Abstract
We describe a new species of Proctoporus from the scientifically unexplored southern sector of the Otishi National Park (Region Cusco) in Peru, on the basis of molecular and morphological characters. Seven type specimens were obtained from six localities between 3241–3269 m a.s.l. within a radius of ca. 1.5 km in a Puna valley. Nine adult specimens (four males, five females) from Chiquintirca (Region Ayacucho, ca. 85 km airline from the type locality) are considered referred specimens. Males of the new species have a snout–vent length of 41.3–53.9 mm ( = 46.7, n = 6), females have a snout–vent length of 43.6–52.6 mm ( = 48.1, n = 8). The new species has dorsal scales striated, four supraoculars, four anterior supralabials, loreal and prefrontal scales absent, two pairs of genials (rarely one or three), three rows of pregulars, and five to seven femoral pores in males (absent in females). Sexual dimorphism is evident in the ventral coloration: males have neck, chest, and belly dark gray to black, whereas females have neck, chest, and belly pale gray with a diffuse dark gray fleck in the center of each scale, and an orange iris with a fringed pupil in both sexes.
1. Introduction
Otishi National Park (ONP) of the Avireri-Vraem Biosphere Reserve in Peru (regions Junín and Cusco) covers 305,973 ha between 750 and 4185 m encompassing five types of montane forests, and two different types of montane grasslands [1]. On its west side, the ONP is flanked by the Asháninka Communal Reserve, and on its east side by the Machiguenga Communal Reserve (Figure 1). ONP is located in the VRAEM (Valley of Rivers Apurímac, Ene, Mantaro) area, which is the center of Peru’s coca production and narco-trafficking. Based on its remote location in a dangerous area and steep mountains of the Cordillera de Vilcabamba, the ONP is the least scientifically surveyed national park in Peru, its animal and plant diversity are predominantly unknown, with many new species waiting to be discovered.
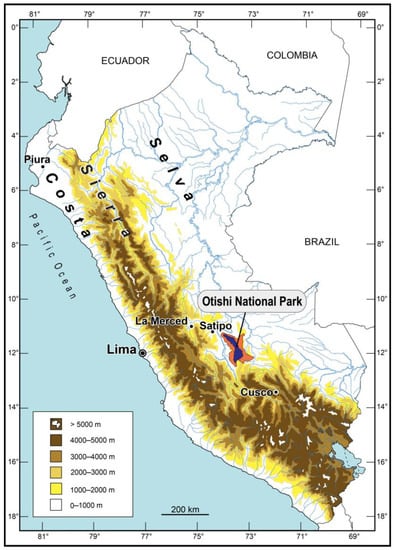
Figure 1.
Peru with the Otishi National Park in dark blue, the Asháninka Communal Reserve on its west side (in pink), and the Machiguenga Communal Reserve on its east side (in pink).
In 1963, the vast unexplored territory of the Cordillera de Vilcabamba attracted attention. The Zoological Society of New York and the National Geographic Society funded a research expedition to explore this area. A team of researchers used parachutes to reach high elevations near the Laguna Parodi and walked adventurously a 150-mile trek by foot northwards down to the Urubamba River in the Amazonian lowlands [2]. In 1997 and 1998, the Smithsonian Institution/Monitoring and Assessment Biodiversity Program team of botanists, zoologists, and anthropologists conducted rapid program assessment surveys at six sites in the northern and southern Cordillera de Vilcabamba [3], two of which (Camp 1 and Camp 2) are today located inside ONP (Figure 2). A detailed report was published in 2001 which documented the rich biodiversity and mentioned several new species [3] some of which were later described (e.g., Lehr 2007 [4]: Pristimantis seorsus, P. vilcabambae, and P. tanyrhynchus; Mendoza 2005 [5]: Polylepis canoi; Mamani and Rodríguez 2022 [6]: Proctoporus otishi from Camp 1). The creation of ONP followed in 2003, and its recognition as an UNESCO Biosphere Reserve in 2021 [7]. Instituto Nacional de Rercursos Naturales (INRENA) [7] mentions that 11 species of amphibians and six species of reptiles (two lizards, five snakes) were recorded within ONP, but without details on their identifications or localities. Lack of knowledge regarding the biodiversity of the Cordillera de Vilcabamba has also been recognized by Rodríguez and Young [8], and Fajardo et al. [9] categorize the ONP with the highest priority for conservation. A research thesis completed by Barboza Sánchez [10] recorded 15 species of amphibians between 658 and 2087 m a.s.l. along three transects (A01, A02, A03) in a total of 30 days between July, September, and November 2013 (Figure 2).
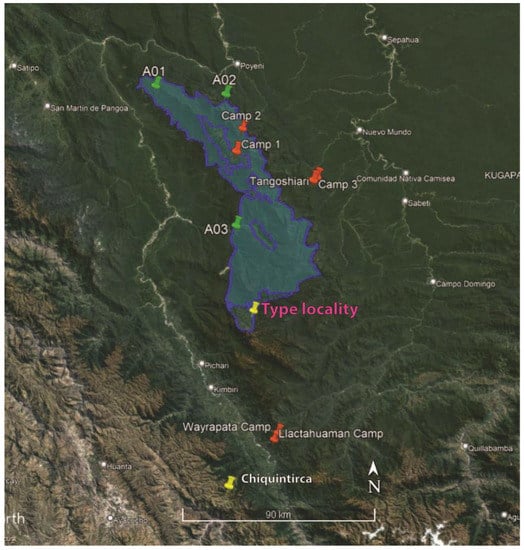
Figure 2.
Google Earth image with the border of the Otishi National Park in blue. In orange are the six RAP collecting sites by Alonso et al. [3], in green are the three collecting sites by Barboza Sánchez [10], in yellow is the type locality of Proctoporus titans sp. nov. and the referred specimens from Chiquintirca.
A team of four biologists (E. Lehr, J.C. Cusi, M.I. Fernandez, and R.J. Vera) and one national park guard (M.A. Mayta) reached a remote area by helicopter inside the southern portion of the ONP that is inaccessible by foot and has never been explored (Figure 2). We landed in a valley close to a marsh with scattered ponds at 3250 m a.s.l. on 16 May 2022, for the duration of two weeks to survey the herpetofauna. Among the recorded taxa is a new species of the genus Proctoporus Tschudi, 1845 [11] which currently has the following 17 species (Mamani et al. 2022 [12], Uetz et al. 2022 [13]): P. bolivianus Werner, 1910 [14]; P. carabaya Goicoechea, Padial, Chaparro, Castroviejo-Fisher and De la Riva, 2013 [15]; P. chasqui (Chávez, Siu-Ting, Duran and Venegas, 2011) [16]; P. guentheri Boettger, 1891 [17]; P. iridescens Goicoechea, Padial, Chaparro, Castroviejo-Fisher and De la Riva, 2013 [15]; P. katerynae Mamani, Cruz, Mallqui and Catenazzi, 2022 [12]; P. kiziriani Goicoechea, Padial, Chaparro, Castroviejo-Fisher and De la Riva, 2013 [15]; P. lacertus Stejneger, 1913 [18]; P. laudahnae Köhler and Lehr, 2004 [19]; P. machupicchu Mamani, Goicoechea and Chaparro, 2015 [20]; P. optimus Mamani, Cruz, Mallqui and Catenazzi, 2022 [12]; P. oreades (Chávez, Siu-Ting, Duran and Venegas, 2011) [16]; P. otishi Mamani and Rodríguez, 2022 [6]; P. pachyurus Tschudi, 1845 [11]; P. rahmi De Grijs, 1936 [21]; P. spinalis Boulenger, 1911 [22]; P. sucullucu Doan and Castoe, 2003 [23]; P. unsaacae Doan and Castoe, 2003 [23]; P. xestus (Uzzell, 1969) [24]. All species are known to occur in Peru except for P. xestus whose distribution in Peru is doubtful ([12]) but is known from Argentina and Bolivia ([13]), and P. bolivianus and P. guentheri, which also occur in Bolivia ([13]).
