Odontogenic Keratocyst in an Edentulous Patient: Report of an Unusual Case
Abstract
:1. Introduction
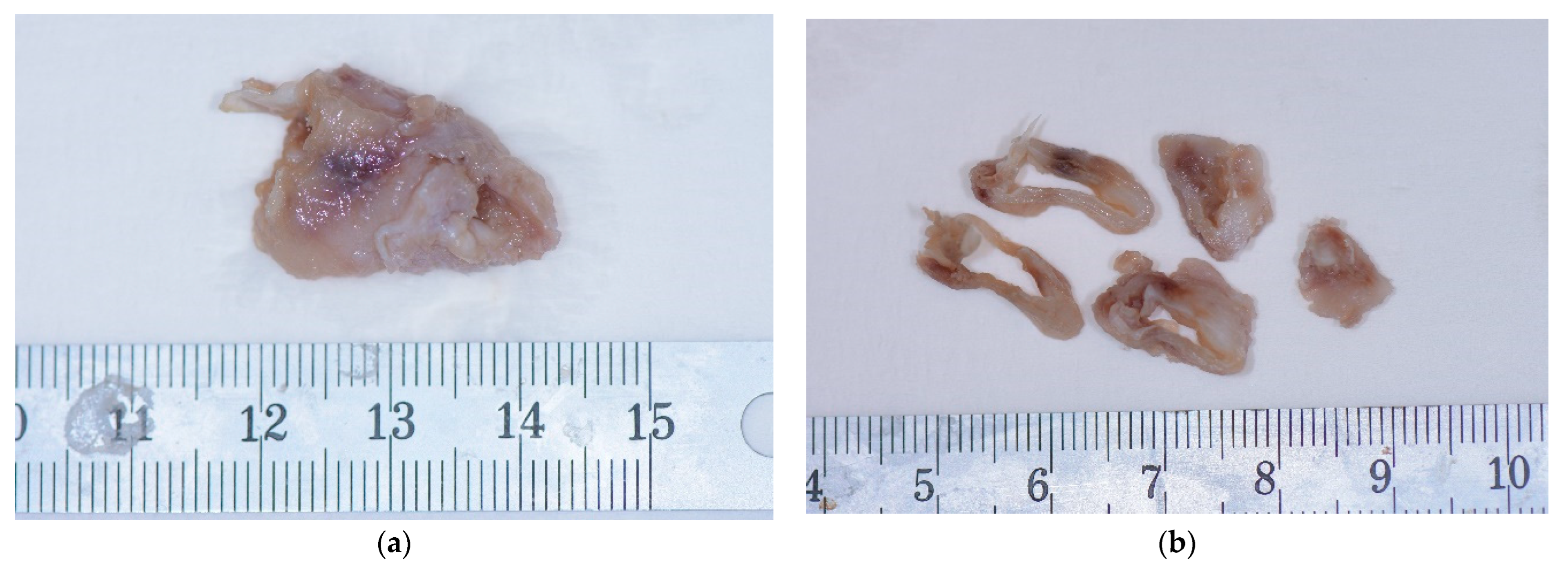
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madras, J.; Lapointe, H. Keratocystic odontogenic tumour: Reclassification of the odontogenic keratocyst from cyst to tumour. J. Can. Dent. Assoc. 2008, 74, 165. [Google Scholar] [PubMed]
- Avril, L.; Lombardi, T.; Ailianou, A.; Burkhardt, K.; Varoquaux, A.; Scolozzi, P.; Becker, M. Radiolucent lesions of the mandible: A pattern-based approach to diagnosis. Insights Imaging 2014, 5, 85–101. [Google Scholar] [CrossRef] [Green Version]
- Manfredi, M.; Vescovi, P.; Bonanini, M.; Porter, S. Nevoid basal cell carcinoma syndrome: A review of the literature. Int. J. Oral Maxillofac. Surg. 2004, 33, 117–124. [Google Scholar] [CrossRef]
- Gianfranco, F.; Spirito, F.; Lo Muzio, E.; Capodiferro, S.; Tempesta, A.; Limongelli, L.; Lo Muzio, L.; Maiorano, E. Histopathological Comparative Analysis between Syndromic and Non-Syndromic Odontogenic Keratocysts: A Retrospective Study. Oral 2022, 3, 198–204. [Google Scholar]
- Passi, D.; Singhal, D.; Singh, M.; Mishra, V.; Panwar, Y.; Sahni, A. Odontogenic keratocyst (OKC) or keratocystic odontogenic tumor (KCOT)—Journey of OKC from cyst to tumor to cyst again: Comprehensive review with recent updates on WHO classification. Int. J. Curr. Res. 2017, 9, 54080–54086. [Google Scholar]
- Myoung, H.; Hong, S.P.; Hong, S.D.; Lee, J.l.; Lim, C.Y.; Choung, P.H.; Lee, J.H.; Choi, J.Y.; Seo, B.M.; Kim, M.J. Odontogenic keratocyst: Review of 256 cases for recurrence and clinicopathologic parameters. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 91, 328–333. [Google Scholar] [CrossRef] [PubMed]
- White, S.C.; Pharoah, M.J. Oral Radiology: Principles and Interpretation; Mosby Elsevier: St. Louis, MO, USA, 2009. [Google Scholar]
- Thamizhchelvan, H.; Malathi, N.; Radhika, T.; Padmanabhan, V.; Nandakumar, N.; Santhosh Kumar, K. Incidental discovery of odontogenic keratocyst in an edentulous patient: Importance of routine pre-prosthetic radiographic evaluation. J. Indian Prosthodont. Soc. 2011, 3, 199–201. [Google Scholar] [CrossRef]
- Nurhan, G.; Sençift, K.; Demirkol, O. Conservative management of keratocystic odontogenic tumors of jaws. Sci. World J. 2012, 2012, 680397. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, A.; Baughman, R.A. Maxillary odontogenic keratocyst: A common and serious clinical misdiagnosis. J. Am. Dent. Assoc. 2003, 134, 877–883. [Google Scholar]
- Chow, H.T. Odontogenic keratocyst: A clinical experience in Singapore. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 86, 573–577. [Google Scholar]
- Zachariades, N.; Papanicolaou, S.; Triantafyllou, D. Odontogenic keratocysts: Review of the literature and report of sixteen cases. J. Oral Maxillofac. Surg. 1985, 43, 177–182. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Sun, Y.; Liu, B.; JiaJia, J. Pathological fractures of the mandible: A report of 27 cases. Clin. Surg. 2017, 2, 1839. [Google Scholar]
- Fidele, N.B.; Bing, L.; Sun, Y.; Wu, T.; Zheng, Y.; Zhao, Y. Management of mandibular odontogenic keratocyst through radical resection: Report of 35 cases. Oncol. Lett. 2019, 18, 733–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoelinga, P.J. The treatment of odontogenic keratocysts by excision of the overlying, attached mucosa, enucleation, and treatment of the bony defect with Carnoy solution. J. Oral Maxillofac. Surg. 2005, 63, 1662–1666. [Google Scholar] [CrossRef] [PubMed]
