Disentangling the Taxonomic History of the Widespread and Overlooked Centric Diatom Stephanodiscus makarovae and Its Transfer to Cyclostephanos
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling, Cultivation and Electron Microscopy
2.2. DNA Extraction, Purification, Amplification
3. Results
3.1. Molecular Phylogeny
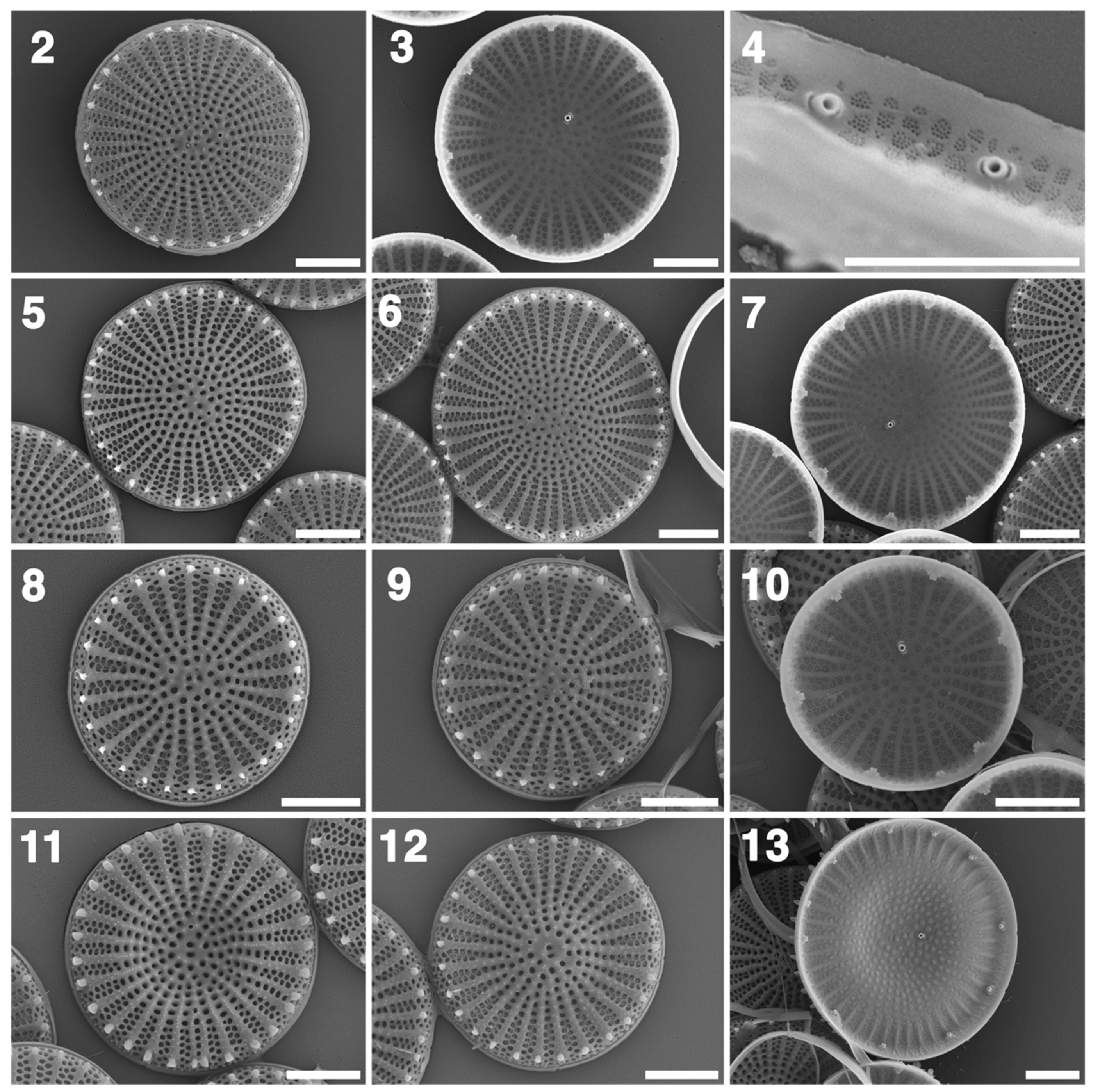
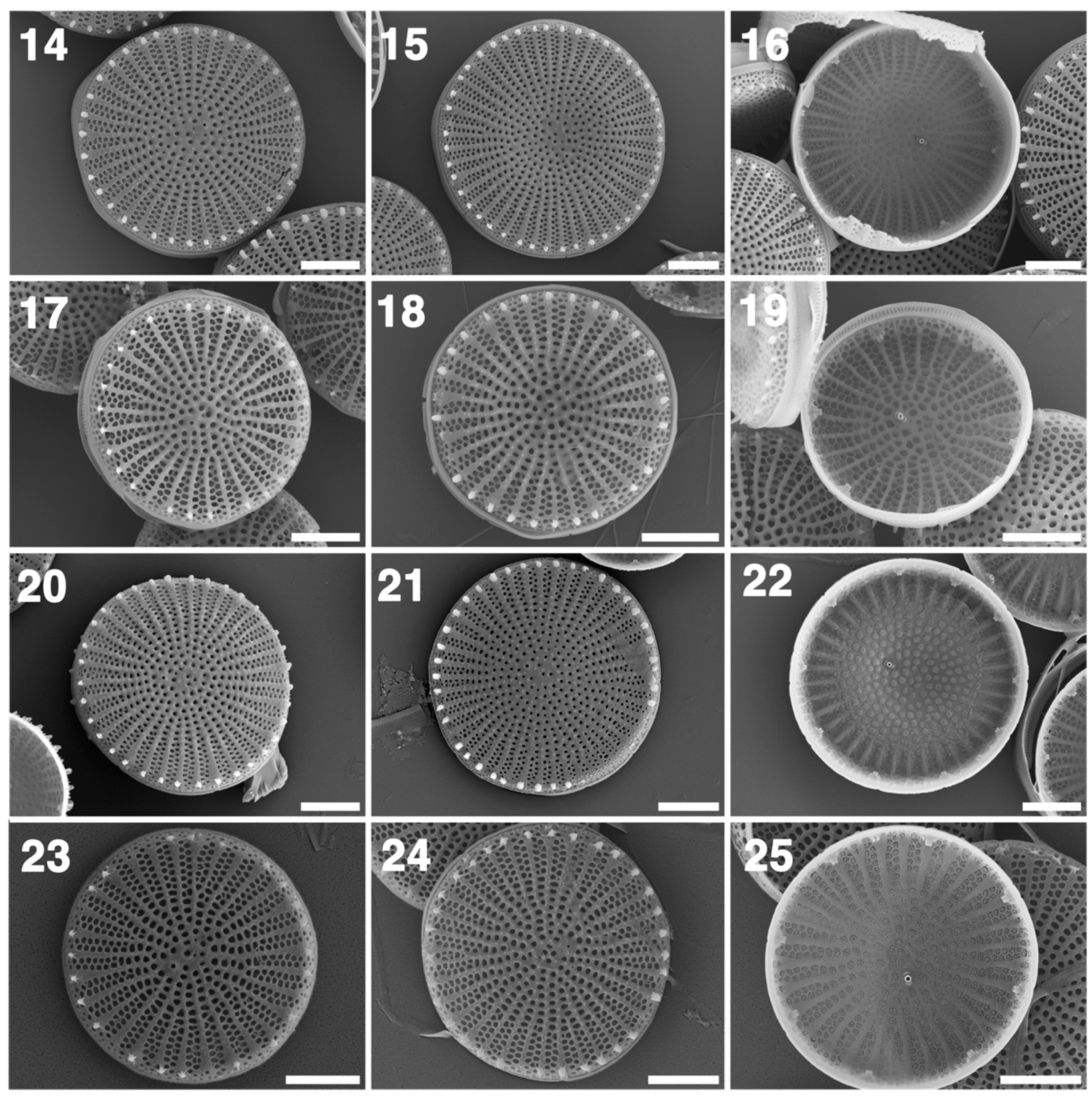
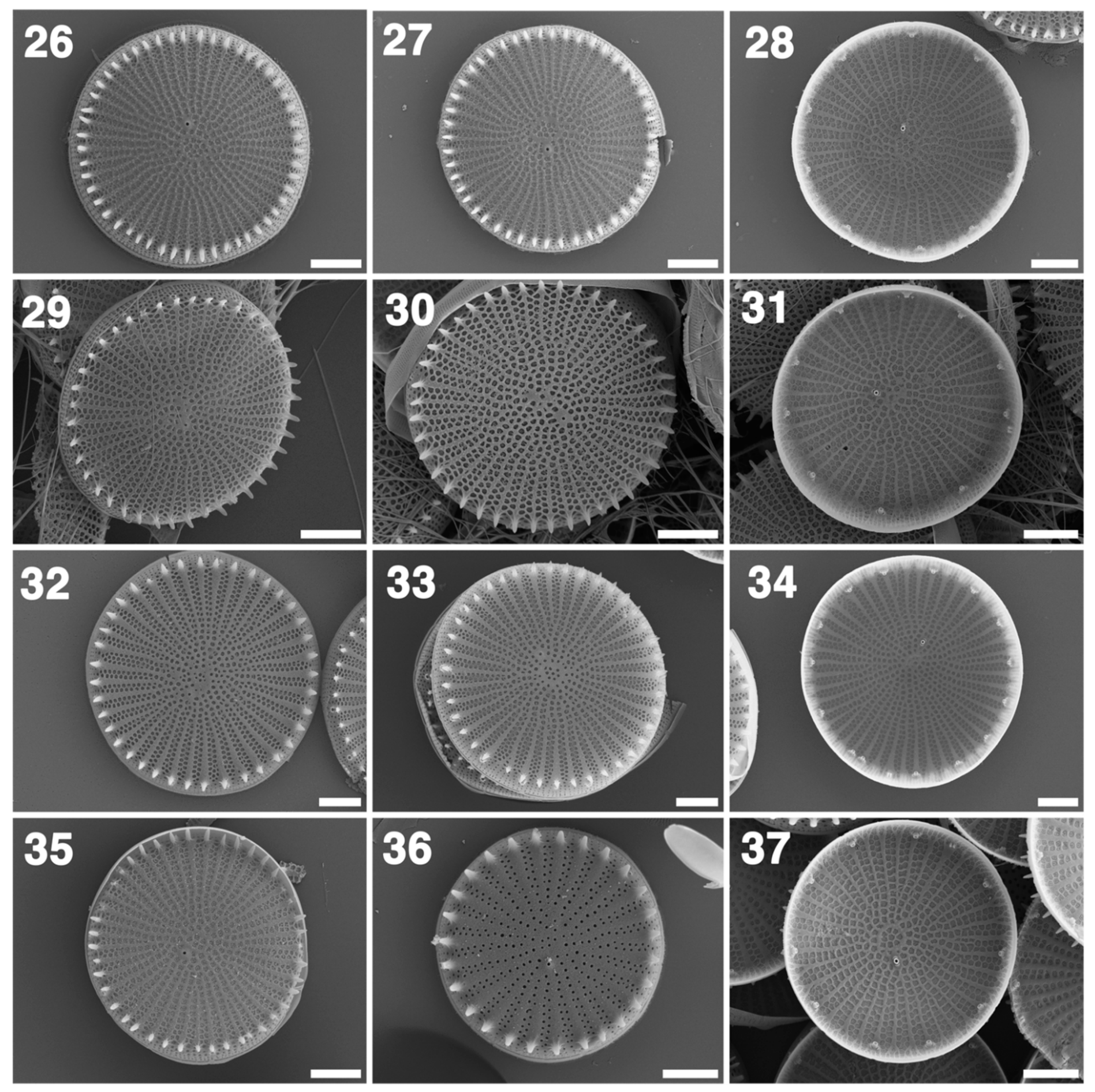
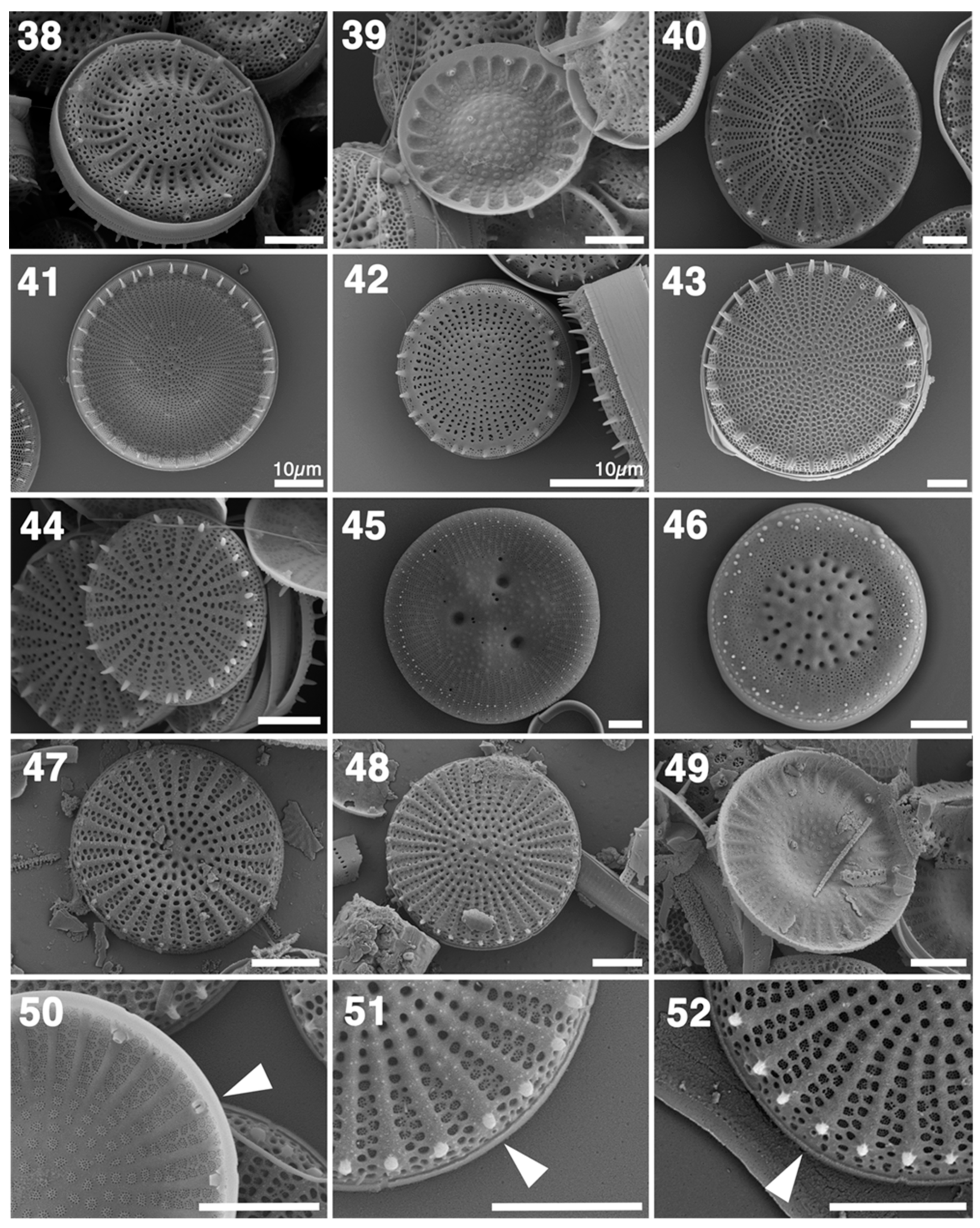
3.2. LM and SEM
4. Discussion
4.1. The Transfer of S. makarovae into Cyclostephanos
4.2. Reprioritizing the Name of C. delicatus
4.3. Comparative Morphology
4.4. Environmental Distribution
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ehrenberg, C.G. Neue Untersuchungen Uber Das Kleinste Leben Als Geologisches Moment. Mit Kurzer Charakteristik von 10 Neuen Genera Und 66 Neuen Arten. Ber. Uber Die Zur Bekanntm. Geeigneten Verh. Der K.-Preuss. Akad. Der Wiss. Zu Berl. 1846, 1845, 53–88. [Google Scholar]
- Boyer, C.S. Supplement: Synopsis of North American Diatomaceae. Part II. Naviculatae, Surirellatae. Proc. Acad. Nat. Sci. Phila. 1927, 79, 228–583. [Google Scholar]
- Round, F.E. Cyclostephanos—A New Genus within the Sceletonemaceae. Arch. Für Protistenkd. 1982, 125, 323–329. [Google Scholar] [CrossRef]
