Periodontitis and Alzheimer’s Disease: Is There a Connection?
Abstract
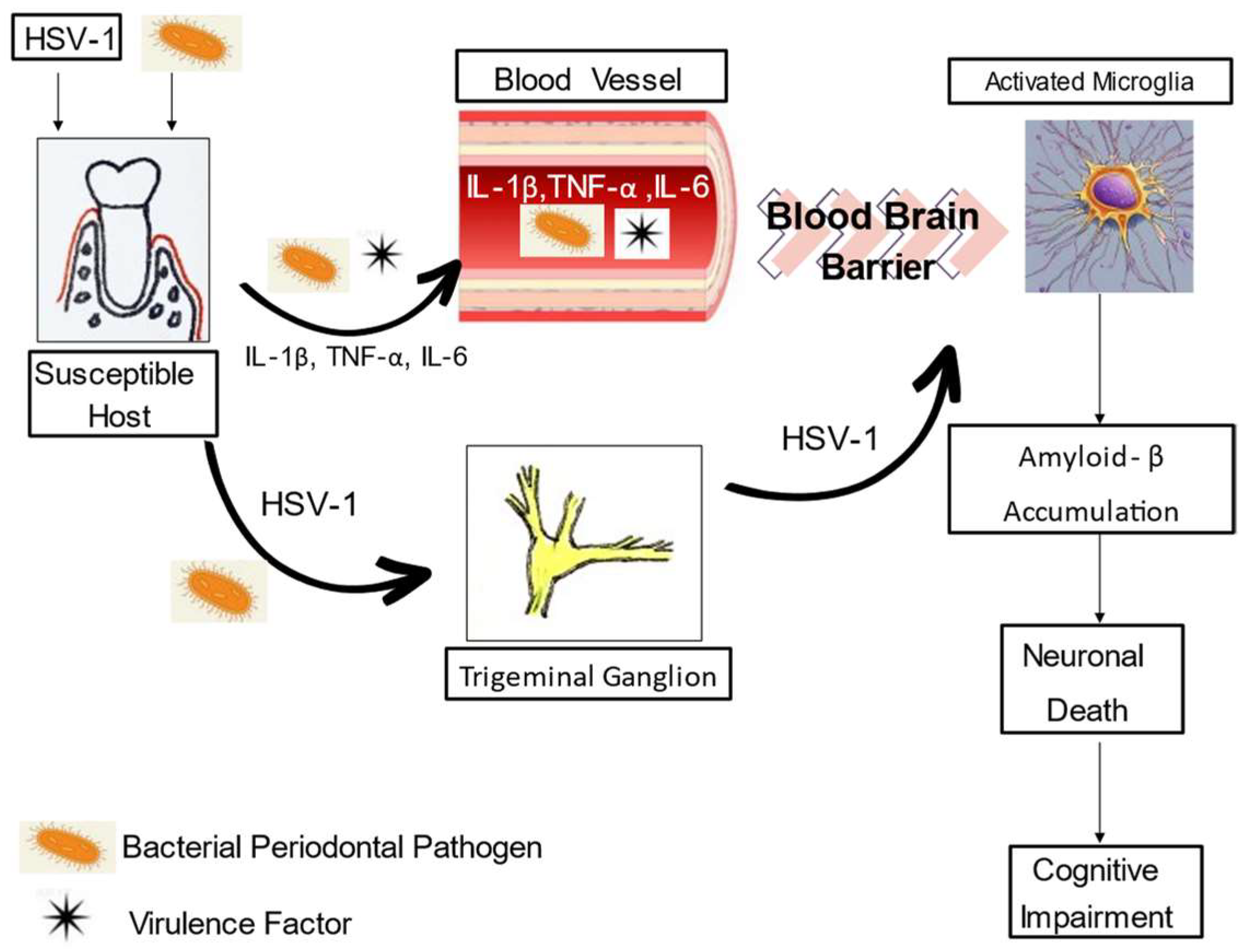
1. Introduction
2. Biologic Plausibility
3. Human Association Studies
4. Animal Studies
5. Therapeutic Intervention
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Association. 2023 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2023, 19, 1598–1695. [Google Scholar] [CrossRef] [PubMed]
- Rostagno, A.A. Pathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 107. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Dye, B.A.; Wei, L.; Slade, G.D.; Thorton-Evans, G.O.; Borgnakke, W.S.; Taylor, G.W.; Page, R.C.; Beck, J.D.; Genco, R.J. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J. Periodontol. 2015, 86, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Page, R.C. The role of inflammatory mediators in the pathogenesis of periodontal disease. J. Periodont. Res. 1991, 26, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T. The potential role of chemokines and inflammatory cytokines in periodontal disease progression. Clin. Infect. Dis. 1999, 28, 482–490. [Google Scholar] [CrossRef]
- Kornman, K.S.; Page, R.C.; Tonetti, M.S. The host response to the microbial challenge in periodontitis: Assembling the players. Periodontology 2000, 14, 33–53. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.D.; Papapanou, P.N.; Phillips, K.H.; Offenbacher, S. Periodontal Medicine: 100 Years of Progress. J. Dent. Res. 2019, 98, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Bui, Q.F.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, D.; Liu, S.; Zhang, S.; Pan, Y. The role of Porphyromonas gingivalis outer membrane vesicles in periodontal disease and related systemic diseases. Front. Cell. Infect. Microbiol. 2021, 10, 585917. [Google Scholar] [CrossRef]
- Kamer, A.R.; Craig, R.G.; Dasanayake, A.P.; Brys, M.; Glodzik-Sobanska, L.; de Leon, M.J. Inflammation and Alzheimer’s disease: Possible role of periodontal diseases. Alzheimer’s Dement. 2008, 4, 242–250. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Fatima, M.; Mondal, A.C. Influence of microglia and astrocyte activation in the neuroinflammatory pathogenesis of Alzheimer’s disease: Rational insights for the therapeutic approaches. J. Clin. Neurosci. 2019, 59, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Forner, L.; Larsen, T.; Kilian, M.; Holmstrup, P. Incidence of bacteremia after chewing, tooth brushing, and scaling in individuals with periodontal inflammation. J. Clin. Periodontal. 2006, 33, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Haraszthy, V.I.; Zambon, J.J.; Trevisan, M.; Zeid, M.; Genco, R.J. Identification of periodontal pathogens in atheromatous plaques. J. Periodontol. 2000, 71, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Nara, P.L.; Sindelar, D.; Penn, M.S.; Potempa, J.; Griffin, W.; Sue, T. Porphyromonas gingivalis outer membrane vesicles as the major driver of and explanation for neuropathogenesis, the chlorine hypothesis, iron dyshomeostasis, and salivary lactoferrin in Alzheimer’s disease. J. Alzheimer’s Dis. 2021, 82, 1417–1450. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Cools, L.; Van Imschoot, G.; Van Wonterghem, E.; Pauwels, M.J.; Vlaeminck, I.; De Witte, C.; Andaloussi, S.E.; Wierda, K.; De Groef, L.; et al. Helicobacter pylori derived outer membrane vesicles contribute to Alzheimer’s disease pathogenesis via C3-C3aR signaling. J. Extacell Vesicles 2023, 12, 12306. [Google Scholar] [CrossRef] [PubMed]
