Sexual Dimorphism in Wing Shape and Its Impact on Conspecific Identification of Neotropical Fannia Species (Diptera: Fanniidae)
Abstract
1. Introduction
2. Materials and Methods
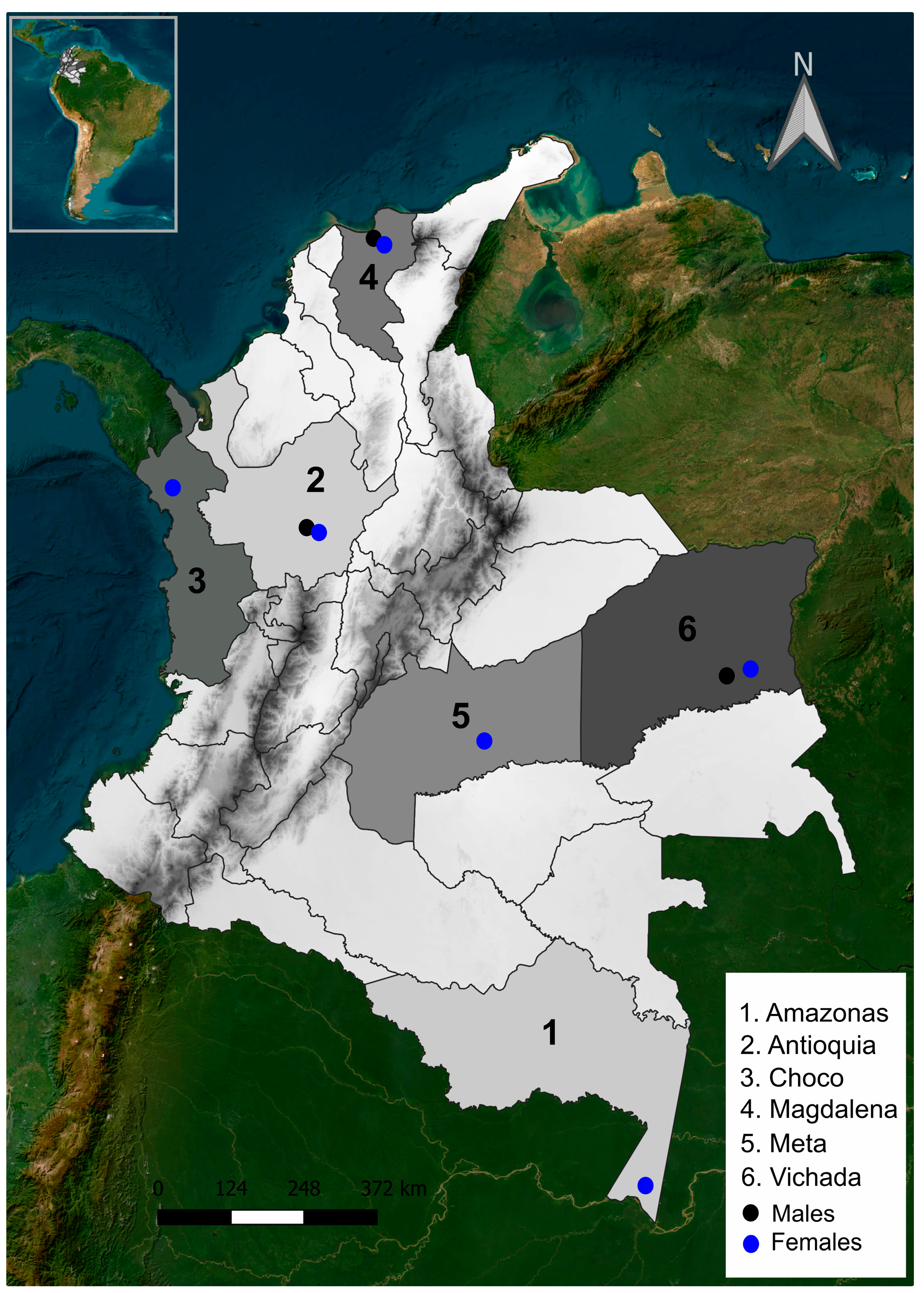
2.1. Specimens
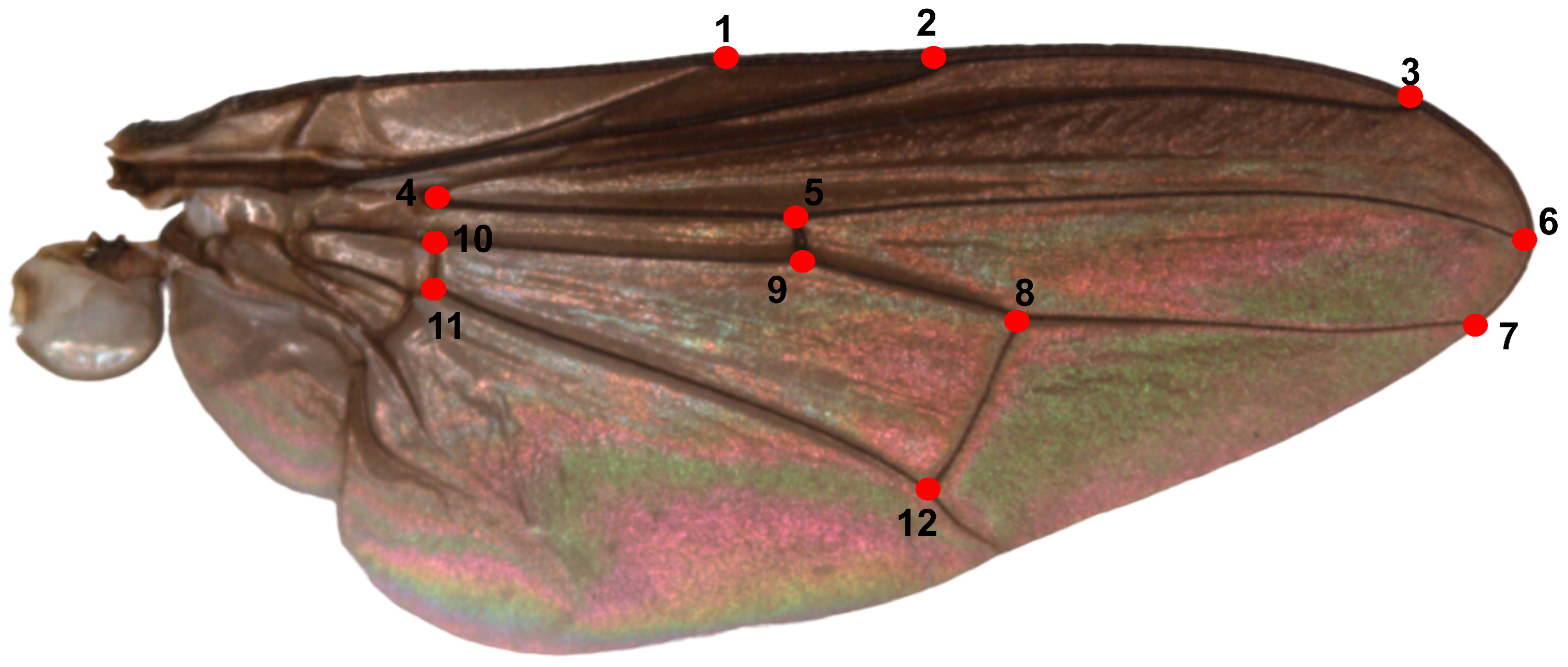
2.2. Slide Preparation and Landmark Digitization
2.3. Molecular Reference Data
2.4. Landmark-Based Geometric Morphometrics
3. Results
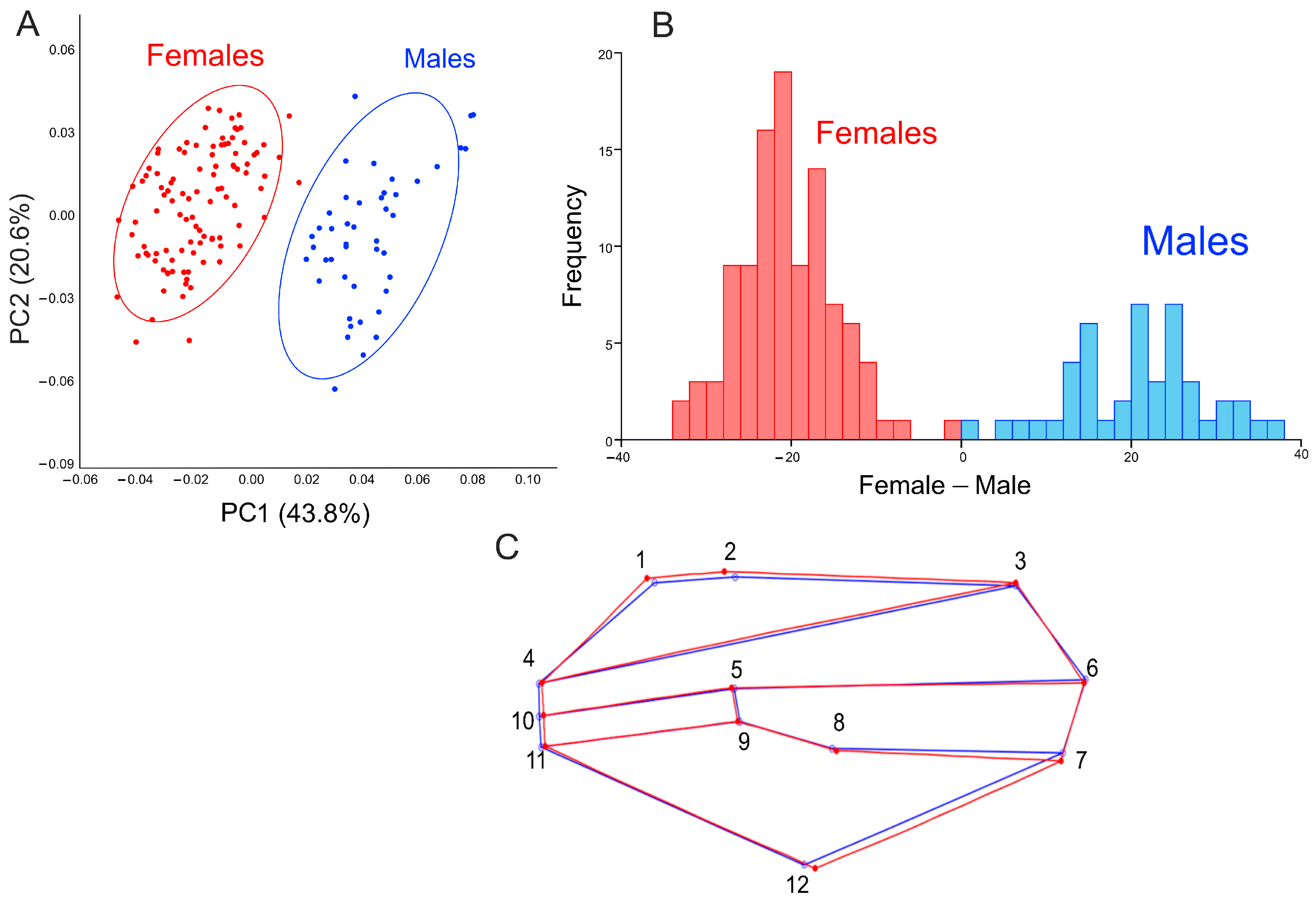
3.1. Sexual Dimorphism
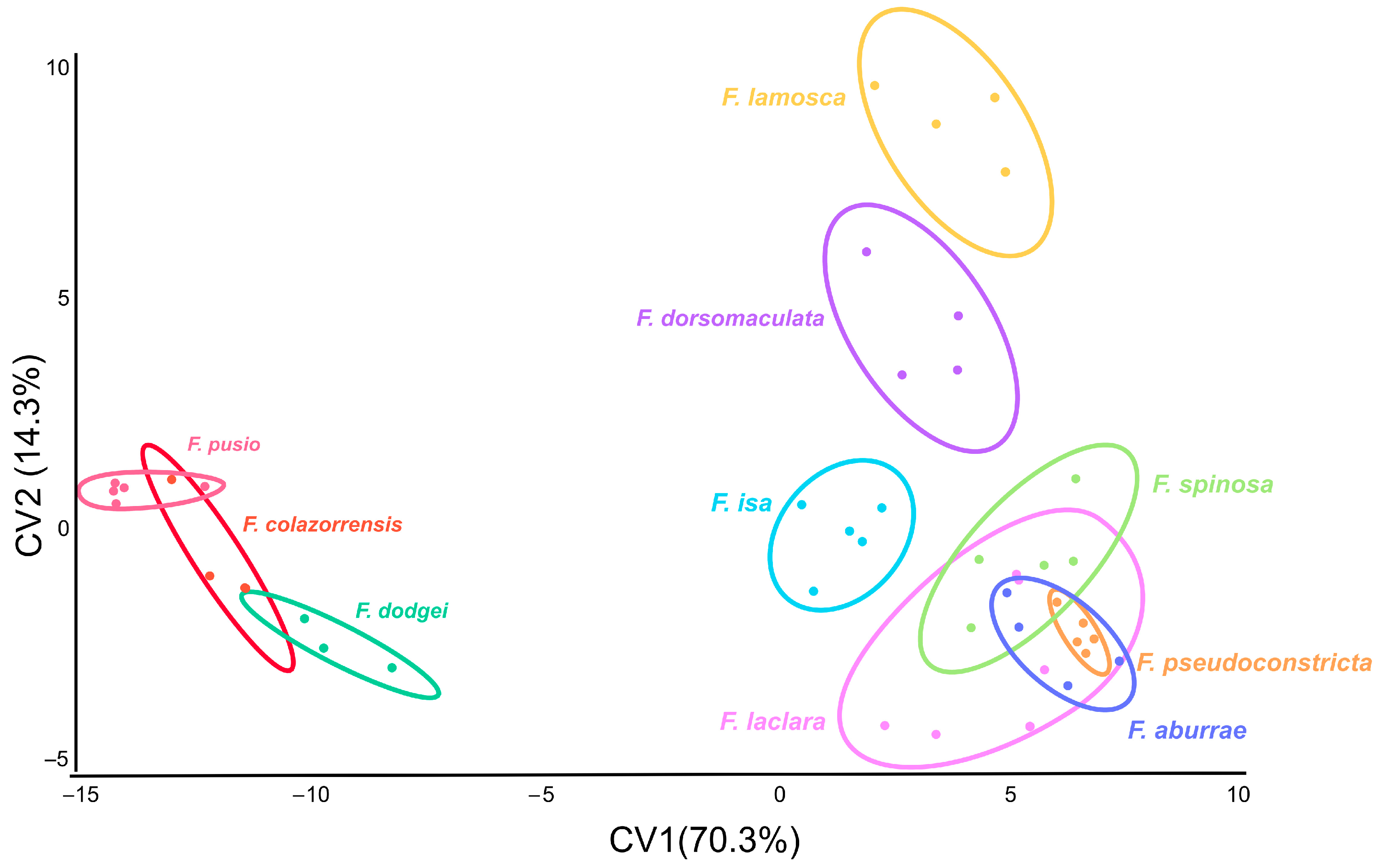
3.2. Interspecific Differences in Wing Size and Shape in Males
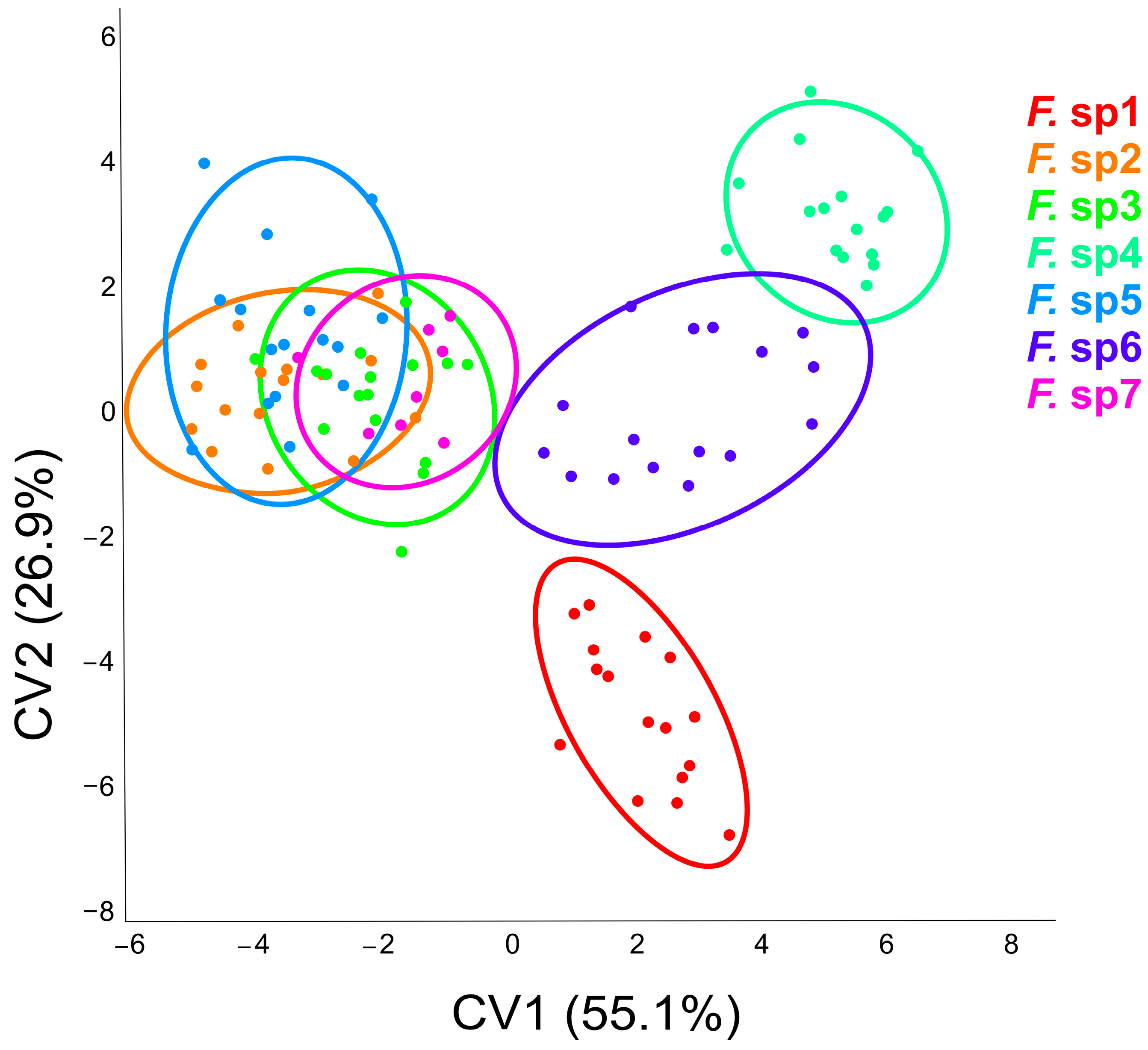
3.3. Differences in Wing Size and Shape in Females
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grzywacz, A.; Jarmusz, M.; Walczak, K.; Skowronek, R.; Johnston, N.P.; Szpila, K. DNA Barcoding identifies unknown females and larvae of Fannia R.-D. (Diptera: Fanniidae) from carrion succession experiment and case report. Insects 2021, 12, 381. [Google Scholar] [CrossRef] [PubMed]
- Vanin, S.; Gherardi, M.; Bugelli, V.; Di Paolo, M. Insects found on a human cadaver in Central Italy including the blowfly Calliphora loewi (Diptera, Calliphoridae), a new species of forensic interest. Forensic Sci. Int. 2011, 207, e30–e33. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, Y.; Magaña, C.; Martínez-Sánchez, A.; Rojo, S. Diptera of forensic importance in the Iberian Peninsula: Larval identification key. Med. Vet. Entomol. 2010, 24, 293–308. [Google Scholar] [CrossRef]
- Benecke, M.; Lessig, R. Child neglect and forensic entomology. Forensic Sci. Int. 2001, 120, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Urabe, S.; Kurahashi, H.; Inokuchi, G.; Chiba, F.; Motomura, A.; Hoshioka, Y.; Torimitsu, S.; Yamaguchi, R.; Tsuneya, S.; Iwase, H. Carrion flies (Insecta: Diptera) found on human cadavers in Chiba Prefecture, Honshu, Japan, with the first record of Fannia prisca from a human corpse. J. Forensic Sci. 2022, 67, 2469–2478. [Google Scholar] [CrossRef]
