Safety and Efficacy of Pemphigus Treatments: A Subtype-Specific Review of Conventional and Emerging Therapies
Abstract
1. Introduction
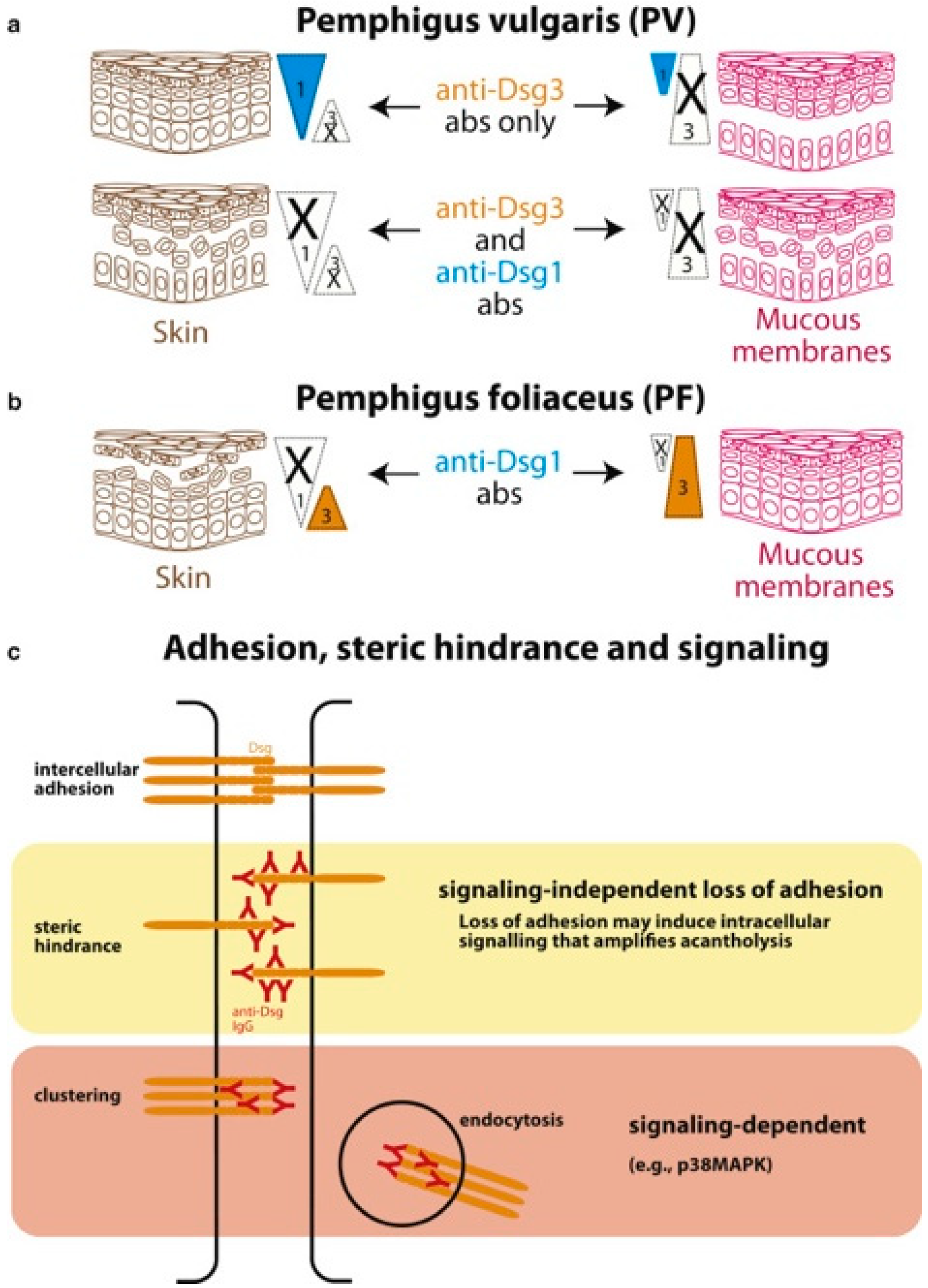
2. Pathophysiology
3. Conventional Treatments
3.1. Glucocorticoids
3.1.1. Efficacy
Pemphigus Vulgaris
Pemphigus Foliaceus
Pemphigus Vulgaris/Pemphigus Foliaceus
Paraneoplastic Pemphigus
| First Author (Year) | Study Design | Study Population | Results/Conclusion |
|---|---|---|---|
| Sharma et al. (2013) [27] | Non-blinded, prospective RCT | 60 PV | 60% relapse rate after 9 weeks 71% relapse rate after 1 year |
| Almugairen et al. (2013) [29] | Multi-centre retrospective study | 80 PV 47 PF | 47 out of 80 (58.7%) of patients achieved CRoffT after 36 ± 39 months 21 out 47 (44.7%) of patients achieved CRoffT after 36 ± 39 months |
| Schmidt et al. (2020) [32] | Controlled prospective trial | 90 PV/PF | 34% of patients achieved CRoffT after 2 years |
| Leger et al. (2012) [37] | Multi-centre retrospective cohort study | 37 PNP | 26 out of 37 (70%) did not achieve complete epithelisation |
3.1.2. Safety
Pemphigus Vulgaris
Pemphigus Foliaceus
Paraneoplastic Pemphigus
3.2. Mycophenolate Mofetil
3.2.1. Efficacy
Pemphigus Vulgaris
Pemphigus Foliaceus
Pemphigus Vulgaris/Pemphigus Foliaceus
Paraneoplastic Pemphigus
3.2.2. Safety
Pemphigus Vulgaris
Pemphigus Vulgaris/Pemphigus Foliaceus
Paraneoplastic Pemphigus
3.3. Azathioprine
3.3.1. Efficacy
Pemphigus Vulgaris
Pemphigus Foliaceus
Paraneoplastic Pemphigus
3.3.2. Safety
Pemphigus Vulgaris
Pemphigus Foliaceus
Paraneoplastic Pemphigus
4. Emerging Treatment
4.1. Rituximab
4.1.1. Efficacy
Pemphigus Vulgaris
Pemphigus Foliaceus
Pemphigus
Paraneoplastic Pemphigus
4.1.2. Safety
Pemphigus Vulgaris
Pemphigus Foliaceus
Paraneoplastic Pemphigus
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Didona, D.; Maglie, R.; Eming, R.; Hertl, M. Pemphigus: Current and Future Therapeutic Strategies. Front. Immunol. 2019, 10, 1418. [Google Scholar] [CrossRef] [PubMed]
- Prüßmann, W.; Prüßmann, J.; Koga, H.; Recke, A.; Iwata, H.; Juhl, D.; Görg, S.; Henschler, R.; Hashimoto, T.; Schmidt, E.; et al. Prevalence of pemphigus and pemphigoid autoantibodies in the general population. Orphanet J. Rare Dis. 2015, 10, 63. [Google Scholar] [CrossRef]
- Timóteo, R.P.; Pessoa-Gonçalves, Y.M.; do Carmo Neto, J.R.; Rodrigues, W.F.; da Silva, M.V.; Oliveira, C.J.F. A Global View of Pemphigus: Geographical Variations. Clin. Rev. Allergy Immunol. 2024, 66, 14–29. [Google Scholar] [CrossRef]
- Kridin, K.; Schmidt, E. Epidemiology of Pemphigus. JID Innov. 2021, 1, 100004. [Google Scholar] [CrossRef] [PubMed]
- Aryanian, Z.; Balighi, K.; Esmaeli, N.; Daneshpazhooh, M.; Mazloomi Tootoonchi, N.; Razavi, Z.; Beigmohammadi, F.; Gul, U.; Khayyat, A.; Hatami, P. A Comparative Study of Demographic and Clinical Criteria Between Male and Female Patients with Pemphigus Referred to a Referral Hospital in Iran. Dermatol. Res. Pract. 2024, 2024, 9572303. [Google Scholar] [CrossRef]
