Abstract
The construction industry is recognized for its high consumption of natural resources, resulting in significant environmental impacts. Given this reality, it is essential to seek new methods and solutions that minimize the impact of this activity on the environment. An innovative approach consists of using pigments derived from acid mine drainage (AMD) as a sustainable alternative in the production of mortar for decorative façade cladding. In this context, the main objective of this paper was to evaluate the physical/mechanical properties of decorative mortars developed by partially replacing natural sand with pigment from acid mine drainage. Initially, the pigment (yellow) was produced, characterized, and compared with a commercial pigment. Sequentially, decorative mortars were developed with different pigment concentrations (0%, 2%, 4%, and 6%). The mortars were subjected to compressive strength, flexural tensile strength, shrinkage, loss of mass, and colorimetry tests. The results showed that compressive strength, flexural tensile strength, weight loss, and dimensional variation were significantly affected by the partial addition of pigment to replace natural aggregate. In other words, there was a decrease in strength and an increase in mass loss and expansion of the mortars. However, the main factor influencing these variables was the greater amount of water added in the higher substitution cases. The addition of water was necessary to keep the consistency constant. A possible solution to maintain the same amount of water and avoid negative effects on the mortar properties would be to use additives in the mortar formulation in future work. Therefore, this research contributes to the search for more sustainable solutions in civil construction, exploring the use of pigments from AMD as a viable alternative to reduce the environmental impacts associated with this industry.
1. Introduction
The generation of coal tailings is a significant environmental concern due to the negative impacts it has on the environment. One of the main problems associated with this practice is the formation of acid mine drainage, a phenomenon resulting from the exposure of sulphide minerals present in coal to water and air. This process triggers the release of acids and heavy metals, making groundwater and surface water highly acidic and contaminated. Acid mine drainage poses a serious threat to water quality, aquatic ecosystems, and wildlife, requiring stringent mitigation measures and sustainable solutions to minimize its harmful effects on the environment [1,2,3].
In Brazil, coal reserves are located in the South Region, in the states of Paraná, Rio Grande do Sul, and Santa Catarina, accounting for 4%, 29%, and 67% of production, respectively [4]. Despite being one of the main sources of energy, coal mining generates a considerable amount of waste, which has led to reflections on the sustainability of its processes. In the Santa Catarina coal region, for example, around 65% of the material extracted from underground is discarded as tailings [5]. This reality has driven the search for alternatives that promote more sustainable use of the waste generated by coal mining.
Coal tailings contain approximately 10% pyrite [5,6]. When this sulphide comes into contact with oxygen and rainwater, it undergoes an oxidation process, resulting in a highly acidic effluent with a large amount of dissolved metals known as acid mine drainage (AMD). The AMD generated by pyrite leaching requires treatment, in which the metals are precipitated in the form of metal oxides, separated from the aqueous phase, and safely disposed of in the environment, which entails high treatment and final disposal costs [7,8].
One form of reuse is the production of pigment through the appropriate treatment of acid mine drainage, which can be carried out in the following steps: collection of acid mine drainage, neutralization of acid, precipitation of metals, oxidation of sulphides, formation of yellow pigment, filtering, and grinding [9,10].
Several examples of the use of acid mine drainage in pigments can be found in the literature [11,12,13,14]. In Hedin’s work [15], a significant amount of sludge containing acid mine drainage (around 2000 tons) was used to generate pigments, specifically burnt sienna. The latter was used to develop different colorations of other pigments. In a study by Ryan et al. [16], iron oxide pigments of various colors and properties were recovered from a synthetic AMD solution by means of a selective precipitation process using oxidation, pH adjustment, and filtration. The factor that exerted the greatest influence on the coloration of the iron oxide pigments was the pH during the recovery process. In particular, the pigments obtained at a lower pH (pH < 3) exhibited a distinct yellow color. Marcello et al. [17] used sedimented-treated sludge from coal mine drainage to obtain inorganic pigments, which were later evaluated in the manufacture of ceramic tiles. In this study, the predominant color of the pigments produced was brown.
This type of pigment, obtained by leaching pyrite, has already been tested in the pigmentation of ceramic materials, showing promising results [6]. Another possible application is the use of this pigment in decorative mortars, which may represent a viable alternative to expand the range of applications for this material and, consequently, offer a solution to the environmental problem caused by acid mine drainage [18,19].
Colored pigment mortar is a building material that incorporates mineral or synthetic pigments to add color to the cement or mortar matrix [20]. This pigmentation process is carried out with the aim of achieving and specifically controlling the desired shade in the final mix. In addition to its aesthetic function, the addition of pigments to mortar can influence its physical and chemical properties, such as abrasion resistance, durability, and weather resistance [21,22]. This type of mortar is often used in decorative applications, cladding, flooring, and in other contexts where aesthetics play a fundamental role in architectural design [23,24].
In this way, the transformation of waste from coal mining into products or by products for the development of construction materials represents an innovative and sustainable approach to dealing with the environmental and economic challenges associated with the construction industry. In this context, waste that was previously disposed of in an environmentally damaging way is being intelligently reused and recycled to create high quality materials. This approach not only reduces waste and the demand for limited natural resources, but can also contribute to reducing carbon emissions [25,26,27].
This work presents innovations in the development of decorative mortar, highlighted by the origin of pigments from acid mine drainage, a source of polluting waste. In addition, the use of pigments obtained from acid drainage generated by controlled leaching in pyrite, a by product of coal mining, makes production more sustainable and contributes to the responsible management of mining waste. The transformation of coal mining waste, such as pyrite, into valuable pigments exemplifies a circular economy approach, reinforcing the commitment to sustainability and the reduction of industrial waste in the construction industry. Controlled pyrite leaching produces a ferrous sulfate solution with reduced concentrations of contaminants, such as aluminum, chromium, zinc, and copper, which are present in tailings ponds or deposits. In this controlled leaching process, the pyrite is purified over several successive leaching stages [9,28].
In this context, the main aim of this work was to evaluate the physical and mechanical properties of decorative mortars developed with the addition of pigments generated from acid mine drainage. Decorative mortars with the addition of industrial pigments were also developed for comparison purposes.
