Low-Temperature Synthesis and Photoluminescence Properties of Mg2TiO4:Mn4+ Phosphor Prepared by Solid-State Reaction Methods Assisted by LiCl Flux
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Property Characterization
2.3. Device Fabrication and Performance Measurements
3. Results and Discussion
3.1. Structural and Morphological Properties
3.2. Optical Properties
3.3. Device Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, D.; Zhou, Y.; Zhong, J. A review on Mn4+ activators in solids for warm white light-emitting diodes. RSC Adv. 2016, 6, 86285–86296. [Google Scholar] [CrossRef]
- Fan, N.; Du, Q.; Guo, R.; Luo, L.; Wang, L. Sol-Gel Synthesis and Photoluminescence Properties of a Far-Red Emitting Phosphor BaLaMgTaO6:Mn4+ for Plant Growth LEDs. Materials 2023, 16, 4029. [Google Scholar] [CrossRef] [PubMed]
- Borkovska, L.; Kozoriz, K.; Gudymenko, O.; Vorona, I.; Marchylo, O.; Kryshtab, T. Comparative study of Mn4+ activated magnesium titanate red phosphors synthesized by sol–gel and solid-state reaction methods. Emergent Mater. 2025, 8, 1267–1278. [Google Scholar] [CrossRef]
- He, S.A.; Xu, F.F.; Wu, D.; Wang, Z.W.; Peng, J.Q.; Ye, X.Y. Effects of introducing cations on the luminescence performance of CsPF6:Mn4+ phosphor. Nonferr. Met. Sci. Eng. 2020, 11, 111–117. [Google Scholar]
