Abstract
White sponge nevus (WSN) is an uncommon, hereditary benign keratinization defect that primarily affects the oral mucosa and occasionally, though rarely, the skin or other mucosal sites, such as the nose, esophagus and anogenital area. Sporadic cases of vaginal WSN have been reported. In the oral cavity, the buccal mucosa is prominently affected. Lesions have been reported at birth but are more commonly noted later during adolescent years. We present three cases of WSN with a discussion of the clinical appearance and histopathology, along with a brief review of the literature.
1. Introduction
White sponge nevus (WSN) is a rare, autosomal dominant disorder that primarily affects the oral mucosa [1]. It is a benign genodermatosis characterized by high penetrance and wide variability [2]. The first WSN case was reported in 1909 by Hyde and Cannon, who published a detailed report in 1935 [3]. The genetic transmission involves mutations in the cytokeratin 4 and cytokeratin 13 genes, which cause a defect in the normal keratinization of the mucosa. This defect causes keratin instability and results in hyperkeratotic lesions on non-keratinized epithelial surfaces [4].
The onset is usually before 20 years of age, with both sexes affected equally [5]. Clinically, WSN is characterized by symmetric, white, thickened, corrugated, asymptomatic plaques located predominately on the oral mucosa [2]. The condition most commonly affects the buccal mucosa bilaterally, followed by the lips, alveolar ridges and the floor of the mouth [6]. The expression of WSN is variable, with the size of the plaques varying from patient to patient, and the distribution can change with time [7]. Unlike leukoedema, the white coloration does not diminish or disappear when the mucosa is stretched.
Differentiating WSN from other malignant, reactive, familial and congenital disorders is important. The diagnosis of WSN is often solely based on the distinct clinical presentation and subsequent biopsy of the mucosal lesions, which is usually unnecessary. However, since the oral manifestations of WSN may clinically resemble other white lesions, a biopsy followed by microscopic evaluation often provides a definitive diagnosis [8]. In this report, we contribute three additional cases to the literature that were clinically and microscopically consistent with WSN.
2. Case Report
The three WSN cases were received as incisional biopsies at the University of Florida Oral Diagnostic Biopsy Service for microscopic examination. Table 1 presents the summary of the clinical findings for the three cases.

Table 1.
Demographics, clinical locations and duration reported for 3 cases of WSN.
In comparison, all three cases had similar oral clinical appearance and location; however, the patient for case one additionally reported lesions bilaterally on the lateral/ventral tongue. As seen in Figure 1, Figure 2 and Figure 3, all lesions clinically appeared as corrugated, white plaques with a spongy texture. All three cases reported similar lesions in some other family members.
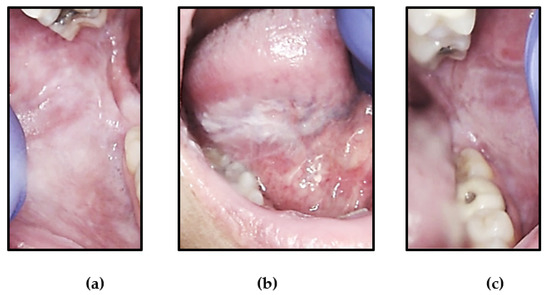
Figure 1.
Intra-oral photographs of lesions of Case 1 that clinically appeared as corrugated, white plaques with a spongy texture of: (a) right buccal mucosa, (b) right ventral-lateral tongue and (c) left buccal mucosa.
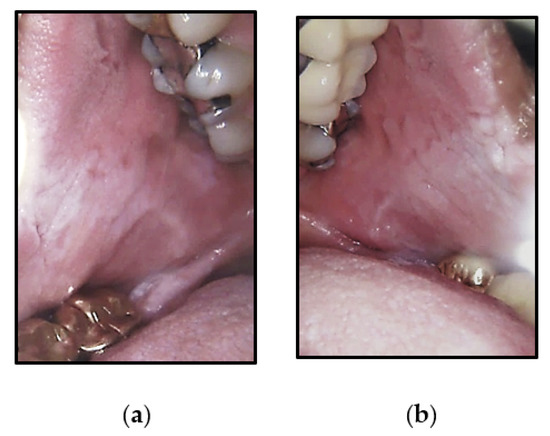
Figure 2.