- Padniewski, J.J.; Shaver, R.L.; Schultz, B.; Pearson, D.R. Patient Quality of Life Improvement in Bullous Disease: A Review of Primary Literature and Considerations for the Clinician. Clin. Cosmet. Investig. Dermatol. 2022, 15, 27–42. [Google Scholar] [CrossRef]
- Quintarelli, L.; Coi, A.; Maglie, R.; Corrà, A.; Mariotti, E.B.; Aimo, C.; Ruffo di Calabria, V.; Verdelli, A.; Bianchi, B.; Del Bianco, E.; et al. Clinical Patterns, Survival, Comorbidities, and Treatment Regimens in 149 Patients With Pemphigus in Tuscany (Italy): A 12-Year Hospital-Based Study. Front. Immunol. 2022, 13, 895490. [Google Scholar] [CrossRef] [PubMed]
- Kiran, K.C.; Madhukara, J.; Abraham, A.; Muralidharan, S. Cutaneous Bacteriological Profile in Patients with Pemphigus. Indian J. Dermatol. 2018, 63, 301–304. [Google Scholar] [CrossRef]
- Wang, J.; Wu, H.; Cong, W.; Zhu, H.; Zheng, J.; Li, X.; Pan, M. Psychological morbidity in patients with pemphigus and its clinicodemographic risk factor: A comparative study. J. Dermatol. 2023, 50, 1237–1245. [Google Scholar] [CrossRef]
- DiMarco, C. Pemphigus: Pathogenesis to Treatment. Rhode Isl. Med. J. 2016, 99, 28–31. [Google Scholar]
- Kasperkiewicz, M.; Ellebrecht, C.T.; Takahashi, H.; Yamagami, J.; Zillikens, D.; Payne, A.S.; Amagai, M. Pemphigus. Nat. Rev. Dis. Primers 2017, 3, 17026. [Google Scholar] [CrossRef] [PubMed]
- Carver, C.A.; Kalesinskas, M.; Ahmed, A.R. Current biologics in treatment of pemphigus foliaceus: A systematic review. Front. Immunol. 2023, 14, 1267668. [Google Scholar] [CrossRef]
- Cholera, M.; Chainani-Wu, N. Management of Pemphigus Vulgaris. Adv. Ther. 2016, 33, 910–958. [Google Scholar] [CrossRef]
- Luo, Y.; Fei, X.; Wang, M.; Yang, H.; Zhang, Y.; Chen, Y.; Luo, Y.; Ding, X.; Gao, C.; Shen, F.; et al. Epidemiology of malignant tumors in patients with pemphigus: An analysis of trends from 1955 to 2021. Clin. Exp. Med. 2024, 24, 100. [Google Scholar] [CrossRef]
- Moro, F.; Sinagra, J.L.M.; Salemme, A.; Fania, L.; Mariotti, F.; Pira, A.; Didona, B.; Di Zenzo, G. Pemphigus: Trigger and predisposing factors. Front. Med. 2023, 10, 1326359. [Google Scholar] [CrossRef]
- Vuong, T.B.T.; Do, D.M.; Ong, P.T.; Thanh Le, T.V. HLA-DRB1 and DQB1 genetic susceptibility to pemphigus vulgaris and pemphigus foliaceus in Vietnamese patients. Dermatol. Rep. 2022, 14, 9286. [Google Scholar] [CrossRef] [PubMed]
- Stone, C.; Bak, G.; Oh, D.; Zhao, C.; Venugopal, S.; Kumar, K.; Murrell, D.F. Environmental triggers of pemphigus vulgaris and bullous pemphigoid: A case control study. Front. Med. 2024, 11, 1441369. [Google Scholar] [CrossRef]
- Lepe, K.; Yarrarapu, S.N.S.; Zito, P.M. Pemphigus Foliaceus. In StatPearls (StatPearls Publishing Copyright © 2025); StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Sardana, K.; Garg, V.K.; Agarwal, P. Is there an emergent need to modify the desmoglein compensation theory in pemphigus on the basis of Dsg ELISA data and alternative pathogenic mechanisms? Br. J. Dermatol. 2013, 168, 669–674. [Google Scholar] [CrossRef]
