Osteoporosis: Focus on Bone Remodeling and Disease Types
Abstract
1. Introduction
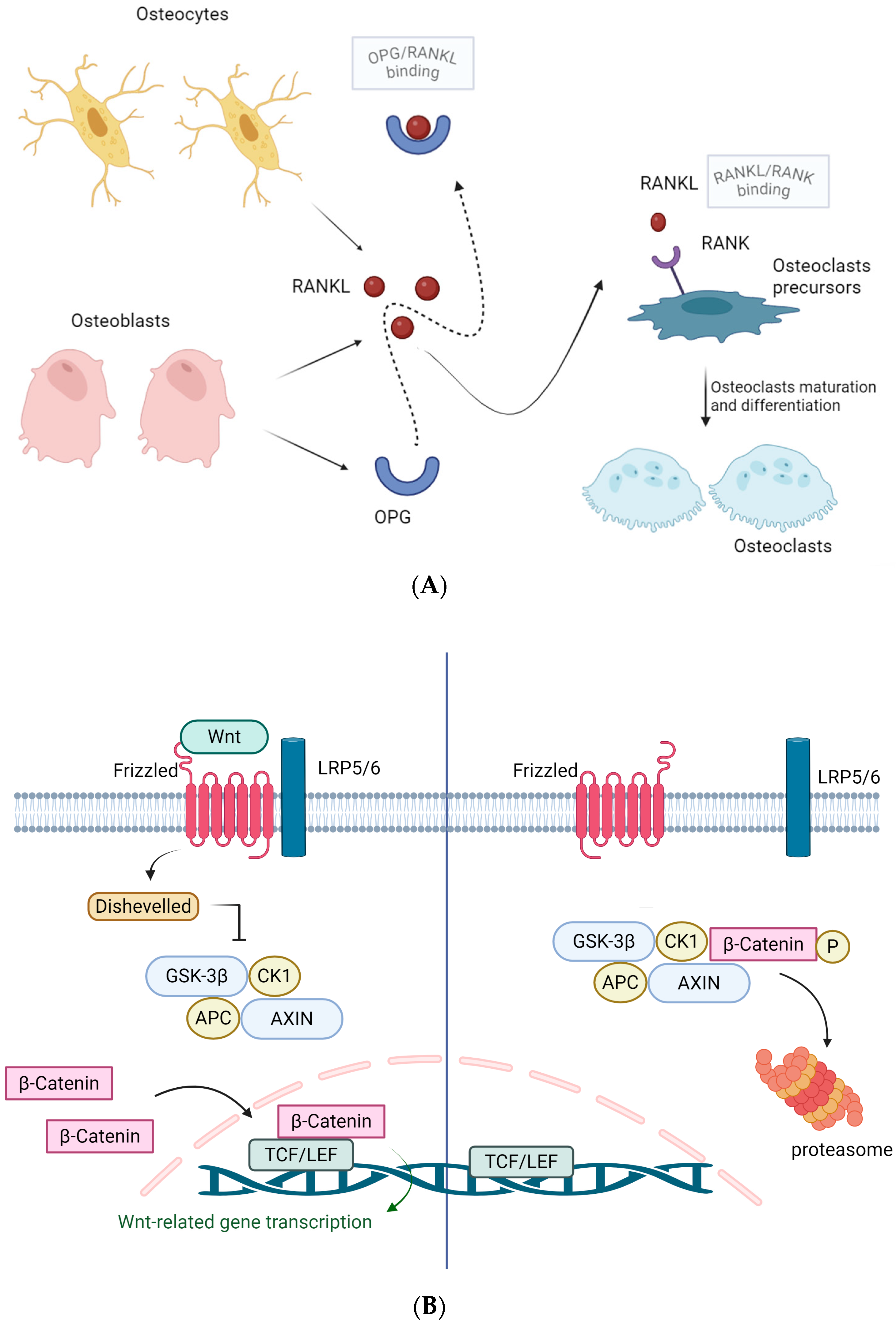
2. Bone Physiology
3. Osteoporosis Definition and Epidemiology
4. Primary Osteoporosis
5. Male Osteoporosis
6. Secondary Osteoporosis
6.1. Glucocorticoids
6.2. Endocrine Disorders
6.2.1. Diabetes Mellitus
6.2.2. Thyroid and Parathyroid
6.2.3. Hypogonadism
6.2.4. Obesity
6.2.5. Miscellaneous
6.3. GI Disease
6.4. Transplantation
6.5. Cancer
6.6. Other Drugs
6.7. Skeletal Development Disorders and Genetic Causes
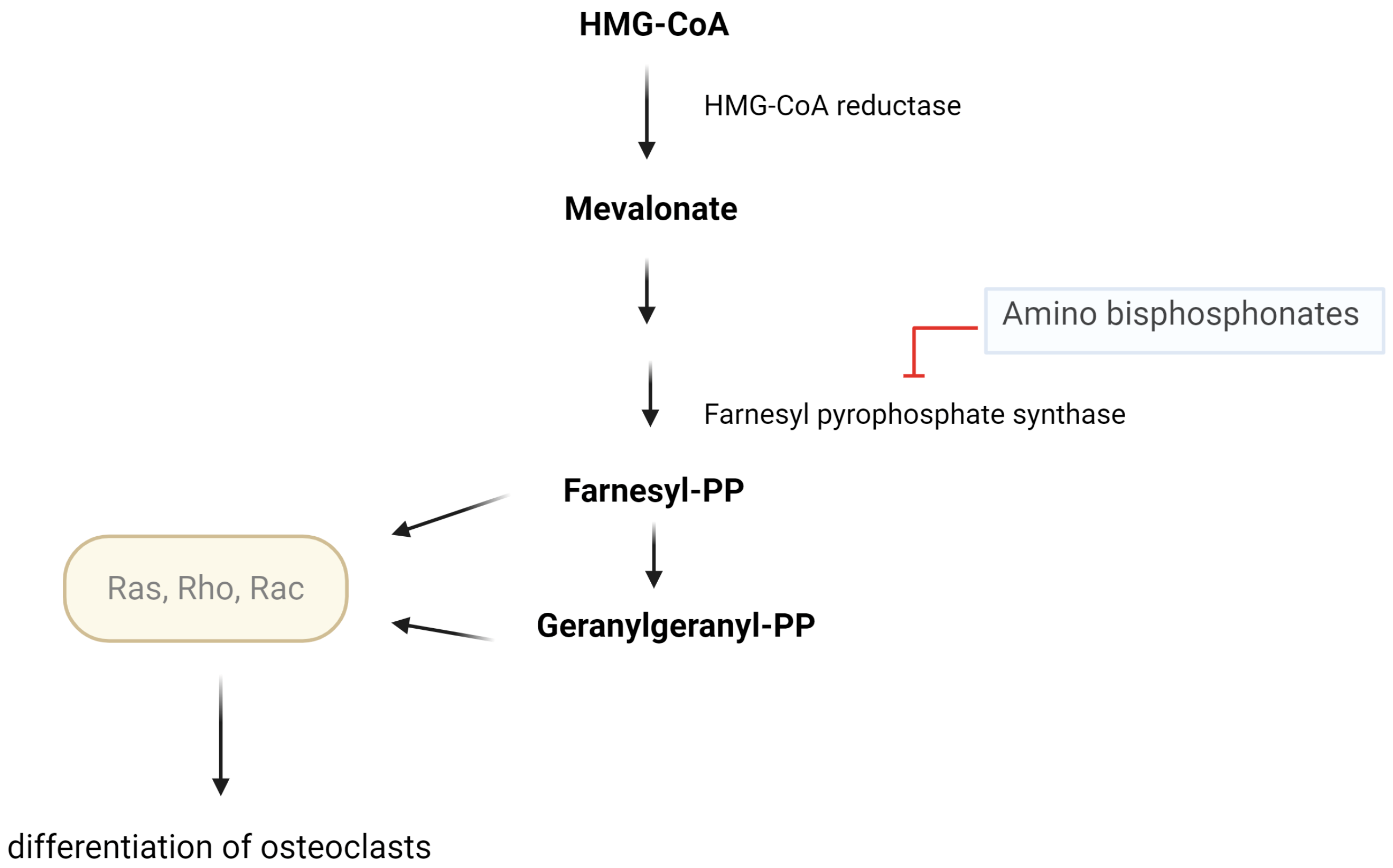
7. Treatment
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Consensus Development Conference. Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Preclinical Evaluation and Clinical Trials in Osteoporosis; World Health Organization: Geneva, Switzerland, 1998; Available online: https://iris.who.int/handle/10665/42088 (accessed on 20 April 2025).
- Wu, A.M.; Bisignano, C.; James, S.L.; Abady, G.G.; Abedi, A.; Abu-Gharbieh, E.; Alhassan, R.K.; Alipour, V.; Alipour, J.; Asaad, M.; et al. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the global burden of disease study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef]
- Murray, S.L.; Wolf, M. Calcium and Phosphate Disorders: Core Curriculum 2024. Am. J. Kidney Dis. 2024, 83, 241–256. [Google Scholar] [CrossRef]
- Khan, M.; Jose, A.; Sharma, S. Physiology, Parathyroid Hormone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Leung, E.K.Y. Parathyroid hormone. Adv. Clin. Chem. 2021, 101, 41–93. [Google Scholar]
- Srinivasan, A.; Wong, F.K.; Karponis, D. Calcitonin: A useful old friend. J. Musculoskelet. Neuronal Interact. 2020, 20, 600–609. [Google Scholar] [PubMed]
- Lung, B.E.; Komatsu, D.E.E. Calcitriol. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Columbu, C.; Rendina, D.; Gennari, L.; Pugliese, F.; Carnevale, V.; Salcuni, A.S.; Chiodini, I.; Battista, C.; Tabacco, P.; Guarnieri, V.; et al. Phosphate metabolism in primary hyperparathyroidism: A real-life long-term study. Endocrine 2025, 88, 571–580. [Google Scholar] [CrossRef]
- Ho, B.B.; Bergwitz, C. FGF23 signalling and physiology. J. Mol. Endocrinol. 2021, 66, R23–R32. [Google Scholar] [CrossRef]
- Edmonston, D.; Grabner, A.; Wolf, M. FGF23 and klotho at the intersection of kidney and cardiovascular disease. Nat. Rev. Cardiol. 2024, 21, 11–24. [Google Scholar] [CrossRef]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Eng. Part B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef]
- Nahian, A.; AlEssa, A.M. Histology, Osteocytes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kitazawa, S.; Haraguchi, R.; Kitazawa, R. Roles of osteoclasts in pathological conditions. Pathol. Int. 2025, 75, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Hasan, W.N.W.; Chin, K.Y.; Jolly, J.J.; Ghafar, N.A.; Soelaiman, I.N. Identifying potential therapeutics for osteoporosis by exploiting the relationship between the mevalonate pathway and bone metabolism. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 450–457. [Google Scholar] [CrossRef]