Intra-oral photographs of Case 2 that were widespread, corrugated and slightly stippled white lesions of the: (a) right buccal mucosa and (b) left buccal mucosa.
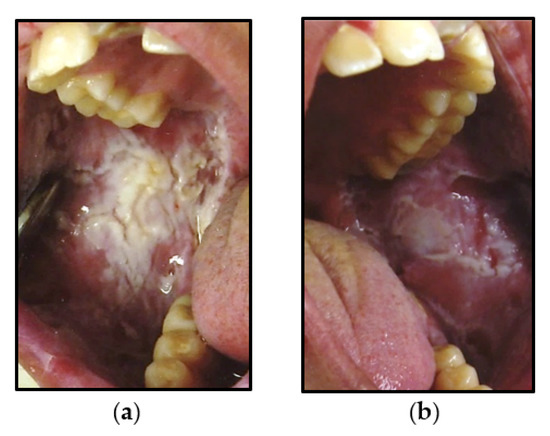
Figure 3.
Intra-oral photographs of Case 3 that were symptomatic, thickened, bilateral white corrugated plaque lesions of: (a) right posterior buccal mucosa and (b) left posterior buccal mucosa.
2.1. Case 1
A healthy 48-year-old white female presented with bilateral, slightly thickened white lesions of the buccal mucosa and tongue (Figure 1). She stated that the lesions were asymptomatic, but she occasionally traumatized the affected areas while chewing. She recalled her father and nephew having similar lesions but reported that their lesions were much smaller and discrete compared to hers. The patient was aware of her father’s WSN diagnosis but still requested a biopsy for verification.
2.2. Case 2
A 63-year-old healthy white female, presented with widespread, bilateral, corrugated and slightly stippled white lesions on the buccal mucosa (Figure 2). She also reported her family history, stating that her brother and possibly her father had similar lesions of the oral cavity.
2.3. Case 3
An 18-year-old Native-American male presented with bilateral, thickened, asymptomatic, white corrugated plaques of the buccal mucosa, which did not rub off. (Figure 3). The clinician reported that the patient had poor oral hygiene. The clinician who performed the biopsy also considered candidiasis in the differential diagnosis. The patient reported a family history of diagnosis of WSN in several family members. Details of the family history could not be obtained, as the patient was lost to follow-up after the initial visit.
3. Discussion
WSN is a rare, hereditary disorder that affects approximately one in 200,000 people [9]. WSN is inherited as an autosomal dominant trait that exhibits an irregular penetrance and variable expressivity [10]. Interestingly, all three of our patients had at least one or more family members affected, which correlates with other published cases [4,10,11]. The onset of WSN is usually in infancy or childhood, and the disease reaches its peak in early adulthood, with the duration of the disease course largely unknown [5].
WSN is a benign condition resulting in defects in keratinization of the surface mucosa, predominately the oral mucosa [12]. Less frequently reported mucosal sites involved include nasal, esophageal, and genital mucosa. None of our three patients reported involvement of extraoral sites.
Keratins are a family of around 30 proteins that are classified as type I and type II and are differentially expressed according to the tissue of origin and cell contents. WSN typically affects the epithelium that covers mucosal surfaces, particularly the oral cavity and the anogenital region. These surfaces express type II keratin 4 and type I keratin 13. WSN is attributed to point mutations in the gene coding for cytokeratin 4 and/or cytokeratin 13 [13].
The microscopic findings of WSN are characteristic, but not entirely pathognomonic. All three cases presented here had almost identical histopathologic findings that correlate with published literature. As seen in Figure 4, microscopic examination revealed hyperkeratotic stratified squamous epithelium surfaced by a severely thickened layer of parakeratin. The surface epithelium is edematous and contains numerous superficial keratohyaline granules (Figure 4c). Extensive vacuolization of the suprabasilar keratinocytes is seen. The characteristic dyskeratotic cells, which demonstrate dense perinuclear eosinophilic condensation of the cytoplasm and perinuclear keratinization, are prominent [14]. This feature is unique to WSN and is attributed to the ultrastructural concentration of tonofilaments around the nucleus.