Herein, we describe this new species of Proctoporus from the ONP based on molecular and morphological data. We complement its description with a previously unidentified series of Proctoporus spp. from the Region Ayacucho encountered in the Natural History Museum of the National University of San Marcos (Lima, Peru) that we assign to this taxon.
2. Materials and Methods
2.1. Molecular Genetics
We used finger tissue from MUSM 40915–16, 40922, and 40924 to obtain 16S rRNA DNA sequences for the new species (see Appendix A for GenBank accession codes). All four specimens returned identical sequences, and we used the longest one, 508 bp for phylogenetic analyses (holotype MUSM 40916). We used the alignment from Goicoechea et al. [25], and sequences of species of Proctoporus and related genera described since 2013 from GenBank. We used a commercial extraction kit (IBI Scientific, Peosta, IA, USA) to extract DNA, and standard protocols for 16S amplification and sequencing. We conducted the polymerase chain reaction (PCR) with a ProFlex thermal cycler (Applied Biosystems) at the Catenazzi Lab at Florida International University. Purified PCR products (Exosap-IT, Affymetrix, Santa Clara, CA, USA) were mailed to MCLAB (San Francisco, CA, USA) for sequencing. We used Geneious R11, version 11.1.5 (Biomatters Ltd., Auckland, New Zealand) to align sequences with the MAFFT v7.017 alignment program [26], and trimmed sequences to a length of 462 bp. We estimated uncorrected p-distances (i.e., the proportion of nucleotide sites at which any two sequences are different) for the 16S rRNA mitochondrial fragment with the software MEGA [27], and uploaded the table to Figshare (https://doi.org/10.6084/m9.figshare.21335010, accessed on 28 December 2022).
We conducted an analysis using Maximum Likelihood with IQ-TREE v1.6.12 [28] for phylogenetic inference of 16S using the GTR + I + G model and the ultrafast bootstrap method (10,000 bootstrap alignments). We determined the best substitution model for 16S by using the default options in MEGA.
2.2. Morphology
The IACUC Committee of Illinois Wesleyan University approved this study (Protocol 19-011) on 5 November 2019 and the Ministerio del Ambiente, Lima, Peru granted research permits (N° 002-2021-SERNANP-JPNO). The format of the descriptions and terminology of the morphological characters follow mostly Oftedal [29], Chávez et al. [30], Sánchez-Pacheco et al. [31,32], Moravec et al. [33], and Mamani et al. [12]. The definition for anterior infralabials and anterior supralabials follows Mamani et al. [12] and Mamani and Rodríguez [6]. The characterization of other head scales, groin scales, and scales of the lateral body side follows Mamani and Rodríguez [6] (p. 3, Figure 1). Specimens were fixed in 10% formol and stored in 70% ethanol. Deposited eggs were preserved in 70% ethanol. Sex (male = m, female = f) and maturity of specimens were identified through sexual dimorphic characters (size, femoral pores, coloration). The following metric characters were taken by E.L. using a digital caliper and dissecting microscope: snout–vent length (SVL)—distance from the snout tip to cloaca; head length (HL)—distance from the snout tip to the angle of jaw; head width (HW)—greatest width of the head; head depth (HD)—greatest depth of the head; tail length (TL)—distance from cloaca to the tail tip; eye-nose distance (E–N)—straight distance from the snout tip to anterior corner of eye; forelimb length (FLL)––from axilla to tip of distal claw; hind limb length (HLL) from groin to tip of distal claw; axilla-groin distance (AGD)––distance between limbs (left/right). Whether forelimbs and hindlimbs extremities overlap or not was revealed by addressing them laterally against the body. The length and width of two deposited eggs were measured with a digital caliper. All examined characters were measured to the nearest 0.1 mm.
Meristic and qualitative pholidotic characters were counted and evaluated by E.L. as follows: number of supralabials—from the rostral to the mouth corner, last labial defined by its considerably larger size compared with the posteriorly adjacent shields; number of infralabials––from the mental to the mouth corner, last labial defined by its considerably larger size compared with the posteriorly adjacent shields; anterior infralabials––in contact with the mental and genial scales; anterior supralabials––anterior to the posteroventral angle of the subocular; genial scales––posteriorly to the post mental, in contact medially and in contact with infralabials, genials not in contact medially are separated by pregulars; gular scale rows––number of transverse scale rows in a straight median series; collar scale rows––number of transverse scale rows in a median series; dorsal scales––number of transverse rows of dorsal scales from the third row behind the interparietal to the level of the rear edge of the hind limb; longitudinal dorsal scale rows––measured at the 10th transverse ventral scale row; ventral scales––number of transverse rows of ventral scales from plane between forelimbs to row anterior to anterior preanal plate scales; longitudinal ventral scale rows––measured at the 10th transverse ventral scale row; lateral scales––number of considerably smaller lateral scales situated between larger dorsal and ventral scales at the 10th transverse ventral scale row (left/right); scales around midbody at the 10th transverse ventral scale row; posterior cloacal plate scales (=anal scales)—number of large scales; anterior preanal plate scales (=preanals)––number of large scales; number of lamellae under Finger IV—number of single and divided lamellae (left/right, lamella divided into segments counted as one individual lamella); number of lamellae under Toe IV—number of single and divided lamellae (left/right, lamella divided into segments counted as one individual lamella); number of femoral pores (left/right). Drawings were made by E.L. using a stereomicroscope with a drawing tube attachment. The preserved holotype was photographed submersed in ethanol to avoid reflections, and small beads helped to keep the head in position. All maps and photos of life specimens and habitats by E.L.
Notes on the coloration in life were taken from field notes and photographs. Collection acronym is: MUSM = Museo de Historia Natural Universidad Nacional Mayor de San Marcos, Lima, Peru. Field number code is: IWU = Illinois Wesleyan University. For comparative material examined, see Appendix B. Threat status was evaluated using the IUCN criteria [34]. Google Earth was accessed on 4 September 2022 to design the maps.
3. Results
3.1. Molecular Genetics
Based on the phylogeny shown in Figure 3, the new species is assigned to Proctoporus. There is generally weak support for most of the nodes in the genus, including for the group with the new species, which comprises specimens of P. oreades and P. pachyurus. Uncorrected p-distances (https://doi.org/10.6084/m9.figshare.21335010, accessed on 28 December 2022) are lowest (1.06%) between the fragment sequence of the new species and specimens of the two species, supporting the idea that the new taxon is closely related to P. oreades and P. pachyurus.
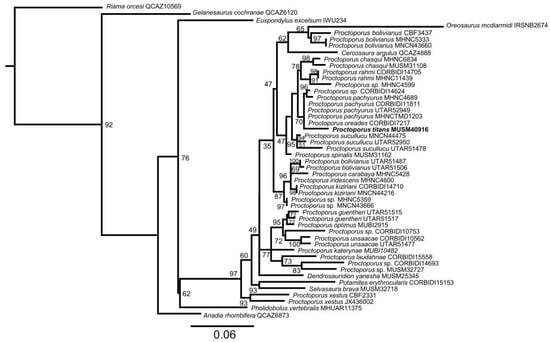
Figure 3.
Maximum Likelihood phylogeny inferred for species of Proctoporus and related genera of the family Gymnophthalmidae included in this study based on a 462-bp gene fragment of 16S rRNA (ML bootstrap values are indicated at each node). The new species P. titans sp. nov. is in bold.