- Jung, J.; Park, J.S.; Chun, J.; Al-Nawas, B.; Ziebart, T.; Kwon, Y.D. Geranylgeraniol application in human osteoblasts and osteoclasts for reversal of the effect of bisphosphonates. Life 2023, 13, 1353. [Google Scholar] [CrossRef]
- Xu, W.; Gong, L.; Tang, W.; Lu, G. Nitrogen-containing bisphosphonate induces enhancement of OPG expression and inhibition of RANKL expression via inhibition of farnesyl pyrophosphate synthase to inhibit the osteogenic differentiation and calcification in vascular smooth muscle cells. BMC Cardiovasc. Disord. 2024, 24, 494. [Google Scholar] [CrossRef]
- McDonald, M.M.; Khoo, W.H.; Ng, P.Y.; Xiao, Y.; Zamerli, J.; Thatcher, P.; Kyaw, W.; Pathmanandavel, K.; Grootveld, A.K.; Moran, I.; et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell 2021, 184, 1330–1347.e13. [Google Scholar] [CrossRef]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG pathway: A mechanism involved in exercise-induced bone remodeling. Biomed. Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Zhang, Q.; Zhang, L. Effect of OPG gene mutation on protein expression and biological activity in osteoporosis. Exp. Ther. Med. 2017, 14, 1475–1480. [Google Scholar] [CrossRef]
- Wang, T.; He, C. TNF-alpha and IL-6: The link between immune and bone system. Curr. Drug Targets 2020, 21, 213–227. [Google Scholar]
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of osteoclast differentiation by cytokine networks. Immune Netw. 2018, 18, e8. [Google Scholar] [CrossRef]
- Marahleh, A.; Kitaura, H.; Ohori, F.; Kishikawa, A.; Ogawa, S.; Shen, W.R.; Qi, J.; Noguchi, T.; Nara, Y.; Mizoguchi, I. TNF-alpha directly enhances osteocyte RANKL expression and promotes osteoclast formation. Front. Immunol. 2019, 10, 2925. [Google Scholar] [CrossRef] [PubMed]
- Agostino, M.; Pohl, S. The structural biology of canonical Wnt signalling. Biochem. Soc. Trans. 2020, 48, 1765–1780. [Google Scholar] [CrossRef] [PubMed]
- Sawakami, K.; Robling, A.G.; Ai, M.; Pitner, N.D.; Liu, D.; Warden, S.J.; Li, J.; Maye, P.; Rowe, D.W.; Duncan, R.L.; et al. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J. Biol. Chem. 2006, 281, 23698–23711. [Google Scholar] [CrossRef]
- Komori, T. Molecular mechanism of Runx2-dependent bone development. Mol. Cells 2020, 43, 168–175. [Google Scholar]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Oden, A.; Melton, L.J., 3rd; Khaltaev, N. A reference standard for the description of osteoporosis. Bone 2008, 42, 467–475. [Google Scholar] [CrossRef]
- Osteoporosis Foundation. Epidemiology of Osteoporosis and Fragility Fractures. Available online: https://www.osteoporosis.foundation/facts-statistics/epidemiology-of-osteoporosis-and-fragility-fractures (accessed on 20 April 2025).