- Malik, V.S.; Hu, F.B. Obesity Prevention. In Cardiovascular, Respiratory, and Related Disorders; Prabhakaran, D., Anand, S., Gaziano, T.A., Mbanya, J.C., Wu, Y.F., Nugent, R., Eds.; The International Bank for Reconstruction and Development/The World Bank © 2017 International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2017. [Google Scholar]
- Zhao, L.; Chen, Y.; Wang, M. The Global Incidence Rate of Pemphigus Vulgaris: A Systematic Review and Meta-Analysis. Dermatology 2023, 239, 514–522. [Google Scholar] [CrossRef]
- Lim, Y.L.; Bohelay, G.; Hanakawa, S.; Musette, P.; Janela, B. Autoimmune Pemphigus: Latest Advances and Emerging Therapies. Front. Mol. Biosci. 2021, 8, 808536. [Google Scholar] [CrossRef] [PubMed]
- Maruta, C.W.; Miyamoto, D.; Aoki, V.; Carvalho, R.G.R.D.; Cunha, B.M.; Santi, C.G. Paraneoplastic pemphigus: A clinical, laboratorial, and therapeutic overview. An. Bras. Dermatol. 2019, 94, 388–398. [Google Scholar] [CrossRef]
- Kappius, R.H.; Ufkes, N.A.; Thiers, B.H. Paraneoplastic Pemphigus. In StatPearls (StatPearls Publishing Copyright © 2025); StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Hammers, C.M.; Stanley, J.R. Recent Advances in Understanding Pemphigus and Bullous Pemphigoid. J. Investig. Dermatol. 2020, 140, 733–741. [Google Scholar] [CrossRef]
- Kridin, K. Emerging treatment options for the management of pemphigus vulgaris. Ther. Clin. Risk Manag. 2018, 14, 757–778. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Khandpur, S. Evaluation of cyclophosphamide pulse therapy as an adjuvant to oral corticosteroid in the management of pemphigus vulgaris. Clin. Exp. Dermatol. 2013, 38, 659–664. [Google Scholar] [CrossRef]
- Fang, S.Y.; Li, C.L.; Liu, X.S.; Chen, F.; Hua, H. Correlation between polymorphisms of the NR3C1 gene and glucocorticoid effectiveness in patients with pemphigus vulgaris. Sci. Rep. 2017, 7, 11890. [Google Scholar] [CrossRef]
- Almugairen, N.; Hospital, V.; Bedane, C.; Duvert-Lehembre, S.; Picard, D.; Tronquoy, A.F.; Houivet, E.; D’incan, M.; Joly, P. Assessment of the rate of long-term complete remission off therapy in patients with pemphigus treated with different regimens including medium- and high-dose corticosteroids. J. Am. Acad. Dermatol. 2013, 69, 583–588. [Google Scholar] [CrossRef]
- Gregoriou, S.; Efthymiou, O.; Stefanaki, C.; Rigopoulos, D. Management of pemphigus vulgaris: Challenges and solutions. Clin. Cosmet. Investig. Dermatol. 2015, 8, 521–527. [Google Scholar] [CrossRef]