2. Materials and Methods
The methodology adopted in this study was divided into three distinct stages. In the first stage, acid mine drainage was generated, and the pigment to be incorporated into decorative mortars was developed. In this phase, the pigment extraction and treatment processes were carried out on acid mine drainage in order to obtain a material suitable for application in coatings. In the second stage, the essential materials for the mortars were characterized, such as sand, cement, and the pigment itself. Tests and analyses were carried out to assess the physical and chemical properties of each component in order to guarantee the quality and compatibility of the materials used. Finally, the third stage involved making the decorative mortars in which the pigment obtained was incorporated in different proportions, and then the samples were subjected to a series of tests to characterize their physical and mechanical properties. Figure 1 provides a schematic illustration of the three methodological stages described, giving a brief description of each one.
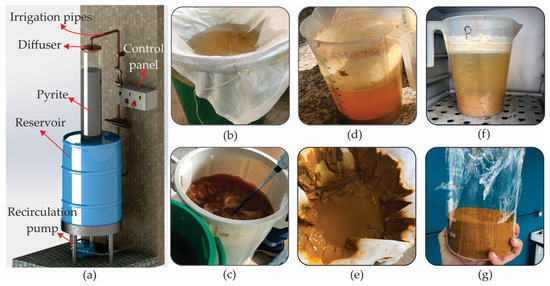
Figure 1.
Main pigment production stages: (a) AMD production device, (b) AMD filtration, (c) pH adjustment with sodium carbonate, (d) decantation, (e) sludge filtration, (f) heating in an oven, and (g) pigment after grinding.
2.1. Production of AMD and Pigment
The pigment based on iron oxide is obtained from AMD, which is a fluid characterized by its excessive acidity (pH < 4.0) and high dissolved iron content, as well as containing sulphate and other metals in lower concentration. To produce the pigment, AMD was used as a precursor and prepared by means of controlled leaching on an increased scale. The experiment was carried out according to the work of [19] using a benchtop device shown in Figure 1a. The method consisted of the percolation and recirculation of water over a PVC column 180 mm in diameter and 1 m high, filled with 10 kg of pyrite concentrate with granulometry between 2 and 10 mm in diameter.
The concentrate containing pyrite was collected from a coal mining company located in the state of Santa Catarina, in the coal seam known as Barro Branco, the same geological formation described by [28]. This concentrate contained 62% pyrite (FeS2), which is susceptible to oxidation to generate AMD. The leachate storage system consisted of a reservoir with a capacity of 240 L, and circulation was carried out by a recirculation pump. The experiment lasted 8 weeks, with a nominal recirculation flow rate of 1.2 L/min. During this period, the column was partially filled with water, allowing oxygen to enter the system.
After preparing the AMD, the iron oxide-based pigment was prepared, following the method of Cornell and Schwertmann [29,30,31] based on the studies of [9,32,33]. The AMD was filtered (Figure 1b), resulting in a concentration of approximately 1.74 g/L of iron in a total of 138 L. The sample was divided into four parts, and the pH was adjusted (Figure 1c) to 3.6 ± 0.1 using sodium carbonate (NaCO3) at a concentration of 50%, promoting the precipitation of iron with high purity in the form of ferric hydroxide, with an iron recovery of around 80% [9]. After multiple washes, the iron hydroxide precipitate was decanted (Figure 1d), filtered (Figure 1e) and dissolved in nitric acid. Precipitation of the ferric nitrate solution occurred with the addition of KOH, giving rise to ferrihydrite. The precipitate was then dissolved in water, forming ferric ion species (), and heated at 70 °C for 60 h (Figure 1f), resulting in the formation of goethite crystalline powder (Figure 1g).
After the reaction period, the samples were filtered, dried, ground, and then characterized for use in decorative mortar. A total of 337.40 g of pigment was obtained from 138 L of AMD. In addition to the pigment produced from AMD, an industrial goethite pigment supplied by Lanxess® was used for colorimetry comparison.
The use of nitric acid to dissolve the iron hydroxide precipitate was selected in order to obtain better quality goethite. In other words, goethite can be produced in two ways: one involves the use of ferrous sulphate, but this method is complicated and results in a product with low crystallinity. The other approach uses ferric nitrate, which makes it possible to obtain goethite with greater crystallinity and higher quality.
2.2. Pigment Characterization
The pigments were characterized in terms of various fundamental aspects, including analysis of their morphology, chemical composition, mineralogical composition, unit mass, specific mass, and colorimetry.
The morphology of the pigments was evaluated using scanning electron microscopy (SEM) using a Hitachi TM3000 TableTop Microscope, Hitachi High-Technologies Corporation, Tokyo, Japan.
The chemical compositions of the AMD pigment and the industrial pigment were analyzed in the LC SATC solid fuels laboratory. A Shimadzu model EDX-7000 energy dispersive X-ray fluorescence (EDXRF) spectrometer, Shimadzu Manufacturing Company, Kyoto, Japan, was used for this analysis.
To identify the crystalline phases using the X-ray diffraction (XRD) technique, a Shimadzu XRD 6100 diffractometer, Shimadzu Manufacturing Company, Kyoto, Japan, was used with CuKa radiation (λ = 1.5418 Å), operating at 40 kV and 30 mA. Data collection was carried out with a step interval of 0.02° and a step duration of 0.6 s, covering the range of 2θ angles from 0 to 80°. The information collected was identified using the HighScore Plus software (Version 3.0.5), which is used to index ICDD (International Center for Diffraction Data) standards.
The colors of the pigment samples, obtained from AMD (AMD-Pi) and industrial (IND-Pi), were characterized using differential colorimetry (ΔLab), using the colorimetric parameters “L”, “a”, and “b”. The sample tablets were made from 2 g of the material, compacted at a pressure of 74 N/cm2. The color evaluation was conducted using a Minolta 2600d spectrophotometer, Konica Minolta Inc., Tokyo, Japan, at the Mineral Processing Laboratory (LAPROM) of the Federal University of Rio Grande do Sul in Brazil.