- Theriot, E.; Håkansson, H.; Kociolek, J.P.; Round, F.E.; Stoermer, E.F. Validation of the Centric Diatom Genus Name Cyclostephanos. Br. Phycol. J. 1987, 22, 345–347. [Google Scholar] [CrossRef] [Green Version]
- Theriot, E.; Stoermer, E.; Håkansson, H. Taxonomic interpretation of the rimoportula of freshwater genera in the centric diatom family Thalassiosiraceae. Diatom Res. 1987, 2, 251–265. [Google Scholar] [CrossRef]
- Casper, S.J.; Scheffler, W. Cyclostephanos delicatus (Genkal) Casper et Scheffler Comb. Nov. from Waters in the Northern Part of Germany. Arch. Für Protistenkd. 1990, 138, 304–312. [Google Scholar] [CrossRef]
- Håkansson, H.; Kling, H. The current status of some very small freshwater diatoms of the genera Stephanodiscus and Cyclostephanos. Diatom Res. 1990, 5, 273–287. [Google Scholar] [CrossRef]
- Dreßler, M.H.; Hübener, T. Morphology and Ecology of Cyclostephanos delicatus (Genkal) Casper Scheffler (Bacillariophyceae) in Comparison with C. tholiformis Stoermer, Hakansson Theriot. Nova Hedwig. 2006, 82, 409–434. [Google Scholar] [CrossRef]
- Genkal, S.I. New Taxa of the Genus Stephanodiscus Ehr. (Bacillariophyta). Nov. Sist. Nizshykh Rasteniy 1978, 15, 11–14. [Google Scholar]
- Genkal, S.I. Method of Calculation of Some Taxonomically Important Structural Elements of the Diatom Valve from Family Thalassiosiraceae Lebour Emend Hasle (Bacillariophyta). Bot. Zhurnal 1977, 62, 848–851. [Google Scholar]