- Han, M.; Tang, H.; Liu, L.; Wang, Y.; Zhang, X.; Lv, L.J. Tuning the Mn4+ coordination environment in Mg2TiO4 through a codoping strategy for enhancing luminescence performance. J. Phys. Chem. C. 2021, 125, 15687–15695. [Google Scholar] [CrossRef]
- Li, C.; Fang, Z.; Yan, Y.; Li, H.; Luo, X.; Wang, X.; Zhou, P. Study on the Performance of Deep Red to Near-Infrared pc-LEDs by the Simulation Method Considering the Distribution of Phosphor Particles. Micromachines 2024, 15, 1035. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, C.; Zhang, Q.; Yin, P.; Sun, X.; Wang, K.; Wang, J.; Feng, X. Change from La2Ti2O7 to LaTiO3 induced by Li2CO3 addition: Higher local symmetry and particle uniformity achieved an efficient Mn4+ activated far red phosphor for agricultural cultivation. J. Lumin. 2022, 248, 119000. [Google Scholar] [CrossRef]
- Chai, X.J.; Wang, M.H.; Li, J.Q.; Han, Z.; Peng, G.H.; Liao, J.S.; Wen, H.R. Preparation and near-infrared luminescence properties of Y2MgTiO6: Mn4+/ Nd3+. Nonferr. Met. Sci. Eng. 2020, 11, 48–56. [Google Scholar]