3.2. Generic Assignment
Our phylogeny (Figure 3) supports the assignment to Proctoporus. Furthermore, following Castoe et al. [35] and Mamani et al. [12], the presence of imbricate and scale-like papillae on the tongue; smooth head scales lacking striations or rugosities; eyelids with an undivided translucent disc; dorsal scales quadrangular elongate, keeled, juxtaposed, and forming transversal series; and posterior gulars squarish support the generic assignment based on morphological characters.
3.3. Systematics
Family Gymnophthalmidae Fitzinger, 1826 [36].
Subfamily Cercosaurinae Gray, 1838 [37].
Genus Proctoporus Tschudi, 1845 [11].
Proctoporus titans sp. nov. Lehr, Cusi, Fernandez, Vera and Catenazzi, 2023.
ZooBank LSID: zoobank.org:pub:2B11D9C4-8B21-491D-B307-86E5A84685AA.

Figure 4.
Life holotype of Proctoporus titans sp. nov. (MUSM 40916) in dorsolateral (A), dorsal (B), and ventral views (C). SVL 53.9 mm.

Figure 5.
Preserved holotype of Proctoporus titans sp. nov. (MUSM 40916, male) in dorsal (A) and ventral (B) views. SVL 53.9 mm.
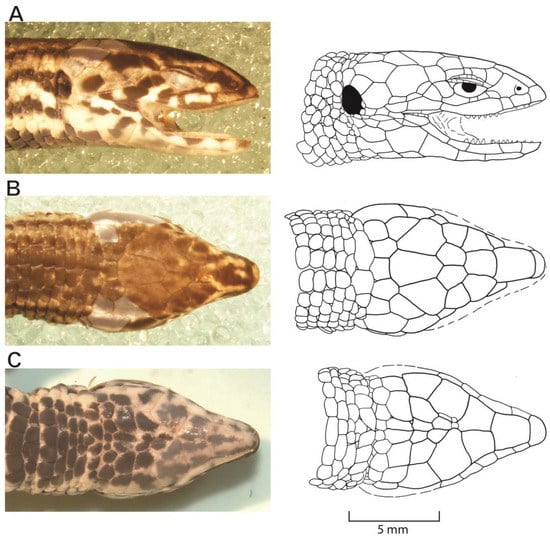
Figure 6.
Photos (left column) and drawings (right column with scale) of the head of Proctoporus titans sp. nov. (MUSM 40916, holotype) in lateral (A), dorsal (B), and ventral (C) views. Head length 10.7 mm.
Holotype (Figure 4, Figure 5 and Figure 6): MUSM 40916 (field number IWU 395), an adult male from the puna of the Otishi National Park (12°19′25.54″ S, 73°35′44.14″ W; WGS84), 3241 m a.s.l., Distrito Echarate, Provincia La Convención, Region Cusco, Peru, collected on 17 May 2022 by E. Lehr, J, C., Cusi, M. I. Fernandez, and R. J. Vera.
Paratypes: 6 (1 male, 3 females, 2 juveniles, Figure 7 and Figure 8) all collected in the Otishi National Park, Distrito Echarate, Provincia La Convención, Region Cusco, Peru by E. Lehr, J. C., Cusi, M. I. Fernandez, and R. J. Vera: MUSM 40921 (field number IWU 400), adult male, 12°19′36.4″ S, 73°35′50.0″ W, 3257 m a.s.l. on 19 May 2022; MUSM 40915 (IWU 394), adult female, 12°19′26.91″ S, 73°35′44.63″ W, 3249 m a.s.l. on 17 May 2022; MUSM 40922 (IWU 401), adult female, 12°19′19.24″ S, 73°35′49.20″ W, 3269 m a.s.l. on 20 May 2022; MUSM 40924 (IWU 403), adult female, 12°19′28.35″ S, 73°35′43.36″ W, 3250 m a.s.l., on 22 May 2022; MUSM 40917 (IWU 396), MUSM 40918 (IWU 397), juveniles, 12°19′27.98″ S, 73°35′43.94″ W, 3248 m a.s.l. on 17 May 2022.

Figure 7.
Life female of Proctoporus titans sp. nov. (MUSM 40915, paratype) in dorsolateral (A), dorsal (B), and ventral views (C). SVL 46.0 mm.

Figure 8.
Life hatchling (MUSM 40918, male paratype) of Proctoporus titans sp. nov. in dorsolateral (A), dorsal (B), and ventral views (C). SVL 22.9 mm.
Referred specimens: 9 adults (4 males, 5 females), MUSM 35637 (f), 35638 (m), 35639 (f), 37754 (m), 37755 (m), 37756 (f), 37757 (m), 37758 (f), 37789 (f), all collected at Chiquintirca (Figure 2), Region Ayacucho by A. Guzmán.
Diagnosis: (1) Frontonasal equal in size or longer than frontal; (2) nasoloreal suture incomplete or absent; (3) three supraoculars, the anteriormost supraocular fused with the anteriormost superciliary; (4) four superciliaries, the anteriormost superciliary fused with the anteriormost supraocular; (5) two postoculars; (6) palpebral disc undivided and transparent; (7) three to five supralabials anterior to the posteroventral angle of the subocular; (8) four to six anterior infralabials; (9) two pairs of genials in medial contact (rarely one or three); (10) three (rarely four) rows of pregulars; (11) dorsal body scales, rectangular, striated (two centrally positioned, parallel and longitudinal distinct furrows), and subimbricate; (12) 34–40 scales around the midbody; (13) 33–41 transverse dorsal rows; (14) 19–23 transverse ventral rows; (15) 20–25 longitudinal dorsal rows; (16) 10–12 longitudinal ventral rows; (17) a continuous series of small lateral scales separating the dorsals from the ventrals; (18) two anterior preanal plate scales; (19) two to six posterior cloacal plate scales; (20) five to seven femoral pores per hind limb in males, absent in females; (21) preanal pores absent; (22) 10–14 subdigital lamellae on Finger IV; 15–20 sudigital lamellae on Toe IV; (23) limbs not overlapping when pressed against the body; (24) pentadactyl, digits clawed; (25) sexual dimorphism in coloration is evident: in adult males, the dorsum is pale reddish to greenish brown with small and irregular black flecks irregularly distributed and a distinct pale greenish yellow dorsolateral strip bordered with narrow black margins longitudinally; the flanks are pale orange to orange brown with a continuous line of conspicuous ocelli of black blotches with white flecks from the neck to hind legs; the ventral surface of neck, chest, and belly are dark gray to black; the throat is black with pale orange brown to yellow flecks; in adult females, the dorsum is pale reddish brown to pale olive with small and irregular black flecks irregularly distributed and a distinct pale greenish yellow dorsolateral strip bordered with narrow black margins longitudinally; the flanks are pale olive to reddish brown with a continuous line of conspicuous ocelli of black blotches with white flecks from the neck to hind legs; the ventral surface of neck, chest, and belly are pale gray with a diffuse dark gray fleck in the center of each scale; the throat is pale gray to pale cream with few gray flecks; the iris is orange with a fringed pupil in both sexes (Figure 4 and Figure 8).