- Mariani, R.; García-Mancuso, R.; Varela, G.L.; Inda, A.M. Entomofauna of a buried body: Study of the exhumation of a human cadaver in Buenos Aires, Argentina. Forensic Sci. Int. 2014, 237, 19–26. [Google Scholar] [CrossRef]
- Hodecek, J.; Fumagalli, L.; Jakubec, P. All Insects Matter: A review of 160 entomology cases from 1993 to 2007 in Switzerland—Part I (Diptera). J. Med. Entomol. 2024, 61, 400–409. [Google Scholar] [CrossRef]
- Durango-Manrique, Y.; López-Rubio, A.; Gómez, G.F. Una revisión del género Fannia Robineau-Desvoidy, 1830 en el neotrópico. Boletín Mus. Entomológico Fr. Luis Gallego 2023, 15, 9–31. [Google Scholar]
- Byrd, J.H.; Tomberlin, J.K. Forensic Entomology: The Utility of Arthropods in Legal Investigations, 3rd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; ISBN 9780815350163. [Google Scholar]
- Klingenberg, C.P. Evolution and development of shape: Integrating quantitative approaches. Nat. Rev. Genet. 2010, 11, 623–635. [Google Scholar] [CrossRef]
- Carreira, V.P.; Soto, I.M.; Mensch, J.; Fanara, J.J. Genetic basis of wing morphogenesis in Drosophila: Sexual dimorphism and non-allometric effects of shape variation. BMC Dev. Biol. 2011, 11, 32. [Google Scholar] [CrossRef]
- Jiménez-Martín, F.J.; Cabrero, F.J.; Martínez-Sánchez, A. Wing Morphometrics for identification of forensically important blowflies (Diptera: Calliphoridae) in Iberian Peninsula. J. Forensic Leg. Med. 2020, 75, 102048. [Google Scholar] [CrossRef] [PubMed]
- Limsopatham, K.; Klong-klaew, T.; Fufuang, N.; Sanit, S.; Sukontason, K.L.; Sukontason, K.; Somboon, P.; Sontigun, N. Wing Morphometrics of medically and forensically important muscid flies (Diptera: Muscidae). Acta Trop. 2021, 222, 106062. [Google Scholar] [CrossRef] [PubMed]