2.3. Production of Decorative Coating Mortar
According to the literature, the most suitable ratio for coating mortars is 1:1:6 [34]. However, we opted for a higher proportion of lime, i.e., 1:2:6 (binder:lime:fine aggregate). This choice resulted in a lighter mortar, which allowed for better pigmentation over the gray color of the cement.
The binder used to produce the mortar was Portland cement with added pozzolan (CP II-Z 32). This type of cement was chosen due to its frequent use in laying and coating mortars. This cement has a particle size of less than 41 μm and a composition containing 6% to 15% pozzolan. The chemical and physical characteristics of this cement are detailed in Table 1.

Table 1.
CP II-Z 32 Portland cement characteristics.
The lime used in this experimental research is CH-II with CaO = 41.54% and MgO = 28.77%. The Brazilian Standards do not classify lime as calcium or dolomitic. It is classified as CH-I, CH-II, or CH-III. There is no mention if it is dolomitic or not. According to BS EN 459–1 [35], there is a clear difference between calcium lime and dolomitic lime. The standard establishes a maximum MgO content of 5% in calcium lime and two minimum content of MgO to dolomitic lime (>5% or ≥30%). In this work, it was decided to maintain the Brazilian Standard to classify the lime. Table 2 shows the characteristics of type CH-II lime.

Table 2.
CH-II hydrated lime characteristics.
The fine aggregate used was quartz river sand passing the 4.75 mm sieve. Table 3 shows the characteristics of the fine aggregate.

Table 3.
Granulometry and properties of sand.
The mortars were prepared with different pigment contents replacing the sand, namely: 0% (as a reference), 2%, 4%, and 6%. These proportions are in line with the study [36], which states that the maximum recommended pigment content is 6%. This is because exceeding this value can have an impact on the workability of the material. Table 4 shows the quantities of materials used in each mortar composition.

Table 4.
Quantity of material for each decorative mortar mix.
The dosage, preparation, and manufacture of the specimens followed a standardized procedure. Initially, the materials were weighed in the recommended quantities, separated, and then mixed using a mechanical mortar. To ensure effective homogenization, the dry materials were mixed before adding the water. Figure 2 shows the mixing sequence and the resulting specimens.
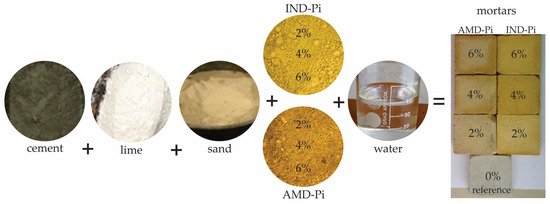
Figure 2.
Schematic diagram of the mixing sequence for mortar production.
The amount of water in the mix was varied to keep the consistency of the mortar constant, i.e., a consistency of 260 ± 10 mm was accepted. The cone trunk test was carried out on the mortar to ensure consistency, which followed the guidelines of NBR 13276 [37].
The specimens were molded with dimensions of 40 × 40 × 160 mm and 50 × 50 × 10 mm and demolded after 24 h. After demolding, they were kept at room temperature in the open air, simulating real curing conditions for 28 days. Three specimens were produced for each test to ensure repeatability of the results.
2.4. Mortar Characterization
The specimens were characterized in terms of flexural tensile strength, compressive strength, dimensional variation, loss of mass, and colorimetry.
The compressive strength and flexural tensile strength tests were carried out by molding specimens for each composition and type of pigment, with dimensions of 40 × 40 × 160 mm. These specimens were manually compacted and subjected to rupture after 28 days of curing, following the guidelines established by standard NBR 13279 [38]. Compressive strength was assessed using an EMIC model PC 100 C hydraulic press (EMIC-PC100C, Instron, Norwood, MA, USA). For the flexural tensile strength test, the Instron EMIC EL 2000 universal testing machine was used.
For the dimensional variation test, three specimens were made for each composition, each with dimensions of 40 × 40 × 160 mm. The dimensions were measured at 7, 14, and 28 days of age, using high-precision digital equipment, with a margin of error of just 0.001 mm, strictly following the specifications established by the NBR 15261 standard [39]. Also, for the same curing times as the dimensional variation assessment, the masses of each specimen were determined in order to assess the loss of mass of the specimens.
The loss of mass was evaluated because it may explain the behavior of the mortar in relation to shrinkage due to the reduction in hydration water. The main factor responsible for this loss of water is the phenomenon of shrinkage. In addition, the composition of the pigment can lead to different reactions when it interacts with sulfate and other salts, such as sodium and sulfate.
For the colorimetry tests, 55 readings were taken on 11 specimens, which were specially made for the color test. These specimens had dimensions of 50 × 50 × 10 mm and were evaluated after 28 days of curing. Measurements were taken at each end of the sample, as well as at its center. It is important to note that the distribution of the pigment in the mass was uniform, thanks to the process of mixing the pigment with the fine aggregate before adding the mixing water. Differences in shade were detected with a ΔE*Lab value above 0.2–0.5 (according to [40]). The same equipment used to characterize the pigments was used to characterize the mortars.
3. Results and Discussion
3.1. Characteristics of the Pigment Produced
The pigments were subjected to granulometric characterization using a laser system. This analysis revealed that the pigment obtained from AMD (AMD-Pi) has 10% particles with diameters of less than 0.75 μm (D10), a median particle diameter (D50) of 2.81 μm, and 10% particles with diameters of more than 11.09 μm (D90). In the case of the industrial pigment (IND-PI), the D10, D50, and D90 values were 2.63 μm, 84.72 μm, and 214.78 μm, respectively. These results are in line with previous study [41]. Figure 3 shows the particle size curves corresponding to the different pigments.
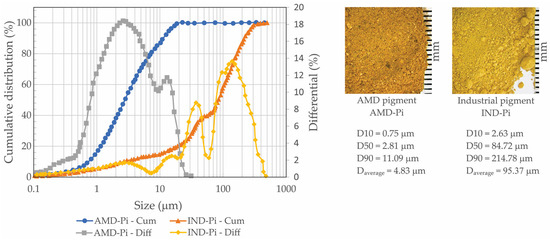
Figure 3.
Particle size distribution for the pigment extracted from AMD (AMD-Pi) and the industrial pigment (IND-Pi).