- Theriot, E.; Serieyssol, K. Phylogenetic Systematics as a Guide to Understanding Features and Potential Morphological Characters of the Centric Diatom Family Thalassiosiraceae. Diatom Res. 1994, 9, 429–450. [Google Scholar] [CrossRef]
- Aljanabi, S.M.; Martinez, I. Universal and Rapid Salt-Extraction of High Quality Genomic DNA for PCR-Based Techniques. Nucleic Acids Res. 1997, 25, 4692–4693. [Google Scholar] [CrossRef] [PubMed]
- Hamsher, S.E.; Evans, K.M.; Mann, D.G.; Poulíčková, A.; Saunders, G.W. Barcoding Diatoms: Exploring Alternatives to COI-5P. Protist 2011, 162, 405–422. [Google Scholar] [CrossRef] [PubMed]
- Wawrik, B.; Paul, J.H.; Tabita, F.R. Real-Time PCR Quantification of RbcL (Ribulose-1, 5-Bisphosphate Carboxylase/Oxygenase) MRNA in Diatoms and Pelagophytes. Appl. Environ. Microbiol. 2002, 68, 3771–3779. [Google Scholar] [CrossRef] [Green Version]
- Iwatani, N.; Murakami, S.; Suzuki, Y. A Sequencing Protocol of Some DNA Regions in Nuclear, Chloroplastic and Mitochondrial Genomes with an Individual Colony of Thalassiosira nordenskioeldii Cleve (Bacillariophyceae). Polar Biosci. 2005, 18, 35–45. [Google Scholar]
- Kistenich, S.; Dreßler, M.; Zimmermann, J.; Hübener, T.; Bastrop, R.; Jahn, R. An Investigation into the Morphology and Genetics of Cyclotella comensis and Closely Related Taxa. Diatom Res. 2014, 29, 423–440. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Genkal, S.I. Morphology, Taxonomy, Ecology and Distribution of Small-Sized Stephanodiscus Species (Bacillariophyta). 2. Stephanodiscus Makarovae. Bot. Zhurnal 2007, 92, 241–248. [Google Scholar]