- Sontigun, N.; Samerjai, C.; Sukontason, K.; Wannasan, A.; Amendt, J.; Tomberlin, J.K.; Sukontason, K.L. Wing morphometric analysis of forensically important flesh flies (Diptera: Sarcophagidae) in Thailand. Acta Trop. 2019, 190, 312–319. [Google Scholar] [CrossRef] [PubMed]
- López-García, J.; Angell, C.; Martín-Vega, D. Wing morphometrics for the identification of Nearctic and Palaearctic Piophilidae (Diptera) of forensic relevance. Forensic Sci. Int. 2020, 309, 110192. [Google Scholar] [CrossRef] [PubMed]
- Amat García, E.C.; Ramírez Mora, M.A.; Pérez Hoyos, A.M.; Cadavid Sánchez, I.C.; Pérez Pérez, J.; Varela Pineda, A.M.; Pérez Cardona, A.; Durango Manrique, Y.S.; López Rubio, A.; Gómez García, G.F.; et al. Del Campo al Laboratorio: Integración de Procedimientos para el Estudio de Moscas; Gómez, L.M., Gómez, G.F., Eds.; Primera; Sello Editorial Publicar-T: Medellin, Colombia, 2018; ISBN 978-958-59925-97. [Google Scholar]
- Durango, Y.; Ramírez-Mora, M. Fannia Robineau-Desvoidy (Diptera: Fanniidae) of Colombia: New species, identification key and updated checklist. Zootaxa 2019, 4604, 301–325. [Google Scholar] [CrossRef]
- Grisales, D.; Wolff, M.; De Carvalho, C.J.B. Neotropical Fanniidae (Insecta, Diptera): New species of Fannia from Colombia. Zootaxa 2012, 3591, 1–46. [Google Scholar] [CrossRef]
- Grisales, D.; de Carvalho, C.J.B. Highland biodiversity of Fanniidae (Insecta, Diptera): Fourteen new species from the Andes and Central America. Zootaxa 2019, 4551, 330–360. [Google Scholar] [CrossRef]
- Wendt, L.D.; De Carvalho, C.J.B. Taxonomia de Fanniidae (Diptera) do Sul Do Brasil—I: Nova espécie e chave de identificação de Euryomma Stein. Rev. Bras. Entomol. 2007, 51, 197–204. [Google Scholar] [CrossRef]
- Cumming, J.M.; Wood, D.M. Adult morphology and terminology. In Manual of Central American Diptera; Brown, B.V., Borkent, A., Cumming, J.M., Wood, D.M., Woodley, N.E., Zumbado, M.A., Eds.; National Research Council Press: Ottawa, Canada, 2009; pp. 9–502. [Google Scholar]