Comparisons: Proctoporus titans sp. nov., P. laudahnae, P. machupicchu, and P. unsaacae have four anterior supralabials. However, P. titans sp. nov. and P. laudahnae have dorsal scales striated, but P. laudahnae is larger (max SVL 65.0 vs. 53.9 mm) and females have 6–7 femoral pores (absent), [19,38]. Proctoporus titans sp. nov. and P. machupicchu both have the venter black or dark gray but P. machupicchu has a loreal scale present (absent), three pairs of genials in medial contact (one to two, rarely three), limbs overlapping (not overlapping), and first supraocular not fused with first supraciliary (fused), [20]. All examined specimens of Proctoporus titans sp. nov. have dorsal scales striated, whereas P. unsaacae have dorsal scales keeled and sometimes striated, but P. unsaacae has a loreal scale present (absent), nasal divided (undivided), and femoral pores in females present or absent (absent), [23]. Five anterior supralabials are present in P. bolivianus, P. carabaya, P. chasqui, P. iridescens, P. kiziriani, P. lacertus, P. machupicchu, P. oreades, P. pachyurus, P. rahmi, P. spinalis, P. sucullucu, P. unsaacae, and P. xestus whereas P. katerynae, P. optimus, and P. otishi have three anterior supralabials (rarely four in P. optimus). Proctoporus optimus has the venter orange or cream (black to pale gray), two rows of pregulars (three), and four to eight femoral pores in females (absent), [12]. Proctoporus titans sp. nov. differs from P. pachyurus which has four supraoculars (three), and 47–60 transverse dorsal scale rows (33–41), [15].
Proctoporus titans sp. nov. and P. otishi both occur in the ONP, and their type localities are 75 km (airline) apart. Proctoporus titans sp. nov. is known between 3241–3269 m a.s.l. and P. otishi from 3350 m a.s.l. [6]. Both species can be distinguished as follows (characters of P. otishi following Mamani and Rodríguez [6] are in parenthesis). Proctoporus titans sp. nov. has dorsal scales striated (keeled), four supraoculars (two), four to six anterior infralabials (three), four anterior supralabials (three), five to seven femoral pores (four) in males, dorsum pale reddish to greenish brown (purple brown) with small irregular black flecks (tiny cream-colored spots) and a pale greenish yellow dorsolateral stripe (absent), and the flanks have a continuous line of conspicuous ocelli of black blotches with white flecks (enlarged cream-colored spots irregularly distributed).
Description of holotype: Adult male with incompletely everted hemipenes and cut-off right forelimb, SVL 53.9 mm, TL (reg.) 56.8 mm (Figure 5). Body slender; extremities short, tail regenerated; head length 19.9% of SVL, head width 15.2% of SVL; snout pointed, moderately long, eye-nose distance 35.5% of HL; neck distinct, collar present; head scales smooth; rostral scale wider than tall (Figure 6B), slightly higher than adjacent supralabials, in contact with frontonasal, nasals, and first supralabials; frontonasal longer than wide, longer than frontal, prefrontals absent; frontal longer than wide, in contact with second supraoculars; frontoparietals in contact with second and third supraoculars, parietals and interparietal; supraoculars three, the anteriormost supraocular fused with the anteriormost superciliary, none in contact with ciliaries; superciliary series complete, consisting of four shields (anteriormost superciliary fused with anteriormost supraocular), in contact with frontal and loreal anteriorly; parietals in contact with frontoparietal, third supraocular, dorsalmost postocular, one temporal and one postparietal; interparietal longer than wide, in contact with three postparietals posteriorly; postparietals five; nasal shield fused with loreal forming a nasoloreal shield (Figure 6A); nasal shield incompletely divided, nasal suture vertically from the posterior margin of the nostril to first supralabial, in contact with rostral and first and second supralabial; frenocular quadrangular, in contact with nasoloreal and second and third supralabial ventrally; palpebral disc oval, translucent, undivided; postoculars two; supratympanic temporals polygonal, 2/4; supralabials 5/5, three supralabials anterior to the posteroventral angle of the subocular, third supralabial longest, covering the entire surface below the eye; infralabials 5/5, anterior infralabials 4/3; mental wider than long, in contact with first infralabials and postmental posteriorly; postmental single, in contact with first and second infralabials; four genials, first pair of genials in full contact medially, second left genial in contact medially with first right genial (Figure 5C); first genial pair in contact with second and third infralabials, second pair separated by two pregular scales, in contact with third and fourth infralabials; three transverse rows of pregular scales; two gular scale rows; seven collar scale rows forming a distinct collar fold; dorsal body scales rectangular, striated, and subimbricate, in 34 transverse rows, irregularly shaped (trapezoid) and smaller than surrounding dorsals along vertebral line from transverse rows 17 to 24; dorsals (enlarged scales) across body at fifth transverse row 10, at 10th transverse ventral scale row 7, at 15th transverse ventral scale row 14; continuous series of lateral scales (smaller than dorsals) separating the dorsals from the ventrals; laterals at fifth transverse ventral scale row 7/8, at 10th transverse ventral scale row 3/2, at 15th transverse ventral scale row 3/2; ventrals smooth, squared to rectangular, juxtaposed, in 21 transverse rows; ventrals across 5th transverse scale row 8, at 10th transverse scale row 20, at 15th transverse scale row 10; scales around midbody 35; two anterior preanal scales, four posterior cloacal scales; dorsal scales on tail rectangular to quadrangular, strongly striated from base to middle of tail, then getting weakly striated posteriorly, subimbricate; caudal scales quadrangular to rectangular, smooth; forelimbs pentadactyl with clawed digits; subdigital lamellae under Finger IV –/13; subdigital lamellae under Toe IV 19/19; femoral pores 5/5.
Measurements (in mm) of the holotype: SVL 53.9; TL 56.8 (regenerated); HL 10.7; HW 8.2; HD 5.7; E-N 3.8; FLL –/10.4; HLL 14.1/13.7; AGD 28.0/26.7.
Coloration of holotype in life (Figure 4): Dorsal surfaces of head and body are greenish brown and the tail is orange brown with small and irregular black flecks irregularly distributed (Figure 4A). A distinct pale greenish-yellow dorsolateral strip bordered with narrow black margins longitudinally starts on the neck and extends to the tail, more prominent on the anterior body half (Figure 4B). The flanks are pale orange with a continuous line of conspicuous ocelli of black blotches with white flecks from the neck to the hind leg (Figure 4A). Ventral surface of neck, chest, and belly are black with an irregular-shaped ventrolateral yellow to orange stripe covering one to two ventral scales on each side of the body (Figure 4C). The throat is black with pale orange-brown and yellowish-cream flecks. Arms and hindlimbs are colored dorsally and laterally similarly to the flanks and have a few ocelli of black blotches with white flecks. The arms are ventrally pale greenish yellow with pale gray blotches and are pale gray hands, the hindlimbs are black with some pale greenish-yellow to orange flecks, pale orange-brown femoral pores, and pale gray and pale orange feet. The frontonasal is greenish brown and yellowish-cream on its lateral margins. The sides of the head are colored similarly to the flanks with a dark gray stripe extending over the canthus rostralis, super ciliaris, upper postoculars, and postparietals (Figure 4A). The supralabials and infralabials are yellowish cream to orange with diagonal dark gray blotches. The iris is orange with a fringed pupil.
Coloration of holotype in alcohol (Figure 5 and Figure 6): The dorsum is grayish brown with pale brown dorsolateral stripes lined with black. The flanks are slightly paler than the dorsum with contrasting ocelli consisting of black blotches with white flecks. The neck, chest, belly, and tail are ventrally black with a pale cream irregular-shaped ventrolateral stripe, and the throat is pale cream with pale gray blotches.
Variation: Measurements and scutellation data of the type series are given in Appendix C (Table A2) and for referred specimens in Appendix D (Table A3). Females are slightly larger than males: SVL 41.3–53.9 mm ( = 46.7 ± 4.8, n = 6) in males, SVL 43.6–52.6 mm ( = 48.1 ± 3.3, n = 8) in females. One hatchling is a male (MUSM 40918, SVL 22.9, TL 27.8) with preformed 5/5 femoral pores, the other is a female (MUSM 40917, no femoral pores, SVL 21.4, TL 25.0).