- Houk, V.; Klee, R.; Tanaka, H. Atlas of Freshwater Centric Diatoms with a Brief Key and Descriptions: Stephanodiscus, Cyclostephanos, Pliocaenicus, Hemistephanos, Stephanocostis, Mesodictyon & Spicaticribra. Stephanodiscaceae B; Czech Phycological Society: Prague, Czech Republic, 2014. [Google Scholar]
- Genkal, S. Morphological Variability and Taxonomy of Diatoma Tenue Ag. (Bacillariophyta). Int. J. Algae 2004, 6, 319–330. [Google Scholar] [CrossRef]
- Genkal, S.; Makarova, V. Diatomvye Vodorosli, Novye Dlya Planktona Kapiiiskogo i Azoskogo Morei (Bacillariophyta Planctonica Nova e Maribus Caspio et Maeotico). Nov. Sist. Nizhi. Rastenii 1985, 22, 35–37. [Google Scholar]
- Stoermer, E.F.; Håkansson, H.; Theriot, E.C. Cyclostephanos Species Newly Reported from North America: C. tholiformis Sp. Nov. and C. Costatilimbus Comb. Nov. Br. Phycol. J. 1987, 22, 349–358. [Google Scholar] [CrossRef] [Green Version]

| Taxon | Strain | Locality | Coordinates | Collector | Isolation | D2D3 LSU | rbcL | cox1 |
|---|---|---|---|---|---|---|---|---|
| Cyclostephanos makarovae | MIC7 | Lake Mickowsee, Germany | 53.70459°, 11.62498° | K. Schultz | K. Schultz | OL436661 | OL493032 | OL628836 |
