Characteristics and Evolution of Leaf Epidermis in the Genus Amana Honda (Liliaceae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Observation of Leaf Epidermis
2.2. Reconstruction of Leaf Epidermis Features
3. Results
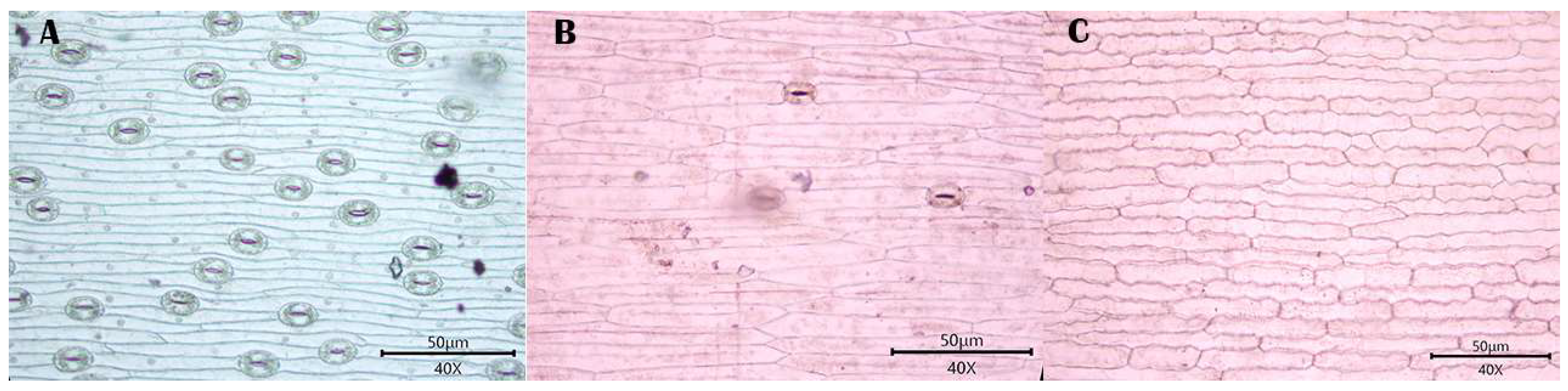
3.1. Observations
3.1.1. Stomatal Distribution
3.1.2. Morphology of Epidermal Cells
3.1.3. Morphology of the Anticlinal Wall of Epidermal Cells
3.2. Reconstruction of Epidermal Features
3.2.1. Reconstruction of Stomata Distribution
3.2.2. Reconstruction of Epidermal Cell Morphology
3.2.3. Reconstruction of the Anticlinal Wall Morphology of Epidermal Cells
4. Discussion
4.1. The Significance of Leaf Epidermis Characteristics in the Classification Andrelationships of Amana
4.2. Evolution of Leaf Epidermal Characteristics of Amana
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, J.; He, R.; Zhang, Y.; Jin, X. Systematic Significance of Leaf Epidermal Features in Holcoglossum (Orchidaceae). PLoS ONE 2014, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Wang, Y.; Qi, Q.; He, S.; Mi, Z.; Zhu, X. Leaf Epidermal Morphology of Ten Wild Tree Peonies in China and Its Taxonomic Significance. Horticulturae 2022, 8, 502. [Google Scholar] [CrossRef]
- Tan, D.; Li, X.; Hong, D. Neotypification and additional description of Amana anhuiensis (X. S. Shen) D. Y. Tan & D. Y. Hong (Liliaceae) from Anhui, China. Acta Bot. Boreali-Occident. Sin. 2008, 28, 393–395. [Google Scholar]
- Wang, L.; Xing, Q.; Lu, G.; Lu, X.; Zhao, Q.; Song, X.; Han, B. Amana baohuaensis (Liliaceae), a new species from East China. Phytotaxa 2019, 427, 43–50. [Google Scholar] [CrossRef]
- Tan, D.; Li, X.; Hong, D. Amana kuocangshanica (Liliaceae) a new species from south–east China. Bot. J. Linn. Soc. 2007, 154, 435–442. [Google Scholar] [CrossRef]
- Han, B.; Zhang, K.; Huang, L. Amana wanzhensis (Liliaceae), a new species from Anhui, China. Phytotaxa 2014, 177, 118–124. [Google Scholar] [CrossRef][Green Version]
- Honda, M. Amana a new genus of Liliaceae. Biogeogr. Soc. 1935, 6, 19–21. [Google Scholar]
- Ohwi, J.; Kitagawa, M. New Flora of Japan; Shibundo Co., Ltd.: Tokyo, Japan, 1992. [Google Scholar]
- Chen, X.; Mordak, H.V. Tulipa Linnaeus. In Flora of China; Wu, Z.-Y., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2000; Volume 24, pp. 123–126. [Google Scholar]
- Wang, M.; Fan, X.; Zhang, Y.; Wu, J.; Mao, L.; Zhang, S.; Cai, M.; Li, M.; Zhu, Z.; Zhao, M.; et al. Phylogenomics and integrative taxonomy reveal two new species of Amana (Liliaceae). Plant Divers. 2023, 45, 54–68. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, S.; Wu, J.; Zhu, X.; Liu, Z.; Lu, G.; Li, P. Amana hejiaqingii (Liliaceae), a New Species from the Dabie Mountains, China. Taxonomy 2022, 2, 279–290. [Google Scholar] [CrossRef]