- Pathak, G.N.; Agarwal, P.; Wolfe, S.M.; Patel, K.H.; Dhillon, J.; Rao, B.K. Pemphigus relapse: Mechanisms, risk factors, and agents associated with disease recurrence. J. Dermatol. 2024, 51, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Sticherling, M.; Sárdy, M.; Eming, R.; Goebeler, M.; Hertl, M.; Hofmann, S.C.; Hunzelmann, N.; Kern, J.S.; Kramer, H.; et al. S2k guidelines for the treatment of pemphigus vulgaris/foliaceus and bullous pemphigoid: 2019 update. J. Dtsch. Dermatol. Ges. 2020, 18, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Atzmony, L.; Hodak, E.; Gdalevich, M.; Rosenbaum, O.; Mimouni, D. Treatment of pemphigus vulgaris and pemphigus foliaceus: A systematic review and meta-analysis. Am. J. Clin. Dermatol. 2014, 15, 503–515. [Google Scholar] [CrossRef]
- Ratnam, K.V.; Phay, K.L.; Tan, C.K. Pemphigus therapy with oral prednisolone regimens. A 5-year study. Int. J. Dermatol. 1990, 29, 363–367. [Google Scholar] [CrossRef]
- Porro, A.M.; Caetano Lde, V.; Maehara Lde, S.; Enokihara, M.M. Non-classical forms of pemphigus: Pemphigus herpetiformis, IgA pemphigus, paraneoplastic pemphigus and IgG/IgA pemphigus. An. Bras. Dermatol. 2014, 89, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.; Czernik, A. Paraneoplastic pemphigus: A short review. Clin. Cosmet. Investig. Dermatol. 2016, 9, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Leger, S.; Picard, D.; Ingen-Housz-Oro, S.; Arnault, J.P.; Aubin, F.; Carsuzaa, F.; Chaumentin, G.; Chevrant-Breton, J.; Chosidow, O.; Crickx, B.; et al. Prognostic Factors of Paraneoplastic Pemphigus. Arch. Dermatol. 2012, 148, 1165–1172. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.C. Paraneoplastic pemphigus. Dermatol. Sin. 2010, 28, 1–14. [Google Scholar] [CrossRef]
- Chen, D.M.; Odueyungbo, A.; Csinady, E.; Gearhart, L.; Lehane, P.; Cheu, M.; Maho-Vaillant, M.; Prost-Squarcioni, C.; Hebert, V.; Houivet, E.; et al. Rituximab is an effective treatment in patients with pemphigus vulgaris and demonstrates a steroid-sparing effect. Br. J. Dermatol. 2020, 182, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.A.P.; Wilson, A.; Sheriff, T.; Murrell, D.F. Side effects of steroid-sparing agents in patients with bullous pemphigoid and pemphigus: A systematic review. JAAD Int. 2022, 9, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Liu, H.; Solbrig, H. Mining Severe Drug-Drug Interaction Adverse Events Using Semantic Web Technologies: A Case Study. BioData Min. 2015, 8, 12. [Google Scholar] [CrossRef]
- Goodale, E. Pemphigus foliaceous. Can. Vet. J. 2019, 60, 311–313. [Google Scholar]
- Antiga, E.; Bech, R.; Maglie, R.; Genovese, G.; Borradori, L.; Bockle, B.; Caproni, M.; Caux, F.; Chandran, N.S.; Corrà, A.; et al. S2k guidelines on the management of paraneoplastic pemphigus/paraneoplastic autoimmune multiorgan syndrome initiated by the European Academy of Dermatology and Venereology (EADV). J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1118–1134. [Google Scholar] [CrossRef]
- Sarma, N.; Ghosh, S. Mycophenolate mofetil as adjuvant in pemphigus vulgaris. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Beissert, S.; Mimouni, D.; Kanwar, A.J.; Solomons, N.; Kalia, V.; Anhalt, G.J. Treating Pemphigus Vulgaris with Prednisone and Mycophenolate Mofetil: A Multicenter, Randomized, Placebo-Controlled Trial. J. Investig. Dermatol. 2010, 130, 2041–2048. [Google Scholar] [CrossRef]