| QC2 | Lake Lac Saint-Augustin, Canada | 46.75047°–71.39313° | O. Jacques | K. Schultz | OL436662 | OL493031 | OL628834 | |
| SN29 | Lake Schweriner See, Germany | 53.63405°, 11.46698° | S. Schultz | K. Schultz | OL436665 | OL493033 | OL628837 | |
| US5 | Lake Untersee, Germany | 52.94595°, 12.44498° | M. Dreßler | K. Schultz | OL436664 | OL493034 | OL628835 | |
| STB1 | Lake Sternberger See, Germany | 53.71738°, 11.84062° | K. Schultz | K. Schultz | OL436668 | OL493037 | OL628832 | |
| M13 | Lake Mälaren, Sweden | 59.4325°, 17.70446° | S. Gottschalk | M. Dreßler | OL436666 | OL493036 | OL628833 | |
| RIE1 | Lake Scharmützelsee, Germany | 52.21957°, 14.02552° | M. Knie | T. Hübener | OL436663 | OL493038 | OL628831 | |
| W36 | River Warnow, Germany | 54.04522°, 12.16332° | T. Hübener | T. Hübener | OL436667 | OL493035 | OL628838 | |
| Cyclostephanos invisitatus | W13 | River Warnow, Germany | 54.04522°, 12.16332° | T. Hübener | T. Hübener | OL436670 | OL493042 | OL628841 |
| M10 | Lake Mälaren, Sweden | 59.4325°, 17.70446° | S. Gottschalk | M. Dreßler | OL436671 | OL493041 | OL628840 | |
| GC4 | River Guadalquivir, Spain | 37.86848°, 4.78506° | S. Haupt | K. Schultz | OL436672 | OL493040 | OL628842 | |
| SN34 | Lake Schweriner See, Germany | 53.63405°, 11.46698° | S. Schultz | K. Schultz | OL436669 | OL493039 | OL628839 | |