- Cortés-Suarez, L.; Durango, Y.S.; Gómez, G.F. Sexual dimorphism in the wing geometry of Musca domestica L. (Diptera: Muscidae) from Colombia. Rev. Soc. Entomol. Argent. 2021, 80, 81–88. [Google Scholar] [CrossRef]
- Rohlf, F.J. TpsDig2, Digitize Landmarks and Outlines; Version 2.32; Department of Ecology and Evolution, State University of New York: Stony Brook, NY, USA, 2006. [Google Scholar]
- Arnqvist, G.; Mårtensson, T. Measurement error in geometric morphometrics: Empirical strategies to assess and reduce its impact on measures of shape. Acta Zool. Acad. Sci. Hung. 1998, 44, 73–96. [Google Scholar]
- Watanabe, A. How many landmarks are enough to characterize shape and size variation? PLoS ONE 2018, 13, e0198341. [Google Scholar] [CrossRef] [PubMed]
- Durango-Manrique, Y.; López-Rubio, A.; Gómez, G.F. Molecular differentiation analysis of ten putative species of Fannia (Diptera: Fanniidae) collected in carrion-baited traps from Colombia. Med. Vet. Entomol. 2024; early view. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Analyzing fluctuating asymmetry with geometric morphometrics: Concepts, methods, and applications. Symmetry 2015, 7, 843–934. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Size, Shape, and Form: Concepts of allometry in geometric morphometrics. Dev. Genes. Evol. 2016, 226, 113–137. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, S.; Dujardin, J.P. Geometric morphometrics in the cloud. Infect. Genet. Evol. 2019, 70, 189–196. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9–18. [Google Scholar] [CrossRef]
- Espra, A.S.; Tabugo, S.R.M.; Torres, M.A.J.; Gorospe, J.G.; Manting, M.M.E.; Demayo, C.G. Describing dimorphism in wing shapes in the blowfly Lucilia sericata Meigen (Diptera: Calliphoridae) using geometric morphometrics. Adv. Environ. Biol. 2015, 9, 64–70. [Google Scholar]