- Ioannides, D.; Apalla, Z.; Lazaridou, E.; Rigopoulos, D. Evaluation of mycophenolate mofetil as a steroid-sparing agent in pemphigus: A randomized, prospective study. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 855–860. [Google Scholar] [CrossRef]
- Chams-Davatchi, C.; Esmaili, N.; Daneshpazhooh, M.; Valikhani, M.; Balighi, K.; Hallaji, Z.; Barzegari, M.; Akhyani, M.; Ghodsi, S.Z.; Seirafi, H.; et al. Randomized controlled open-label trial of four treatment regimens for pemphigus vulgaris. J. Am. Acad. Dermatol. 2007, 57, 622–628. [Google Scholar] [CrossRef]
- Mimouni, D.; Anhalt, G.J.; Cummins, D.L.; Kouba, D.J.; Thorne, J.E.; Nousari, H.C. Treatment of pemphigus vulgaris and pemphigus foliaceus with mycophenolate mofetil. Arch. Dermatol. 2003, 139, 739–742. [Google Scholar] [CrossRef]
- Sukanjanapong, S.; Thongtan, D.; Kanokrungsee, S.; Suchonwanit, P.; Chanprapaph, K. A Comparison of Azathioprine and Mycophenolate Mofetil as Adjuvant Drugs in Patients with Pemphigus: A Retrospective Cohort Study. Dermatol. Ther. 2020, 10, 179–189. [Google Scholar] [CrossRef]
- Beissert, S.; Werfel, T.; Frieling, U.; Böhm, M.; Sticherling, M.; Stadler, R.; Zillikens, D.; Rzany, B.; Hunzelmann, N.; Meurer, M.; et al. A comparison of oral methylprednisolone plus azathioprine or mycophenolate mofetil for the treatment of pemphigus. Arch. Dermatol. 2006, 142, 1447–1454. [Google Scholar] [CrossRef]
- Powell, A.M.; Albert, S.; Al Fares, S.; Harman, K.E.; Setterfield, J.; Bhogal, B.; Black, M.M. An evaluation of the usefulness of mycophenolate mofetil in pemphigus. Br. J. Dermatol. 2003, 149, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.V.; Marks, J.G., Jr.; Billingsley, E.M. Use of mycophenolate mofetil in the treatment of paraneoplastic pemphigus. Br. J. Dermatol. 2000, 142, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Bredlich, R.O.; Grundmann-Kollmann, M.; Behrens, S.; Kerscher, M.; Peter, R.U. Mycophenolate mofetil monotherapy for pemphigus vulgaris. Br. J. Dermatol. 1999, 141, 934. [Google Scholar] [CrossRef]
- Doukaki, S.; Platamone, A.; Alaimo, R.; Bongiorno, M.R. Mycophenolate mofetil and enteric-coated mycophenolate sodium in the treatment of pemphigus vulgaris and pemphigus foliaceus. J. Dermatolog. Treat. 2015, 26, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Surjushe, A.; Saple, D.G. Mycophenolate mofetil. Indian J. Dermatol. Venereol. Leprol. 2008, 74, 180–184. [Google Scholar] [CrossRef]
- Mignogna, M.D.; Fortuna, G.; Leuci, S.; Ruoppo, E. Oropharyngeal pemphigus vulgaris and clinical remission: A long-term, longitudinal study. Am. J. Clin. Dermatol. 2010, 11, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Davarmanesh, M.; Zahed, M.; Sookhakian, A.; Jehbez, S. Oral Pemphigus Vulgaris Treatment with Corticosteroids and Azathioprine: A Long-Term Study in Shiraz, Iran. Evid. Based Complement. Alternat. Med. 2022, 2022, 7583691. [Google Scholar] [CrossRef]