| Cyclostephanos dubius | HZ1 | Lake Herzsee, Austria | 47.24854°, 11.45539° | E. Rott | M. Dreßler | OL436674 | OL493045 | OL628843 |
| DOL38 | Lake Dolgener See, Germany | 53.95012°, 12.24597° | T. Hübener | T. Hübener | OL436673 | OL493044 | OL628844 | |
| Cyclostephanos delicatus | QN14 | St. Lawrence River, Canada | 46.75062°–71.26741° | O. Jacques | K. Schultz | OL436675 | OL493043 | OL628845 |
| Stephanodiscus niagarae | LD1 | Lake Lac Daviault, Canada | 52.81044°–67.07239° | O. Jacques | K. Schultz | OL436676 | OL493051 | OL628851 |
| Stephanodiscus neoastraea | DOL10 | Lake Dolgener See, Germany | 53.95012°, 12.24597° | T. Hübener | T. Hübener | OL436679 | OL493046 | OL628848 |
| Stephanodiscus hantzschii | CAS3 | Lake Cambser See, Germany | 53.68989°, 11.53306° | M. Dreßler | M. Dreßler | OL436677 | OL493049 | OL628846 |
| TO6 | Lake Lago di Toblino, Italy | 46.05274°, 10.96444° | K. Fink | M. Dreßler | OL436678 | OL493050 | OL628847 | |
| Stephanodiscus binatus | QC3 | Lake Lac Saint-Augustin, Canada | 46.75047°–71.39313° | O. Jacques | K. Schultz | OL436681 | OL493048 | OL628849 |
| S4 | Lake Stechlinsee, Germany | 53.15284°, 13.02772° | L. Krienitz | T. Hübener | OL436680 | OL493047 | OL628850 | |
| Pantocsekiella ocellata | RL1 | Ross Lake, Ireland | 53.37257°–9.21186° | U. Nitzschke | T. Hübener | OL436682 | OL493052 | OL628852 |