- Nuñez-Rodríguez, J.A.; Liria, J. Geometric morphometrics sexual dimorphism in three forensically important species of blow fly (Diptera: Calliphoridae). Life Excit. Biol. 2017, 4, 272–284. [Google Scholar] [CrossRef]
- Changbunjong, T.; Sumruayphol, S.; Weluwanarak, T.; Ruangsittichai, J.; Dujardin, J.-P. Landmark and outline-based geometric morphometrics analysis of three Stomoxys flies (Diptera: Muscidae). Folia Parasitol. 2016, 63, 037. [Google Scholar] [CrossRef]
- Reis, M.; Siomava, N.; Wimmer, E.A.; Posnien, N. Conserved and divergent aspects of plasticity and sexual dimorphism in wing size and shape in three Diptera. Front. Ecol. Evol. 2021, 9, 660546. [Google Scholar] [CrossRef]
- Ardkhongharn, N.; Ravichotikul, R.; Aksornchai, P.; Weluwanarak, T.; Chaiphongpachara, T.; Changbunjong, T. Wing geometric morphometrics to distinguish and identify Haematobosca flies (Diptera: Muscidae) from Thailand. Int. J. Parasitol. Parasites Wildl. 2023, 21, 74–82. [Google Scholar] [CrossRef]
- Rodríguez, J.N.; Liria, J. Sexual Wing shape dimorphism in Piophila casei (Linnaeus, 1758 Diptera: Piophilidae). Indian J. Forensic Med. Toxicol. 2017, 11, 217–221. [Google Scholar] [CrossRef]
- Pinto, J.; Magni, P.A.; O’Brien, R.C.; Dadour, I.R. Chasing Flies: The use of wingbeat frequency as a communication cue in calyptrate flies (Diptera: Calyptratae). Insects 2022, 13, 822. [Google Scholar] [CrossRef] [PubMed]
- Virginio, F.; Oliveira Vidal, P.; Suesdek, L. Wing sexual dimorphism of pathogen-vector culicids. Parasit. Vectors 2015, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.H. Multiple modes of canalization: Links between genetic, environmental canalizations and developmental stability, and their trait-specificity. Semin. Cell Dev. Biol. 2019, 88, 14–20. [Google Scholar] [CrossRef]
- Costa Nascimento, L.; Das Chagas Roque Machado, F.; Tidon, R. Wing symmetry in wild drosophilids (Insecta, Diptera) is not affected by season in the Brazilian Cerrado. Heringeriana 2021, 15, 17–26. [Google Scholar] [CrossRef]