- Hu, M.; Liao, C.; Xia, L.; You, W.; Li, Z. Low temperature synthesis and photoluminescence properties of Mn4+-doped La2MgTiO6 deep-red phosphor with a LiCl flux. J. Lumin. 2019, 211, 114–120. [Google Scholar] [CrossRef]
- Sasaki, T.; Fukushima, J.; Hayashi, Y.; Takizawa, H. Photoluminescence Properties of the Magnetoplumbite-Type BaMg6Ti6O19:Mn4+ and Spinel-Type Mg2TiO4:Mn4+. Mater. Sci. Forum 2016, 868, 73–78. [Google Scholar] [CrossRef]
- Lv, L.; Wang, S.; Wang, X.; Han, L. Compounds. Inducing luminescent properties of Mn4+ in magnesium titanate systems: An experimental and theoretical approach. J. Alloy. Compd. 2018, 750, 543–553. [Google Scholar] [CrossRef]
- Ye, T.; Li, S.; Wu, X.; Xu, M.; Wei, X.; Wang, K.; Bao, H.; Wang, J.; Chen, J. Sol–gel preparation of efficient red phosphor Mg2TiO4:Mn4+ and XAFS investigation on the substitution of Mn4+ for Ti4+. J. Mater. Chem. C 2013, 1, 4327–4333. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, H.; Zhang, D.; Shen, Y.; Li, Y.; Hong, R.; Tao, C.; Han, Z.; Chen, L.; Zhou, S. Deep-red emitting Mg2TiO4:Mn4+ phosphor ceramics for plant lighting. J. Adv. Ceram. 2021, 10, 88–97. [Google Scholar] [CrossRef]
- Park, J.Y.; Jung, J.Y.; Ramaraju, G.S.; Yang, H.K. Development of Mg2TiO4:Mn4+ phosphors for enhanced red LED emission and forensic fingerprint analysis. Mater. Today Chem. 2024, 41, 102308. [Google Scholar] [CrossRef]