- Patel, D.; Saxena, B. Decoding osteoporosis: Understanding the disease, exploring current and new therapies, and emerging targets. J. Orthop. Rep. 2025, 4, 100472. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z. Ovarian aging and osteoporosis. Adv. Exp. Med. Biol. 2018, 1086, 199–215. [Google Scholar]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef]
- Cauley, J.A. Estrogen and bone health in men and women. Steroids 2015, 99, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Vilaca, T.; Eastell, R.; Schini, M. Osteoporosis in men. Lancet Diabetes Endocrinol. 2022, 10, 273–283. [Google Scholar] [CrossRef]
- Kassem, M.; Marie, P.J. Senescence-associated intrinsic mechanisms of osteoblast dysfunctions. Aging Cell 2011, 10, 191–197. [Google Scholar] [CrossRef]
- Zhou, S.; Greenberger, J.S.; Epperly, M.W.; Goff, J.P.; Adler, C.; Leboff, M.S.; Glowacki, J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell 2008, 7, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M. Aging mechanisms in bone. Bonekey Rep. 2012, 1, 102. [Google Scholar] [CrossRef] [PubMed]
- Jilka, R.L.; Almeida, M.; Ambrogini, E.; Han, L.; Roberson, P.K.; Weinstein, R.S.; Manolagas, S.C. Decreased oxidative stress and greater bone anabolism in the aged, as compared to the young, murine skeleton by parathyroid hormone. Aging Cell 2010, 9, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C.; Almeida, M. Gone with the Wnts: Beta-catenin, T-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol. Endocrinol. 2007, 21, 2605–2614. [Google Scholar] [CrossRef]
- Pfeilschifter, J.; Diel, I.; Pilz, U.; Brunotte, K.; Naumann, A.; Ziegler, R. Mitogenic responsiveness of human bone cells in vitro to hormones and growth factors decreases with age. J. Bone Miner. Res. 1993, 8, 707–717. [Google Scholar] [CrossRef]
- Campisi, J. Senescent cells, tumor suppression, and organismal aging: Good citizens, bad neighbors. Cell 2005, 120, 513–520. [Google Scholar] [CrossRef]
- Han, M.H.; Kwon, H.S.; Hwang, M.; Park, H.H.; Jeong, J.H.; Park, K.W.; Kim, E.J.; Yoon, S.J.; Yoon, B.; Jang, J.W.; et al. Association between osteoporosis and the rate of telomere shortening. Aging 2024, 16, 11151–11161. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, J.C.; Jiang, Q.; Lee, W.Y. Role of sirtuins in bone biology: Potential implications for novel therapeutic strategies for osteoporosis. Aging Cell 2021, 20, e13301. [Google Scholar] [CrossRef]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef]
- Xing, Y.; Liu, C.; Zhou, L.; Li, Y.; Wu, D. Osteogenic effects of rapamycin on bone marrow mesenchymal stem cells via inducing autophagy. J. Orthop. Surg. Res. 2023, 18, 129. [Google Scholar] [CrossRef] [PubMed]
- Suarjana, I.N.; Isbagio, H.; Soewondo, P.; Rachman, I.A.; Sadikin, M.; Prihartono, J.; Malik, S.G.; Soeroso, J. The role of serum expression levels of microRNA-21 on bone mineral density in hypostrogenic postmenopausal women with osteoporosis: Study on level of RANKL, OPG, TGFβ-1, sclerostin, RANKL/OPG ratio, and physical activity. Acta Med. Indones 2019, 51, 245–252. [Google Scholar]
- Zuo, B.; Zhu, J.F.; Li, J.; Wang, C.D.; Zhao, X.Y.; Cai, G.Q.; Li, Z.; Peng, J.; Wang, P.; Shen, C.; et al. MicroRNA-103a functions as a mechanosensitive microRNA to inhibit bone formation through targeting Runx2. J. Bone Miner. Res. 2015, 30, 330–345. [Google Scholar] [CrossRef]
- Liu, H.; Yue, X.; Zhang, G. Downregulation of miR-146a inhibits osteoporosis in the jaws of ovariectomized rats by regulating the Wnt/β-catenin signaling pathway. Int. J. Mol. Med. 2021, 47, 6. [Google Scholar] [CrossRef]
- Trojniak, J.; Sendera, A.; Banaś-Ząbczyk, A.; Kopańska, M. The microRNAs in the pathophysiology of osteoporosis. Int. J. Mol. Sci. 2024, 25, 6240. [Google Scholar] [CrossRef]
- Zamani, A.; Zamani, V.; Heidari, B.; Parsian, H.; Esmaeilnejad-Ganji, S.M. Prevalence of osteoporosis with the World Health Organization diagnostic criteria in the Eastern Mediterranean Region: A systematic review and meta-analysis. Arch. Osteoporos. 2018, 13, 129. [Google Scholar] [CrossRef]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef]
- Chen, P.; Li, Z.; Hu, Y. Prevalence of osteoporosis in China: A meta-analysis and systematic review. BMC Public Health 2016, 16, 1039. [Google Scholar] [CrossRef]
- Bliuc, D.; Nguyen, N.D.; Milch, V.E.; Nguyen, T.V.; Eisman, J.A.; Center, J.R. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. J. Am. Med. Assoc. 2009, 301, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, L.; Bilezikian, J.P. Novel Therapies for Postmenopausal Osteoporosis. Endocrinol. Metab. Clin. N. Am. 2017, 46, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Riggs, B.L.; Atkinson, E.J.; Oberg, A.L.; McDaniel, L.J.; Holets, M.; Peterson, J.M.; Melton, L.J., 3rd. Effects of sex and age on bone microstructure at the ultradistal radius: A population-based noninvasive in vivo assessment. J. Bone Miner. Res. 2006, 21, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Monroe, D.G. Regulation of Bone Metabolism by Sex Steroids. Cold Spring Harb. Perspect. Med. 2018, 8, a031211. [Google Scholar] [CrossRef]
