Report on Eight Unrecorded Species of Freshwater Oligochaetes in Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Areas
2.2. Sample Collection
2.3. Specimen Processing and Preservation
2.4. Voucher Specimen Deposition
2.5. Morphological Analysis
3. Results

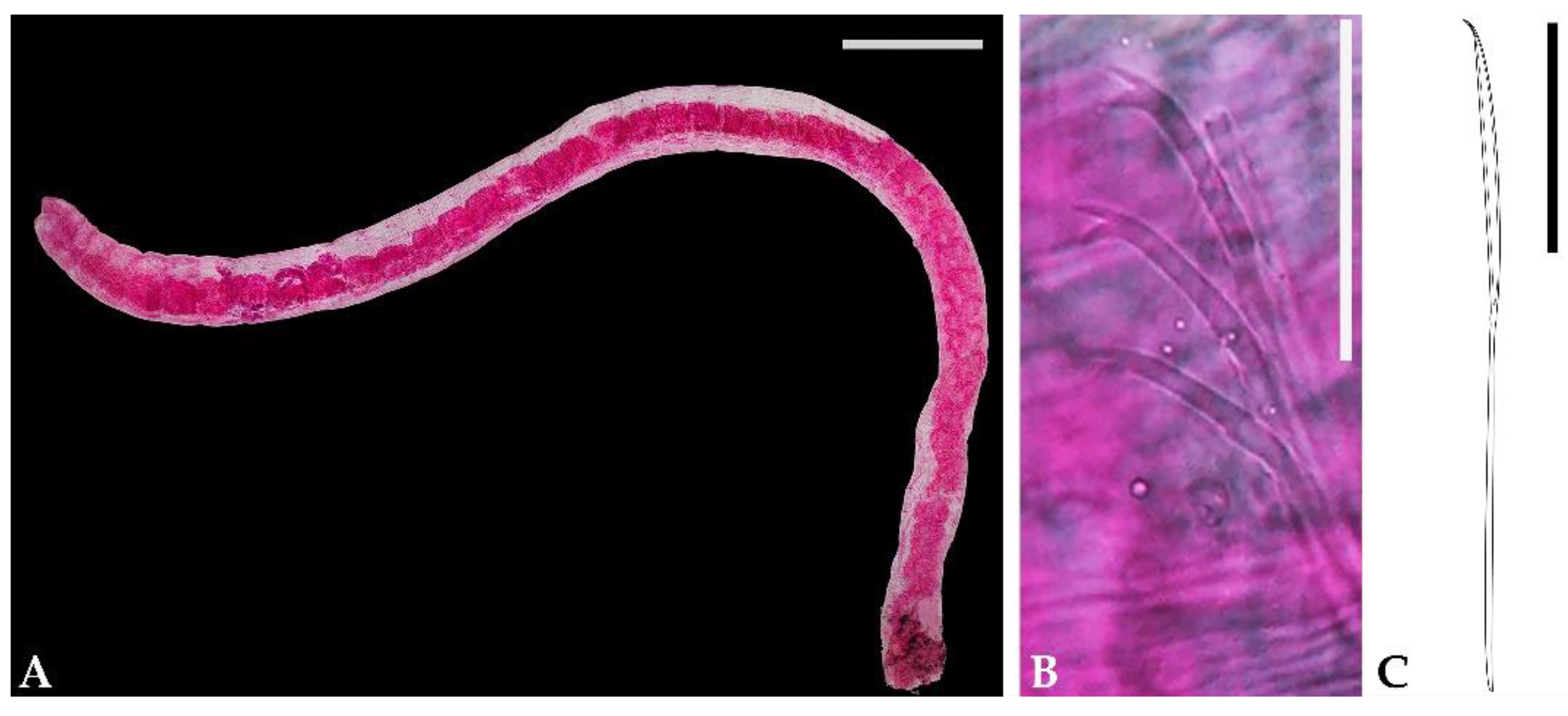
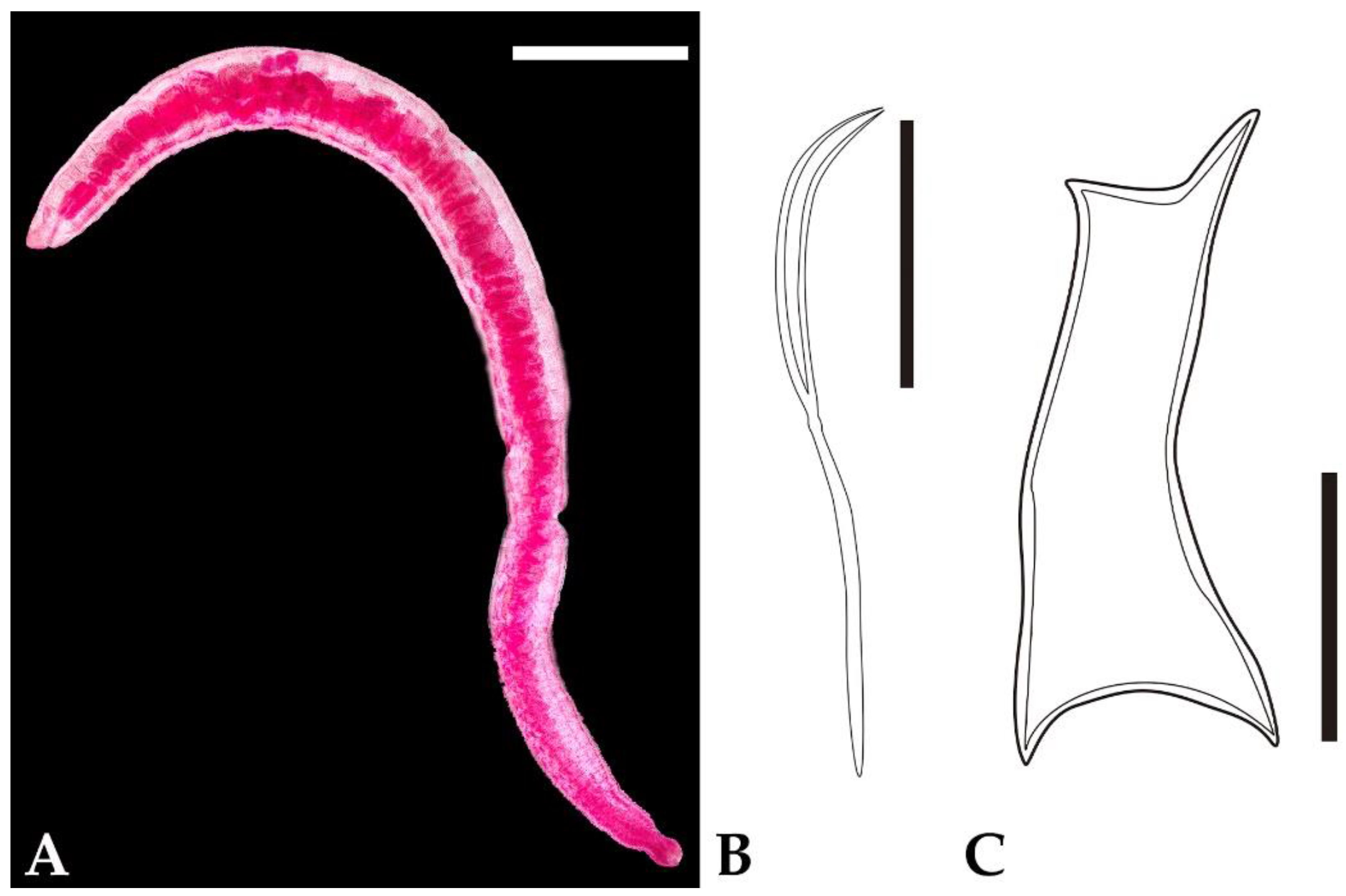
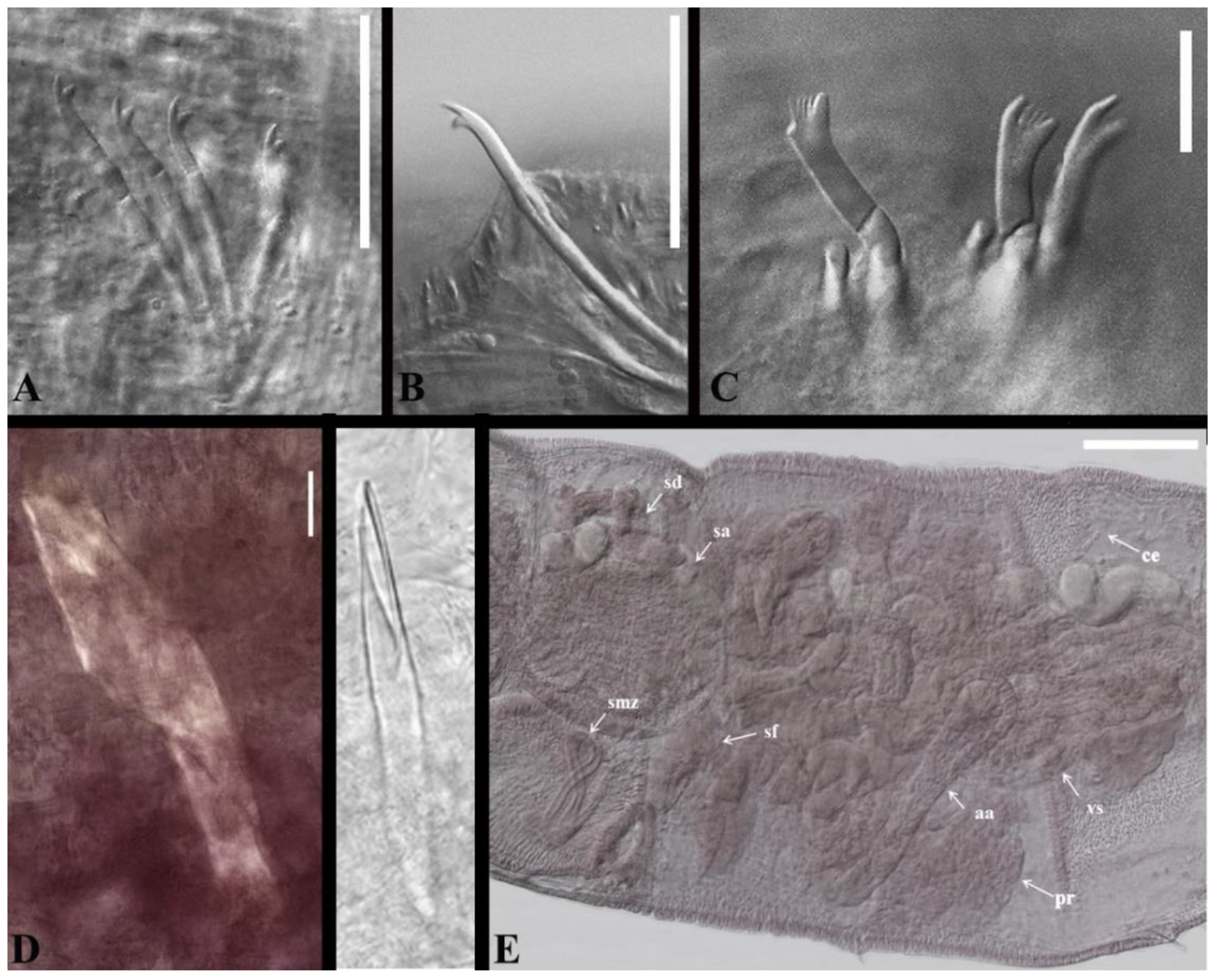
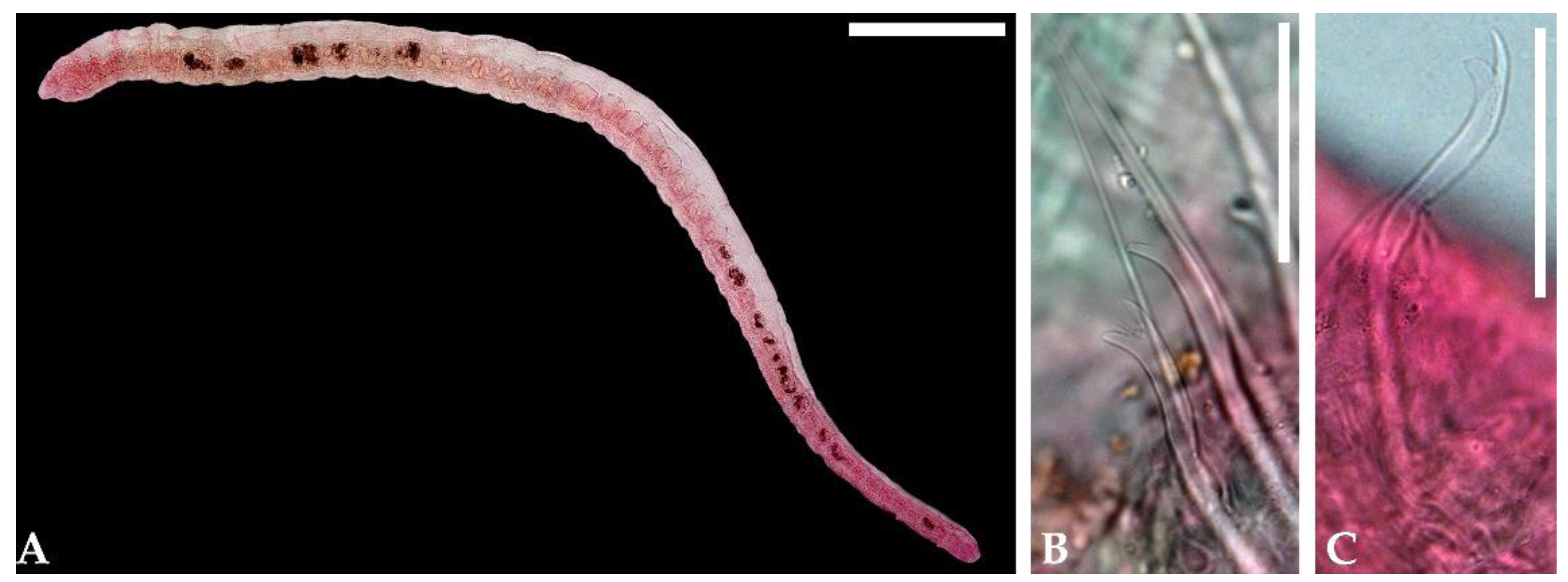
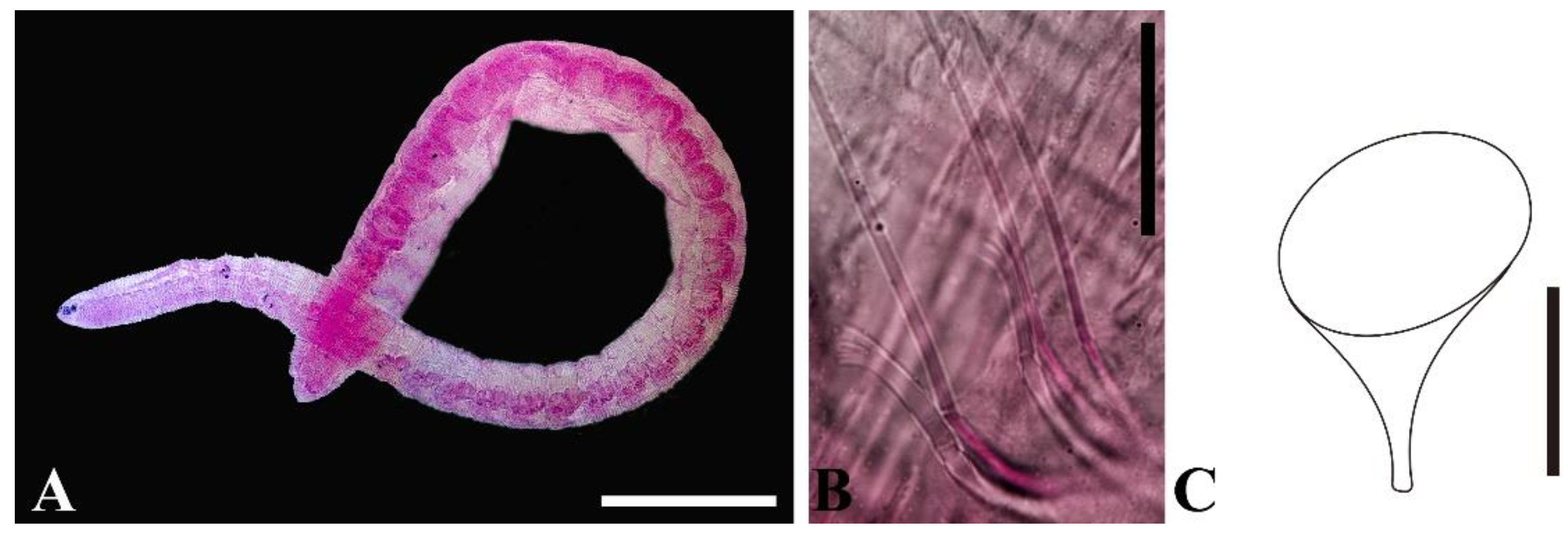

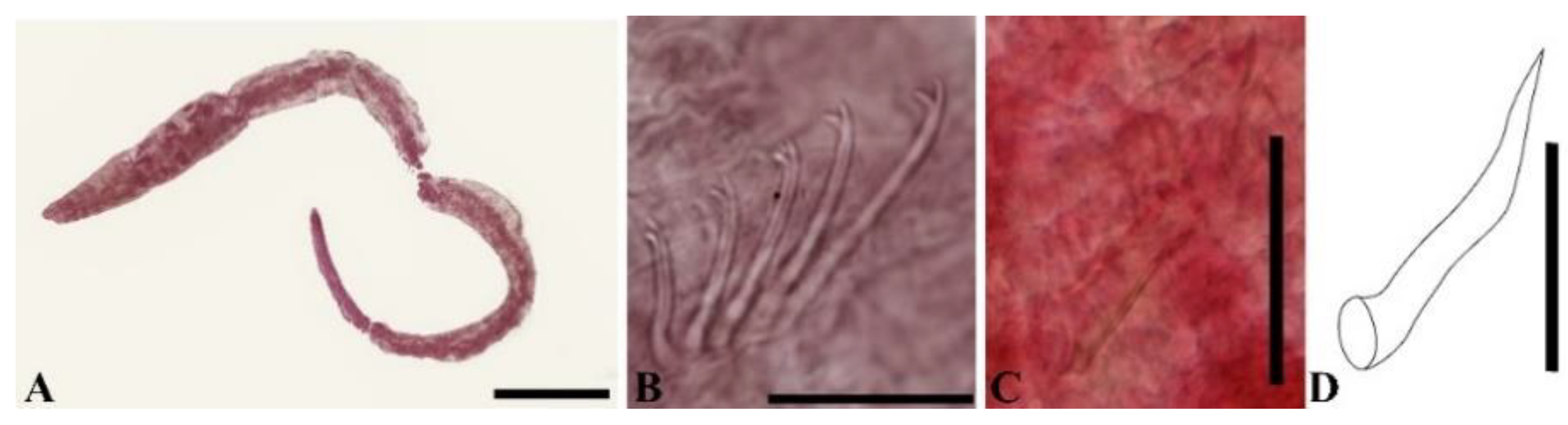