- Poole, S.; Singhrao, S.K.; Kesavalu, L.; Curtis, M.A.; Crean, S. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J. Alzheimer’s Dis. 2013, 6, 665–677. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Haditsch, U.; Raha, D.; Griffin, C.; Holsinger, L.J.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef]
- Parra-Torres, V.; Melgar-Rodríguez, S.; Muñoz-Manríquez, C.; Sanhueza, B.; Cafferata, E.A.; Paula-Lima, A.C.; Díaz-Zúñiga, J. Periodontal bacteria in the brain—Implication for Alzheimer’s disease: A systematic review. Oral Dis. 2023, 29, 21–28. [Google Scholar] [CrossRef]
- Riviere, G.R.; Riviere, K.H.; Smith, K.S. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer’s disease. Oral Microbiol. Immunol. 2002, 17, 113–118. [Google Scholar] [CrossRef]
- Chen, C.; Feng, P.; Slots, J. Herpesvirus-bacteria synergistic interaction in periodontitis. Periodontol. 2000 2020, 82, 42–64. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, G.A.; Maitland, N.J.; Wilcock, G.K.; Craske, J.; Itzhaki, R.F. Latent herpes simplex virus type 1 in normal and Alzheimer’s disease brains. J. Med. Virol. 1991, 33, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.A.; Harris, E.A. Molecular mechanisms for herpes simplex virus type 1 pathogenesis in Alzheimer’s disease. Front. Aging Neurosci. 2018, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.S.; Desrosiers, M.; Donegan, S.J.; Yepes, J.F.; Kryscio, R.J. Tooth loss, dementia and neuropathology in the Nun Study. J. Am. Dent. Assoc. 2007, 138, 1314–1322. [Google Scholar] [CrossRef]
- Okamoto, N.; Morikawa, M.; Tomioka, K.; Yanagi, M.; Amano, N.; Kurumatani, N. Association between tooth loss and the development of mild memory impairment in the elderly: The Fujiwara-kyo study. J. Alzheimer’s Dis. 2015, 44, 777–786. [Google Scholar] [CrossRef]
- Stewart, R.; Stenman, U.; Hakeberg, M.; Hägglin, C.; Gustafson, D.; Skoog, I. Associations between oral health and risk of dementia in a 37-year follow-up study: The prospective population study of women in Gothenburg. J. Am. Geriatr. Soc. 2015, 63, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Kaye, E.K.; Valencia, A.; Baba, N.; Spiro III, A.; Dietrich, T.; Garcia, R.I. Tooth loss and periodontal disease predict poor cognitive function in older men. J. Am. Geriatr. Soc. 2010, 58, 713–718. [Google Scholar] [CrossRef]
- Gil-Montoya, J.A.; Sanchez-Lara, I.; Carnero-Pardo, C.; Fornieles, F.; Montes, J.; Vilchez, R.; Burgos, J.S.; Gonzalez-Moles, M.A.; Barrios, R.; Bravo, M. Is periodontitis a risk factor for cognitive impairment and dementia? A case-control study. J. Periodontal. 2015, 86, 244–253. [Google Scholar] [CrossRef]
- Ide, M.; Harris, M.; Stevens, A.; Sussams, R.; Hopkins, V.; Culliford, D.; Fuller, J.; Ibbett, P.; Raybould, R.; Thomas, R.; et al. Periodontitis and cognitive decline in Alzheimer’s disease. PLoS ONE 2016, 11, e0151081. [Google Scholar] [CrossRef]