- Szulc, P.; Claustrat, B.; Marchand, F.; Delmas, P.D. Increased risk of falls and increased bone resorption in elderly men with partial androgen deficiency: The MINOS study. J. Clin. Endocrinol. Metab. 2003, 88, 5240–5247. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A.; Ewing, S.K.; Taylor, B.C.; Fink, H.A.; Ensrud, K.E.; Bauer, D.C.; Barrett-Connor, E.; Marshall, L.; Orwoll, E.S.; Osteoporotic Fractures in Men Study (MrOS) Research Group. Sex steroid hormones in older men: Longitudinal associations with 4.5-year change in hip bone mineral density—The osteoporotic fractures in men study. J. Clin. Endocrinol. Metab. 2010, 95, 4314–4323. [Google Scholar] [CrossRef]
- LeBlanc, E.S.; Nielson, C.M.; Marshall, L.M.; Lapidus, J.A.; Barrett-Connor, E.; Ensrud, K.E.; Hoffman, A.R.; Laughlin, G.; Ohlsson, C.; Orwoll, E.S.; et al. The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. J. Clin. Endocrinol. Metab. 2009, 94, 3337–3346. [Google Scholar] [CrossRef] [PubMed]
- Kirby, D.J.; Buchalter, D.B.; Anil, U.; Leucht, P. DHEA in bone: The role in osteoporosis and fracture healing. Arch. Osteoporos. 2020, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, P.; Roato, I.; D’Amico, L.; Veneziano, L.; Suman, E.; Sassi, F.; Bisignano, G.; Ferracini, R.; Gargiulo, G.; Castoldi, F.; et al. Bone and bone marrow pro-osteoclastogenic cytokines are up-regulated in osteoporosis fragility fractures. Osteoporos. Int. 2011, 22, 2869–2877. [Google Scholar] [CrossRef]
- Lips, P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr. Rev. 2001, 22, 477–501. [Google Scholar] [CrossRef]
- Metter, E.J.; Conwit, R.; Tobin, J.; Fozard, J.L. Age-associated loss of power and strength in the upper extremities in women and men. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1997, 52, B267–B276. [Google Scholar] [CrossRef]
- Izquierdo, M.; Ibañez, J.; Gorostiaga, E.; Garrues, M.; Zúniga, A.; Antón, A.; Larriòn, J.L.; Hakkinen, K. Maximal strength and power characteristics in isometric and dynamic actions of the upper and lower extremities in middle-aged and older men. Acta Physiol. Scand. 1999, 167, 57–68. [Google Scholar] [CrossRef]
- Compston, J. Glucocorticoid-induced osteoporosis: An update. Endocrine 2018, 61, 7–16. [Google Scholar] [CrossRef]
- van Staa, T.P.; Leufkens, H.G.M.; Abenhaim, L.; Zhang, B.; Cooper, C. Oral corticosteroids and fracture risk: Relationship to daily and cumulative doses. Rheumatology 2000, 39, 1383–1389. [Google Scholar] [CrossRef]
- Wu, Z.; Bucher, N.L.R.; Farmer, S.R. Induction of peroxisome proliferator-activated receptor γ during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPβ, C/EBPγ, and glucocorticoids. Mol. Cell Biol. 1996, 16, 4128–4136. [Google Scholar] [CrossRef]
- Weinstein, R.S.; Jilka, R.L.; Parfitt, A.M.; Manolagas, S.C. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids—Potential mechanisms of their deleterious effects on bone. J. Clin. Investig. 1998, 102, 274–282. [Google Scholar] [CrossRef]
- Ohnaka, K.; Tanabe, M.; Kawate, H.; Nawata, H.; Takayanagi, R. Glucocorticoid suppresses the canonical Wnt signal in cultured human osteoblasts. Biochem. Biophys. Res. Commun. 2005, 329, 177–181. [Google Scholar] [CrossRef]
- Swanson, C.; Lorentzon, M.; Conaway, H.H.; Lerner, U.H. Glucocorticoid regulation of osteoclast differentiation and expression of receptor activator of nuclear factor-kappaB (NF-kappaB) ligand, osteoprotegerin, and receptor activator of NF-kappaB in mouse calvarial bones. Endocrinology 2006, 147, 3613–3622. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Gori, F.; Riggs, B.L.; Lacey, D.L.; Dunstan, C.R.; Spelsberg, T.C.; Khosla, S. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblasts: Potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology 1999, 140, 4382–4389. [Google Scholar] [CrossRef]
- Mazziotti, G.; Formenti, A.M.; Adler, R.A.; Bilezikian, J.P.; Grossman, A.; Sbardella, E.; Minisola, S.; Giustina, A. Glucocorticoid-induced osteoporosis: Pathophysiological role of GH/IGF-I and PTH/vitamin D axes, treatment options and guidelines. Endocrine 2016, 54, 603–611. [Google Scholar] [CrossRef]
- Sato, A.Y.; Richardson, D.; Cregor, M.; Davis, H.M.; Au, E.D.; McAndrews, K.; Zimmers, T.A.; Organ, J.M.; Peacock, M.; Plotkin, L.I.; et al. Glucocorticoids induce bone and muscle atrophy by tissue-specific mechanisms upstream of E3 ubiquitin ligases. Endocrinology 2017, 158, 664–677. [Google Scholar] [CrossRef] [PubMed]
- Skowrońska-Jóźwiak, E.; Lewandowski, K. Editorial: Osteoporosis secondary to endocrine disorders. Front. Endocrinol. 2023, 14, 1194241. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, Q. Trends in osteoporosis and mean bone density among type 2 diabetes patients in the US from 2005 to 2014. Sci. Rep. 2021, 11, 3693. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Shepherd, S.; McMillan, M.; McNeilly, J.; Foster, J.; Wong, S.C.; Robertson, K.J.; Ahmed, S.F. Skeletal fragility and its clinical determinants in children with type 1 diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 3585–3594. [Google Scholar] [CrossRef]