- Tan, D.; Zhang, Z.; Li, X.; Hong, D. Restoration of the genus Amana Honda (Liliaceae) based on a cladistic analysis of morphological characters. Acta Phytotaxon 2005, 43, 262–270. [Google Scholar]
- Li, P.; Lu, R.; Xu, W.; Cai, M.; Qiu, Y.; Fu, C. Comparative Genomics and Phylogenomics of East Asian Tulips (Amana, Liliaceae). Front. Plant Sci. 2017, 8, 12. [Google Scholar] [CrossRef]
- Christenhusz, M.; Govaerts, R.; David, J.; Hall, T.; Borland, K.; Roberts, P.; Tuomisto, A.; Buerki, S.; Chase, M.; Fay, M. Tiptoe through the tulips—Cultural history, molecular phylogenetics and classification of Tulipa (Liliaceae). Bot. J. Linn. Soc. 2013, 172, 280–328. [Google Scholar] [CrossRef]
- Yu, Y.; Harris, A.J.; Blair, C.; He, X. RASP (Reconstruct Ancestral State in Phylogenies): A tool for historical biogeography. Mol. Phylogenet. Evol. 2015, 87, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Rudall, P.J.; Hilton, J.; Bateman, R.M. Several developmental and morphogenetic factors govern the evolution of stomatal patterning in land plants. New Phytol. 2013, 200, 598–614. [Google Scholar] [CrossRef]
- Vatén, A.; Bergmann, D.C. Mechanisms of stomatal development: An evolutionary view. Evo. Devo. 2012, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Casson, S.A.; Franklin, K.A.; Gray, J.E.; Grierson, C.S.; Whitelam, G.C.; Hetherington, A.M. Phytochrome B and PIF4 regulate stomatal development in response to light quantity. Curr. Biol. 2009, 19, 229–234. [Google Scholar] [CrossRef]
- Lake, J.A.; Quick, W.P.; Beerling, D.J.; Woodward, F.I. Plant development: Signals from mature to new leaves. Nature 2001, 411, 154. [Google Scholar] [CrossRef]
- Wang, H.; Guo, S.; Wang, P.; Song, C. Research Progress in Stomatal Development Mechanism. Chin. J. Ecol. 2018, 53, 164–174. [Google Scholar]
- Kim, T.W.; Michniewicz, M.; Bergmann, D.C.; Wang, Z. Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature 2012, 482, 419–422. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nose, T.; Jikumaru, Y.; Kamiya, Y. ABA inhibits entry into stomatal-lineage development in Arabidopsis leaves. Plant J. 2013, 74, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Le, J.; Liu, X.; Yang, K.; Chen, X.; Zou, J.; Wang, H.; Wang, M.; Vanneste, S.; Morita, M.; Tasaka, M.; et al. Auxin transport and activity regulate stomatal patterning and development. Nat. Commun. 2014, 5, 3090. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wu, C.; Zhang, Y.; Mu, K. Relationship between Leaf Structure and Drought Resistance of Four Species of North American Begonia. Bot. Res. 2020, 9, 196–204. [Google Scholar]
- Wu, J.; Wang, M.; Zhu, Z.; Cai, M.; Lee, J.; Li, P. Cytogography of the East Asian Tulips (Amana, Liliaceae). Taxonomy 2022, 2, 145–159. [Google Scholar] [CrossRef]
- Lin, Z.; Li, S.; Lin, G. The distribution of stomata and photosynthetic pathway in leaves. Acta Bot. Sin. 1986, 28, 387–395. [Google Scholar]