This difference in particle size between pigments can have a considerable impact on the production of decorative mortars, affecting their workability, resistance, texture and final appearance. Very fine particles can make the mortar more fluid, while coarser particles can make it rough and difficult to handle. Inadequate distribution of particle sizes can result in voids, compromising strength. In addition, particle size influences the texture of the surface and the uniformity of the pigment dispersion, affecting the final appearance.
For morphological comparison purposes, images were taken using a scanning electron microscope at 5000× magnification. The images show that both samples are composed of acicular crystals. However, in the commercial sample (IND-Pi), seen in Figure 4a, the crystals are more dispersed and have a better defined shape, whereas in the sample obtained from AMD (AMDP-Pi—Figure 4b), the crystals are more “compact” and their shape is not so clearly delineated. The discrepancy in the aspects of the crystals may be related to the crystallization process, the drying process, and/or the comminution stages. These results are in line with the work carried out by Silva [41].
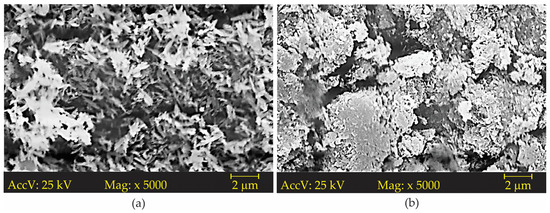
Figure 4.
Scanning electron microscopy for (a) the industrial pigment (IND-Pi) and (b) the pigment obtained from AMD.
X-ray fluorescence was carried out on the solids from acid mine drainage (AMD), the precursor to the pigment, as well as on samples of the industrial pigment (IND-Pi) and the pigment obtained from AMD (AMD-Pi). The purpose of the chemical composition analysis was to quantify the presence of iron, a constituent element of the pigment. In addition, we sought to identify other elements considered to be contaminants, which could modify or even prevent conversion into pigment [9]. These elements include Al, Zn, Mn, Ca, Cu, Ni, Cd, and Pb, which are listed in Table 5.

Table 5.
Chemical composition of pigments (IND-Pi, AMD-Pi, and AMD).
After analysis, it was found that the contaminating/unwanted metals were present in quantities of less than 1% in both samples, when compared to the raw AMD. The process of iron recovery by selective precipitation (pH 3.6 ± 0.1) indicates that the contaminating metals may remain in the supernatant, since their levels decreased in relation to the pigment and AMD values. It is important to recognize the inherent uncertainty associated with X-ray fluorescence (XRF) analyses for trace elements (Pb, Zn and Cu), as highlighted in the study. This uncertainty factor significantly affects any conclusions drawn regarding differences in analysis between the pigments.
The difference in chemical composition between raw AMD and AMD-Pi is determined by the precipitation diagram as a function of pH found at EPA 625/8-80-003 [42]. It can be seen that practically all the metals in solution are retained, with the exception of iron III. The K2O content is explained by the addition of this reagent to convert ferric nitrate into goethite, in accordance with Cornell and Schwertmann’s second method [29].
One observation that should be made is the absence of aluminum oxide in the XRF analysis. Aluminum was analyzed, but not detected in the samples, due to the acid mine drainage used in the formation of goethite. This drainage was produced by controlled leaching of beneficiated pyrite, which significantly reduced the possibility of aluminum contamination. Furthermore, goethite is an iron mineral (FeOOH) and therefore its chemical composition is dominated by iron and oxygen, with the presence of hydroxyls. Aluminum oxide is not a common component of goethite, and its concentration in this mineral is generally very low.
After this analysis, it was found that the amount of iron (%) recovered from AMD was 86.5%, a result 10% higher than that obtained in the studies by [6], which indicated a recovery of around 70% for the production of the pigment. To compare the iron content between the pigments, the amount was determined based on the stoichiometric ratios. The industrial pigment had a iron content of 68.7%, while the AMD goethite sample had a iron content of 63.8%.
According to [41], the stoichiometric percentage of iron in the composition of the Goethite pigment (FeOOH) is 62.85% of iron content. A small but significant difference was therefore identified in relation to the pigment obtained by AMD. These results suggest that the pigment obtained from AMD was achieved, since the difference of iron are around 1.5%.
Based on the X-ray diffraction analysis carried out on the industrial pigment (IND-Pi) and AMD pigment (AMD-Pi) samples (Figure 5), goethite (FeOOH) was found in both pigments. For the AMD-Pi pigment, it was also possible to observe the presence of jarosite, which is a potassium iron sulphate mineral that can have yellow tones and is commonly found in acidic environments associated with the oxidation of iron sulphide minerals. It should be noted that the peak indicated as 1 could not be identified with the appropriate degree of certainty, since the diffractogram lines did not coincide with the standards available in the analysis system. However, it can be said that it is a compound derived from arsenic, with a lesser degree of importance, based on the chemical quantification carried out by XRF.
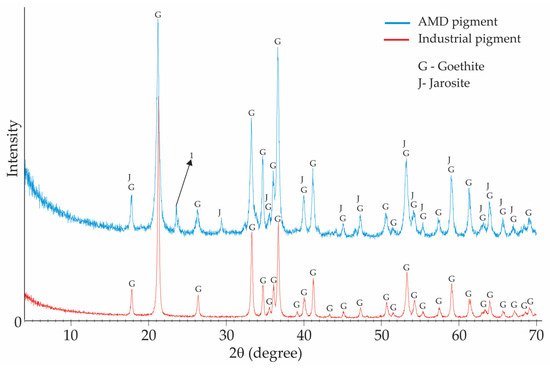
Figure 5.
X-ray diffractogram for AMD-Pi and IND-Pi pigments.
The IND-Pi and AMD-Pi samples, both in powder form, were analyzed by differential colorimetry. The values of the colorimetric criteria of the ICES-Lab* system are shown in Table 6, where L* shows the values from black to white (0% = black and 100% = white); a* shows the colors green to red (a “negative” = green and a “positive” = red); and b* shows the colors blue to yellow (b “negative” = blue and b “positive” = yellow). Based on these values, the color differences (ΔE*Lab) between the two samples were calculated, with the IND-Pi sample as the reference.