- Shah, V.N.; Harrall, K.K.; Shah, C.S.; Gallo, T.L.; Joshee, P.; Snell-Bergeon, J.K.; Kohrt, W.M. Bone mineral density at femoral neck and lumbar spine in adults with type 1 diabetes: A meta-analysis and review of the literature. Osteoporos. Int. 2017, 28, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Busse, B.; Eastell, R.; Ferrari, S.; Frost, M.; Müller, R.; Burden, A.M.; Rivadeneira, F.; Napoli, N.; Rauner, M. Bone fragility in diabetes: Novel concepts and clinical implications. Lancet Diabetes Endocrinol. 2022, 10, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Holloway-Kew, K.L.; De Abreu, L.L.F.; Kotowicz, M.A.; Sajjad, M.A.; Pasco, J.A. Bone turnover markers in men and women with impaired fasting glucose and diabetes. Calcif. Tissue Int. 2019, 104, 599–604. [Google Scholar] [CrossRef]
- Kitaura, H.; Ogawa, S.; Ohori, F.; Noguchi, T.; Marahleh, A.; Nara, Y.; Pramusita, A.; Kinjo, R.; Ma, J.; Kanou, K.; et al. Effects of incretin-related diabetes drugs on bone formation and bone resorption. Int. J. Mol. Sci. 2021, 22, 6578. [Google Scholar] [CrossRef]
- Schacter, G.I.; Leslie, W.D. Diabetes and osteoporosis: Part I, epidemiology and pathophysiology. Endocrinol. Metab. Clin. N. Am. 2021, 50, 275–285. [Google Scholar] [CrossRef]
- Sheu, A.; Greenfield, J.R.; White, C.P.; Center, J.R. Assessment and treatment of osteoporosis and fractures in type 2 diabetes. Trends Endocrinol. Metab. 2022, 33, 333–344. [Google Scholar] [CrossRef]
- Martínez-Montoro, J.I.; García-Fontana, B.; García-Fontana, C.; Muñoz-Torres, M. Evaluation of quality and bone micro-structure alterations in patients with type 2 diabetes: A narrative review. J. Clin. Med. 2022, 11, 2206. [Google Scholar] [CrossRef] [PubMed]
- Sihota, P.; Yadav, R.N.; Dhaliwal, R.; Bose, J.C.; Dhiman, V.; Neradi, D.; Karn, S.; Sharma, S.; Aggarwal, S.; Goni, V.G.; et al. Investigation of mechanical, material, and compositional determinants of human trabecular bone quality in type 2 diabetes. J. Clin. Endocrinol. Metab. 2021, 106, e2271–e2289. [Google Scholar] [CrossRef]
- Yuan, S.; Wan, Z.H.; Cheng, S.L.; Michaëlsson, K.; Larsson, S.C. Insulin-like growth factor-1, bone mineral density, and fracture: A Mendelian randomization study. J. Clin. Endocrinol. Metab. 2021, 106, e1552–e1558. [Google Scholar] [CrossRef]
- Ouquerke, A.; Blulel, J. Osteoblasts and insulin: An overview. J. Biol. Regul. Homeost. Agents 2021, 35, 27–35. [Google Scholar]
- Govoni, K.E. Insulin-like growth factor-I molecular pathways in osteoblasts: Potential targets for pharmacological manipulation. Curr. Mol. Pharmacol. 2012, 5, 143–152. [Google Scholar] [CrossRef]
- Karim, L.; Bouxsein, M.L. Effect of type 2 diabetes-related non-enzymatic glycation on bone. Bone 2016, 82, 21–27. [Google Scholar] [CrossRef]
- Gortázar, A.R.; Ardura, J.A. Osteocytes and diabetes: Altered function of diabetic osteocytes. Curr. Osteoporos. Rep. 2020, 18, 796–802. [Google Scholar] [CrossRef]
- Kalaitzoglou, E.; Popescu, I.; Bunn, R.C.; Fowlkes, J.L.; Thrailkill, K.M. Effects of type 1 diabetes on osteoblasts, osteocytes, and osteoclasts. Curr. Osteoporos. Rep. 2016, 14, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Palermo, A.; D’Onofrio, L.; Buzzetti, R.; Manfrini, S.; Napoli, N. Pathophysiology of bone fragility in patients with diabetes. Calcif. Tissue Int. 2017, 100, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Skowrońska-Jóźwiak, E.; Krawczyk-Rusiecka, K.; Lewandowski, K.C.; Adamczewski, Z.; Lewiński, A. Successful treatment of thyrotoxicosis is accompanied by a decrease in serum sclerostin levels. Thyroid Res. 2012, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Skowrońska-Jóźwiak, E.; Lewandowski, K.C.; Adamczewski, Z.; Krawczyk-Rusiecka, K.; Lewiński, A. Mechanisms of normalisation of bone metabolism during recovery from hyperthyroidism: Potential role for sclerostin and parathyroid hormone. Int. J. Endocrinol. 2015, 2015, 948384. [Google Scholar] [CrossRef]
- Yeh, M.W.; Ituarte, P.H.; Zhou, H.C.; Nishimoto, S.; Liu, I.L.A.; Harari, A.; Haigh, P.I.; Adams, A.L. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J. Clin. Endocrinol. Metab. 2013, 98, 1122–1129. [Google Scholar] [CrossRef]
- Pallan, S.; Rahman, M.O.; Khan, A.A. Diagnosis and management of primary hyperparathyroidism. BMJ 2012, 344, e1013. [Google Scholar] [CrossRef]
- Thakker, R.V. Genetics of parathyroid tumours. J. Intern. Med. 2016, 280, 574–583. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Brandi, M.L.; Eastell, R.; Silverberg, S.J.; Udelsman, R.; Marcocci, C.; Potts, J.T., Jr. Guidelines for the management of asymptomatic primary hyperparathyroidism: Summary statement from the Fourth International Workshop. J. Clin. Endocrinol. Metab. 2014, 99, 3561–3569. [Google Scholar] [CrossRef]
- Stein, E.; Shane, E. Secondary osteoporosis. Endocrinol. Metab. Clin. N. Am. 2003, 32, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Lespessailles, E.; Paccou, J.; Javier, R.M.; Thomas, T.; Cortet, B.; GRIO Scientific Committee. Obesity, bariatric surgery, and fractures. J. Clin. Endocrinol. Metab. 2019, 104, 4756–4768. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, C.; Schafer, A.L. Bone health after bariatric surgery. J. Bone Miner. Res. Plus 2018, 2, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Garvey, W.T.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures—2019 update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg. Obes. Relat. Dis. 2020, 16, 175–247. [Google Scholar]