- Sochocka, M.; Sobczyński, M.; Sender-Janeczek, A.; Zwolińska, K.; Blachowicz, O.; Tomczyk, T.; Ziętek, M.; Leszek, J. Association between periodontal health status and cognitive abilities. The role of cytokine profile and systemic inflammation. Curr. Alzheimer Res. 2017, 14, 978–990. [Google Scholar] [CrossRef]
- Lee, Y.-T.; Lee, H.-C.; Hu, C.-J.; Huang, L.-K.; Chao, S.-P.; Lin, C.-P.; Su, E.C.-Y.; Lee, Y.-C.; Chen, C.-C. Periodontitis as a modifiable risk factor for dementia: A nationwide population-based cohort study. J. Am. Geriatr. Soc. 2017, 65, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Holmer, J.; Eriksdotter, M.; Schultzberg, M.; Pussinen, P.J.; Buhlin, K. Association between periodontitis and risk of Alzheimer’s disease, mild cognitive impairment and subjective cognitive decline: A case-control study. J. Clin. Periodontol. 2018, 45, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Kimura, Y.; Ogawa, H.; Yamaga, T.; Ansai, T.; Wada, T.; Sakamoto, R.; Ishimoto, Y.; Fujisawa, M.; Okumiya, K.; et al. Periodontitis, periodontal inflammation, and mild cognitive impairment: A 5-year cohort study. J. Periodont. Res. 2019, 54, 233–240. [Google Scholar] [CrossRef]
- Hategan, S.I.; Kamer, S.A.; Craig, R.G.; Sinescu, C.; de Leon, M.J.; Jianu, D.C.; Marian, C.; Bora, B.I.; Dan, T.-F.; Birdac, C.D.; et al. Cognitive dysfunction in young subjects with periodontal disease. Neurol. Sci. 2021, 42, 4511–4519. [Google Scholar] [CrossRef]
- Guo, H.; Li, B.; Yao, H.; Liu, D.; Chen, R.; Zhou, S.; Ji, Y.; Zeng, L.; Du, M. Profiling the oral microbiomes in patients with Alzheimer’s disease. Oral Dis. 2023, 29, 1341–1355. [Google Scholar] [CrossRef]
- Fonseca, M.I.; Zhou, J.; Botto, M.; Tenner, A.J. Absence of C1q leads to less neuropathology in transgenic mouse models of Alzheimer’s disease. J. Neurosci. 2004, 24, 6457–6465. [Google Scholar] [CrossRef]
- Fonseca, M.I.; Ager, R.R.; Chu, S.-H.; Yazan, O.; Sanderson, S.D.; LaFerla, F.M.; Taylor, S.M.; Woodruff, T.M.; Tenner, A.J. Treatment with a C5aR antagonist decreases pathology and enhances behavioral performance in murine models of Alzheimer’s disease. J. Immunol. 2009, 183, 1375–1383. [Google Scholar] [CrossRef]
- Hong, S.; Beja-Glasser, V.F.; Nfonoyim, B.M.; Frouin, A.; Li, S.; Ramakrishnan, S.; Merry, K.M.; Shi, Q.; Rosenthal, A.; Barres, B.A.; et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 2016, 352, 712–716. [Google Scholar] [CrossRef]
- Hao, X.; Li, Z.; Li, W.; Katz, J.; Michalek, S.M.; Barnum, S.R.; Pozzo-Miller, L.; Saito, T.; Saido, T.C.; Wang, Q.; et al. Periodontal infection aggravates C1q-mediated microglial activation and synapse pruning in Alzheimer’s mice. Front. Immunol. 2022, 13, 816640. [Google Scholar] [CrossRef]
- Poole, S.; Singhrao, S.K.; Chukkapalli, S.; Rivera, M.; Velsko, I.; Kesavalu, L.; Crean, S. Active invasion of Porphyromonas gingivalis and infection-induced complement activation in ApoE−/− mice brains. J. Alzheimer’s Dis. 2015, 43, 67–80. [Google Scholar] [CrossRef]