- Morshedi, B.; Ring, K. Cutaneous paraneoplastic pemphigus syndrome associated with undifferentiated uterine sarcoma. Gynecol. Oncol. Rep. 2020, 32, 100534. [Google Scholar] [CrossRef]
- Ojaimi, S.; O’Connor, K.; Lin, M.; Schifter, M.; Fulcher, D. Treatment Outcomes in a Cohort of Patients with Mucosal-predominant Pemphigus Vulgaris. Intern. Med. J. 2014, 45, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Porro, A.M.; Seque, C.A.; Ferreira, M.C.C.; Simões, M.M.; Enokihara, S. Pemphigus vulgaris. An. Bras. De Dermatol. 2019, 94, 264–278. [Google Scholar] [CrossRef]
- Lee, M.S.; Yeh, Y.C.; Tu, Y.K.; Chan, T.C. Network meta–analysis–based comparison of first-line steroid-sparing adjuvants in the treatment of pemphigus vulgaris and pemphigus foliaceus. J. Am. Acad. Dermatol. 2021, 85, 176–186. [Google Scholar] [CrossRef]
- Zakka, L.R.; Shetty, S.S.; Ahmed, A.R. Rituximab in the treatment of pemphigus vulgaris. Dermatol. Ther. 2012, 2, 17. [Google Scholar] [CrossRef]
- Nili, A.; Mahmoudi, H.; Heidari, N.; Tavakolpour, S.; Salehi Farid, A.; Balighi, K.; Daneshpazhooh, M. Rituximab monotherapy in mild pemphigus. J. Dermatol. Treat. 2022, 33, 1784–1786. [Google Scholar] [CrossRef]
- de Sena Nogueira Maehara, L.; Huizinga, J.; Jonkman, M.F. Rituximab therapy in pemphigus foliaceus: Report of 12 cases and review of recent literature. Br. J. Dermatol. 2015, 172, 1420–1423. [Google Scholar] [CrossRef]
- Joly, P.; Maho-Vaillant, M.; Prost-Squarcioni, C.; Hebert, V.; Houivet, E.; Calbo, S.; Caillot, F.; Golinski, M.L.; Labeille, B.; Picard-Dahan, C.; et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): A prospective, multicentre, parallel-group, open-label randomised trial. Lancet 2017, 389, 2031–2040. [Google Scholar] [CrossRef]
- Colliou, N.; Picard, D.; Caillot, F.; Calbo, S.; Le Corre, S.; Lim, A.; Lemercier, B.; Le Mauff, B.; Maho-Vaillant, M.; Jacquot, S.; et al. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci. Transl. Med. 2013, 5, 175ra30. [Google Scholar] [CrossRef]
- Gregoriou, S.; Giatrakou, S.; Theodoropoulos, K.; Katoulis, A.; Loumou, P.; Toumbis-Ioannou, E.; Papadavid, E.; Avgerinou, G.; Stavrianeas, N.; Rigopoulos, D. Pilot study of 19 patients with severe pemphigus: Prophylactic treatment with rituximab does not appear to be beneficial. Dermatology 2014, 228, 158–165. [Google Scholar] [CrossRef]
- Palacios-Álvarez, I.; Riquelme-Mc Loughlin, C.; Curto-Barredo, L.; Iranzo, P.; García-Díez, I.; España, A. Rituximab treatment of pemphigus foliaceus: A retrospective study of 12 patients. J. Am. Acad. Dermatol. 2021, 85, 484–486. [Google Scholar] [CrossRef]
- Aryanian, Z.; Balighi, K.; Emadi, S.N.; Hatami, P. Rituximab as a maintenance treatment in patients with pemphigus vulgaris: When is the right time for discontinuation? J. Cosmet. Dermatol. 2024, 23, 406–408. [Google Scholar] [CrossRef]