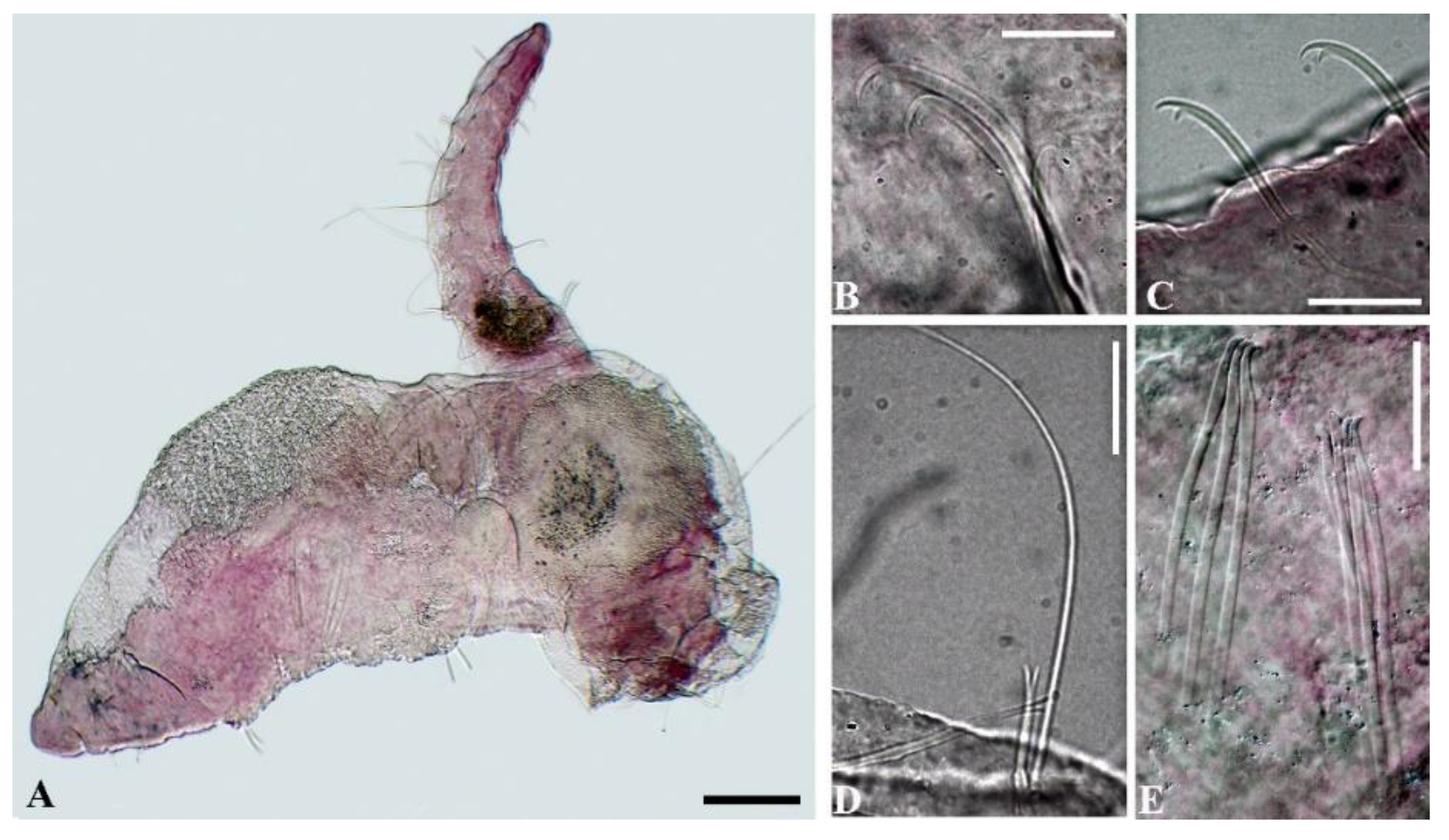
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brinkhurst, R.O.; Jamieson, B.G.M. Aquatic Oligochaeta of the World; Oliver and Boyd: Edinburgh, UK, 1971; p. 860. [Google Scholar]
- Brinkhurst, R.O.; Wetzel, M.J. Aquatic Oligochaeta in the World: Supplement. A Catalogue of New Freshwater Species, Descriptions, and Revisions; Canadian Technical Report of Hydrography and Ocean Sciences; Fisheries and Oceans Canada: Ottawa, ON, Canada, 1984; Volume 44, p. 101.