- Lo Sasso, G.; Schlage, W.K.; Boué, S.; Veljkovic, E.; Peitsch, M.C.; Hoeng, J. The Apoe−/− mouse model: A suitable model to study cardiovascular and respiratory diseases in the context of cigarette smoke exposure and harm reduction. J. Transl. Med. 2016, 14, 146. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Ishihara, Y.; Ishida, K.; Tada, H.; Funaki-Kato, Y.; Hagiwara, M.; Ferdous, T.; Abdullah, M.; Mitani, A.; Michikawa, M.; et al. Periodontitis induced by bacterial infection exacerbates features of Alzheimer’s disease in transgenic mice. Aging Mech. Dis. 2017, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Bahar, B.; Kanagasingam, S.; Tambuwala, M.M.; Aljabali, A.A.A.; Dillon, S.A.; Doaei, S.; Welbury, R.; Chukkapalli, S.S.; Singhrao, S.K. Porphyromonas gingivalis (W83) infection induces Alzheimer’s disease-like pathophysiology in obese and diabetic mice. J. Alzheimer’s Dis. 2021, 82, 1259–1275. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Zhang, S.; Duan, L.; Yang, F.; Zhang, K.; Yan, F.; Ge, S. Periodontitis deteriorates cognitive function and impairs neurons and glia in a mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2021, 79, 1785–1800. [Google Scholar] [CrossRef] [PubMed]
- Dewey, C.W.; Rishniw, M. Periodontal disease is associated with cognitive dysfunction in aging dogs: A blinded prospective comparison of visual periodontal and cognitive questionnaire scores. Open Vet. J. 2021, 11, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Kuramoto, E.; Dhar, A.; Wang, R.P.-H.; Seki, H.; Iwai, H.; Yamanaka, A.; Matsumoto, S.-E.; Hara, H.; Michikawa, M.; et al. Neurodegeneration of trigeminal mesencephalic neurons by the tooth loss triggers the progression of Alzheimer’s disease in 3 x Tg-AD model mice. J. Alzheimer’s Dis. 2020, 76, 1443–1459. [Google Scholar] [CrossRef] [PubMed]
- Oue, H.; Miyamoto, Y.; Okada, S.; Koretake, K.; Jung, C.-G.; Michikawa, M.; Akagawa, Y. Tooth loss induces memory impairment and neuronal cell loss in APP transgenic mice. Behav. Brain Res. 2013, 252, 318–325. [Google Scholar] [CrossRef]
- Luo, B.; Pang, Q.; Jiang, Q. Tooth loss causes spatial cognitive impairment in rats through decreased cerebral blood flow and increased glutamate. Arch. Oral Bio. 2019, 102, 225–230. [Google Scholar] [CrossRef]
- Taslima, F.; Jung, C.-G.; Zhou, C.; Abdelhamid, M.; Abdullah, M.; Goto, T.; Saito, T.; Saido, T.C.; Michikawa, M. Tooth loss induces memory impairment and gliosis in App knock-in mouse models of Alzheimer’s disease. J. Alzheimer’s Dis. 2021, 80, 1687–1704. [Google Scholar] [CrossRef]
- Taslima, F.; Abdelhamid, M.; Zhou, C.; Chen, Y.; Jung, C.-G.; Michikawa, M. Tooth loss induces memory impairment and glial activation in young wild-type mice. J. Alzheimer’s Dis. Rep. 2022, 6, 663–675. [Google Scholar] [CrossRef]
- Sakamoto, S.; Hara, T.; Kurozumi, A.; Oka, M.; Kuroda-Ishimine, C.; Araki, D.; Iida, S.; Minagi, S. Effect of occlusal rehabilitation on spatial memory and hippocampal neurons after long-term loss of molars in rats. J. Oral Rehab. 2014, 41, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, J.; Jiang, Q. Tooth loss-associated mechanisms that negatively affect cognitive function: A systematic review of animal experiments based on occlusal support loss and cognitive impairment. Front. Neurosci. 2022, 16, 811335. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.S.; Hasturk, H.; Carreras, I.; Dedeoglu, A.; Veeravalli, J.J.; Huang, J.Y.; Kantarci, A.; Wei, J.C. Dementia and the risk for periodontitis: A population-based cohort study. J. Dent. Res. 2022, 101, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Schwahn, C.; Frenzel, S.; Holtfreter, B.; Van der Auwera, S.; Pink, C.; Bülow, R.; Friedrich, N.; Völzke, H.; Biffar, R.; Kocher, T.; et al. Effect of periodontal treatment on preclinical Alzheimer’s disease—Results of a trial emulation approach. Alzheimer’s Dement. 2022, 18, 127–141. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Wu, X.; Xu, X.; Ji, X.; Wang, B.; Zhang, P.; Li, H. Effects of oral health intervention strategies on cognition and microbiota alterations in patients with mild Alzheimer’s disease: A randomized controlled trial. Geriatr. Nurs. 2022, 48, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.; Tuijt, R.; Burton, A.; Walters, K.; Cooper, C. Supporting self-care of long-term conditions in people with dementia: A systematic review. Int. J. Nurs. Stud. 2021, 116, 103432. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Kim, S.; Nam, Y.; Jung, U.J.; Kim, S.R. Mitochondrial dysfunction as a driver of cognitive impairment in Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 4850. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/study/NCT03823404 (accessed on 17 July 2023).
- Zhao, Y.; Quan, Y.; Lei, T.; Fan, L.; GE, X.; Hu, S. The role of inflammasome NLRP3 in the development and therapy of periodontitis. Int. J. Me. Sci. 2022, 19, 1603–1614. [Google Scholar] [CrossRef]
- Van Zellar, M.; Dias, D.; Sebastio, A.M.; Valente, C.A. NLRP3 Inflammasome: A starring role in amyloid-β- and tau-driven pathological events in Alzheimer’s disease. J. Alzheimer’s Dis. 2021, 83, 939–961. [Google Scholar] [CrossRef]
- Merchant, A.T.; Yi, F.; Vidanapathirana, N.P.; Lohman, M.; Zhang, J.; Newman-Norlund, R.D.; Fridriksson, J. Antibodies against periodontal microorganisms and cognition in older adults. JDR Clin. Trans. Res. 2023, 8, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.A. The environment and disease: Association or causation? JR Soc. Med. 2015, 108, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Fedak, K.M.; Bernal, A.; Capshaw, Z.A.; Gross, S. Applying the Bradford Hill criteria in the 21st century: How data integration has changed causal inference in molecular epidemiology. Emerg. Themes Epidemiol. 2015, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N. Systemic effects of periodontitis: Lessons learned from research on atherosclerotic vascular disease and adverse pregnancy outcomes. Int. Dent. J. 2015, 65, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Ryder, M.I.; Xenoudi, P. Alzheimer disease and the periodontal patient: New insights, connections and therapies. Periodontol. 2000 2021, 87, 32–42. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Periodontol. 2018, 89 (Suppl. S1), S173–S182. [Google Scholar] [CrossRef]
| Author | Year Published | Type of Study | Outcome |
|---|---|---|---|
| Kaye et al. [27] | 2010 | Prospective cohort study | Periodontal disease related to cognitive decline |
| Gil-Montoya et al. [28] | 2015 | Case-control study | Periodontitis appears to be associated with cognitive impairment |
| Ide et al. [29] | 2016 | Cohort study | Periodontitis associated with six-fold increase in rate of cognitive decline |
| Lee et al. [31] | 2017 | Cohort study | Periodontitis is associated with greater risk of developing dementia |