- Sharif, F.N.; Oliver, R.; Sweet, C.; Sharif, M.O. Interventions for the treatment of keratocystic odontogenic tumours. Cochrane Database Syst. Rev. 2015, 2016, CD008464. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, D.K. Mechanisms of tissue destruction following cryosurgery. Ann. R. Coll. Surg. Engl. 1984, 66, 313–318. [Google Scholar]
- Pogrel, M.A. The use of liquid nitrogen cryotherapy in the management of locally aggressive bone lesions. J. Oral Maxillofac. Surg. 1993, 51, 269–273. [Google Scholar] [CrossRef]
- Janas-Naze, A.; Zhang, W.; Szuta, M. Modified Carnoy’s Versus Carnoy’s Solution in the Management of Odontogenic Keratocysts—A Single Center Experience. J. Clin. Med. 2023, 3, 1133. [Google Scholar] [CrossRef]
- Kinard, B.E.; Chuang, S.K.; August, M.; Dodson, T.B. How well do we manage the odontogenic keratocyst? J. Oral Maxillofac. Surg. 2013, 71, 1353–1358. [Google Scholar] [CrossRef]
- Tarakji, B.; Baroudi, K.; Hanouneh, S.; Azzeghaiby, S.N.; Nassani, M.Z. Possible recurrence of keratocyst in nevoid basal cell carcinoma syndrome: A review of literature. Eur. J. Dent. 2013, 7, S126–S134. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.D.; Lim, J.H.; Kim, H.J.; Nam, W.; Cha, I.H. Appropriate follow-up period for odontogenic keratocyst: A retrospective study. Maxillofac. Plast. Reconstr. Surg. 2021, 43, 16. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.L.; Dias-Ribeiro, E.; Honfi, E.S.; De Araujo, T.N.; Honorio de Goes, K.K.; Do Socorro Aragao, M. Odontogenic keratocyst of mandible. Indian J. Otolaryngol. Head. Neck Surg. 2006, 58, 373–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallejo-Rosero, K.A.; Camolesi, G.V.; de Sá, P.L.D.; Bernaola-Paredes, W.E. Conservative management of odontogenic keratocyst with long-term 5-year follow-up: Case report and literature review. Int. J. Surg. Case Rep. 2020, 66, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Saponaro, P.C. Management of Edentulous Patients. Dent. Clin. N. Am. 2019, 63, 249–261. [Google Scholar] [CrossRef]
- Feine, J.S.; Carlsson, G.E.; Awad, M.A. The McGill consensus statement on overdentures. Mandibular two-implant overdentures as first choice standard of care for edentulous patients. Gerodontology 2002, 19, 3–4. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez, A.; Calcoen, V.; Lombardi, T. Odontogenic Keratocyst in an Edentulous Patient: Report of an Unusual Case. Oral 2023, 3, 307-315. https://doi.org/10.3390/oral3030025
Perez A, Calcoen V, Lombardi T. Odontogenic Keratocyst in an Edentulous Patient: Report of an Unusual Case. Oral. 2023; 3(3):307-315. https://doi.org/10.3390/oral3030025
Chicago/Turabian StylePerez, Alexandre, Valentina Calcoen, and Tommaso Lombardi. 2023. "Odontogenic Keratocyst in an Edentulous Patient: Report of an Unusual Case" Oral 3, no. 3: 307-315. https://doi.org/10.3390/oral3030025
APA StylePerez, A., Calcoen, V., & Lombardi, T. (2023). Odontogenic Keratocyst in an Edentulous Patient: Report of an Unusual Case. Oral, 3(3), 307-315. https://doi.org/10.3390/oral3030025