Table 6.
Colorimetric parameters for AMD-Pi and IND-Pi pigments.
It was observed that the AMD-Pi sample had a darker color (lower L* value), with more reddish tones (higher a* value) and less yellowish tones (lower b* value) when compared to the industrial pigment. This is the ochre shade that corresponds to the natural color of goethite contaminated by jarosite, suggesting that its formation may have been triggered by the same reaction in the system, in the presence of potassium salts [16]. When potassium comes into contact with jarosite, it can replace part of the iron ions (Fe3+) present in the jarosite structure, resulting in a series of chemical changes. Potassium tends to form complexes with iron ions, which can cause the reduction of iron ions from Fe3+ to Fe2+. This results in a change in the color of the jarosite, which can turn from yellow or red to a darker color, such as brown.
In the present work, the value found for ΔEL*a*b* was 15.38, while in the study by [41], the value was 15.47, which is very close. These results fall within the classification, according to the criteria of DIN 6174 [40], as “very large”, i.e., greater than six. This indicates that the color difference is significant when comparing the two types of pigment.
Figure 6 is a comparison between the reflectance curves of IND-Pi and AMD-Pi. It can be seen that the IND-Pi pigment has a higher reflectance than the AMD-Pi pigment and the curves have the same behavior, i.e., the peaks are in the same wavelength range, indicating the formation of yellow color in the pigments. However, there is a difference in the shade of yellow between the pigments, i.e., the IND-Pi pigment is apparently brighter than the AMD-Pi pigment (Figure 3).
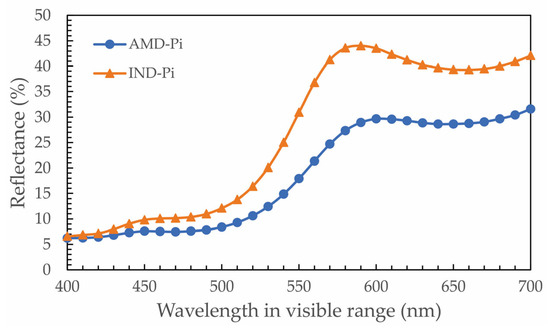
Figure 6.
Percentage reflectance curves for AMD-Pi and IND-Pi.
3.2. Decorative Coating Mortars
3.2.1. Mechanical Resistance
Figure 7 shows the graphs representing the compressive strength and flexural tensile strength as a function of the different pigment percentages, as illustrated in Figure 7a AMD-Pi and Figure 7b IND-Pi. In the case of both AMD-Pi and IND-Pi, as the amount of pigment substituted for natural sand increases, the strengths decrease for substitution values above 4%. With a 2% substitution, AMD-Pi has slightly higher strength values (compression and traction) than the reference mortar. In the case of IND-Pi, a 2% substitution results in a slight increase in tensile strength and a small reduction in compressive strength compared to the reference mortar. These differences were significant, as corroborated by Tukey’s statistical test.
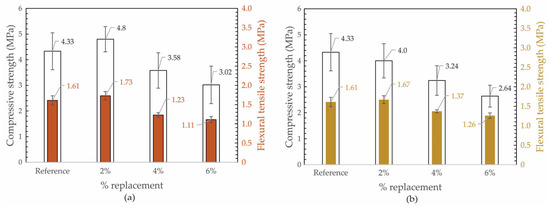
Figure 7.
Compressive strength and flexural tensile strength for different replacement for (a) AMD-Pi and (b) IND-Pi.
The reduction in mechanical strength in relation to the increase in pigment may be related to the water/binder ratio, which increased as the percentage of substitution increased. This increase in the amount of water was necessary to keep the consistency constant. Another factor that could contribute to a reduction in strength is the replacement of fine aggregate with pigment, but when sand is replaced with pigment, the amount of water needed to produce the mortar is adjusted. The percentage of 6% should not significantly affect the strength of the mortar, especially if the substitution is less than 10% [43].
On the other hand, the differences found in resistance between the pigment types may be related to their particle size, since AMD-Pi has smaller particles than IND-Pi. In this case, the pigment may have acted as a filer in the mortar, filling the voids.
3.2.2. Dimensional Variation
The dimensional variation results are shown in Figure 8, which shows the dimensional variation as a function of the percentage of pigment added, showing the influence of two types of pigment (Figure 8a) and curing time (Figure 8b). Compared to the reference mortar, there is shrinkage at 7 and 14 days of curing and expansion at 28 days of curing. With regard to the effect of the type of pigment, it can be seen that AMD-Pi showed expansion as the percentage of substitution in relation to the reference mortar increased. IND-Pi, on the other hand, showed irregular behavior, i.e., with 2% substitution, there was a shrinkage in relation to the reference material; with 4% substitution, there was an expansion in relation to 2%; and with 6%, there was a shrinkage in relation to the material with 4% substitution. With regard to the effect of curing time, little impact could be observed over the study period, however, there was a tendency for expansion as the percentage of substitution increased.
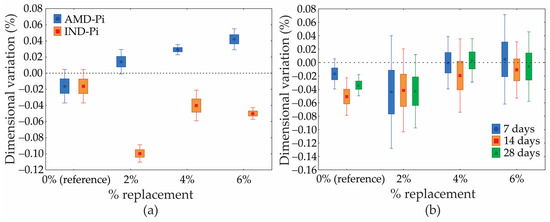
Figure 8.
Dimensional variation as a function of the percentage of addition for (a) the two types of pigment and (b) different curing times.
The main reason for the influence of pigments on mortar is probably the interference of the shape and dimensions of the pigment on the microstructure and packing density of the mixtures [23]. For example, if the pigments are long needles or flat particles, they can disturb the organization of the particles in the mortar matrix, creating spaces or channels between them. This can result in a nonuniform distribution of voids in the matrix, which in turn can lead to expansion or contraction. Packing density refers to the efficiency with which the particles organize themselves and fill the available space. When pigments are added, they occupy part of this space. The way the pigments fit together with the cement and sand particles affects the overall packing density. If the pigments have shapes that allow for more efficient packing, the density of the mixture can increase, resulting in expansion. On the other hand, if the pigments introduce significant voids between themselves and the matrix particles, the density can decrease, resulting in contraction.