- Liao, C.; Ma, Z.; Dong, G.; Qiu, J. Flexible porous SiO2–Bi2WO6 nanofibers film for visible-light photocatalytic water purification. J. Am. Ceram. Soc. 2015, 98, 957–964. [Google Scholar] [CrossRef]
- Hu, Z.; Ma, Z.; He, X.; Liao, C.; Li, Y.; Qiu, J. Preparation and characterization of flexible and thermally stable CuO nanocrystal-decorated SiO2 nanofibers. J. Sol.-Gel. Sci. Techn. 2015, 76, 492–500. [Google Scholar] [CrossRef]
- Liao, C.; Ma, Z.; Chen, X.; He, X.; Qiu, J. Controlled synthesis of bismuth oxyiodide toward optimization of photocatalytic performance. Appl. Surf. Sci. 2016, 387, 1247–1256. [Google Scholar] [CrossRef]
- Meng, X.; Wang, Z.; Yan, Z.; Guo, D.; Liu, M.; Gong, J.; Feng, X.; Zhang, T.; Li, X.; Li, P. Flux induced highly efficient and stable phosphor Sr2ScSbO6: Mn4+ for plant growth lighting. J. Mater. Chem. C 2023, 11, 5064–5072. [Google Scholar] [CrossRef]
- Wang, L.S.; Chen, J.J.; Zhou, J.; Lai, H.S. Influences of Flux on Properties of Y3Al5O12:Ce Phosphor. Nonferr. Met. Sci. Eng. 2011, 2, 28–31. [Google Scholar]