- Atanacković, A.; Popović, N.; Marinković, N.; Tomović, J.; Đuknić, J.; Stanković, J.; Paunović, M. Effects of environmental factors on the distribution and diversity of aquatic oligochaetes. Water 2023, 15, 3873. [Google Scholar] [CrossRef]
- Patra, S.B. Observations on the abundance of Oligochaeta along with some environmental factors in an unmanaged freshwater wetland of West Bengal, India. Sustain. Agri Food Environ. Res. 2023, 12, 1. [Google Scholar] [CrossRef]
- Timm, T. Observations on the life cycles of aquatic Oligochaeta in aquaria. Zoosymposia 2020, 17, 102–120. [Google Scholar] [CrossRef]
- Girolli, D.A.; Lima, M.F.D.; Sanches, N.A.D.O.; Colombo-Corbi, V.; Corbi, J.J.; Gorni, G.R. Aquatic oligochaetes (Annelida: Clitellata) in reservoirs in São Paulo State: List of occurrence and ecological observations on the species. Biota Neotrop. 2021, 21, e20201152. [Google Scholar] [CrossRef]
- Guimarães, L.P.; do Amaral, P.H.M.; da Gama Alves, R. Taxonomic and functional diversity of oligochaetes in Neotropical springs: The influence of eucalyptus monocultures. Hydrobiologia 2024, 1–12. [Google Scholar] [CrossRef]
- Verdonschot, P.F.M. Oligochaeta and climate change. In Proceedings of the 10th International Symposium on Aquatic Oligochaeta, Wuhan, China, 16–21 October 2006; p. K-8. [Google Scholar]
- Ji, C.W.; Cui, Y.; Wang, H.; Park, Y.M.; Chon, T.S. Computational analysis of responsable behaviors of indicator specimens exposed to toxic chemicals for water quality monitoring. In Proceedings of the 10th International Symposium on Aquatic Oligochaeta, Wuhan, China, 16–21 October 2006; p. O-22. [Google Scholar]
- Hirabayashi, K.; Yamamoto, M.; Oga, K. Influence of water temperature and dissolved oxygen concentration on hatchability of aquatic Oligochaeta cocoons in Lake Kizaki. In Proceedings of the 10th International Symposium on Aquatic Oligochaeta, Wuhan, China, 16–21 October 2006; p. P-12. [Google Scholar]
- Chunchukova, M.; Zaharieva, R.; Zaharieva, P. Biodiversity and ecological assessment of the freshwater ecosystem of the Osam River, Bulgaria. In Proceedings of the International May Conference on Strategic Management (IMCSM20), Bor, Serbia, 25–27 September 2020; Volume XVI, Issue 1. pp. 182–193. [Google Scholar]
- Sokolskaja, N.L. Freshwater Oligochaeta of the Amur basin. Tr. Amur. Ikhtiol. Eksped. 1958, 4, 287–358. [Google Scholar]
- Cernosvitov, L. Oligochaeta from various parts of the world. Proc. Zool. Soc. Lond. 1941, 111, 197–236. [Google Scholar]
- Sperber, C. A taxonomical study of the Naididae. Zool. Bidr. Fran Upps. 1948, 28, 1–297. [Google Scholar]
- Poddubnaya, T.L. Materialy po pitaniyu massovykh vidov tubificid Rybinskogo vodokhranilischa. Tr. Instituta Biol. Vodokhranilisch 1961, 4, 219–231. [Google Scholar]
- Brinkhurst, R.O. Notes on the brackish-water and marine species of Tubificidae [Annelida, Oligochaeta]. J. Mar. Biol. Assoc. UK 1963, 43, 709–715. [Google Scholar] [CrossRef]
- Brinkhurst, R.O. Taxonomical studies on the Tubificidae (Annelida, Oligochaeta). Int. Rev. Gesamten Hydrobiol. Suppl. 1963, 2, 1–89. [Google Scholar] [CrossRef]
- Brinkhurst, R.O.; Austin, M.J. Assimilation by aquatic oligochaeta. Int. Rev. Gesammte Hydrobiol. 1979, 64, 245–250. [Google Scholar] [CrossRef]
- Verdonschot, P.F.M. Some notes on the ecology of aquatic oligochaetes in the Delta Region of the Netherlands. Arch. Hydrobiol. 1981, 92, 53–70. [Google Scholar]
- Timm, T. Oligochaeta of the small lakes of Southern Karelia. Hydrobiol. Res. Gidrobiol. Issled. 1983, 14, 30–39. [Google Scholar]
- Poddubnaya, T.L.; Bakanov, A.J. Spatial distribution of aquatic oligochaetes. In Vodnye Maloshchetinkovye Chervi (= Aquatic oligochaeta); Kurashvili, B.E., Ed.; Metsniereba Publishing House: Tbilisi, Georgia, 1983; pp. 5–10. [Google Scholar]
- Semernoi, V.P. Oligochaeta of the Maloye More of Lake Baikal. In Proceedings of the 6th All-Union Symposium, Salaspils, Latvia, 27–30 April 1987; pp. 123–127. [Google Scholar]
- Wagner, B. Population dynamics of oligochaetes in a high mountain lake. Hydrobiologia 1987, 155, 191. [Google Scholar]
- Ali, S.; Chakraborti, T. Freshwater Invertebrates of Bangladesh; Bangla Academy: Dhaka, Bangladesh, 1992; p. 207. [Google Scholar]
- Juget, J.; Lafont, M. Distribution of Oligochaeta in some lakes and pools of Bolivia. Hydrobiologia 1994, 278, 125–127. [Google Scholar] [CrossRef]
- Erséus, C.; Hsieh, H.L. Records of estuarine Tubificidae (Oligochaeta) from Taiwan. Species Divers. 1997, 2, 97–104. [Google Scholar] [CrossRef]
- Sambugar, B.; Giani, N.; Martínez-Ansemil, E. Groundwater oligochaetes from Southern-Europe. Tubificidae with marine phyletic affinities: New data with description of a new species, review and consideration on their origin. Mémoires Biospéologie 1999, 26, 107–116. [Google Scholar]
- Healy, B. Ecology of Enchytraeidae in Irish brackishwater habitats. In Book of Abstracts, Proceedings of the VIII International Symposium on Aquatic Oligochaeta, Bilbao, Spain, 18–22 July 2000; Rodriguez, P., Martinez-Madrid, M., Arrate-Jorrin, J.A., Eds.; Servicie de Publicaciones de la Universidad del Pais Vasco: Bilbao, Spain, 2000; p. 48. [Google Scholar]