- Mott, K.A.; O’Leary, J.W. Stomatal behavior and CO2 exchange characteristics in amphistomatous leaves. Plant Physiol. 1984, 74, 47–51. [Google Scholar] [CrossRef]
- Beerling, D.J.; Kelly, C.K. Evolutionary comparative analyses of the relationship between leaf structure and function. New Phytol. 1996, 134, 35–51. [Google Scholar] [CrossRef]
- Xiong, D.; Jaume, F. From one side to two sides: The effects of stomatal distribution on photosynthesis. New Phytol. 2020, 228, 1754–1766. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Y.; Peng, K. Micro-morphological Characters of Leaf Epidermis of Ten Species in Genus Paphiopedilum. Bull. Bot. Res. 2014, 34, 723–729. [Google Scholar]












| Species Name | Sample Number | Latitude | Longitude | Elevation/m | Locality |
|---|---|---|---|---|---|
| A. anhuiensis (X.S. Shen) Christenh. | LJK61 | 30.7235 | 116.4537 | 1711 | China, Anhui Province, Qianshan County, Mt. Tianzhu |
| LJK62 | 30.7235 | 116.4537 | 1711 | China, Anhui Province, Qianshan County, Mt. Tianzhu | |
| LJK63 | 29.0967 | 115.5767 | 703 | China, Jiangxi Province, Yongxiu County, Mt. Yunju | |
| WMZ1499 | 29.0968 | 115.5768 | 711 | China, Jiangxi Province, Yongxiu County, Mt. Yunju | |
| A. baohuaensis B.X. Han, Long Wang and G.Y. Lu, | LJK31 | 31.7883 | 119.2961 | 107 | China, Jiangsu Province, Jurong City, Mt. Mao |
| LP207885 | 32.1385 | 119.2762 | 259 | China, Jiangsu Province, Zhenjiang City, Mt. Chaohuang | |
| LP207895 | 32.0559 | 118.5476 | 346 | China, Jiangsu Province, Nanjing City, Doushuai Temple | |
| LP207904 | 31.2614 | 119.7528 | 82 | China, Jiangsu Province, Yixing City, Songshan Village | |
| WMZ1060 | 31.4657 | 117.7861 | 76 | China, Anhui Province, Wuwei City, Yanqiao Town | |
| WMZ1417 | 32.1007 | 118.5875 | 114 | China, Jiangsu Province, Nanjing City, Mt. Lao | |
| WMZ1423 | 31.6714 | 118.0888 | 74 | China, Anhui Province, Hanshan County, Chaibulao Village | |
| A. edulis (Miq.) Honda | LJK12 | 29.4536 | 120.2869 | 331 | China, Zhejiang Province, Zhuji City, Banqiu Village |
| LJK38 | 31.2146 | 119.6959 | 247 | China, Jiangsu Province, Zhangzhu County, Mt. Longchi | |
| LJK54 | 31.0870 | 119.3347 | 308 | China, Anhui Province, Guangde City, Xiaojiawan | |
| LJK9 | 30.3864 | 118.2289 | 97 | China, Zhejiang Province, Changxing County, Biyan Temple | |
| LP173055A | 30.9435 | 120.0380 | 305 | China, Henan Province, Nanyang City, Mt. Du | |
| LP173072 | 33.0600 | 112.5786 | 183 | China, Jiangsu Province, Lianyungang City, Mt. Yuntai | |
| LP207881 | 34.7128 | 119.4206 | 99 | China, Anhui Province, Chuzhou City, Mt. Langya | |
| LP207884 | 32.2558 | 118.2789 | 177 | China, Henan Province, Tongbai City, Huaiyuan Town | |
| LP207887 | 32.4292 | 113.3197 | 269 | China, Jiangsu Province, Zhenjiang City, Mt. Mao | |
| LP207891 | 31.8154 | 119.3090 | 458 | China, Zhejiang Province, Ningbo City, Mt. Jin’e | |