- Zhu, F.; Gao, Y.; Lu, X.; Qiu, J. Orthoboric acid as sintering flux improved emission efficiency and thermal stability of Ni2+-doped SrTiO3 perovskite NIR-II phosphor. Ceram. Int. 2025, 51, 9124–9130. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Y.; Yan, X.; Li, G.; Li, X.; Zhang, J.; Chen, B. Outstanding pure green upconversion luminescence in LaZrTa3O11: Er3+/Yb3+ phosphors prepared by molten salt synthesis with B2O3 flux. J. Am. Ceram. Soc. 2025, 108, e20262. [Google Scholar] [CrossRef]
- Borkovska, L.; Stara, T.; Vorona, I.; Nosenko, V.; Gudymenko, O.; Kladko, V.; Kozoriz, K.; Labbé, C.; Cardin, J.; Doualan, J.-L.; et al. Optical and structural properties of Mn4+-activated (ZnxMg1–x)2TiO4 Red Phosphors. Phys. Status Solidi A 2021, 219, 509. [Google Scholar] [CrossRef]
- Cao, R.; Wang, W.; Zhang, J.; Jiang, S.; Chen, Z.; Li, W.; Yu, X. Compounds. synthesis and luminescence properties of Li2SnO3: Mn4+ red-emitting phosphor for solid-state lighting. J. Alloy. Compd. 2017, 704, 124–130. [Google Scholar] [CrossRef]
- Borkovska, L.; Khomenkova, L.; Markevich, I.; Osipyonok, M.; Stara, T.; Gudymenko, O.; Kladko, V.; Baran, M.; Lavoryk, S.; Portier, X. Effect of Li+ co-doping on structural and luminescence properties of Mn4+ activated magnesium titanate films. J. Mater. Sci. 2018, 29, 15613–15620. [Google Scholar] [CrossRef]