The nasofrontal is distinctly longer than the frontal in six specimens (males MUSM 37754, 37757, 40916, females MUSM 35639, 37756, 40924) and about equal in length in the other specimens. The majority of the specimens have two pairs of genials medially in contact; two specimens (holotype MUSM 40916 and female MUSM 40924) have the first pair of genials medially in contact, and one specimen (female MUSM 37789) has a third pair of genials partially in contact medially. Males have five to seven femoral pores (MUSM 35638: 6/6, 37754: 5/6, 37755: 7/7, 37757: 7/6, 40921: 6/6). Everted hemipenes are only present in the two males of the type series. The number of postanal scales ranges from two to six but a lower number is rare, one male (MUSM 37757) has two, and two specimens (male MUSM 37755, female MUSM 37758) have three postanal scales.
The completely everted right hemipenis (MUSM 40921) measures approximately 6.2 mm. The hemipenial body has a conical shape with proximal region distinctly thinner than the distal region. It is a stout acapitate organ without a medial welt. About 15 spinulike flounces forming two chevrons on distal half of the hemipenis while the asculate side in its center has four short transverse flounces that do not reach the lateral sides. The distal chevrons are separated by a small expansion pleat. The sulcus spermaticus is single, bordered by well-developed sulcal lips and flanked by a broad naked expansion pleat widened distally; sulcus spermaticus divided by a small protrusion distally.
Sexual dimorphism in coloration is evident in the type series and the referred specimens with males having dark gray to black throat, chest, belly, and tail (Figure 4C), whereas females have the dorsum pale reddish brown (MUSM 40922) to pale olive (MUSM 40915, 40924, Figure 7A,B) with small and irregular black flecks irregularly distributed and a distinct pale greenish yellow dorsolateral strip bordered with narrow black margins longitudinally; the flanks are pale olive (MUSM 40915, 40922) to reddish brown (MUSM 40924) with a continuous line of conspicuous ocelli of black blotches with white flecks from the neck to hind legs; the ventral surface of neck, chest, and belly are pale gray with a diffuse dark gray fleck in the center of each scale; the throat is pale gray (MUSM 40922, 40924) to pale cream (MUSM 40915, Figure 7C) with few gray flecks. In juveniles, the color pattern is generally brighter than in adults and consists of distinct cream-brown dorsolateral stipes on pale copper-brown background coloration (Figure 8A). The flanks in one juvenile (MUSM 409018, Figure 8B) are orange-brown, the throat is pale orange, and chest and anterior half of belly are pale red with gray flecks, and the tail is gray (Figure 8C).
Etymology: The species epithet titans is used in reference to the Titan community of Illinois Wesleyan University, including former, current, and future students, faculty, and staff. We dedicate this species in recognition of the Titans’ mission “To go out and do well, but more importantly, to do good.”
Distribution, natural history, and threat status (Figure 9 and Figure 10): The type series of Proctoporus titans sp. n. is known from six localities (Figure 9) between 3241–3269 m a.s.l. within a radius of ca. 1.5 km in a humid puna (Holdridge life zone “tropical subalpine pluvial páramo”, [7,39] valley in the southern sector of the Otishi National Park, an area that is in a strict protection zone (Zona de Protección Estricta, [7]). The valley is confined transversely in the south by a waterfall that we name the Kitamarapó Waterfall (Asháninka meaning “white hand” due to its appearance, Figure 10A,C). We name the mountain ridge east and west of the water fall the Shirampari Matsiri Mountain Ridge (Asháninka meaning “sleeping man” due to its appearance, Figure 10A–C). The valley has several shallow ponds that hold temporary water forming an extended swamp that we name Pantano La Esperanza (Spanish meaning “Swamp Hope”, Figure 10A,C). The habitat of P. titans sp. nov. is a humid puna valley consisting of tall Peruvian feather grass (Figure 10B,D), mosses, bushes, small and scattered trees, and dense Polylepis forests and bamboo patches along the mountain slopes. The absence of stones on the soil, except for very few large rocks at the type locality was noticeable. All specimens were found during the day under moss and grass. One female (MUSM 40924) was found active at the campsite at 8:00 AM on a sunny day, and one gravid female (MUSM 40922, one egg on each side of the belly) was found under moss along with five empty eggs likely in the process of egg deposition. Eggs were found in pairs under moss in a depth of ca. 10 cm in open areas exposed to the sun. Nesting grounds often contained multiple pairs of eggs (some developing, others hatched) indicating that females either return to the same nesting places or that popular nesting places are frequented by multiple females. Two intact eggs (MUSM 40919), oval-shaped and white-colored, were collected at 12°19’27.98”S, 73°35’43.94”W, 3248 m a.s.l. on 17 May 2022 (EL = 11.7–12.0 mm, EW = 6.3–6.4 mm, n = 2). Two eggs started hatching (hatchlings MUSM 40917, 40918) likely due to being disturbed by us (Figure 9E) on a rainy day at 18 °C at 9:45 AM on 17 May 2022. Two empty eggs (MUSM 40920) were collected at the same locality. At the type locality, a potential predator of P. titans sp. nov. is a new species of colubrid snake that we will describe shortly.
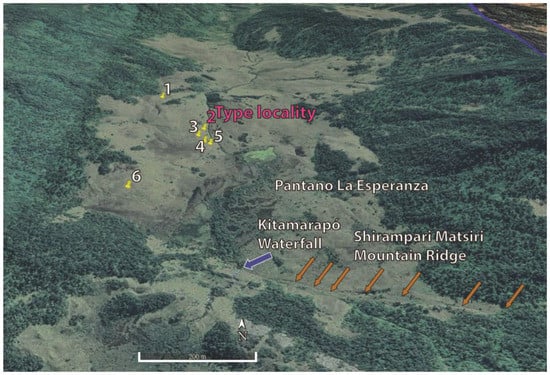
Figure 9.
Google Earth image with collecting sites (yellow pins 1–6) of Proctoporus titans sp. nov. The blue arrow marks the Kitamarapó Waterfall, and the brown arrows the Shirampari Matsiri Mountain Ridge.

Figure 10.
Habitat and natural history of Proctoporus titans sp. nov. (A): Panorama of the valley in southern view over the Pantano la Esperanza swamp toward the mountain ridge and swamp, taken 25 May 2022 at 11:44 AM. (B): western slope of the valley with Peruvian feathergrass in southeastern view taken from the campsite on 21 May 2022 at 8:22 AM. (C): Kitamarapó Waterfall in close aspect taken 25 May 2022 at 11:20 AM. (D): type locality of P. titans sp. nov. in western view taken on 17 May 2022 at 11:10 AM. (E): Hatchling (MUSM 40917) of P. titans sp. nov., taken on 17 May 2022 at 9:46 AM.
A series of nine adult specimens of P. titans sp. nov. herein classified as referred specimens from Chiquintirca, Region Ayacucho is located approximately 85 km southwest of the type locality (Figure 2).
We suggest classifying P. titans sp. n. as “least concern” according to the IUCN Red List criteria [34] due to its relatively large area of distribution that is partially protected in the ONP.
4. Discussion
The taxonomic history of Proctoporus is complex which has been pointed out by various authors (e.g., [15,23]). Lack of synapomorphic characters, along with morphological convergences, has led to incorrect generic classifications that were recognized and corrected based on molecular characters (e.g., [15]). Consequently, Proctoporus spp. enter natural history collections often without identifications (e.g., the referred specimens of Proctoporus titans sp. nov.) or with incorrect identifications despite the publication of Proctoporus species keys [15,20].