| LP207905 | 29.6386 | 121.5596 | 247 | China, Jiangsu Province, Yixing City, Mt. Longchi | |
| LP207914 | 31.2146 | 119.6959 | 597 | China, Zhejiang Province, Jinhua City, Shuanglong | |
| WMZ1488 | 29.2092 | 119.6267 | 394 | China, Hubei Province, Guangshui City, Heilongtan | |
| WMZ1490 | 31.7980 | 114.0873 | 177 | China, Henan Province, Tongbai City, Huaiyuan Town | |
| A. erythronioides (Baker) D.Y. Tan and D.Y. Hong | LJK24 | 29.7339 | 121.3428 | 169 | China, Zhejiang Province, Ningbo City, Fenghua District |
| LJK26 | 29.7398 | 121.0869 | 845 | China, Zhejiang Province, Yuyao City, Mt. Siming | |
| LJK5 | 29.3735 | 121.5854 | 509 | China, Zhejiang Province, Ninghai County, Mt. Cha | |
| A. hejiaqingii M.Z. Wang and P. Li, | LP173093 | 31.7207 | 115.5035 | 496 | China, Henan Province, Shangcheng County, Liluocheng Village |
| LP207883 | 32.4036 | 113.3078 | 196 | China, Henan Province, Tongbai County, Chengjiao Village | |
| WMZ1487 | 31.7973 | 114.0872 | 529 | China, Hubei Province, Guangshui City, Heilongtan | |
| WMZ1489 | 32.3954 | 113.2989 | 248 | China, Henan Province, Tongbai County, Tayuan Temple | |
| WMZ1492 | 32.3066 | 113.4548 | 157 | China, Henan Province, Tongbai County, Mt. Bijia | |
| WMZ1714 | 31.9792 | 113.9154 | 185 | China, Henan Province, Xinyang City, Shihe District | |
| WMZ1716 | 31.8552 | 113.9298 | 199 | China, Hubei Province, Guangshui City, Santan | |
| WMZ1721 | 31.6234 | 115.4832 | 118 | China, Henan Province, Shangcheng County, Mt. Daniu | |
| ZXX19043 | 31.9792 | 113.9154 | 185 | China, Henan Province, Xinyang City, Shihe District | |
| A. kuocangshanica D.Y. Tan and D.Y. Hong | LJK22 | 28.8150 | 120.9432 | 868 | China, Zhejiang Province, Linhai City, Mt. Kuocang |
| LJK3 | 29.3752 | 121.5909 | 460 | China, Zhejiang Province, Ninghai County, Liyang Town | |
| LJK7 | 29.2582 | 121.3078 | 195 | China, Zhejiang Province, Ninghai County, Qiantong Town | |
| A. kuocangshanica × A. latifolia | WMZ1448 | 28.5512 | 120.7998 | 413 | China, Zhejiang Province, Yongjia County, Mt. Sihai |
| A. latifolia (Makino) Honda | DSL01 | 27.9079 | 120.6970 | 324 | China, Zhejiang Province, Wenzhou City, Mt. Daluo |
| WMZ1446 | 27.8273 | 120.3295 | 336 | China, Zhejiang Province, Rui’an City, Gaolou Town | |
| A. nanyueensis P. Li and L.X. Liu | LP196220 | 27.2767 | 112.6746 | 1063 | China, Hunan Province, Hengyang City, Mt. Heng |
| WMZ1464 | 27.2881 | 112.6932 | 1067 | China, Hunan Province, Hengyang City, Mt. Heng | |
| A. polymorpha ined. | LJK10 | 29.5069 | 120.4369 | 438 | China, Zhejiang Province, Zhuji City, Liaozhai Village |
| LJK11 | 29.4500 | 120.2860 | 413 | China, Zhejiang Province, Zhuji City, Banqiu Village | |
| LJK21 | 28.9799 | 120.5393 | 747 | China, Zhejiang Province, Pan’an County, Mt. Dapan | |
| LJK23 | 29.2525 | 121.0977 | 990 | China, Zhejiang Province, Tiantai County, Mt. Huading | |