- Zhou, X.; Zhan, T.; Xu, X.; Lan, T.; Hu, H.; Zhou, Y.; Xia, D.; Wang, J.; Wang, Y.; Xiao, Y.; et al. The efficacy and safety of low-dose rituximab in the treatment of pemphigus vulgaris: A cohort study. J. Dermatol. Treat. 2024, 35, 2302071. [Google Scholar] [CrossRef]
- Carranza, M.C. Paraneoplastic Pemphigus: A Comprehensive Review of Pathophysiology, Clinical Manifestations, Diagnostic Challenges, and Therapeutic Approaches. Int. J. Med. Sci. Clin. Res. Stud. 2024, 4, 1658–1665. [Google Scholar] [CrossRef]
- Rodriguez, R.; Sivesind, T.E.; Murrell, D.; Dellavalle, R.P. From the Cochrane Library: Interventions for Pemphigus Vulgaris and Pemphigus Foliaceus. JMIR Dermatol. 2023, 6, e46812. [Google Scholar] [CrossRef] [PubMed]
- Izumi, K.; Bieber, K.; Ludwig, R.J. Current Clinical Trials in Pemphigus and Pemphigoid. Front. Immunol. 2019, 10, 978. [Google Scholar] [CrossRef] [PubMed]
| Grade | Definition |
|---|---|
| Grade 1 | Mild; asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated. |
| Grade 2 | Moderate; minimal, local or non-invasive intervention indicated; limiting age-appropriate instrumental ADL *. |
| Grade 3 | Severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care ADL **. |
| Grade 4 | Life-threatening consequences: urgent intervention indicated. |
| Grade 5 | Death related to AE. |
| First Author (Year) | Study Design | Study Population | Results/Conclusion |
|---|---|---|---|
| Beissert et al. (2010) [45] | Prospective, multi-centre, double-blinded RCT | 94 PV | 21.8% relapse rate in MMF vs. 44.5% relapse rate in placebo at 24 weeks. |
| Beissert et al. (2010) [45] Chams-Davatchi et al. (2007) [47] | Prospective, multi-centre, double-blinded RCT Randomised controlled open-label trial | 94 PV 60 PV | MMF has no steroid-sparing effect compared to oral glucocorticoids alone. |
| Mimouni et al. (2003) [48] | Historical prospective study | 94 PF | 45% of patients achieved complete remission. 36% of patients achieved complete remission. |
| Beissert et al. (2006) [50] | Prospective RCT | 19 PV/3 PF | MMF had better results over azathioprine in maintaining disease in a trial (n = 40; RR 0.72; 95% CI 0.52 to 0.99, NNT 3.7). |
| Sukanjanapong et al. (2020) [49] | Retrospective cohort study | 62 PV/PF | MMF showed better results in disease control than azathioprine (n = 40; RR 0.72; 95% CI 0.52 to 0.99, NNT 3.7). |
| Beissert et al. (2010) [45] Chams-Davatchi et al. (2007) [47] Ioannides et al. (2012) [46] | Prospective, multi-centre, double-blinded RCT Randomised controlled open-label trial Randomised, prospective, non-blinded trial | 94 PV 60 PV 47 PV/PF | MMF does not have better response rate compared to glucocorticoid monotherapy. |
| Williams et al. (2000) [52] | Case report | 1 PNP | Prednisone, cyclosporin and azathioprine discontinued after 5 months with sustained resolution of skin and oral lesions. |