Currently, 20 species of Proctoporus are known, of which 18 occur in Peru (this paper, [6]). Eight new species (40%) have been described within the last decade (2012–2022) by a small group of authors (Goicoechea et al. 2013 [15], Mamani et al. 20015 [20], Mamani et al. 2022 [12], Mamani and Rodríguez 2022 [6], this paper), and all new species came from southern Peru (Region Cusco). This is an apparent artifact of fieldwork as we can expect additional new species of Proctoporus from other unexplored montane forest or puna regions in Peru or Bolivia.
The National Service of Natural Protected Areas in Peru (SERNANP = Servicio Nacional de Áreas Naturales Protegidas por el Estado, Peru) preserves 17.53% of its national territory, including 15 national parks ([1]). The ONP remains one of the least investigated regions in Peru. With its location inside the VRAEM, fieldwork is becoming increasingly dangerous due to coca production in remote areas and narco-traffickers who transport cocaine paste through the ONP to remote Andean “airports” from which it is flown out of the country (EL personal observation). Reaching the ONP requires air transportation, and military airplanes are the only means of legal transportation in this area. Unfortunately, the Peruvian military does not currently collaborate with SERNANP as done in the past when costs for transportation were reasonable. Nowadays, tourist agencies can charter military helicopters to transport researchers or tourists. The chartered military helicopter of the past has now been replaced by a costly private helicopter. We urge SERNANP to re-establish collaboration efforts with the Peruvian military to facilitate research that depends on air transportation and to make research costs transparent and affordable. Furthermore, we encourage SERNANP and the military not to tolerate drug activities within protected areas, to the benefit of biodiversity conservation, and the safety of indigenous people of the Asháninka and Mashinguenga.
Author Contributions
Conceptualization, E.L.; Funding acquisition, E.L.; Investigation, E.L., J.C.C., M.I.F., R.J.V. and A.C.; Methodology, E.L., J.C.C., M.I.F., R.J.V. and A.C.; Project administration, E.L.; Writing—original draft, E.L.; Writing—review and editing, E.L., J.C.C., M.I.F., R.J.V. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Illinois Wesleyan University including an ASD grant to E.L.
Institutional Review Board Statement
Ethical review and approval (protocol 19-011) were granted by the IACUC committee of Illinois Wesleyan University.
Data Availability Statement
All DNA sequences used in this study are in GenBank (https://www.ncbi.nlm.nih.gov), accessed on 15 September 2022.
Acknowledgments
We thank C. Aguilar (MUSM, Lima) for cataloging the Proctoporus specimens, for the loan of specimens, and for working in his herpetological collection. We thank the director of the ONP Ing. C.A. Barrientos Allca for granting us research permits and for his support during our expedition. ONP specialist K. Meza Villacorta kindly helped with the research permit proposal. We thank ONP guard M.A. Mayta for his help and companionship during the expeditions. E. Ventocilla kindly helped us to translate English names into Asháninka and M. Sheldon (IWU 70’) helped to phrase the etymology. The fieldwork of EL was supported by a grant from Illinois Wesleyan University. We thank the Ministerio del Ambiente, Lima, Peru for research permits. We thank three reviewers for their helpful feedback that improved our manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
GenBank accession codes for new sequences used in the molecular genetic analysis in this study.
Table A1.
GenBank accession codes for new sequences used in the molecular genetic analysis in this study.
| Species | Voucher | Accession Code (16S) |
|---|---|---|
| Proctoporus titans sp. nov. | MUSM 40915 | OQ148481 |
| Proctoporus titans sp. nov. | MUSM 40916 | OQ148482 |
| Proctoporus titans sp. nov. | MUSM 40922 | OQ148483 |
| Proctoporus titans sp. nov. | MUSM 40924 | OQ148484 |
Appendix B
The following specimens were examined: Euspondylus excelsum: Peru: Region Junín: coffee plantation on the trail leading to the Pui Pui Protected Forest, 1550 m: MUSM 31949; Proctoporus bolivianus: Peru: Region Cusco: Provincia Lares: MUSM 2790, 35583–91, 37857; Proctoporus chasqui: Peru: Region Junín: near road leading to Comas, 3038 m: MUSM 31159; Region Junín: Pui Pui Protected Forest, Hito 3, 1615 m: MUSM 31172; Proctoporus guentheri: Peru: Region Huánuco: Provincia Huánuco: Acomayo: MUSM 13903–4; Region Junín: Provincia Tarma: Palca: 3905–14; Region Junín: Provincia Tarma: Tarma: MUSM 13915–16; Proctoporus laudahnae: Peru: Region Huánuco: Palma Pampa, 3010 m a.s.l.: MUSM 20116 (holotype); Proctoporus spinalis: Peru: Region Junín: near road leading to Comas: MUSM 31162; Region Pasco: Oxapampa: MUSM 17725–28; Proctoporus sucullucu: Peru: Region Cotambambas: MUSM 27769–70, 27789; Region Apurimac: Chinchay: MUSM 27987–89, 2796–98; Proctoporus unsaacae: Peru: Cusco: MUSM 35029–31; Proctoporus xestus: Peru: Region Junín: Provincia Chancamayo: Palomar: MUSM 13881; Wilsonosaura josyi: Peru: Region Junín: Pui Pui Protected Forest: MUSM 31978.
Appendix C

Table A2.
Measurements (in mm) and pholidotic characters of adult type specimens of Proctoporus titans sp. nov. A plus sign indicates the presence of a character, a dash indicates its absence, and a diagonal bar separates counts from the left and right body side. TL condition: b = broken, i = incomplete, reg = regenerated. For other abbreviations see materials and methods.
Table A2.
Measurements (in mm) and pholidotic characters of adult type specimens of Proctoporus titans sp. nov. A plus sign indicates the presence of a character, a dash indicates its absence, and a diagonal bar separates counts from the left and right body side. TL condition: b = broken, i = incomplete, reg = regenerated. For other abbreviations see materials and methods.
| Characters | MUSM 40916 | MUSM 40921 | MUSM 40915 | MUSM 40922 | MUSM 40924 |
|---|---|---|---|---|---|
| Sex | m | m | f | f | f |
| SVL | 53.9 | 41.3 | 46.0 | 44.6 | 48.4 |
| TL | 56.8 r | 35.6 r | 60.8 | 63.8 | 48.8 b, r |
| HL | 10.7/10.9 | 8.9/8.9 | 9.0/9.6 | 8.8/9.6 | 9.2/8.4 |
| HW | 8.2 | 5.4 | 5.1 | 4.9 | 5.7 |
| HD | 5.7 | 4.7 | 3.9 | 3.8 | 4.4 |
| E-N | 3.8/3.6 | 2.8/2.8 | 2.9/2.7 | 3.1/2.9 | 2.9/2.9 |
| FLL | –/10.4 | 8.2/6.9 | 8.0/6.6 | 9.7/– | 9.8/– |
| HLL | 14.1/13.7 | 11.7/12.1 | –/12.9 | 11.3/10.8 | 13.9/10.6 |
| AGD | 28.0/26.7 | 21.7/21.5 | 21.5/22.4 | 23.5/23.2 | 26.9/25.2 |
| Supralabials | 5/5 | 6/6 | 6/6 | 6/6 | 6/6 |
| Infralabials | 5/5 | 6/6 | 5/5 | 5/5 | 5/6 |
| Anterior infralabials | 4/3 | 5/3 | 4/4 | 4/4 | 4/4 |
| Nasal scales | 1/1 inc | 1/1 inc | 1/1 und | 1/1 und | 1/1 inc |
| Pairs of genials in contact | 1 | 1 | 2 | 2 | 1 |
| Gular scale rows | 2 | 2 | 2 | 3 | 2 |
| Collar scale rows | 7 | 6 | 6 | 7 | 7 |
| Dorsal scales | 34 | 33 | 35 | 36 | 36 |
| Longitudinal dorsal scale rows | 21 | 20 | 21 | 20 | 21 |
| Lateral scales | 3/2 | 3/3 | 3/3 | 2/3 | 2/2 |
| Scales around midbody | 35 | 34 | 35 | 34 | 35 |
| Ventral scales | 21 | 21 | 21 | 20 | 23 |
| Longitudinal ventral scale rows | 10 | 10 | 10 | 10 | 10 |
| Anterior preanal plate scales | 2 | 2 | 2 | 2 | 2 |
| Posterior cloacal plate scales | 4 | 4 | 4 | 6 | 5 |
| Lamellae under Finger IV | –/13 | 11/10 | 12/12 | 10/– | 10/– |
| Lamellae under Toe IV | 19/19 | 17/17 | –/17 | 15/15 | 17/17 |
| Femoral pores | 5/5 | 6/6 | – | – | – |
Appendix D

Table A3.