| LP207908 | 29.3514 | 121.0259 | 629 | China, Zhejiang Province, Xinchang County, Xiaojiang Town | |
| WMZ1458 | 29.4500 | 120.2860 | 413 | China, Zhejiang Province, Zhuji City, Banqiu Village | |
| A. tianmuensis P. Li and M.Z. Wang | LJK49 | 30.3884 | 118.2182 | 629 | China, Anhui Province, Huangshan City, Yuxiang Village |
| WMZ1473 | 30.4728 | 117.8345 | 736 | China, Anhui Province, Qingyang County, Mt. Jiuhua | |
| WMZ1506 | 30.3884 | 118.2182 | 1620 | China, Anhui Province, Huangshan City, Mt. Huang | |
| A. wanzhensis Lu Q. Huang, B.X. Han and K. Zhang | LJK39 | 31.2146 | 119.6959 | 272 | China, Jiangsu Province, Yixing City, Mt. Longchi |
| LJK41 | 31.0864 | 119.3342 | 307 | China, Anhui Province, Guangde County, Xiasi Village | |
| LJK8 | 30.9452 | 120.0388 | 22 | China, Zhejiang Province, Changxing City, Biyan Temple | |
| WMZ1455 | 31.0871 | 119.3349 | 307 | China, Zhejiang Province, Zhuji City, Huangshan Town | |
| WMZ1460 | 29.4565 | 120.2906 | 572 | China, Zhejiang Province, Zhuji City, Huangshan Town | |
| A. yunmengensis ined. | WMZ1707-1L | 29.5990 | 113.2556 | 161 | China, Hubei Province, Jianli County, Mt. Yanglin |
| WMZ1707-2L | 29.5990 | 113.2556 | 161 | China, Hubei Province, Jianli County, Mt. Yanglin | |
| WMZ1709-1L | 29.6698 | 113.3250 | 8 | China, Hubei Province, Honghu County, Luoshan Town | |
| WMZ1709-3L | 29.6698 | 113.3250 | 8 | China, Hubei Province, Honghu County, Luoshan Town |
| Species Name | Sample Number | Adaxial Epidermis | Abaxial Epidermis | ||||
|---|---|---|---|---|---|---|---|
| Shape | Stoma | Anticlinal Wall | Shape | Stoma | Anticlinal Wall | ||
| A. anhuiensis (X.S.Shen) Christenh. | LJK61 | R, N | +++ | L | R | +++ | L |
| LJK62 | R, N | +++ | L | R | +++ | L | |
| LJK63 | R, L | +++ | L | L | +++ | L | |
| WMZ1499 | R, L | +++ | L | L, D | +++ | L | |
| A. baohuaensis B.X.Han, Long Wang and G.Y.Lu, | LJK31 | R | +++ | L | L, R, N | +++ | L |
| LP207885 | R | +++ | L | L, D | +++ | L | |
| LP207895 | R | +++ | L | R | +++ | L | |
| LP207904 | R | +++ | L | L | +++ | L | |
| WMZ1060 | R | +++ | L | R, D | +++ | P | |
| WMZ1417 | D | +++ | L | N | +++ | L | |
| WMZ1423 | L | +++ | L | L, D | +++ | L | |
| A. edulis (Miq.) Honda | LJK12 | R | +++ | P | R, L | +++ | L |
| LJK38 | N | +++ | P | N | +++ | L | |
| LJK54 | R, L | +++ | L | L | +++ | L | |
| LJK9 | L | +++ | P | L | +++ | L | |
| LP173055A | R | +++ | L | L | +++ | L | |
| LP173072 | R, L | +++ | P | L | +++ | L | |
| LP207881 | R, L | +++ | P | L | +++ | L | |
| LP207884 | R, L | +++ | L | L | +++ | L | |
| LP207887 | L | +++ | L | L, R, N | +++ | L | |
| LP207891 | R | +++ | P | L | +++ | P | |
| LP207905 | R | +++ | P | R, L | +++ | L | |
| LP207914 | L | +++ | L | L | +++ | L | |
| WMZ1488 | D | +++ | L | L | +++ | L | |
| WMZ1490 | R, L | +++ | L | L | +++ | L | |
| A. erythronioides (Baker) D.Y.Tan and D.Y.Hong | LJK24 | N | + | W | L | +++ | L |