| First Author (Year) | Study Design | Study Population | Results/Conclusion |
|---|---|---|---|
| Mignogna et al. (2010) [56] | Longitudinal cohort study | 37 PV | Over the course of 2–13 years, 34 were able to reach complete healing of lesions in 4.7 ± 2.57 months. |
| Davarmanesh et al. (2022) [57] | Retrospective cohort study | 47 PV | 46/47 patients achieved complete remission in a mean time of 150 ± 224 days. 36 patients relapse at least once over the course of the treatment but 55% was due to self-discontinuation. |
| Pathak et al. (2024) [31] | RCT | 56 PV | Azathioprine had a lower relapse rate of 39.3% compared to glucocorticoid-only group with 64.3%. |
| Morshedi et al. (2020) [58] | Case report | 1 PNP | Resistant to azathioprine, but stabilised with prednisone and rituximab. |
| First Author (Year) | Study Design | Study Population | Results/Conclusion |
|---|---|---|---|
| Chen et al. (2020) [39] | Phase III, open-label RCT | 38 PV | 90% of participants treated with rituximab and prednisone demonstrated complete remission off glucocorticoids at month 24 compared to 28% in the prednisone-only group. 24% (9/38) of rituximab patients relapsed compared to 50% (18/36) of patients in the prednisone-only group. |
| Nili et al. (2020) [63] | Retrospective cohort study | 16 severe Pemphigus 5 moderate PV 15 mild Pemphigus | 50% achieved complete remission. 5 moderate PV patients had a 40% complete remission rate. Majority of patients (87%, 13/15) achieved complete remission. |
| Palacios-Álvarez et al. (2021) [68] | Retrospective case series | 12 PF | 6/12 (50%) of patients showed CRoffT. 5/12 (42%) showed partial response on and off minimal therapy. 50% relapse rate after 12 months. |
| Joly et al. (2017) [65] | Prospective RCT | 46 Pemphigus | 89% of patients treated with rituximab achieved remission compared to just 34% in the prednisolone group. |
| Colliou et al. (2020) [66] | Prospective cohort study | 22 Pemphigus | 81% of patients relapse by 7 years. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christjaroon, P.; Wagon, O.; Tatian, A.H. Safety and Efficacy of Pemphigus Treatments: A Subtype-Specific Review of Conventional and Emerging Therapies. BioChem 2025, 5, 28. https://doi.org/10.3390/biochem5030028
Christjaroon P, Wagon O, Tatian AH. Safety and Efficacy of Pemphigus Treatments: A Subtype-Specific Review of Conventional and Emerging Therapies. BioChem. 2025; 5(3):28. https://doi.org/10.3390/biochem5030028
Chicago/Turabian StyleChristjaroon, Pokphazz, Orli Wagon, and Artiene H. Tatian. 2025. "Safety and Efficacy of Pemphigus Treatments: A Subtype-Specific Review of Conventional and Emerging Therapies" BioChem 5, no. 3: 28. https://doi.org/10.3390/biochem5030028
APA StyleChristjaroon, P., Wagon, O., & Tatian, A. H. (2025). Safety and Efficacy of Pemphigus Treatments: A Subtype-Specific Review of Conventional and Emerging Therapies. BioChem, 5(3), 28. https://doi.org/10.3390/biochem5030028