- Giani, N.; Sambugar, B.; Rodriguez, P.; Martínez-Ansemil, E. Oligochaetes in southern European groundwater: New records and overview. Hydrobiologia 2001, 463, 65–74. [Google Scholar] [CrossRef]
- Aston, R.J. Tubificids and water quality: A review. Environ. Pollut. 1970 1973, 5, 1–10. [Google Scholar] [CrossRef]
- Brinkhurst, R.O. Taxonomy, pollution and the sludge worm. Mar. Pollut. Bull. 1980, 11, 248–251. [Google Scholar] [CrossRef]
- Milbrink, G. Oligochaete communities in pollution biology: The European: Situation with special reference to lakes in Scandinavia. In Aquatic Oligochaete Biology; Springer: Berlin/Heidelberg, Germany, 1980; pp. 433–455. [Google Scholar]
- Milbrink, G. Characteristic deformities in tubificid oligochaetes inhabiting polluted bays of Lake Vänern, Southern Sweden. Hydrobiologia 1983, 106, 169–184. [Google Scholar] [CrossRef]
- Williams, D.D.; Williams, N.E.; Cao, Y. Spatial differences in macroinvertebrate community structure in springs in southeastern Ontario in relation to their chemical and physical environments. Can. J. Zool. 1997, 75, 1404–1414. [Google Scholar] [CrossRef]
- Lin, K.J.; Yo, S.P. Distribution and diversity of aquatic oligochaete fauna in the urban watercourses of Taichung Basin in Taiwan. In Proceedings of the Tenth International Symposium on Aquatic Oligochaeta, Wuhan, China, 16–21 October 2006; p. O-19. [Google Scholar]
- Diaz, R.J.P. Pollution and tidal benthic communities of the James River Estuary, Virginia. Hydrobiologia 1989, 180, 195–211. [Google Scholar] [CrossRef]
- Milbrink, G. Oligochaetes and water pollution in two deep Norwegian lakes. Hydrobiologia 1994, 278, 213–222. [Google Scholar] [CrossRef]
- Arimoro, F.O.; Ikomi, R.B.; Iwegbue, C.M. Ecology and abundance of oligochaetes as indicators of organic pollution in an urban stream in southern Nigeria. Pak. J. Biol. Sci. PJBS 2007, 10, 446–453. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, M.; Ding, S.; Liu, L.; Wang, Y.; Xing, X.; Wang, D.; Gong, M.; Zhang, C. Fine-scale bioturbation effects of tubificid worm (Limnodrilus hoffmeisteri) on the lability of phosphorus in sediments. Environ. Pollut. 2016, 219, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.B. Tubificids alter profiles of redox potential and pH in profundal lake sediment. Limnol. Oceanogr. 1974, 19, 342–346. [Google Scholar] [CrossRef]
- Fisher, J.A.; Beeton, A.M. The effect of dissolved oxygen on the burrowing behavior of Limnodrilus hoffmeisteri (Oligochaeta). Hydrobiologia 1975, 47, 273–290. [Google Scholar] [CrossRef]
- Chapman, P.M.; Farrell, M.A.; Brinkhurst, R.O. Relative tolerances of selected aquatic oligochaetes to individual pollutants and environmental factors. Aquat. Toxicol. 1982, 2, 47–67. [Google Scholar] [CrossRef]
- Chapman, P.M.; Farrell, M.A.; Brinkhurst, R.O. Effects of species interactions on the survival and respiration of Limnodrilus hoffmeisteri and Tubifex tubifex (Oligochaeta, tubificidae) exposed to various pollutants and environmental factors. Water Res. 1982, 16, 1405–1408. [Google Scholar] [CrossRef]
- Brinkhurst, R.O.; Chapman, P.M.; Farrell, M.A. A comparative study of respiration rates of some aquatic oligochaetes in relation to sublethal stress. Int. Rev. Gesammte Hydrobiol. 1983, 68, 683–699. [Google Scholar] [CrossRef]
- Jónasson, P.M. Oxygen demand and long term changes of profundal zoobenthos. Hydrobiologia 1984, 115, 121–126. [Google Scholar] [CrossRef]
- Lafont, M. Oligochaete communities as biological descriptors of pollution in the fine sediments of rivers. Hydrobiologia 1984, 115, 127–129. [Google Scholar] [CrossRef]
- Won, J.H.; Lee, J.Y.; Kim, J.W.; Koh, G.W. Groundwater occurrence on Jeju Island, Korea. Hydrogeol. J. 2006, 14, 532–547. [Google Scholar] [CrossRef]
- Cho, J.-S.R.; Kim, Y.M.; Sagong, J.; Lee, J.-H.; Yeo, M.-Y.; Bahn, S.-Y.; Kim, H.-M.; Lee, G.-S.; Lee, D.-H.; Choo, Y.-S. Biodiversity of marine invertebrates on rocky shores of Dokdo, Korea. Zool. Stud. 2012, 51, 710–726. [Google Scholar]
- Lee, W.; Karanovic, T. Biodiversity of invertebrates in Korea. Zootaxa 2012, 3368, 5–6. [Google Scholar]
- Ahn, D.H.; Lee, C.W.; Yang, H.M.; Song, J.H.; Kwon, J.I.; Ji, S.J.; Park, M.H.; Min, G.S. Freshwater Invertebrates of Jindo Island in Korea. Korean J. Syst. Zool. 2016, 37–44. [Google Scholar] [CrossRef]
- Park, Y.S.; Song, M.Y.; Park, Y.C.; Oh, K.H.; Cho, E.; Chon, T.S. Community patterns of benthic macroinvertebrates collected on the national scale in Korea. Ecol. Model. 2007, 203, 26–33. [Google Scholar] [CrossRef]
- Kim, M.H.; Han, M.S.; Nam, H.K.; Kang, K.K.; Kim, M. Geological distribution of aquatic invertebrates living in paddy fields of South Korea. Korean J. Soil Sci. Fertil. 2012, 45, 1136–1142. [Google Scholar] [CrossRef]
- Bae, M.J.; Park, Y.S. Diversity and distribution of endemic stream insects on a nationwide scale, South Korea: Conservation perspectives. Water 2017, 9, 833. [Google Scholar] [CrossRef]
- Kwak, I.S.; Lee, D.S.; Hong, C.; Park, Y.S. Distribution patterns of benthic macroinvertebrates in streams of Korea. Korean J. Ecol. Environ. 2018, 51, 60–70. [Google Scholar] [CrossRef]
- Lim, D.; Lee, Y. Characteristics of aquatic ecosystem environment in Seosan reservoir, Korea. J. Environ. Sci. Int. 2018, 8, 1105–1115. [Google Scholar] [CrossRef]