3.2.3. Loss of Mass
Figure 9 shows the mass loss results for different types of pigment and curing times. It can be seen that as the percentage of addition and curing time increase, the loss of mass also increases. Furthermore, compared to the use of IND-Pi in the mortars, the use of AMD-Pi proved to be slightly more susceptible to mass loss. In other words, mortars containing AMD-Pi, regardless of the percentage added, showed a greater loss of mass compared to mortars developed with IND-Pi.
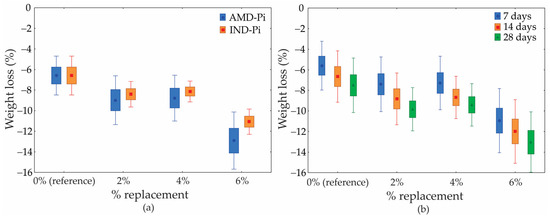
Figure 9.
Loss of mass in relation to the percentage of pigment added. (a) Different types of pigment and (b) curing time.
The difference in mass loss between the pigments (IND-Pi and AMD-Pi) may be related to the salts contained in AMD-Pi, which have the potential to cause expansion and cracking in mortars, thus facilitating water loss during the curing period. On the other hand, a greater loss in mass in relation to the percentage of replacement of natural aggregate with pigment is associated with a greater amount of water present in the mortar formulation. This is because, by increasing the amount of pigment in the mortar, it is necessary to add more water to the mixture in order to maintain a constant consistency. As a result, more water is lost during the curing period [44].
3.2.4. Colorimetry
Figure 10 shows the reflectance curve of the decorative mortars with their respective contents. The image shows a similar colorimetric behavior at wavelengths from 400 to 700 nm, with curves with different values but the same shape in the visible range, with the exception of the reference mortar.
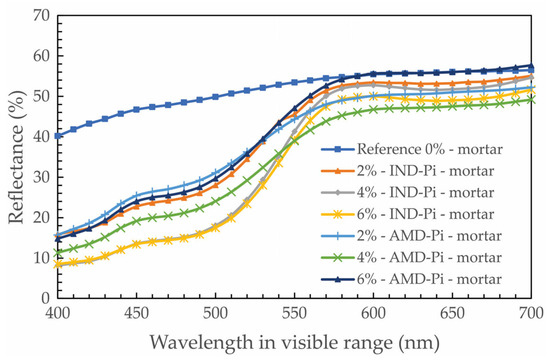
Figure 10.
Reflectance for different mortars.
It can be seen that, in relation to the L* results (Table 7), the mortars with IND-Pi pigment and AMD-PI pigment are similar, with a slight difference in the mortars containing AMD-Pi, which are slightly lighter (higher L* value), especially at the 2%, 6%, and 10% substitution levels, compared to the mortars containing IND-Pi. This is contrary to what was observed in the analysis of the pigments in powder form, where IND-Pi had a higher luminosity value than AMD-Pi.

Table 7.
Average values of the colorimetric parameters and results of the difference in shade of the mortars with their respective contents (ΔE*Lab).
With regard to the a* parameter, it can be seen that the mortars with IND-Pi tend to be less reddish (lower a* value) and more yellowish (higher b* value) compared to the mortars made with AMD-Pi. This may be due to the greater tinting potential of IND-Pi.
4. Conclusions
In summary, the main aim of this study was to evaluate the physical and mechanical properties of decorative mortars developed by partially replacing natural sand with pigment from acid mine drainage. According to the results, the following conclusions can be drawn.
Obtaining this pigment from the process of selective precipitation for metals in acid mine drainage proved to be an effective procedure, recovering around 80% of the iron contained in AMD, transforming it into a yellow pigment (goethite) using hydrometallurgical techniques.
The detailed characterization of the pigment revealed that it is suitable for use as a pigment, although it does show some variations in relation to industrial pigments, especially in terms of tone. However, the possibility of using the AMD-Pi pigment paves the way for using the resource in a more sustainable way and with potential for innovation in the decorative mortar industry.
The workability of the decorative mortars is affected by the increase in the addition of pigment, as an increase in the amount of water, with the increase in the percentage of pigment substitution, was necessary to maintain the desired consistency.
With regard to mechanical strength, the results indicated that the addition of pigment had a negative influence on flexural tensile strength and compressive strength, especially at higher substitution levels. The statistical tests confirmed these trends.
With regard to dimensional variation and loss of mass, the AMD-Pi pigment had a significant influence on both response variables, i.e., an expansion was observed for dimensional variation and a decrease in mass was observed when the percentage of substitution increased.
With regard to the colors of the decorative mortars, the AMD-Pi pigment was similar to the industrial pigment (IND-Pi), with slight variations in shades.
The significant increase in the amount of water incorporated during the mortar production process due to the increase in the proportion of pigments used emerged as one of the main factors responsible for the notable variations in the properties of these materials. However, it is important to note that this influence can be mitigated in future projects by adopting specific additives. The incorporation of such additives provides an effective means of adjusting the water/cement ratio and, consequently, optimizing the characteristics of mortars, ensuring the desired consistency without compromising their strength and durability.
Finally, this study has made a contribution to the field of decorative mortars, exploring an alternative source of pigment and promoting technological innovation while respecting the environment. Despite the limitations observed in terms of mechanical resistance and colorimetry, this work represents an important step towards more sustainable products in the construction industry.
Author Contributions
Conceptualization, R.d.A.S. and R.T.L.; data curation, G.d.O.O. and R.T.L.; formal analysis, R.d.A.S. and R.T.L.; investigation, R.d.A.S. and G.d.O.O.; methodology, R.d.A.S., G.d.O.O. and R.T.L.; project administration, R.d.A.S.; resources, R.T.L.; validation, R.d.A.S., G.d.O.O. and R.T.L.; visualization, R.d.A.S., G.d.O.O. and R.T.L.; writing—original draft preparation, R.T.L.; writing—review and editing, R.d.A.S., G.d.O.O. and R.T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are openly available in article.