- Schafer, A.L.; Kazakia, G.J.; Vittinghoff, E.; Stewart, L.; Rogers, S.J.; Kim, T.Y.; Carter, J.T.; Posselt, A.M.; Pasco, C.; Shoback, D.M.; et al. Effects of gastric bypass surgery on bone mass and microarchitecture occur early and particularly impact postmenopausal women. J. Bone Miner. Res. 2018, 33, 975–986. [Google Scholar] [CrossRef]
- Lindeman, K.G.; Greenblatt, L.B.; Rourke, C.; Bouxsein, M.L.; Finkelstein, J.S.; Yu, E.W. Longitudinal 5-year evaluation of bone density and microarchitecture after Roux-en-Y gastric bypass surgery. J. Clin. Endocrinol. Metab. 2018, 103, 4104–4112. [Google Scholar] [CrossRef]
- Hansen, S.; Jørgensen, N.R.; Hermann, A.P.; Støving, R.K. Continuous decline in bone mineral density and deterioration of bone microarchitecture 7 years after Roux-en-Y gastric bypass surgery. Eur. J. Endocrinol. 2020, 182, 303–311. [Google Scholar] [CrossRef]
- Diamond, T.; Stiel, D.; Posen, S. Osteoporosis in hemochromatosis: Iron excess, gonadal deficiency, or other factors? Ann. Intern. Med. 1989, 110, 430–436. [Google Scholar] [CrossRef]
- Schneider, A.; Shane, E. Osteoporosis secondary to illnesses and medications. In Osteoporosis; Marcus, R., Feldman, D., Kelsey, J., Eds.; Academic Press: San Diego, CA, USA, 2001; pp. 303–327. [Google Scholar]
- Bikle, D. Osteoporosis in gastrointestinal, pancreatic, and hepatic diseases. In Osteoporosis; Marcus, R., Feldman, D., Kelsey, J., Eds.; Academic Press: San Diego, CA, USA, 2001; pp. 237–258. [Google Scholar]
- Sánchez, M.I.; Mohaidle, A.; Baistrocchi, A.; Matoso, D.; Vazquez, H.; Gonzalez, A.; Mazure, R.; Maffei, E.; Ferrari, G.; Smecuol, E.; et al. Risk of fracture in celiac disease: Gender, dietary compliance, or both? World J. Gastroenterol. 2011, 17, 3035–3042. [Google Scholar] [CrossRef]
- Pritchard, L.; Wilson, S.; Griffin, J.; Pearce, G.; Murray, I.A.; Lewis, S. Prevalence of reduced bone mineral density in adults with coeliac disease—Are we missing opportunities for detection in patients below 50 years of age? Scand. J. Gastroenterol. 2018, 53, 1433–1436. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Stavropolous, S.; Diamond, B.; Shane, E.; Green, P.H. Osteoporosis in a North American adult population with celiac disease. Am. J. Gastroenterol. 2001, 96, 112–119. [Google Scholar] [CrossRef]
- Vazquez, H.; Mazure, R.; Gonzalez, D.; Flores, D.; Pedreira, S.; Niveloni, S.; Smecuol, E.; Maurino, E.; Bai, J.C. Risk of fractures in celiac disease patients: A cross-sectional, case-control study. Am. J. Gastroenterol. 2000, 95, 183–189. [Google Scholar] [CrossRef]
- Hernandez, C.J.; Guss, J.D.; Luna, M.; Goldring, S.R. Links between the microbiome and bone. J. Bone Miner. Res. 2016, 31, 1638–1646. [Google Scholar] [CrossRef]
- Bryant, R.V.; Ooi, S.; Schultz, C.G.; Goess, C.; Grafton, R.; Hughes, J.; Lim, A.; Bartholomeusz, F.D.; Andrews, J.M. Low muscle mass and sarcopenia: Common and predictive of osteopenia in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2015, 41, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Haaber, A.B.; Rosenfalck, A.M.; Hansen, B.; Hilsted, J.; Larsen, S. Bone mineral metabolism, bone mineral density, and body composition in patients with chronic pancreatitis and pancreatic exocrine insufficiency. Int. J. Pancreatol. 2000, 27, 21–27. [Google Scholar] [CrossRef]
- Stoltz, D.A.; Meyerholz, D.K.; Welsh, M.J. Origins of cystic fibrosis lung disease. N. Engl. J. Med. 2015, 372, 351–362. [Google Scholar] [CrossRef]
- Paccou, J.; Zeboulon, N.; Combescure, C.; Gossec, L.; Cortet, B. The prevalence of osteoporosis, osteopenia, and fractures among adults with cystic fibrosis: A systematic literature review with meta-analysis. Calcif. Tissue Int. 2010, 86, 1–7. [Google Scholar] [CrossRef]
- Ebeling, P.R. Transplantation osteoporosis. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 9th ed.; John, P., Bilezikian, J.W., Eds.; Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019. [Google Scholar]
- Stein, E.M.; Ortiz, D.; Jin, Z.; McMahon, D.J.; Shane, E. Prevention of fractures after solid organ transplantation: A meta-analysis. J. Clin. Endocrinol. Metab. 2011, 96, 3457–3465. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsdottir, S.; Gislason, G.; Aspelund, T.; Sverrisdottir, I.; Landgren, O.; Turesson, I.; Bjorkholm, M.; Kristinsson, S.Y. Fractures and survival in multiple myeloma: Results from a population-based study. Haematologica 2020, 105, 1067–1073. [Google Scholar] [CrossRef]
- Nador, G.; Ramasamy, K.; Panitsas, F.; Pratt, G.; Sadler, R.; Javaid, M.K. Testing and management for monoclonal gammopathy of uncertain significance and myeloma patients presenting with osteoporosis and fragility fractures. Rheumatology 2019, 58, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Rajkumar, V.; Kyle, R.A.; van Duin, M.; Sonneveld, P.; Mateos, M.V.; Gay, F.; Anderson, K.C. Multiple myeloma. Nat. Rev. Dis. Primers 2017, 3, 17046. [Google Scholar] [CrossRef]
- Hamilton, E.J.; Ghasem-Zadeh, A.; Gianatti, E.; Lim-Joon, D.; Bolton, D.; Zebaze, R.; Seeman, E.; Zajac, J.D.; Grossmann, M. Structural decay of bone microarchitecture in men with prostate cancer treated with androgen deprivation therapy. J. Clin. Endocrinol. Metab. 2010, 95, E456–E463. [Google Scholar] [CrossRef]