- Kim, P.; Kim, D.; Yoon, T.; Shin, S. Early detection of marine invasive species, Bugula neritina (Bryozoa: Cheilostomatida), using species-specific primers and environmental DNA analysis in Korea. Mar. Environ. Res. 2018, 139, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jung, J. Naidid oligochaetes (Annelida: Clitellata) from the Seokhyeoncheon and Changreungcheon streams with new record of Nais variabilis. Korean J. Limnol. 2011, 44, 407–410. [Google Scholar]
- Jung, J. New record of a naidid oligochaete species, Ripistes parasita (Annelida: Clitellata: Naididae) from Korea. Anim. Syst. Evol. Divers. 2012, 28, 137–139. [Google Scholar] [CrossRef]
- Park, H.J.; Timm, T.; Bae, Y.J. Aquatic Oligochaete (Annelida: Clitellata) fauna from the Jungnang stream in Seoul, Korea, with eight new Korean records. Korean J. Ecol. Environ. 2013, 46, 507–512. [Google Scholar] [CrossRef]
- Park, H.J.; Timm, T.; Bae, Y.J. Taxonomy of the Korean freshwater oligochaeta (Annelida) with eight species new to Korea. Entomol. Res. Bull. 2013, 29, 180–188. [Google Scholar]
- Dózsa-Farkas, K.; Felföldi, T.; Hong, Y. New enchytraeid species (Enchytraeidae, Oligochaeta) from Korea. Zootaxa 2014, 4006, 171–197. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, J. Four unrecorded species of Tubificid Oligochaetes (Annelida: Clitellata) in Korea. Anim. Syst. Evol. Divers. 2014, 30, 240–247. [Google Scholar] [CrossRef]
- Lee, J.; Jung, J. Two Aquatic Oligochaete species, Dero dorsalis and Allonais pectinata (Annelida: Clitellata: Naididae), new to Korea. Anim. Syst. Evol. Divers. 2014, 30, 119–123. [Google Scholar] [CrossRef]
- Lee, J.; Jung, J. Faunistic survey on freshwater annelids from Korea. J. Species Res. 2016, 5, 279–288. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, J.W. New record of three aquatic species of Enchytraeidae (Annelida: Clitellata) from Korea. J. Species Res. 2016, 5, 541–546. [Google Scholar] [CrossRef]
- Lee, J.; Lee, T. A new species and four new recorded species of Naididae (Annelida: Oligochaeta) from Korea. Diversity 2023, 16, 7. [Google Scholar] [CrossRef]
- Erséus, C. A new marine species of Smithsonidrilus (Oligochaeta: Tubificidae) from the Florida Keys. Proc. Biol. Soc. Wash. 1993, 106, 587–590. [Google Scholar]
- Hrabě, S. Die Oligochaeten aus den Seen Ochrida und Prespa. Zoologische Jahrbücher. Abteilung für Systematik, Ökologie und Geographie der Tiere. Zool. Jahrb. Syst. 1931, 61, 1–62. [Google Scholar]
- Milligan, M.R. Separation of Haber speciosus (Hrabe) (Oligochaeta: Tubificidae) from its congeners, with a description of a new form from North America. Proc. Biol. Soc. Wash. 1986, 99, 406–416. [Google Scholar]
- Timm, T.; Martin, P.J. Chapter 21—Clitellata: Oligochaeta. In Thorp and Covich’s Freshwater Invertebrates, 4th ed.; Rogers, J.H.T.C., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 529–549. [Google Scholar]
- Michaelsen, W. Zur Kenntniss der Oligochäten des Baikal—Sees. R. Friedländer & Sohn. Russ. Hydrobiol. Z. 1905, V, 153–193. [Google Scholar]
- Brinkhurst, R.O. On the types of Tubificidae (Oligochaeta) described by W. Michaelsen and others in the Zoological Institute and Zoological Museum, University of Hamburg. Mitteilungen Hambg. Zool. Mus. Inst. 1981, 78, 7–17. [Google Scholar]
- Sokolskaya, N.L. Data on the Fauna of Aquatic Oligochaetes of Southern Sakhalin. Lakes of Southern Sakhalin and Their Ichthyofauna; Moscow State University: Moscow, Russia, 1964; pp. 82–96. (In Russian) [Google Scholar]
- Hrabe, S. New or insufficiently known species of the family Tubificidae. Sp. Prirodoved. Fak. Univ. Brne 1966, 470, 57–76. [Google Scholar]
- Michaelsen, W. Neue Tubificiden des Niederelbgebietes. Verhandlungen Naturwiss. Ver. Hambg. 1901, 3, 66–70. [Google Scholar]
- De Visart, E. Tubifex camerani, n. sp. Boll. Musei Zool. Ed Anat. Comp. R. Univ. Torino 1901, 16, 1–4. [Google Scholar]
- Ditlevsen, A. Studien an Oligochäten. Z. Wiss. Zool. 1904, 77, 398–480. [Google Scholar]
- Timm, T. The genus Potamotrix (Annelida, Oligochaeta, Tubificidae): A literature review. Est. J. Ecol. 2013, 62, 121–136. [Google Scholar] [CrossRef]
- Grimm, O.A. Kaspiiskoe more i ego fauna. Tetrad 1. [The Caspian Sea and its fauna, book 1 of 2]. Trudy Aralo-Kaspiiskoi Ekspeditsii. 1976, 1, 1–168. [Google Scholar]
- He, X.; Cui, Y.; Wang, H. Two new species of Tubificinae (Annelida: Clitellata: Naididae) from Tibet, China. Zootaxa 2012, 3458, 159–165. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, H.; Cui, Y. Four species of Tubifex Lamarck (Annelida: Oligochaeta: Naididae) from Tibet, China. Zootaxa 2017, 4320, 366–378. [Google Scholar] [CrossRef]
- Brinkhurst, R.O.; Cook, D.G. Studies on the North American aquatic oligochaeta. III: Lumbriculidae and additional notes and records of other families. Proc. Acad. Nat. Sci. Phila. 1966, 118, 1–33. [Google Scholar]
- Stimpson, K.S.; Klemm, D.J.; Hiltunen, J.K. A Guide to the Freshwater Tubificidae (Annelida: Clitellata: Oligochaeta) of North America; Environmental Monitoring and Support Laboratory, Office of Research and Development, US Environmental Protection Agency: Las Vegas, NV, USA, 1982.