Acknowledgments
The authors of this paper would like to thank ATITUS Educação and Fundação Meridional for their support, for making available the laboratories used to perform the tests.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Weiler, J.; Schneider, I.A.H. Pyrite Utilization in the Carboniferous Region of Santa Catarina, Brazil-Potentials, Challenges, and Environmental Advantages. REM-Int. Eng. J. 2019, 72, 515–522. [Google Scholar] [CrossRef]
- Hadadi, F.; Jodeiri Shokri, B.; Zare Naghadehi, M.; Doulati Ardejani, F. Probabilistic Prediction of Acid Mine Drainage Generation Risk Based on Pyrite Oxidation Process in Coal Washery Rejects—A Case Study. J. Min. Environ. 2021, 12, 127–137. [Google Scholar]
- León, R.; Macías, F.; Cánovas, C.R.; Millán-Becerro, R.; Pérez-López, R.; Ayora, C.; Nieto, J.M. Evidence of Rare Earth Elements Origin in Acid Mine Drainage from the Iberian Pyrite Belt (SW Spain). Ore Geol. Rev. 2023, 154, 105336. [Google Scholar] [CrossRef]
- Caldeira, J.G.; Zancan, F.L.; Gomes, C.J.B.; Dalpont, G. Coal Resources, Production, Use, and Reducing Emissions in Brazil. In The Coal Handbook; Elsevier: Amsterdam, The Netherlands, 2023; pp. 257–300. [Google Scholar]
- Weiler, J.; do Amaral Filho, J.R.; Schneider, I.A.H. Processamento de Rejeito de Carvão Visando a Redução de Custos No Tratamento Da Drenagem Ácida de Minas-Estudo de Caso Na Região Carbonífera de Santa Catarina. Eng. Sanitária Ambient. 2016, 21, 337–345. [Google Scholar] [CrossRef][Green Version]
- Silva, R.d.A.; Secco, M.P.; Menezes, J.C.S.d.S.; Schneider, I.A.H.; Lermen, R.T. Reduction of High-Chromium-Containing Wastewater in the Leaching of Pyritic Waste Rocks from Coal Mines. Sustainability 2022, 14, 11814. [Google Scholar] [CrossRef]
- Tong, L.; Fan, R.; Yang, S.; Li, C. Development and Status of the Treatment Technology for Acid Mine Drainage. Min. Metall. Explor. 2021, 38, 315–327. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Y.; Zhang, Z.; Zhang, Y. Acid Mine Drainage (AMD) in Abandoned Coal Mines of Shanxi, China. Water 2020, 13, 8. [Google Scholar] [CrossRef]
- De Almeida Silva, R.; Secco, M.P.; Lermen, R.T.; Schneider, I.A.H.; Hidalgo, G.E.N.; Sampaio, C.H. Optimizing the Selective Precipitation of Iron to Produce Yellow Pigment from Acid Mine Drainage. Miner. Eng. 2019, 135, 111–117. [Google Scholar] [CrossRef]
- Hedin, R.S. Long-Term Minimization of Mine Water Treatment Costs through Passive Treatment and Production of a Saleable Iron Oxide Sludge. In Mining Meets Water–Conflicts and Solutions, Proceedings of the IMWA 2016 Conference, Leipzig, Germany, 11–15 July 2016; International Mine Water Association: Freiberg, Germany, 2016; pp. 1267–1273. [Google Scholar]
- Masindi, V.; Foteinis, S.; Renforth, P.; Ndiritu, J.; Maree, J.P.; Tekere, M.; Chatzisymeon, E. Challenges and Avenues for Acid Mine Drainage Treatment, Beneficiation, and Valorisation in Circular Economy: A Review. Ecol. Eng. 2022, 183, 106740. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid Mine Drainage Remediation Options: A Review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Kefeni, K.K.; Msagati, T.A.M.; Mamba, B.B. Acid Mine Drainage: Prevention, Treatment Options, and Resource Recovery: A Review. J. Clean. Prod. 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Yuan, J.; Ding, Z.; Bi, Y.; Li, J.; Wen, S.; Bai, S. Resource Utilization of Acid Mine Drainage (AMD): A Review. Water 2022, 14, 2385. [Google Scholar] [CrossRef]
- Hedin, R.S. Recovery of Marketable Iron Oxide from Mine Drainage in the USA. Land Contam. Reclam. 2003, 11, 93–98. [Google Scholar] [CrossRef]
- Ryan, M.J.; Kney, A.D.; Carley, T.L. A Study of Selective Precipitation Techniques Used to Recover Refined Iron Oxide Pigments for the Production of Paint from a Synthetic Acid Mine Drainage Solution. Appl. Geochem. 2017, 79, 27–35. [Google Scholar] [CrossRef]
- Marcello, R.R.; Galato, S.; Peterson, M.; Riella, H.G.; Bernardin, A.M. Inorganic Pigments Made from the Recycling of Coal Mine Drainage Treatment Sludge. J. Environ. Manag. 2008, 88, 1280–1284. [Google Scholar] [CrossRef]
- Masindi, V.; Tekere, M. Innovative Routes for Acid Mine Drainage (AMD) Valorization: Advocating for a Circular Economy. In Recovery of Byproducts from Acid Mine Drainage Treatment; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 189–218. [Google Scholar]
- Tau, A.L.; Maree, J.P.; Letjiane, S.L.; Adeniyi, A.; Onyango, M.S. Pigment Recovered from Iron Rich Mine Water for Use in Colored Concrete. In Proceedings of the 36th Int’l Conference on “Chemical, Biological, Environmental & Natural Resources” (CBENR-23), Kuala Lumpur, Malaysia, 31 May–2 June 2023. [Google Scholar]
- Sadasivam, S.; Thomas, H.R. Colour and Toxic Characteristics of Metakaolinite–Hematite Pigment for Integrally Coloured Concrete, Prepared from Iron Oxide Recovered from a Water Treatment Plant of an Abandoned Coal Mine. J. Solid State Chem. 2016, 239, 246–250. [Google Scholar] [CrossRef]