- Ng, H.S.; Koczwara, B.; Roder, D.; Vitry, A. Development of comorbidities in men with prostate cancer treated with androgen deprivation therapy: An Australian population-based cohort study. Prostate Cancer Prostatic Dis. 2018, 21, 403–410. [Google Scholar] [CrossRef]
- Lassemillante, A.C.; Doi, S.A.; Hooper, J.D.; Prins, J.B.; Wright, O.R. Prevalence of osteoporosis in prostate cancer survivors: A meta-analysis. Endocrine 2014, 45, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Milat, F.; Vincent, A.J. Management of bone disease in women after breast cancer. Climacteric 2015, 18, 47–55. [Google Scholar] [CrossRef]
- Kim, D.; Oh, J.; Lee, H.S.; Jeon, S.; Park, W.C.; Yoon, C.I. Association between tamoxifen and incidence of osteoporosis in Korean patients with ductal carcinoma in situ. Front. Oncol. 2024, 13, 1236188. [Google Scholar] [CrossRef]
- Coates, A.S.; Keshaviah, A.; Thürlimann, B.; Mouridsen, H.; Mauriac, L.; Forbes, J.F.; Paridaens, R.; Castiglione-Gertsch, M.; Gelber, R.D.; Colleoni, M.; et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: Update of study BIG 1-98. J. Clin. Oncol. 2007, 25, 486–492. [Google Scholar] [CrossRef]
- Hadji, P.; Colli, E.; Regidor, P.A. Bone health in estrogen-free contraception. Osteoporos. Int. 2019, 30, 2391–2400. [Google Scholar] [CrossRef] [PubMed]
- Diemar, S.S.; Sejling, A.S.; Eiken, P.; Andersen, N.B.; Jørgensen, N.R. An explorative literature review of the multifactorial causes of osteoporosis in epilepsy. Epilepsy Behav. 2019, 100, 106511. [Google Scholar] [CrossRef]
- Ducy, P.; Karsenty, G. The two faces of serotonin in bone biology. J. Cell Biol. 2010, 191, 7–13. [Google Scholar] [CrossRef]
- Poly, T.N.; Islam, M.M.; Yang, H.C.; Wu, C.C.; Li, Y.J. Proton pump inhibitors and risk of hip fracture: A meta-analysis of observational studies. Osteoporos. Int. 2019, 30, 103–114. [Google Scholar] [CrossRef]
- Hosein-Woodley, R.; Hirani, R.; Issani, A.; Hussaini, A.S.; Stala, O.; Smiley, A.; Etienne, M.; Tiwari, R.K. Beyond the surface: Uncovering secondary causes of osteoporosis for optimal management. Biomedicines 2024, 12, 2558. [Google Scholar] [CrossRef] [PubMed]
- Ebeling, P.R.; Nguyen, H.H.; Aleksova, J.; Vincent, A.J.; Wong, P.; Milat, F. Secondary osteoporosis. Endocr. Rev. 2022, 43, 240–313. [Google Scholar] [CrossRef]
- Trajanoska, K.; Morris, J.A.; Oei, L.; Zheng, H.F.; Evans, D.M.; Kiel, D.P.; Ohlsson, C.; Richards, J.B.; Rivadeneira, F. Assessment of the genetic and clinical determinants of fracture risk: Genome-wide association and mendelian randomisation study. BMJ 2018, 362, k3225. [Google Scholar] [CrossRef]
- Formosa, M.M.; Bergen, D.J.M.; Gregson, C.L.; Maurizi, A.; Kämpe, A.; Garcia Giralt, N.; Zhou, W.; Grinberg, D.; Ovejero Crespo, D.; Zillikens, M.C.; et al. A roadmap to gene discoveries and novel therapies in monogenic low and high bone mass disorders. Front. Endocrinol. 2021, 12, 709711. [Google Scholar] [CrossRef]
- Unger, S.; Ferreira, C.R.; Mortier, G.R.; Ali, H.; Bertola, D.R.; Calder, A.; Cohn, D.H.; Cormier Daire, V.; Girisha, K.M.; Hall, C.; et al. Nosology of genetic skeletal disorders: 2023 revision. Am. J. Med. Genet. A 2023, 191, 1164–1209. [Google Scholar] [CrossRef]
- Gistelinck, C.; Weis, M.; Rai, J.; Schwarze, U.; Niyazov, D.; Song, K.M.; Byers, P.H.; Eyre, D.R. Abnormal bone collagen cross-linking in osteogenesis imperfecta/Bruck syndrome caused by compound heterozygous PLOD2 mutations. J. Bone Miner. Res. Plus 2021, 5, e10454. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, M.; Guterman Ram, G.; Marini, J.C. Osteogenesis Imperfecta: Mechanisms and signaling pathways connecting classical and rare OI types. Endocr. Rev. 2022, 43, 61–90. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Kneissel, M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef]
- Gong, Y.; Slee, R.B.; Fukai, N.; Rawadi, G.; Roman Roman, S.; Reginato, A.M.; Wang, H.; Cundy, T.; Glorieux, F.H.; Lev, D.; et al. LDL receptor related protein 5 (LRP5) affects bone accrual and eye development. Cell 2001, 10, 513–523. [Google Scholar] [CrossRef]
- Hu, J.; Lin, X.; Gao, P.; Zhang, Q.; Zhou, B.; Wang, O.; Jiang, Y.; Xia, W.; Xing, X.; Li, M. Genotypic and phenotypic spectrum and pathogenesis of WNT1 variants in a large cohort of patients with OI/osteoporosis. J. Clin. Endocrinol. Metab. 2023, 108, 1776–1786. [Google Scholar] [CrossRef]
- Peris, P.; Monegal, A.; Mäkitie, R.E.; Guañabens, N.; González Roca, E. Osteoporosis related to WNT1 variants: A not infrequent cause of osteoporosis. Osteoporos. Int. 2023, 34, 405–411. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, F.S.; Zillikens, M.C.; Micha, D.; Riessland, M.; Marcelis, C.L.; de Die Smulders, C.E.; Milbradt, J.; Franken, A.A.; Harsevoort, A.J.; Lichtenbelt, K.D.; et al. PLS3 mutations in X-linked osteoporosis with fractures. N. Engl. J. Med. 2013, 369, 1529–1536. [Google Scholar] [CrossRef]
- Kämpe, A.J.; Costantini, A.; Levy Shraga, Y.; Zeitlin, L.; Roschger, P.; Taylan, F.; Lindstrand, A.; Paschalis, E.P.; Gamsjaeger, S.; Raas Rothschild, A.; et al. PLS3 deletions lead to severe spinal osteoporosis and disturbed bone matrix mineralization. J. Bone Miner. Res. 2017, 32, 2394–2404. [Google Scholar] [CrossRef] [PubMed]