| Lindavia sp. | DR1 | Lago di Landro, Italy | 46.63116°, 12.23037° | K. Fink | K. Schultz | OL436683 | OL493053 | OL628853 |
| Taxon | N | Strain | Und. | Arae/Fas | D | S/D | N MFP | N CFP | MFP/D |
|---|---|---|---|---|---|---|---|---|---|
| C. makarovae | 22 | MIC7 | 1–2 | 2 | 6.7–8.3 | 14.1–18.0 | 7–10 | 1 (–2) | 3.1–4.3 |
| 21 | QC2 | 1–3 | 2 (–3) | 6.0–8.6 | 14.3–18.0 | 5–8 | 1 | 2.5–3.8 | |
| 25 | SN29 | 1–3 | 2 (–3) | 4.9–7.0 | 12.9–16.6 | 5–7 | 1 | 2.7–3.7 | |
| 21 | US5 | 1–3 | 2 (–3) | 6.1–9.3 | 12.7–17.3 | 6–10 | 1 | 2.6–3.7 | |
| 22 | STB1 | 1–3 | 2 | 7.8–10.4 | 13.5–16.2 | 7–9 | 1 | 2.5–3.3 | |
| 26 | M13 | 1–3 | 2 (–3) | 5.8–7.6 | 12.4–16.7 | 5–8 | 1 | 2.5–4.0 | |
| 21 | RIE1 | 1–3 | 2 (–3) | 6.4–9.2 | 12.2–16.2 | 6–11 | 1 (–2) | 2.8–4.8 | |
| 24 | W36 | 1–2 | 2 (–3) | 5.8–7.4 | 13.0–17.1 | 6–9 | 1 | 3.0–4.4 | |
| 182 | all | 1–3 | 2 (–3) | 4.9–10.4 | 12.2–18.0 | 5–11 | 1 (–2) | 2.5–4.8 | |
| C. makarovae [18] | 447 | 2–3 | 3–10 | 14–25 | |||||
| C. invisitatus | 28 | W13 | 1 | 2 (–3) | 9.5–10.0 | 14.3–16.9 | 8–11 | 1 | 2.9–3.7 |
| 36 | M10 | 1 | 2–3 | 8.9–9.7 | 12.5–16.1 | 8–11 | 1 (–2) | 2.6–3.6 | |
| 20 | GC4 | 1 | 2–3 | 11.3–12.2 | 10.6–15.0 | 12–16 | 1 (–2) | 3.2–4.4 | |
| 20 | SN34 | 1 | 2 (–3) | 8.4–9.8 | 10.2–15.5 | 7–11 | 1 | 2.7–3.8 | |
| 104 | all | 1 | 2–3 | 8.4–12.2 | 10.2–16.9 | 7–16 | 1 (–2) | 2.6–4.4 | |
| C. invisitatus [19] | 1 | 2 | 6–18 | 9–19 | 1 | ||||
| C. delicatus [7] | 2–3 | 2–4 | 6–14 | 8–20 | 1 | ||||