Measurements (in mm) and pholidotic characters of referred specimens of adult Proctoporus titans sp. nov. A plus sign indicates the presence of a character, a dash indicates its absence, and a diagonal bar separates counts from the left and right body side. TL condition: b = broken, i = incomplete, reg = regenerated. For other abbreviations see materials and methods.
Table A3.
Measurements (in mm) and pholidotic characters of referred specimens of adult Proctoporus titans sp. nov. A plus sign indicates the presence of a character, a dash indicates its absence, and a diagonal bar separates counts from the left and right body side. TL condition: b = broken, i = incomplete, reg = regenerated. For other abbreviations see materials and methods.
| Characters | MUSM 35638 | MUSM 37754 | MUSM 37755 | MUSM 37757 | MUSM 35637 | MUSM 35639 | MUSM 37756 | MUSM 37758 | MUSM 37789 |
|---|---|---|---|---|---|---|---|---|---|
| Sex | m | m | m | m | f | f | f | f | f |
| SVL | 43.2 | 44.7 | 46.3 | 51.0 | 47.5 | 50.1 | 52.2 | 43.6 | 52.6 |
| TL | 62.2 | 55.2 r | 44.5 r | 65.3 r | 36.5 r, b | 61.4 | 36.6 r | 45.4 r | 52.2 i |
| HL | 11.5/11.7 | 11.0/9.9 | 12.0/12.4 | 12.1/12.7 | 9.8/10.7 | 10.4/11.0 | 10.5/11.3 | 10.9/10.0 | 10.8/11.0 |
| HW | 6.4 | 6.5 | 7.6 | 7.4 | 6.8 | 6.3 | 7.0 | 6.0 | 5.8 |
| HD | 4.7 | 5.4 | 4.9 | 5.3 | 4.4 | 4.9 | 5.0 | 4.5 | 4.7 |
| E-N | 3.3/3.8 | 3.5/3.4 | 3.8/3.5 | 4.0/4.0 | 3.4/3.2 | 3.8/3.7 | 3.2/3.9 | 3.4/3.4 | 3.3/3.8 |
| FLL | 12.4/10.0 | 9.6/11.8 | 11.0/12.7 | 11.3/10.6 | 11.6/10.3 | 10.5/9.9 | 11.2/10.2 | 7.7/8.7 | 11.9/11.6 |
| HLL | 14.0/14.5 | 14.3/16.1 | 17.7/13.4 | 13.6/16.7 | 16.0/15.2 | 14.0/12.9 | 15.6/13.9 | 12.6/15.1 | 16.1/15.1 |
| AGD | 23.6/24.1 | 22.0/24.1 | 23.0/21.6 | 23.3/26.3 | 25.4/21.9 | 25.8/27.9 | 29.5/27.8 | 23.4/22.3 | 28.5/28.4 |
| Supralabials | 7/7 | 7/7 | 5/5 | 7/7 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 |
| Infralabials | 4/4 | 4/4 | 6/6 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 |
| Anterior infralabials | 3/3 | 3/3 | 3/3 | 3/3 | 4/3 | 3/3 | 3/3 | 3/3 | 4/4 |
| Nasal scales | 1/1 inc | 1/1 inc | 1/1 inc | 1/1 inc | 1/1 div | 1/1 inc | 1/1 inc | 1/1 div | 1/1 div |
| Pairs of genials in contact | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 3 |
| Gular scale rows | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 2 |
| Collar scale rows | 6 | 7 | 7 | 7 | 7 | 7 | 7 | 6 | 7 |
| Dorsal scales | 38 | 36 | 38 | 37 | 35 | 37 | 39 | 39 | 41 |
| Longitudinal dorsal scale rows | 22 | 22 | 24 | 25 | 22 | 20 | 21 | 22 | 23 |
| Lateral scales | 2/2 | 3/2 | 2/2 | 2/2 | 3/2 | 3/3 | 2/2 | 2/2 | 4/3 |
| Scales around midbody | 38 | 39 | 38 | 40 | 38 | 35 | 37 | 35 | 39 |
| Ventral scales | 19 | 21 | 20 | 20 | 20 | 21 | 20 | 21 | 22 |
| Longitudinal ventral scale rows | 12 | 12 | 10 | 12 | 11 | 10 | 10 | 10 | 10 |
| Anterior preanal plate scales | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Posterior cloacal plate scales | 4 | 5 | 3 | 2 | 6 | 5 | 6 | 5 | 4 |
| Lamellae under Finger IV | 12/13 | 12/12 | 13/12 | 13/13 | 13/13 | 13/13 | 12/12 | 13/13 | 13/14 |
| Lamellae under Toe IV | 18/18 | 18/18 | 18/19 | 19/18 | 18/19 | 18/19 | 18/18 | 19/19 | 20/19 |
| Femoral pores | 6/6 | 5/6 | 7/7 | 7/7 | – | – | – | – | – |
References
- SERNANP. Guía Oficial De Áreas Naturales Protegidas Del Perú; Ministerio del Ambiente: Lima, Peru, 2010; pp. 1–208.
- Baekeland, G.B. By parachute into Peru’s lost world. Natl. Geogr. 1964, 126, 268–296. [Google Scholar]
- Alonso, L.E.; Alonso, A.; Schulenberg, T.S.; Dallmeier, F. (Eds.) Biological and Social Assessments of the Cordillera de Vilcabamba, Peru. RAP Working Papers 12 and SI/MAB Series 6; Conservation International: Washington, DC, USA, 2001; pp. 1–296. [Google Scholar]
- Lehr, E. New eleutherodactyline frogs (Leptodactylidae: Pristimantis, Phrynopus) from Peru. Bull. Mus. comp. Zool. 2007, 159, 145–178. [Google Scholar] [CrossRef]
- Mendoza, W. Especie nueva de Polylepis (Rosaceae) de la cordillera Vilcabamba (Cusco, Perú). Rev. Peru. Biol. 2005, 12, 103–106. [Google Scholar] [CrossRef][Green Version]
- Mamani, L.; Rodríguez, L. A new species of Andean lizard, Proctoporus (Gymnophthalmidae: Cercosaurinae), from the highland of Parque Nacional Otishi in Peru. Zootaxa 2022, 5213, 75–85. [Google Scholar] [CrossRef]
- INRENA. Parque Nacional Otishi. Plan Maestro 2005–2010; Instituto Nacional de Recursos Naturales—INRENA: Lima, Peru, 2003; pp. 1–118.