- Albuquerque, D.d.O.; Pamplona, D.; De Carvalho, C.J.B. Contribuição ao conhecimento dos Fannia R. D., 1830 da região Neotropical. (Diptera, Fanniidae). Arq. Mus. Nac. 1981, 56, 9–34. [Google Scholar]
- Chillcott, J.G. A Revision of the nearctic species of Fanniinae (Diptera: Muscidae). Can. Entomol. 1960, 92, 5–295. [Google Scholar] [CrossRef]
- Alves, V.M.; Moura, M.O.; de Carvalho, C.J.B. Wing shape is influenced by environmental variability in Polietina orbitalis (Stein) (Diptera: Muscidae). Rev. Bras. Entomol. 2016, 60, 150–156. [Google Scholar] [CrossRef]
- Chaiphongpachara, T.; Duvallet, G.; Changbunjong, T. Wing phenotypic variation among Stomoxys calcitrans (Diptera: Muscidae) populations in Thailand. Insects 2022, 13, 405. [Google Scholar] [CrossRef]
- Klingenberg, C.P.; Gidaszewski, N.A. Testing and quantifying phylogenetic signals and homoplasy in morphometric data. Syst. Biol. 2010, 59, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Rummel, A.D.; Sheehy, E.T.; Schachner, E.R.; Hedrick, B.P. Sample size and geometric morphometrics methodology impact the evaluation of morphological variation. Integr. Org. Biol. 2024, 6, obae002. [Google Scholar] [CrossRef] [PubMed]
- Cardini, A.; Seetah, K.; Barker, G. How Many specimens do I need? Sampling error in geometric morphometrics: Testing the sensitivity of means and variances in simple randomized selection experiments. Zoomorphology 2015, 134, 149–163. [Google Scholar] [CrossRef]
- MacLeod, N.; Price, B.; Stevens, Z. What you sample is what you get: Ecomorphological variation in Trithemis (Odonata, Libellulidae) dragonfly wings reconsidered. BMC Ecol. Evol. 2022, 22, 43. [Google Scholar] [CrossRef]
- Dujardin, J.P.; Kaba, D.; Solano, P.; Dupraz, M.; McCoy, K.D.; Jaramillo-O, N. Outline-based morphometrics, an overlooked method in arthropod studies? Infect. Genet. Evol. 2014, 28, 704–714. [Google Scholar] [CrossRef]