- Hatami, L.; Jamshidi, M.; Yavari, M. Improving Mechanical/Colorimetric Properties of Self-Compacting Mortar Using an Intensively Colored-Nanoparticle Containing Polymeric Paste. J. Build. Eng. 2023, 66, 105841. [Google Scholar] [CrossRef]
- De Oliveira, G.F.F.; de Souza, E.J.; de Lima Gomes, A.J. Study of the Technical Feasibility of Using Colored Mortar for Coating. Int. J. Geosci. Eng. Technol. 2023, 7, 8–15. [Google Scholar]
- Miranda, J.; Costa, H.; Valença, J.; do Carmo, R.; Júlio, E. Design and Durability Assessment of Restoring Mortar for Concrete Heritage. Materials 2021, 14, 4508. [Google Scholar] [CrossRef]
- Miranda, J.; Valenca, J.; Julio, E. Colored Concrete Restoration Method: For Chromatic Design and Application of Restoration Mortars on Smooth Surfaces of Colored Concrete. Struct. Concr. 2019, 20, 1391–1401. [Google Scholar] [CrossRef]
- Lima, L.; Trindade, E.; Alencar, L.; Alencar, M.; Silva, L. Sustainability in the Construction Industry: A Systematic Review of the Literature. J. Clean. Prod. 2021, 289, 125730. [Google Scholar] [CrossRef]
- Norouzi, M.; Chàfer, M.; Cabeza, L.F.; Jiménez, L.; Boer, D. Circular Economy in the Building and Construction Sector: A Scientific Evolution Analysis. J. Build. Eng. 2021, 44, 102704. [Google Scholar] [CrossRef]
- Mohammed, S.A.; Koting, S.; Katman, H.Y.B.; Babalghaith, A.M.; Abdul Patah, M.F.; Ibrahim, M.R.; Karim, M.R. A Review of the Utilization of Coal Bottom Ash (CBA) in the Construction Industry. Sustainability 2021, 13, 8031. [Google Scholar] [CrossRef]
- Menezes, J.; Colling, A.V.; Silva, R.A.S.; Scheneider, I.A.H. Effect of Pyrite Concentration on the Quality of Ferric Sulfate Coagulants Obtained by Leaching from Coal Tailings. Miner. Metall. Process. 2016, 33, 77–81. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences, and Uses; Wiley-VCH: Weinheim, Germany, 2003; Volume 664. [Google Scholar]
- Schwertmann, U.; Murad, E. Effect of PH on the Formation of Goethite and Hematite from Ferrihydrite. Clays Clay Miner. 1983, 31, 277–284. [Google Scholar] [CrossRef]
- Schwertmann, U.; Stanjek, H.; Becher, H.-H. Long-Term in Vitro Transformation of 2-Line Ferrihydrite to Goethite/Hematite at 4, 10, 15 and 25 C. Clay Miner. 2004, 39, 433–438. [Google Scholar] [CrossRef]
- Silva, R.d.A.; Castro, C.D.; Petter, C.O.; Schneider, I.A.H. Production of Iron Pigments (Goethite and Haematite) from Acid Mine Drainage. In Proceedings of the 11th International Mine Water Association Congress, Aachen, Germany, 4–11 September 2011; pp. 469–474. [Google Scholar]
- Akinwekomi, V.; Maree, J.P.; Masindi, V.; Zvinowanda, C.; Osman, M.S.; Foteinis, S.; Mpenyana-Monyatsi, L.; Chatzisymeon, E. Beneficiation of Acid Mine Drainage (AMD): A Viable Option for the Synthesis of Goethite, Hematite, Magnetite, and Gypsum–Gearing towards a Circular Economy Concept. Miner. Eng. 2020, 148, 106204. [Google Scholar] [CrossRef]
- Carasek, H.; Araújo, R.C.; Cascudo, O.; Angelim, R. Parâmetros Da Areia Que Influenciam a Consistência e a Densidade de Massa Das Argamassas de Revestimento. Matéria 2016, 21, 714–732. [Google Scholar] [CrossRef]
- BS EN 459-1; Building Lime—Definitions, Specifications and Conformity Criteria. British Standards Institution: London, UK, 2015.
- Lee, H.-S.; Lee, J.-Y.; Yu, M.-Y. Influence of Inorganic Pigments on the Fluidity of Cement Mortars. Cem. Concr. Res. 2005, 35, 703–710. [Google Scholar] [CrossRef]
- ABNT NBR 13276; Argamassa Para Assentamento e Revestimento de Paredes e Tetos—Determinação Do Índice de Consistência. Associação Brasileira de Normas Técnicas (ABNT): Rio de Janeiro, Brazil, 2016.
- ABNT NBR 13279; Argamassa Para Assentamento e Revestimento de Paredes e Tetos—Determinação Da Resistência à Tração Na Flexão e à Compressão. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2005.
- ABNT NBR 15261; Argamassa Para Assentamento e Revestimento de Paredes e Tetos—Determinação Da Variação Dimensional (Retratação Ou Expansão Linear). Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2005.
- DIN DIN 6174; Farbmetrische Bestimmung von Farbabständen Bei Körperfarben Nach Der CIELAB-Formel. Deutsche Institut für Normung: Berlin, Germany, 1979.
- Silva, R.d.A. Recuperação Hidrometalúrgica de Metais Da Drenagem Ácida de Minas Por Precipitação Seletiva. Ph.D. Thesis, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, Brazil, 2010. [Google Scholar]
- EPA. Summary Report: Control and Treatment Technology for the Metal Finishing Industry; Sulfide Precipitation; Environmental Protection Agency: Boston, MA, USA, 1980; Volume 625.
- Morais, C.F.; Belo, B.R.; Bezerra, A.C.S.; Loura, R.M.; Porto, M.P.; Bessa, S.A.L. Thermal and Mechanical Analyses of Colored Mortars Produced Using Brazilian Iron Ore Tailings. Constr. Build. Mater. 2021, 268, 121073. [Google Scholar] [CrossRef]
- Hall, D.A.; Stevens, R.; Jazairi, B. El Effect of Water Content on the Structure and Mechanical Properties of Magnesia-phosphate Cement Mortar. J. Am. Ceram. Soc. 1998, 81, 1550–1556. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).