| LJK26 | R | + | W | L | +++ | P | |
| LJK5 | R | +++ | W | L | +++ | L | |
| A. hejiaqingii M.Z. Wang and P. Li, | LP173093 | L, N, D | + | L | R, L | +++ | L |
| LP207883 | L | +++ | L | R | +++ | L | |
| WMZ1487 | L | +++ | P | L, D | +++ | L | |
| WMZ1489 | L | +++ | L | R | +++ | L | |
| WMZ1492 | L | +++ | L | R | +++ | L | |
| WMZ1714 | R, N | +++ | P | L | +++ | L | |
| WMZ1716 | R, N | +++ | P | L | +++ | L | |
| WMZ1721 | N | +++ | L | D, N | +++ | L | |
| ZXX19043 | R, N | +++ | P | L | +++ | L | |
| A. kuocangshanica D.Y.Tan and D.Y.Hong | LJK22 | R | - | W | L | +++ | L |
| LJK3 | R, N | - | W | R, L | +++ | L | |
| LJK7 | R, L | - | W | R, L | +++ | L | |
| A. kuocangshanica × A. latifolia | WMZ1448 | R, L | +++ | L | L | +++ | L |
| A. latifolia (Makino) Honda | DSL01 | R, L | +++ | L | L | +++ | L |
| WMZ1446 | R, L | +++ | L | L, D | +++ | L | |
| A. nanyueensis P. Li and L.X. Liu | LP196220 | R | +++ | P | L | +++ | L |
| WMZ1464 | R | +++ | P | L | +++ | L | |
| A. polymorpha ined. | LJK10 | R | - | L | R, L | +++ | L |
| LJK11 | L | + | L | L | +++ | L | |
| LJK21 | R | - | L | R, L | +++ | L | |
| LJK23 | R | - | P | R, D | +++ | L | |
| LP207908 | R | - | L | R, L | +++ | L | |
| WMZ1458 | L | + | L | L | +++ | L | |
| A. tianmuensis P. Li and M.Z. Wang | LJK49 | R, L | +++ | L | D, R | +++ | L |
| WMZ1473 | R | +++ | L | R, L | +++ | L | |
| WMZ1506 | R, L | +++ | L | D, R | +++ | L | |
| A. wanzhensis Lu Q.Huang, B.X.Han and K.Zhang | LJK39 | L, D | +++ | L | L | +++ | L |
| LJK41 | R | +++ | L | L | +++ | L | |
| LJK8 | D, R | +++ | L | L, D | +++ | L | |
| WMZ1455 | R | +++ | L | D, L | +++ | L | |
| WMZ1460 | R | +++ | L | D, L | +++ | L | |
| A. yunmengensis ined. | WMZ1707-1L | R, N | +++ | L | L | +++ | L |
| WMZ1707-2L | R, N | +++ | L | L | +++ | L | |
| WMZ1709-1L | R, L | +++ | L | L | +++ | L | |
| WMZ1709-3L | R, L | +++ | L | L | +++ | L | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.; Wang, M.; Cai, M.; Luo, P.; Pace, M.C.; Li, P. Characteristics and Evolution of Leaf Epidermis in the Genus Amana Honda (Liliaceae). Taxonomy 2023, 3, 435-451. https://doi.org/10.3390/taxonomy3030025
Zeng X, Wang M, Cai M, Luo P, Pace MC, Li P. Characteristics and Evolution of Leaf Epidermis in the Genus Amana Honda (Liliaceae). Taxonomy. 2023; 3(3):435-451. https://doi.org/10.3390/taxonomy3030025
Chicago/Turabian StyleZeng, Xin, Meizhen Wang, Minqi Cai, Pengcheng Luo, Matthew C. Pace, and Pan Li. 2023. "Characteristics and Evolution of Leaf Epidermis in the Genus Amana Honda (Liliaceae)" Taxonomy 3, no. 3: 435-451. https://doi.org/10.3390/taxonomy3030025
APA StyleZeng, X., Wang, M., Cai, M., Luo, P., Pace, M. C., & Li, P. (2023). Characteristics and Evolution of Leaf Epidermis in the Genus Amana Honda (Liliaceae). Taxonomy, 3(3), 435-451. https://doi.org/10.3390/taxonomy3030025