- Han, M.; Liu, L.; Zhang, D.; Du, Y.; Zhao, L.; Wang, Y.; Lv, L. Tuning the morphology of Mg2TiO4: Mn4+ for luminescence performance and latent fingerprint visualization. J. Lumin. 2022, 252, 119417. [Google Scholar] [CrossRef]
- Yuan, J.; Zhao, G.; Ren, S.; Wu, Y.; Hu, F.; Wang, Q.; Shi, Q.; Yang, B.; Li, S.; Zhang, D. Multimode fluorescence intensity ratio thermometer based on synergistic luminescence from Eu3+ to Mn4+ of SrTiO3:Eu3+-ZnTiO3:Mn4+ nanocomposites. Ceram. Inter. 2023, 49, 17699–17708. [Google Scholar] [CrossRef]
- Long, J.; Ma, C.; Wang, Y.; Yuan, X.; Du, M.; Ma, R.; Wen, Z.; Zhang, J.; Cao, Y. Luminescent performances of Mn4+ ions during the phase evolution from MgTiO3 to Mg2TiO4. Mater. Res. Bull. 2017, 85, 234–239. [Google Scholar] [CrossRef]
- Cao, R.; Cheng, X.; Zhang, F.; Su, L.; Chen, T.; Ao, H.; Yu, X.; Ruan, W. Enhanced luminescence properties of MgTO3: Mn4+ red-emitting phosphor by adding Ge4+ ion and H3BO3. J. Mater. Sci.-Mater. Electron. 2018, 29, 13005–13010. [Google Scholar] [CrossRef]
- Wu, X.; Luo, Z.; Zhuang, Y.; Liu, Z.; Sun, P.; Liu, Y.; Zhang, L.; Qin, H.; Jiang, J. The co-optimization of efficiency and emission bandwidth in GSAGG:Cr3+ NIR ceramic phosphors. Ceram. Int. 2023, 49, 21688–21694. [Google Scholar] [CrossRef]