- Rodríguez, L.O.; Young, K.R. Biological diversity of Peru: Determining priority areas for conservation. AMBIO A J. Hum. Environ. 2000, 29, 329–337. [Google Scholar] [CrossRef]
- Fajardo, J.; Lessmann, J.; Bonaccorso, E.; Devenish, C.; Muñoz, J. Combined use of systematic conservation planning, species distribution modeling, and connectivity analysis reveals severe conservation gaps in a megadiverse country (Peru). PLoS ONE 2014, 9, e114367. [Google Scholar] [CrossRef]
- Barboza Sánchez, C.A.N. Composición y Distribución Altitudinal de Los Anfibios del Parque Nacional Otishi, Cordillera Central del Perú. Tesis Para Obtar el Titulo de Biologo; Universidad Nacional de Trujillo: Trujillo, Peru, 2014; pp. 1–75. [Google Scholar]
- von Tschudi, J.J. Reptilium conspectum quae in republica Peruana reperiuntur et pleraque observata vel collecta sunt in itenere. Arch. Für Nat. 1845, 11, 150–170. [Google Scholar]
- Mamani, L.; Cruz, R.; Mallqui, S.; Catenazzi, A. Molecular phylogenetics and comparative examination of voucher museums reveal two new species of gymnophthalmid lizards (Squamata, Gymnophthalmidae) from the Peruvian Andes, with comments on Proctoporus guentheri (Boettger, 1891). Diversity 2022, 14, 215. [Google Scholar] [CrossRef]
- Uetz, P.; Freed, P.; Aguilar, R.; Hošek, J. (Eds.) The Reptile Database. Available online: http://www.reptile-database.org (accessed on 11 December 2022).
- Werner, F. Über neue oder seltene Reptilien des Naturhistorischen Museums in Hamburg. II. Eidechsen. Jahrb. Hamburg. Wiss. Anst. 1910, 27 (Suppl. S2), 1–46. [Google Scholar]
- Goicoechea, N.; Padial, J.M.; Chaparro, J.C.; Castroviejo-Fisher, S.; De la Riva, I. A taxonomic revision of Proctoporus bolivianus Werner (Squamata: Gymnophthalmidae) with the description of three new species and resurrection of Proctoporus lacertus Stejneger. Am. Mus. Nov. 2013, 3786, 1–32. [Google Scholar] [CrossRef][Green Version]
- Chávez, G.; Siu-Ting, K.; Duran, V.; Venegas, P.J. Two new species of Andean gymnophthalmid lizards of the genus Euspondylus (Reptilia, Squamata) from central and southern Peru. ZooKeys 2011, 109, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Boettger, O. Reptilien und Batrachier aus Bolivien. Zool. Anz. 1891, 14, 343–347. [Google Scholar]
- Stejneger, L. Results of the Yale Peruvian Expedition of 1911. Batrachians and reptiles. Proc. United States Natl. Mus. 1913, 45, 541–547. [Google Scholar] [CrossRef][Green Version]
- Köhler, G.; Lehr, E. Comments on Euspondylus and Proctoporus (Squamata: Gymnophthalmidae) from Peru, with the description of three new species and a key to the Peruvian species. Herpetologica 2004, 60, 501–518. [Google Scholar] [CrossRef]
- Mamani, L.; Goicoechea, N.; Chaparro, J.C. A new species of Andean lizard Proctoporus (Squamata: Gymnophthalmidae) from montane forest of the Historic Sanctuary of Machu Picchu, Peru. Amphib. Reptile Conserv. 2015, 9, e96. [Google Scholar]
- De Grijs, P. Prionodactylus rahmi, eine neue Eidechse aus den Anden. Zool. Anz. 1936, 116, 27–30. [Google Scholar]
- Boulenger, G.A. Descriptions of new reptiles from the Andes of South America, preserved in the British Museum. Ann. Mag. Nat. Hist. 1911, 7, 19–25. [Google Scholar] [CrossRef][Green Version]
- Doan, T.M.; Castoe, T.A. Using morphological and molecular evidence to infer species boundaries within Proctoporus bolivianus Werner (Squamata: Gymnopthalmidae). Herpetologica 2003, 59, 432–449. [Google Scholar] [CrossRef]
- Uzzell, T. A new genus and species of teiid lizard from Bolivia. Postilla 1969, 129, 1–15. [Google Scholar] [CrossRef]
- Goicoechea, N.; Padial, J.M.; Chaparro, J.C.; Castroviejo-Fisher, S.; De la Riva, I. Data from: Molecular phylogenetics, species diversity, and biogeography of the Andean lizards Proctoporus (Squamata: Gymnophthalmidae). Dryad Dataset 2013. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Oftedal, O.T. A revision of the genus Anadia (Sauria, Teiidae). Arq. Zool. 1974, 25, 203–265. [Google Scholar] [CrossRef]
- Chávez, G.; Catenazzi, A.; Venegas, P.J. A new species of arboreal microteiid lizard of the genus Euspondylus (Gymnophthalmidae: Cercosaurinae) from the Andean slopes of central Peru with comments on Peruvian Euspondylus. Zootaxa 2017, 4350, 301–316. [Google Scholar] [CrossRef]
- Sánchez-Pacheco, S.J.; Nunes, P.M.S.; Marques-Souza, S.; Rodrigues, M.T.; Murphy, R.W. Formal recognition of the species of Oreosaurus (Reptilia, Squamata, Gymnophthalmidae) from the Sierra Nevada de Santa Marta, Colombia. ZooKeys 2017, 691, 149–162. [Google Scholar] [CrossRef]
- Sánchez-Pacheco, S.J.; Torres-Carvajal, O.; Aguirre-Peñafiel, V.; Nunes, P.M.; Verrastro, L.; Rivas, G.A.; Rodrigues, M.T.; Grant, T.; Murphy, R.W. Phylogeny of Riama (Squamata: Gymnophthalmidae), impact of phenotypic evidence on molecular datasets, and the origin of the Sierra Nevada de Santa Marta endemic fauna. Cladistics 2017, 34, 260–291. [Google Scholar] [CrossRef]
- Moravec, J.; Šmíd, J.; Štundl, J.; Lehr, E. Systematics of Neotropical microteiid lizards (Gymnophthalmidae, Cercosaurinae), with the description of a new genus and species from the Andean montane forests. ZooKeys 2018, 774, 105–139. [Google Scholar] [CrossRef]
- IUCN. IUCN Standards and Petitions Subcommittee. Guidelines for Using the IUCN Red List Categories and Criteria. Version 10.1. Prepared by the Standards and Petitions Subcommittee. 2013. Available online: http://www.iucnredlist.org/documents/RedListGuidelines.pdf (accessed on 15 September 2022).
- Castoe, T.A.; Doan, T.M.; Parkinson, C.L. Data partitions and complex models in Bayesian analyses: The phylogeny of gymnophthalmid lizards. Syst. Biol. 2004, 53, 448–469. [Google Scholar] [CrossRef]
- Fitzinger, L. Neue Classification der Reptilien Nach Ihren Natürlichen Verwandtschaften Nebst Einer Verwandschafts-Tafel Und Einem Verzeichnisse der Reptilien-Sammlung des K. K. Zoologischen Museums zu Wien; Im Verlage von J.G. Heubner: Wien, Austria, 1826; pp. 1–66. [Google Scholar]
- Gray, J.E. Catalogue of the slender-tongued saurians, with descriptions of many new genera and species. Part 1. Ann. Mag. Nat. Hist. London 1838, 1, 274–283. [Google Scholar] [CrossRef]
- Chávez, G.; Chávez-Arribasplata, J.C. Distribution and natural history notes on the Peruvian lizard Proctoporus laudahnae (Squamata: Gymnophthalmidae). Phyllomedusa 2016, 15, 147–154. [Google Scholar] [CrossRef]
- Holdridge, L.R. Life Zone Ecology; Tropical Science Center: San Jose, Costa Rica, 1967; pp. 1–206. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).