- Bravo-Pena, Y.; Herrera-Russert, J.; Romera, E.; Galián, J. The head of Fannia pusio (Fanniidae: Diptera) as a novel source of morphometric data for the assessment of variation along geographic and biological lines. Zool. Stud. 2021, 60, 1–21. [Google Scholar]
- Ling, M.H.; Ivorra, T.; Heo, C.C.; Wardhana, A.H.; Hall, M.J.R.; Tan, S.H.; Mohamed, Z.; Khang, T.F. Machine learning analysis of wing venation patterns accurately identifies Sarcophagidae, Calliphoridae and Muscidae fly species. Med. Vet. Entomol. 2023, 37, 767–781. [Google Scholar] [CrossRef] [PubMed]
| Species/Morphotype | Sex | n |
|---|---|---|
| F. aburrae, Durango and Ramírez-Mora, 2019 | Males | 4 |
| F. colazorrensis, Durango and Ramírez-Mora, 2013 | 4 | |
| F. dodgei, Seago, 1954 | 3 | |
| F. dorsomaculata, Grisales, Wolff and Carvalho, 2012 | 4 | |
| F. isa, Durango and Ramírez-Mora, 2019 | 5 | |
| F. laclara, Durango and Ramírez-Mora, 2013 | 6 | |
| F. lamosca, Grisales, Wolff and Carvalho, 2012 | 4 | |
| F. pseudoconstricta, Durango and Ramírez-Mora, 2019 | 5 | |
| F. pusio, Wiedemann, 1830 | 5 | |
| F. spinosa, Durango and Ramírez-Mora, 2019 | 5 | |
| F. sp1 | Females | 16 |
| F. sp2 | 16 | |
| F. sp3 | 16 | |
| F. sp4 | 16 | |
| F. sp5 | 16 | |
| F. sp6 | 16 | |
| F. sp7 | 8 |
| Species/Morphotype | n | Median CS | IQR | CV |
|---|---|---|---|---|
| F. aburrae | 4 | 10.12 | 9.77–10.90 | 6.00% |
| F. colazorrensis | 4 | 6.62 | 6.07–6.76 | 6.20% |
| F. dodgei | 3 | 6.3 | 5.98–6.53 | 4.40% |
| F. dorsomaculata * | 4 | 9.77 | 8.99–10.54 | 8.20% |
| F. isa | 5 | 9.49 | 8.90–10.45 | 9.30% |
| F. laclara | 6 | 9.59 | 9.25–9.87 | 5.70% |
| F. lamosca * | 4 | 12.04 | 10.86–12.61 | 7.90% |
| F. pseudoconstricta | 5 | 10.25 | 9.84–10.54 | 3.60% |
| F. pusio | 5 | 5.73 | 5.46–7.60 | 26% |
| F. spinosa | 5 | 11.2 | 10.77–11.56 | 3.60% |
| Species | abu | col | dod | dor | isa | lac | lam | pse | pus |