- Pekkinen, M.; Terhal, P.A.; Botto, L.D.; Henning, P.; Mäkitie, R.E.; Roschger, P.; Jain, A.; Kol, M.; Kjellberg, M.A.; Paschalis, E.P.; et al. Osteoporosis and skeletal dysplasia caused by pathogenic variants in SGMS2. JCI Insight 2019, 4, e126180. [Google Scholar] [CrossRef] [PubMed]
- Formosa, M.M.; Christou, M.A.; Mäkitie, O. Bone fragility and osteoporosis in children and young adults. J. Endocrinol. Investig. 2024, 47, 285–298. [Google Scholar] [CrossRef]
- Gray, J.R.; Bridges, A.B.; Mole, P.A.; Pringle, T.; Boxer, M.; Paterson, C.R. Osteoporosis and the Marfan syndrome. Postgrad. Med. J. 1993, 69, 373–375. [Google Scholar] [CrossRef]
- Liu, R.; Weng, G.; Zheng, F.; Chen, J.; Wang, K.; Han, J.; Huang, J.; Yan, L.; Jin, J. Generation of an integration-free induced pluripotent stem cell line, FJMAi001-A, from a Marfan syndrome patient with a heterozygous mutation c.2777G > A (p.Cys926Tyr) in FBN1. Stem Cell Res. 2024, 81, 103591. [Google Scholar] [CrossRef]
- Racine, C.; Callier, P.; Touraine, R.; Vitobello, A.; Hanna, N.; Arnaud, P.; Jondeau, G.; Boileau, C.; Thauvin-Robinet, C.; Creveaux, I.; et al. De novo balanced translocations disrupting the FBN1 gene diagnosed by genome sequencing: An uncommon cause of Marfan syndrome modifying genetic counseling. Am. J. Med. Genet. Part A 2025, 197, e63923. [Google Scholar] [CrossRef]
- Charoenngam, N.; Rittiphairoj, T.; Ponvilawan, B.; Jaroenlapnopparat, A.; Waitayangkoon, P.; Suppakitjanusant, P.; Prasitsumrit, V.; Pongchaiyakul, C.; Holick, M.F. Bone fragility in hereditary connective tissue disorders: A systematic review and meta-analysis. Endocr. Pract. 2023, 29, 589–600. [Google Scholar] [CrossRef]
- Le Parc, J.M.; Plantin, P.; Jondeau, G.; Goldschild, M.; Albert, M.; Boileau, C. Bone mineral density in sixty adult patients with Marfan syndrome. Osteoporos. Int. 1999, 10, 475–479. [Google Scholar] [CrossRef] [PubMed]
- International Osteoporosis Foundation. Modifiable Risk Factors. Available online: https://www.osteoporosis.foundation/health-professionals/about-osteoporosis/risk-factors/modifiable-risks (accessed on 21 April 2025).
- Reid, I.R.; Billington, E.O. Drug therapy for osteoporosis in older adults. Lancet 2022, 399, 1080–1092. [Google Scholar] [CrossRef]
- Tong, G.; Meng, Y.; Hao, S.; Hu, S.; He, Y.; Yan, W.; Yang, D. Parathyroid hormone activates phospholipase C (PLC)-independent protein kinase C signalling pathway via protein kinase A (PKA)-dependent mechanism: A new defined signalling route would induce alternative consideration to previous conceptions. Med. Sci. Monit. 2017, 23, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Vasiliadis, E.S.; Evangelopoulos, D.-S.; Kaspiris, A.; Benetos, I.S.; Vlachos, C.; Pneumaticos, S.G. The role of sclerostin in bone diseases. J. Clin. Med. 2022, 11, 806. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.Y.; Wu, P.W.; Bringhurst, F.R.; Wang, J.T. Estrogen inhibition of PTH-stimulated osteoclast formation and attachment in vitro: Involvement of both PKA and PKC. Endocrinology 2002, 143, 627–635. [Google Scholar] [CrossRef]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef]
- Eriksen, E.F.; Boyce, R.W.; Shi, Y.; Brown, J.P.; Betah, D.; Libanati, C.; Oates, M.; Chapurlat, R.; Chavassieux, P. Reconstruction of remodeling units reveals positive effects after 2 and 12 months of romosozumab treatment. J. Bone Miner. Res. 2024, 39, 729–736. [Google Scholar] [CrossRef]
| Causes of Secondary Osteoporosis |
|---|
| Hormonal deficiency (Androgen deficiency -> hypogonadism, diseases, medications; estrogen and DHEA deficiency) |
| Chronic alcohol use |
| Medications (antiepileptics, SSRIs, PPIs, heparin, glucocorticoids) |
| Vitamin D deficiency |
| Sarcopenia and inflammation |
| Diabetes mellitus (type 1 and type 2) |
| Hyperthyroidism and hyperparathyroidism |
| Hypogonadism (including Turner and Klinefelter syndromes) |
| Obesity and related factors |
| Gastrointestinal disorders causing malabsorption (e.g., celiac disease, inflammatory bowel disease, bariatric surgery) |
| Organ transplants and immunosuppressive therapy |
| Multiple myeloma and other cancers |
| Rare genetic diseases (osteogenesis imperfecta, Marfan syndrome, Ehlers-Danlos syndrome, homocystinuria) |
| Rheumatic and neurological diseases (e.g., Parkinson’s disease, multiple sclerosis) |
| Systemic mastocytosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellani, C.; De Martino, E.; Scapato, P. Osteoporosis: Focus on Bone Remodeling and Disease Types. BioChem 2025, 5, 31. https://doi.org/10.3390/biochem5030031
Castellani C, De Martino E, Scapato P. Osteoporosis: Focus on Bone Remodeling and Disease Types. BioChem. 2025; 5(3):31. https://doi.org/10.3390/biochem5030031
Chicago/Turabian StyleCastellani, Chiara, Erica De Martino, and Paolo Scapato. 2025. "Osteoporosis: Focus on Bone Remodeling and Disease Types" BioChem 5, no. 3: 31. https://doi.org/10.3390/biochem5030031
APA StyleCastellani, C., De Martino, E., & Scapato, P. (2025). Osteoporosis: Focus on Bone Remodeling and Disease Types. BioChem, 5(3), 31. https://doi.org/10.3390/biochem5030031