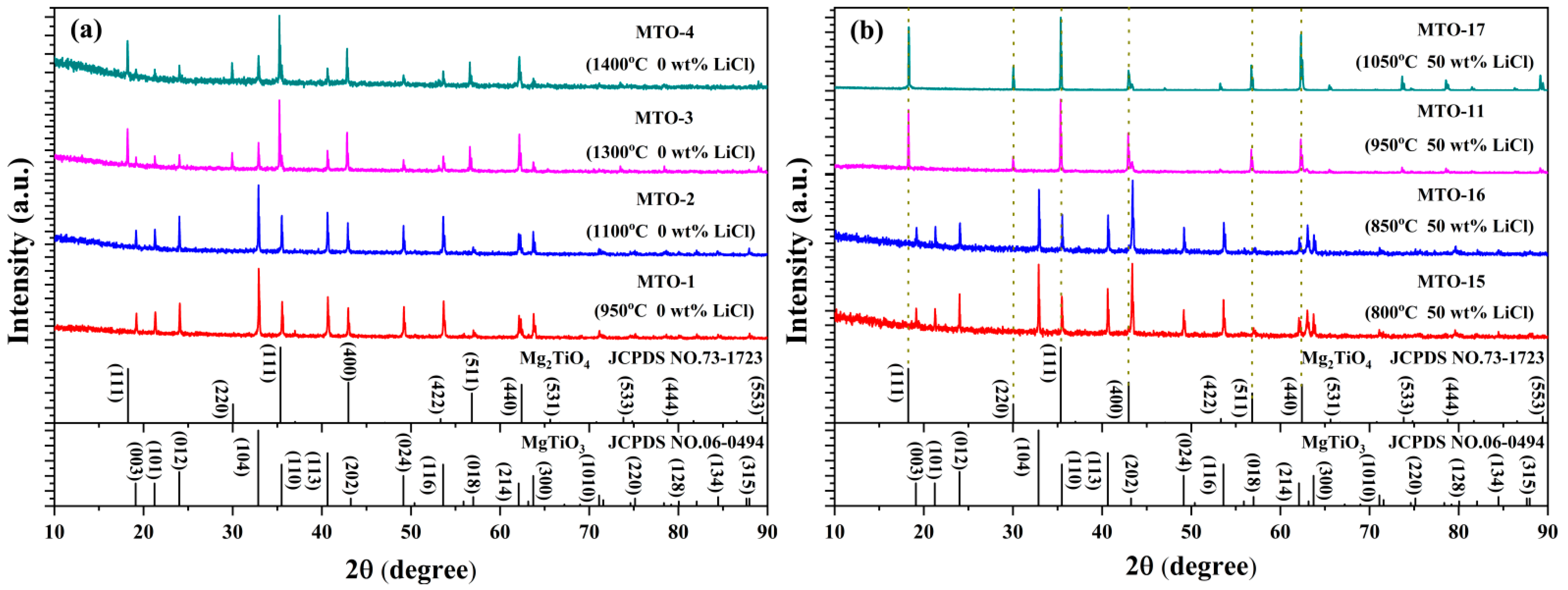
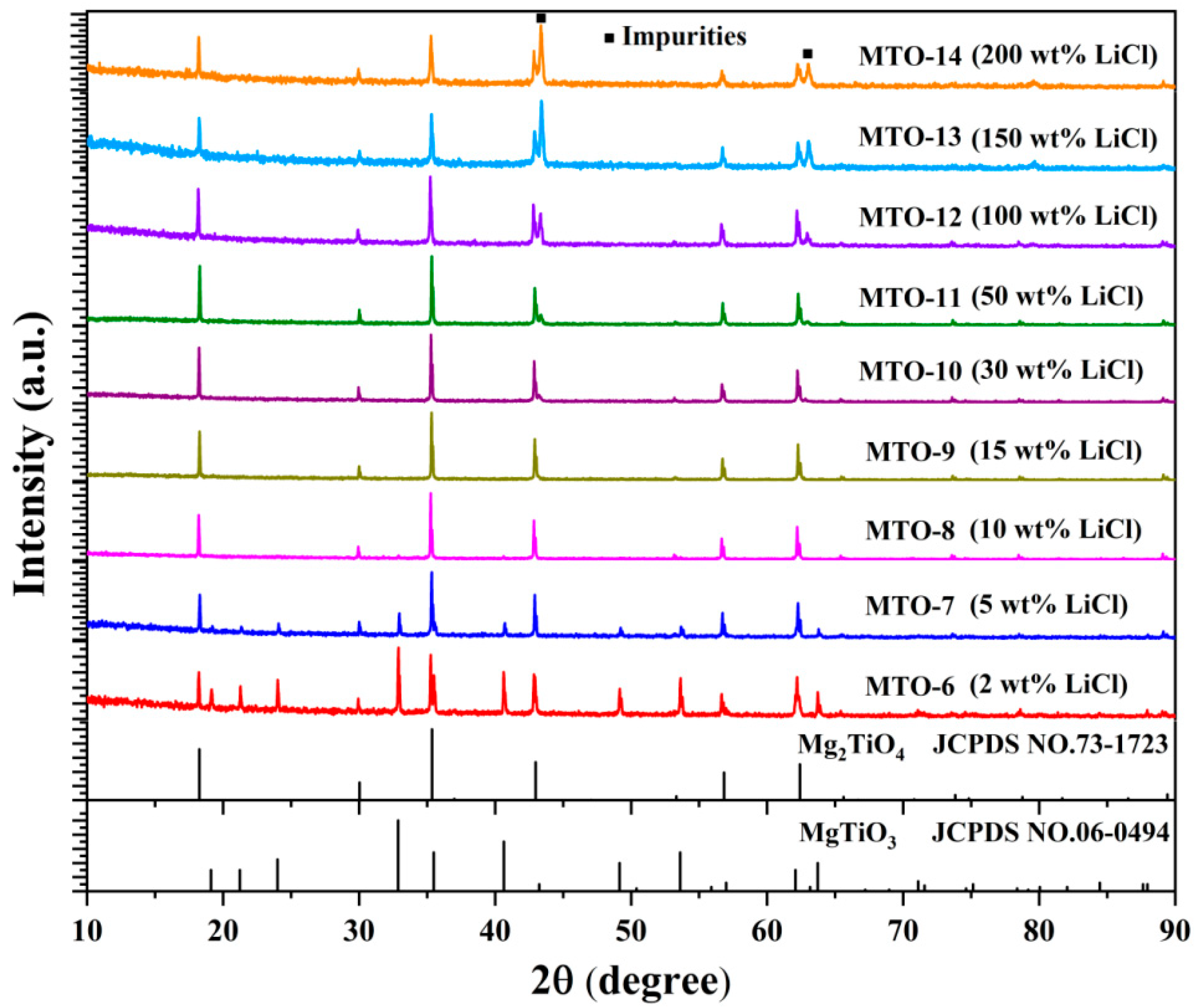
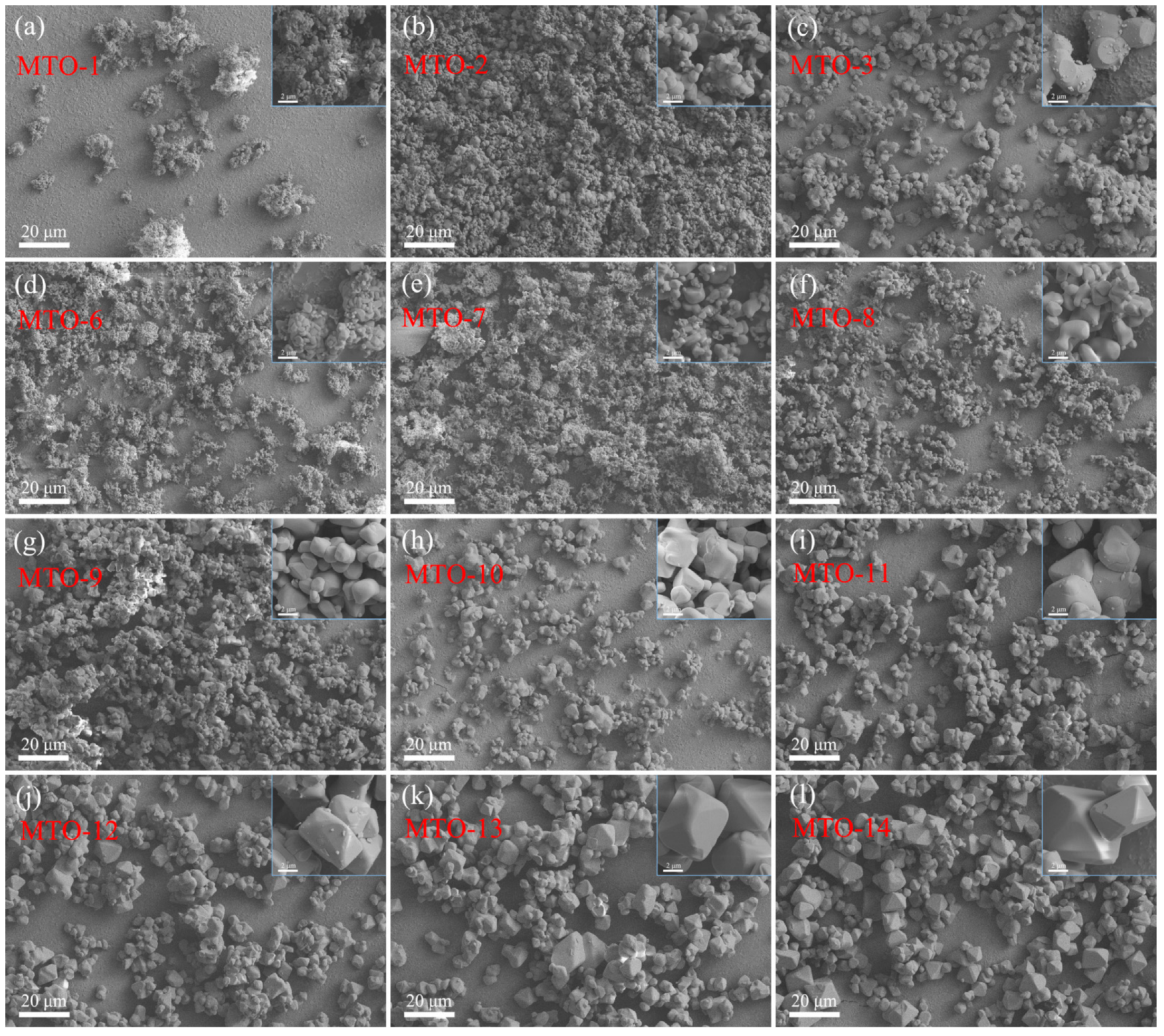
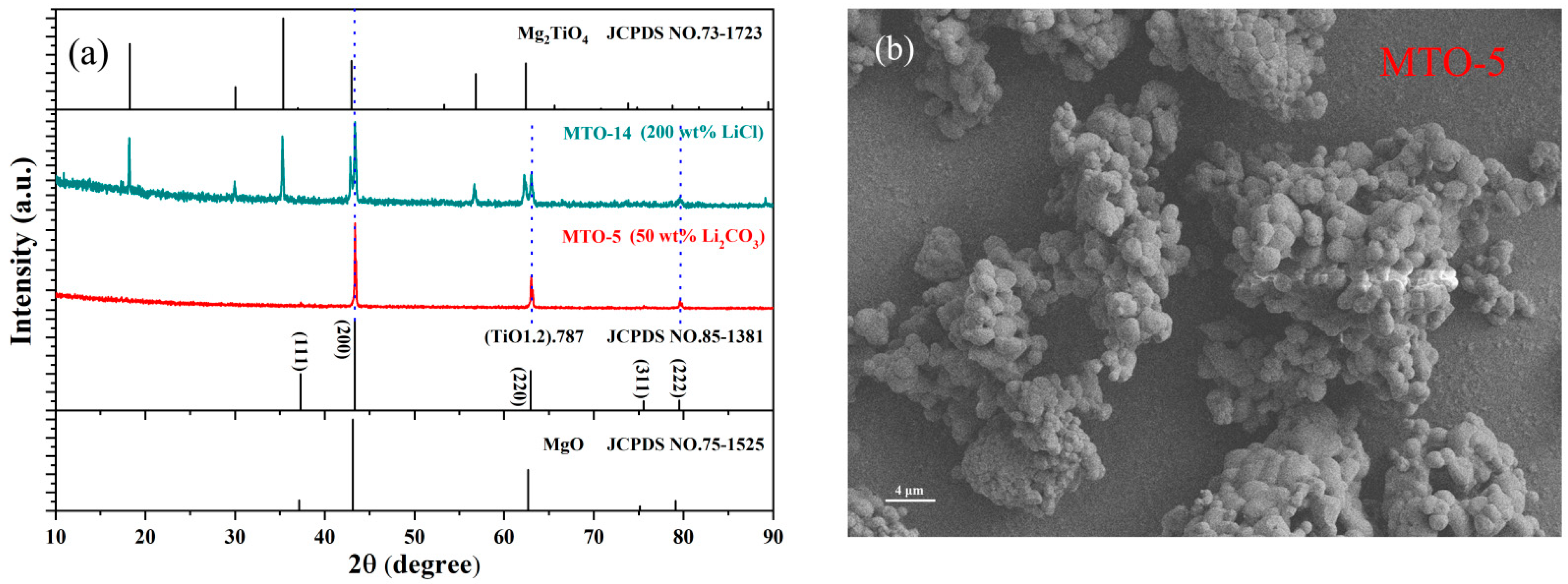
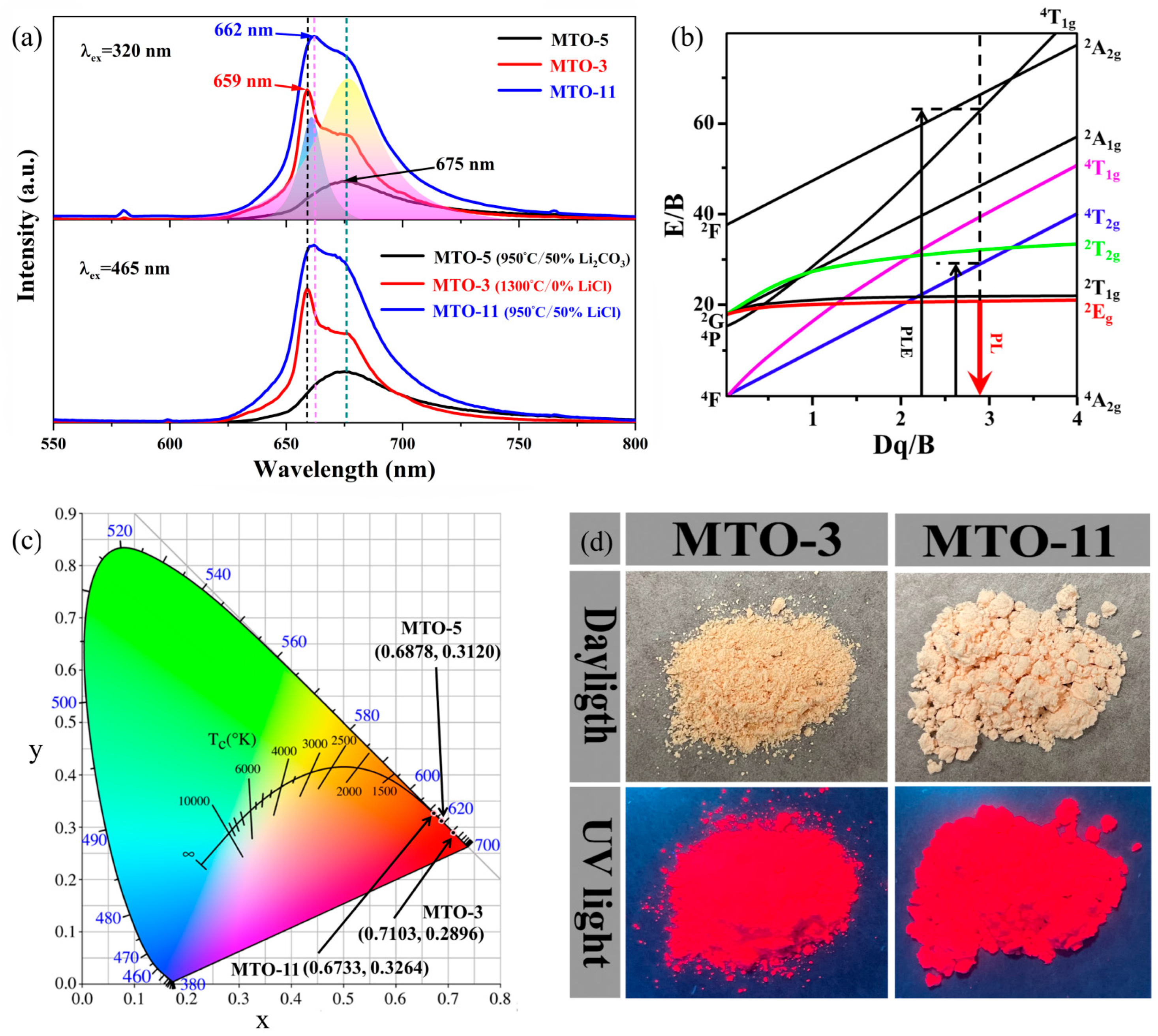
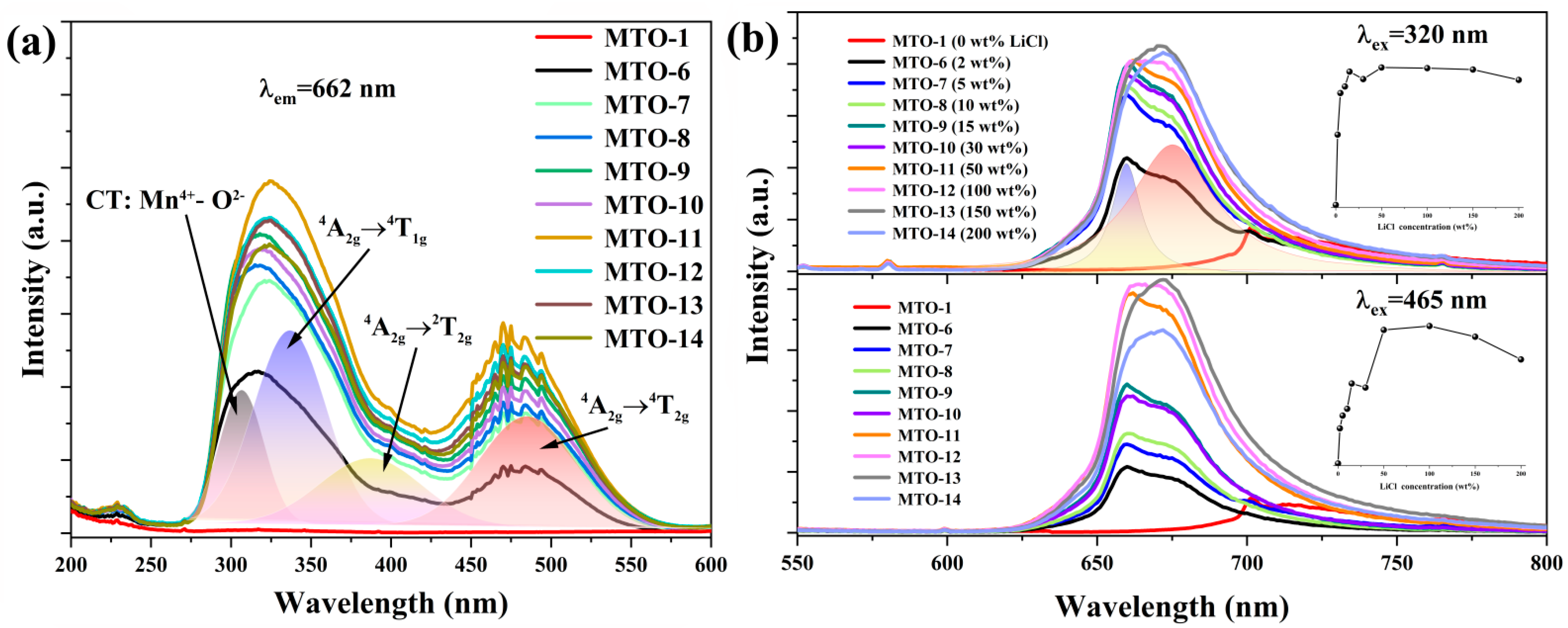

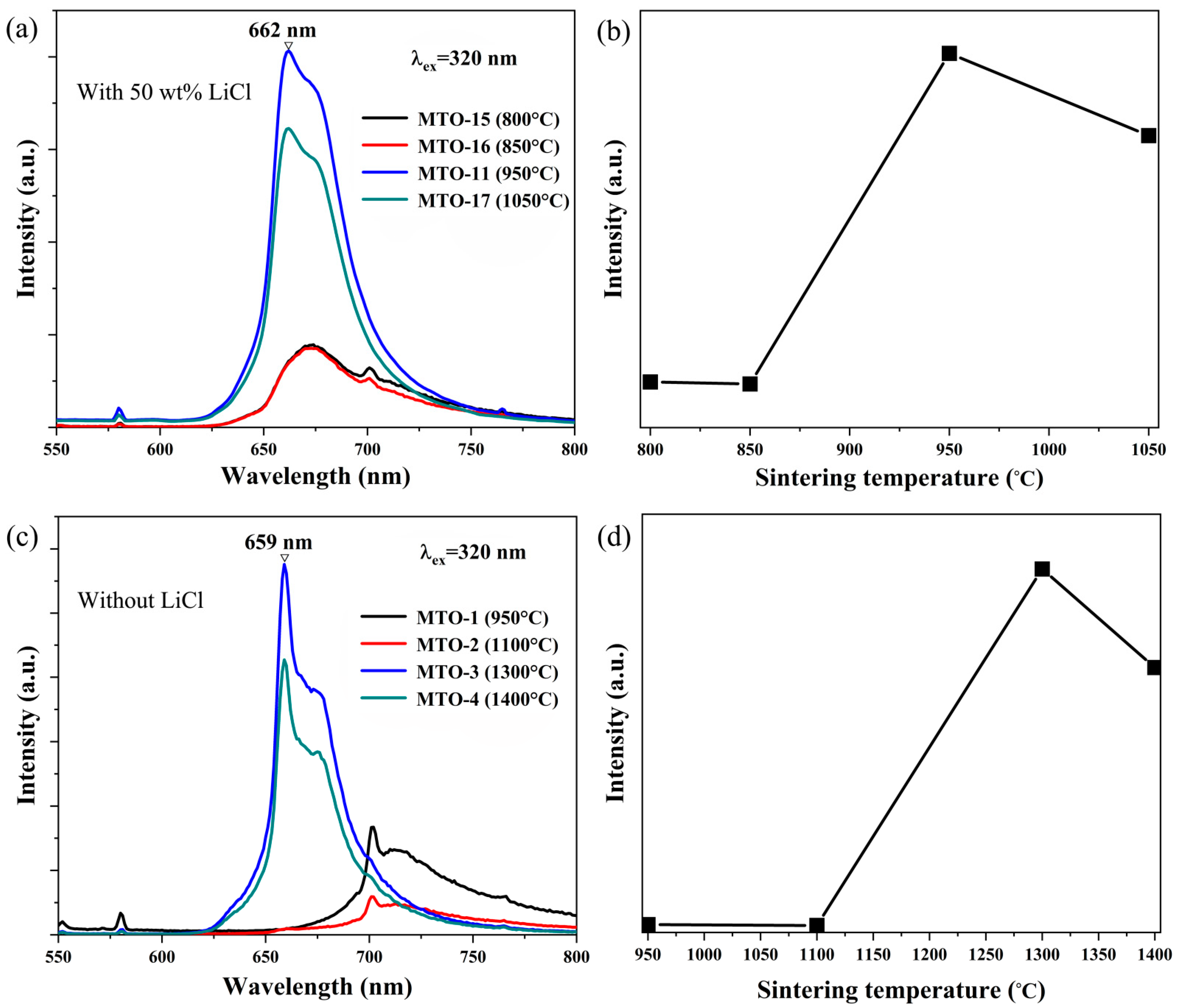

| No. | Main Components | LiCl Content (wt%) | Li2CO3 Content (wt%) | Sintering Temperature (°C) | Doping Content (mol%) |
|---|---|---|---|---|---|
| MTO-1 | MgTiO3:Mn | 0 | 0 | 950 | 0.2 |
| MTO-2 | MgTiO3:Mn | 0 | 0 | 1100 | 0.2 |
| MTO-3 | Mg2TiO4:Mn | 0 | 0 | 1300 | 0.2 |
| MTO-4 | Mg2TiO4:Mn | 0 | 0 | 1400 | 0.2 |
| MTO-5 | (TiO1.2).787/MgO | 0 | 50 | 950 | 0.2 |
| MTO-6 | MgTiO3:Mn | 2 | 0 | 950 | 0.2 |
| MTO-7 | MgTiO3:Mn | 5 | 0 | 950 | 0.2 |
| MTO-8 | Mg2TiO4:Mn | 10 | 0 | 950 | 0.2 |
| MTO-9 | Mg2TiO4:Mn | 15 | 0 | 950 | 0.2 |
| MTO-10 | Mg2TiO4:Mn | 30 | 0 | 950 | 0.2 |
| MTO-11 | Mg2TiO4:Mn | 50 | 0 | 950 | 0.2 |
| MTO-12 | Mg2TiO4:Mn | 100 | 0 | 950 | 0.2 |
| MTO-13 | Mg2TiO4:Mn | 150 | 0 | 950 | 0.2 |
| MTO-14 | Mg2TiO4:Mn | 200 | 0 | 950 | 0.2 |
| MTO-15 | MgTiO3:Mn | 50 | 0 | 800 | 0.2 |
| MTO-16 | MgTiO3:Mn | 50 | 0 | 850 | 0.2 |
| MTO-17 | Mg2TiO4:Mn | 50 | 0 | 1050 | 0.2 |
| MTO-18 | Mg2TiO4:Mn | 50 | 0 | 950 | 0.05 |