|---|---|---|---|---|---|---|---|---|---|
| col | 18.43 | ||||||||
| dod | 16.15 | 7.20 | |||||||
| dor | 8.39 | 16.68 | 14.88 | ||||||
| isa | 7.81 | 14.62 | 13.03 | 7.74 | |||||
| lac | 4.45 | 17.68 | 14.71 | 8.49 | 8.18 | ||||
| lam | 11.86 | 18.96 | 17.99 | 6.61 | 10.62 | 12.46 | |||
| pse | 6.24 | 19.76 | 17.47 | 10.27 | 6.92 | 8.05 | 12.46 | ||
| pus | 20.49 | 4.96 | 7.55 | 18.03 | 15.94 | 19.34 | 19.58 | 21.15 | |
| spi | 4.71 | 17.96 | 16.51 | 7.86 | 8.21 | 6.03 | 10.85 | 8.32 | 20.03 |
| Morphotype | F. sp1 | F. sp2 | F. sp3 | F. sp4 | F. sp5 | F. sp6 |
|---|---|---|---|---|---|---|
| F. sp2 | 7.95 | |||||
| F. sp3 | 7.29 | 3.44 | ||||
| F. sp4 * | 8.61 | 9.54 | 8.44 | |||
| F. sp5 | 8.55 | 3.21 | 4.92 | 9.33 | ||
| F. sp6 * | 5.75 | 7.00 | 5.98 | 5.17 | 7.31 | |
| F. sp7 | 7.03 | 3.79 | 3.66 | 7.95 | 3.97 | 5.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durango-Manrique, Y.; López-Rubio, A.; Gómez, G.F. Sexual Dimorphism in Wing Shape and Its Impact on Conspecific Identification of Neotropical Fannia Species (Diptera: Fanniidae). Taxonomy 2024, 4, 795-804. https://doi.org/10.3390/taxonomy4040043
Durango-Manrique Y, López-Rubio A, Gómez GF. Sexual Dimorphism in Wing Shape and Its Impact on Conspecific Identification of Neotropical Fannia Species (Diptera: Fanniidae). Taxonomy. 2024; 4(4):795-804. https://doi.org/10.3390/taxonomy4040043
Chicago/Turabian StyleDurango-Manrique, Yesica, Andrés López-Rubio, and Giovan F. Gómez. 2024. "Sexual Dimorphism in Wing Shape and Its Impact on Conspecific Identification of Neotropical Fannia Species (Diptera: Fanniidae)" Taxonomy 4, no. 4: 795-804. https://doi.org/10.3390/taxonomy4040043
APA StyleDurango-Manrique, Y., López-Rubio, A., & Gómez, G. F. (2024). Sexual Dimorphism in Wing Shape and Its Impact on Conspecific Identification of Neotropical Fannia Species (Diptera: Fanniidae). Taxonomy, 4(4), 795-804. https://doi.org/10.3390/taxonomy4040043