| Holmer et al. [32] | 2018 | Case-control study | Suggests marginal periodontitis is associated with early cognitive impairment |
| Iwasaki et al. [33] | 2019 | Cohort study | Severe periodontitis and periodontal inflammation associated with mild cognitive impairment |
| Ishida et al. [42] | 2017 | Transgenic mouse model | Cognitive function was significantly impaired in periodontitis-induced mice |
| Qian et al. [44] | 2021 | Mouse model | Periodontitis exacerbated learning and memory impairment in mice |
| Dewey et al. [45] | 2021 | Dog study | Periodontal disease is associated with cognitive dysfunction |
| Schwahn et al. [54] | 2022 | Trial emulation | Periodontal treatment had a favorable effect on brain atrophy |
| Chen et al. [55] | 2022 | Randomized controlled trial | Oral health intervention slowed cognitive decline |
| Author | Year Published | Type of Study | Outcome |
|---|---|---|---|
| Forner et al. [12] | 2006 | Clinical trial | Suggests increased risk for bacteremia in periodontitis patients after chewing and tooth brushing |
| Haraszthy et al. [13] | 2000 | PCR assays of endarterectomy specimens | Periodontal pathogens are found in atherosclerotic plaques |
| Poole et al. [17] | 2013 | Brain histology | Lipopolysaccharide from periodontal bacteria can access Alzheimer’s disease brain |
| Dominy et al. [18] | 2019 | PCR and immunohistochemical assays | Demonstrated P. gingivalis and gingipains in brain tissues and CSF of Alzheimer’s patients |
| Riviere et al. [20] | 2002 | PCR and monoclonal antibody tests of brain tissue | Oral Treponema demonstrated in Alzheimer’s disease brain tissues |
| Jamieson et al. [22] | 1991 | PCR assays of brain tissue | Latent herpes virus found in normal and Alzheimer’s disease brains |
| Guo et al. [35] | 2023 | PCR assays of saliva and gingival crevicular fluid samples | Microbiome community was altered in Alzheimer’s disease patients and the periodontal microbiome was sensitive to cognition changes |
| Hong et al. [38] | 2016 | Mouse model | Complement and microglia mediate synaptic loss in Alzheimer’s disease |
| Hao et al. [39] | 2022 | Mouse model | Porphyromonas gingivalis induced brain overactivation of complement is critical for periodontitis associated acceleration of Alzheimer’s disease |
| Poole et al. [40] | 2015 | Mouse model | Porphyromonas gingivalis was able to access mice brains and contribute to the activation of the complement cascade |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lundergan, W.; Parthasarathy, K.; Knight, N. Periodontitis and Alzheimer’s Disease: Is There a Connection? Oral 2024, 4, 61-73. https://doi.org/10.3390/oral4010006
Lundergan W, Parthasarathy K, Knight N. Periodontitis and Alzheimer’s Disease: Is There a Connection? Oral. 2024; 4(1):61-73. https://doi.org/10.3390/oral4010006
Chicago/Turabian StyleLundergan, William, Kavitha Parthasarathy, and Navid Knight. 2024. "Periodontitis and Alzheimer’s Disease: Is There a Connection?" Oral 4, no. 1: 61-73. https://doi.org/10.3390/oral4010006
APA StyleLundergan, W., Parthasarathy, K., & Knight, N. (2024). Periodontitis and Alzheimer’s Disease: Is There a Connection? Oral, 4(1), 61-73. https://doi.org/10.3390/oral4010006