- Van Haaren, T.; Soors, J. Aquatic Oligochaeta of the Netherlands and Belgium—Identification Key to the Oligochaetes; KNNV Publishing: Zeist, The Netherlands, 2012. [Google Scholar]
- Kokavec, I. Tasserkidrilus cf. americanus (Clitellata, Naididae)—A new record from Slovakia confirms the dissimilarity between the European and North American populations. Biodivers. Data J. 2021, 9, e72846. [Google Scholar] [CrossRef]
- Semernoi, V.P. New oligochaete species from Lake Baikal. In New Information on the Fauna of Lake Baika; Galazii, G.I., Ed.; Academy of Sciences, Siberian Branch, Limnological Institute: Novosibirsk, Russia, 1982; pp. 58–85. [Google Scholar]
- Semyornyi, V.P. Visual observations of Baikal sediments and the study of oligochaetes (Oligochaeta) inhabiting them using the ROV "Mir". News of Irkutsk State University. Series: Biology. Ecology 2010, 3, 87–89. [Google Scholar]
- Černosvitov, L. Oligochseta from Tibet. In Proceedings of the Zoological Society of London; Blackwell Publishing Ltd.: Oxford, UK, 1942; pp. 281–287. [Google Scholar]
- Kaygorodova, I.A.; Verdonschot, P.F.M.; Kravtsova, L.S. Freshwater oligochaetes (Oligochaeta, Clitellata, Annelida) of North Pribaikalye (East Siberia, Russia). Turk. J. Zool. 2012, 36, 47–58. [Google Scholar] [CrossRef]
- Manca, M.; Ruggiu, D.; Panzani, P.; Asioli, A.; Mura, G.; Nocentini, A.M. Report on a collection of aquatic organisms from high mountain lakes in the Khumbu Valley (Nepalese Himalayas). Mem. Dell’istituto Ital. Idrobiol. 1998, 57, 77–98. [Google Scholar]
- Martin, P.; Martens, K.; Goddeeris, B. Oligochaeta from the abyssal zone of Lake Baikal (Siberia, Russia). Hydrobiologia 1999, 406, 165–174. [Google Scholar] [CrossRef]
- Naidu, K.V. Check-list of fresh-water Oligochaeta of the Indian sub-continent and Tibet. Hydrobiologia 1966, 27, 208–226. [Google Scholar] [CrossRef]
- Snimschikova, L.N.; Akinshina, T.W. Oligochaete fauna of Lake Baikal. Hydrobiologia 1994, 278, 27–34. [Google Scholar] [CrossRef]
- Snimschikova, L.N.; Akinshina, T.V. Rhyacodrilus stephensoni (Oligochaeta, Tubificidae) from Lake Baikal. Hydrobiol. J. 1995, 31, 70–76. [Google Scholar]
| No. | GPS | Environment |
|---|---|---|
| St. 1 | N: 37°34′55.22″ E: 127°04′36.14″ | Stream |
| St. 2 | N: 37°34′52.87″ E: 127°04′41.74″ | Stream |
| St. 3 | N: 37°34′51.09″ E: 127°04′38.08″ | Stream |
| St. 4 | N: 36°03′76.27″ E: 126°78′06.71″ | Brook |
| St. 5 | N: 36°04′7.27″ E: 126°51′41.96″ | Farm waterway |
| St. 6 | N: 34°57′26.26″ E: 128°39′16.67″ | Pond |
| St. 7 | N: 34°48′52.38″ E: 128°40′31.15″ | Pond |
| St. 8 | N: 33°31′04.07″ E: 126°57′03.17″ | wetland |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J. Report on Eight Unrecorded Species of Freshwater Oligochaetes in Korea. Taxonomy 2025, 5, 9. https://doi.org/10.3390/taxonomy5010009
Lee J. Report on Eight Unrecorded Species of Freshwater Oligochaetes in Korea. Taxonomy. 2025; 5(1):9. https://doi.org/10.3390/taxonomy5010009
Chicago/Turabian StyleLee, Jeounghee. 2025. "Report on Eight Unrecorded Species of Freshwater Oligochaetes in Korea" Taxonomy 5, no. 1: 9. https://doi.org/10.3390/taxonomy5010009
APA StyleLee, J. (2025). Report on Eight Unrecorded Species of Freshwater Oligochaetes in Korea. Taxonomy, 5(1), 9. https://doi.org/10.3390/taxonomy5010009