| C. delicatus [19] | 2–3 | (2–) 3–4 | 5–15 | 1 (–2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schultz, K.; Hübener, T.; Dreßler, M.; Jacques, O.; Frank, M.; Springer, A.; Van, A.T. Disentangling the Taxonomic History of the Widespread and Overlooked Centric Diatom Stephanodiscus makarovae and Its Transfer to Cyclostephanos. Taxonomy 2021, 1, 425-437. https://doi.org/10.3390/taxonomy1040030
Schultz K, Hübener T, Dreßler M, Jacques O, Frank M, Springer A, Van AT. Disentangling the Taxonomic History of the Widespread and Overlooked Centric Diatom Stephanodiscus makarovae and Its Transfer to Cyclostephanos. Taxonomy. 2021; 1(4):425-437. https://doi.org/10.3390/taxonomy1040030
Chicago/Turabian StyleSchultz, Konrad, Thomas Hübener, Mirko Dreßler, Olivier Jacques, Marcus Frank, Armin Springer, and Anh Tu Van. 2021. "Disentangling the Taxonomic History of the Widespread and Overlooked Centric Diatom Stephanodiscus makarovae and Its Transfer to Cyclostephanos" Taxonomy 1, no. 4: 425-437. https://doi.org/10.3390/taxonomy1040030
APA StyleSchultz, K., Hübener, T., Dreßler, M., Jacques, O., Frank, M., Springer, A., & Van, A. T. (2021). Disentangling the Taxonomic History of the Widespread and Overlooked Centric Diatom Stephanodiscus makarovae and Its Transfer to Cyclostephanos. Taxonomy, 1(4), 425-437. https://doi.org/10.3390/taxonomy1040030






