Scholar]
- Bartoš, E. Die Tardigraden der chinesischen und javanischen Moosproben. Acta Soc. Zool. Bohemoslov. 1963, 27, 108–114. [Google Scholar]
- Stec, D.; Vecchi, M.; Maciejowski, W.; Michalczyk, Ł. Resolving the systematics of Richtersiidae by multilocus phylogeny and an integrative redescription of the nominal species for the genus Crenubiotus (Tardigrada). Sci. Rep. 2020, 10, 19418. [Google Scholar] [CrossRef]
- Schultze, C.A.S. Macrobiotus hufelandii animal e crustaceorum classe novum, reviviscendi post diuturnam asphixiam et aridiatem potens. Berolini Apud Carolum Curths 1834, 1834, 1–8. [Google Scholar]
- Plate, L.H. Beiträge zur Naturgeschichte der Tardigraden. Zool. Jahrbuecher 1888, 3, 487–550. [Google Scholar] [CrossRef]
- Doyère, M. Memoire sur les tardigrades. Annales des Sciences Naturelles. Zool. Ser. 1840, 2.14, 269–362. [Google Scholar]
- Murray, J. Arctiscoida. Proc. R. Ir. Acad. 1911, 31, 1–16. [Google Scholar]
- Claxton, S.K. A revision of the genus Minibiotus (Tardigrada: Macrobiotidae) with descriptions of eleven new species from Australia. Rec. Aust. Mus. 1998, 50, 125–160. [Google Scholar] [CrossRef]
- Pilato, G.; D’urso, V.; Lisi, O. Ramazzottius thulini (Pilato, 1970) bona species and description of Ramazzottius libycus sp. nov. (Eutardigrada, Ramazzottidae). Zootaxa 2013, 3681, 270–280. [Google Scholar] [CrossRef][Green Version]
- Kaczmarek, Ł.; Kayastha, P.; Roszkowska, M.; Gawlak, M.; Mioduchowska, M. Integrative Redescription of the Minibiotus intermedius (Plate, 1888)—The type species of the genus Minibiotus R.O. Schuster, 1980. Diversity 2022, 14, 356. [Google Scholar] [CrossRef]
- Middleton, R.C. Tardigrades in southern Africa. Afr. J. Ecol. 2003, 41, 280–282. [Google Scholar] [CrossRef]
- Mapalo, M.A.; Stec, D.; Mirano-Bascos, D.; Michalczyk, Ł. An integrative description of a limnoterrestrial tardigrade from the Philippines, Mesobiotus insanis, new species (Eutardigrada: Macrobiotidae: harmsworthi group). Raffles Bull. Zool. 2017, 65, 440–454. [Google Scholar]
- Maucci, W. Due nuove specie di tardigradi muscicoli della Spagna. Boll. Mus. Civ. Stor. Nat. Verona 1991, 15, 257–264. [Google Scholar]
- Mapalo, M.A.; Stec, D.; Mirano-Bascos, D.; Michalczyk, Ł. Mesobiotus philippinicus sp. nov., the first limnoterrestrial tardigrade from the Philippines. Zootaxa 2016, 4126, 411–426. [Google Scholar] [CrossRef]
- Stec, D.; Roszkowska, M.; Kaczmarek, Ł.; Michalczyk, Ł. An integrative description of a population of Mesobiotus radiatus (Pilato, Binda and Catanzaro, 1991) from Kenya. Turk. J. Zool. 2018, 42, 523–540. [Google Scholar] [CrossRef]
- Kayastha, P.; Roszkowska, M.; Mioduchowska, M.; Gawlak, M.; Kaczmarek, Ł. Integrative description of two new tardigrade species along with new records of Mesobiotus skorackii Kaczmarek et al. 2018 from Canada. Diversity 2021, 13, 394. [Google Scholar] [CrossRef]
- Warguła, J.; Stec, D.; Rutkowski, T.; Gierlasiński, G.; Kayastha, P.; Kaczmarek, Ł. An integrative description of a new species of the genus Mesobiotus (Tardigrada: Eutardigrada: Macrobiotidae) from Poland. Org. Divers. Evol. 2025, 2025, 1–14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmuchowska, W.; Nawrot, K.; Gawlak, M.; Warguła, J.; Kaczmarek, Ł. New Records of Tardigrades from the Republic of South Africa with Integrative Description of a New Mesobiotus Species (Tardigrada: Eutardigrada: Macrobiotidae). Taxonomy 2025, 5, 20. https://doi.org/10.3390/taxonomy5020020
Dmuchowska W, Nawrot K, Gawlak M, Warguła J, Kaczmarek Ł. New Records of Tardigrades from the Republic of South Africa with Integrative Description of a New Mesobiotus Species (Tardigrada: Eutardigrada: Macrobiotidae). Taxonomy. 2025; 5(2):20. https://doi.org/10.3390/taxonomy5020020
Chicago/Turabian StyleDmuchowska, Wiktoria, Katarzyna Nawrot, Magdalena Gawlak, Jędrzej Warguła, and Łukasz Kaczmarek. 2025. "New Records of Tardigrades from the Republic of South Africa with Integrative Description of a New Mesobiotus Species (Tardigrada: Eutardigrada: Macrobiotidae)" Taxonomy 5, no. 2: 20. https://doi.org/10.3390/taxonomy5020020
APA StyleDmuchowska, W., Nawrot, K., Gawlak, M., Warguła, J., & Kaczmarek, Ł. (2025). New Records of Tardigrades from the Republic of South Africa with Integrative Description of a New Mesobiotus Species (Tardigrada: Eutardigrada: Macrobiotidae). Taxonomy, 5(2), 20. https://doi.org/10.3390/taxonomy5020020