| MTO-19 | Mg2TiO4:Mn | 50 | 0 | 950 | 0.1 |
| MTO-20 | Mg2TiO4:Mn | 50 | 0 | 950 | 0.5 |
| MTO-21 | Mg2TiO4:Mn | 50 | 0 | 950 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, C.; Cai, H.; Dai, D.; Zhang, L. Low-Temperature Synthesis and Photoluminescence Properties of Mg2TiO4:Mn4+ Phosphor Prepared by Solid-State Reaction Methods Assisted by LiCl Flux. Solids 2025, 6, 53. https://doi.org/10.3390/solids6030053
Liao C, Cai H, Dai D, Zhang L. Low-Temperature Synthesis and Photoluminescence Properties of Mg2TiO4:Mn4+ Phosphor Prepared by Solid-State Reaction Methods Assisted by LiCl Flux. Solids. 2025; 6(3):53. https://doi.org/10.3390/solids6030053
Chicago/Turabian StyleLiao, Chenxing, Huihuang Cai, Dongyuan Dai, and Liaolin Zhang. 2025. "Low-Temperature Synthesis and Photoluminescence Properties of Mg2TiO4:Mn4+ Phosphor Prepared by Solid-State Reaction Methods Assisted by LiCl Flux" Solids 6, no. 3: 53. https://doi.org/10.3390/solids6030053
APA StyleLiao, C., Cai, H., Dai, D., & Zhang, L. (2025). Low-Temperature Synthesis and Photoluminescence Properties of Mg2TiO4:Mn4+ Phosphor Prepared by Solid-State Reaction Methods Assisted by LiCl Flux. Solids, 6(3), 53. https://doi.org/10.3390/solids6030053