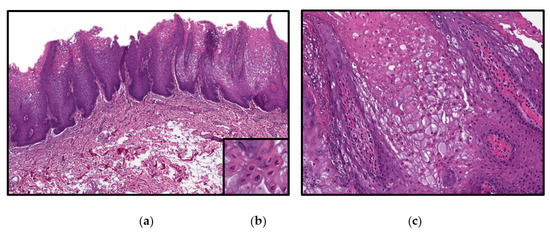
Figure 4.
Photomicrographs from hematoxylin and eosin (H&E) stained from the left buccal mucosa sample of Case 2 demonstrating: (a) a low power view (magnification 10×) (b): numerous dyskeratotic cells with dense perinuclear eosinophilic condensation and perinuclear keratinization (magnification 40×) (c) a higher power view (magnification 20×).
Clinically, WSN has a similar appearance to a spectrum of disorders and processes that present with diffuse white plaques of the oral mucosa. When classifying leukoplakic lesions on clinical appearance, four clinical types of leukoplakia have been identified: (1) homogenous, (2) speckled, (3) white and red patches and (4) verrucous [2,15]. Figure 5 illustrates the common benign clinical differential diagnosis list of leukoplakic lesions based on if the lesion can or cannot be scraped off. All lesions that cannot be scraped off, which includes WSN, are clinically classified as homogeneous leukoplakic lesions. These homogeneous white plaques have no red segments but have a fine, white, grainy texture or a more mottled, rough appearance. Additionally, these lesions are considered low-risk and during the evaluation period, an effort to identify the etiologic factor is key.
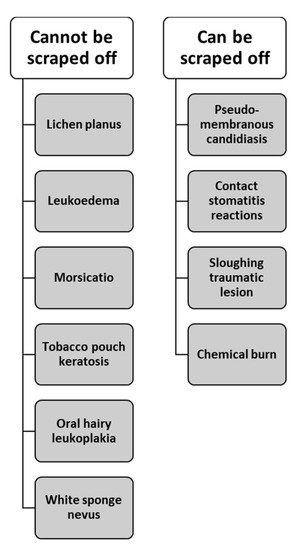
Figure 5.
Common benign leukoplakic lesions of the oral mucosa. These benign white lesions have been categorized based if the lesion can or cannot be scraped off [2].
A top consideration in the differential diagnosis is hereditary benign intraepithelial dyskeratosis (HBID). However, HBID is even a rarer disorder that can be excluded due to the absence of bilateral limbal conjunctival plaques and a lack of a genetic link to a triracial isolate from North Carolina [13]. Leukoplakia can also be considered in the differential, especially proliferative verrucous leukoplakia (PVL). However, PVL typically presents with sharply defined margins, in comparison to the indistinct borders of WSN [16]. Also, PVL is a disease of elderly females and is usually progressive. Darier’s disease (DD) is another important consideration in the differential diagnosis, especially in the case of the second patient, due to the stippled or cobblestone appearance of the buccal mucosa. However, the lack of nail and skin abnormalities, which are characteristic of DD, makes this diagnosis untenable. Similar to DD, dyskeratosis congenita and pachyonychia congenita can present with multi-surface white oral lesions, but also display nail and skin abnormalities [4]. Pseudomembranous candidiasis was considered as a diagnosis for patient three, but the white lesions found in Candidiasis typically can be wiped off and the lack of response to antifungals would also rule it out.
WSN is considered to be a completely benign and harmless condition with no potential for malignant transformation [11]. Patients have reported minor symptoms of discomfort as a result of altered texture of the oral mucosa and esthetic concerns. Usually, there is no treatment required for WSN. Treatment with beta-carotene, various antibiotics (penicillin, azithromycin, etc.), antihistamines, tetracycline mouth rinses, local application of retinoic acid, laser ablation and surgical resection have all been tried to reduce the extent of WSN lesions, as reported in the literature, but without much success [17]. None of the cases reported here received any treatment and the patients were reassured of the benign character of their lesions.
4. Conclusions
We present three cases of WSN, with all cases reporting familial involvement. WSN is a rare and benign condition resulting from a genetic defect in keratinization that most commonly affects the oral mucosa. Clinicians should be cognizant of WSN when presented with a characteristic clinical appearance involving multiple sites of the oral cavity in order to avoid misdiagnosis and unnecessary treatment. The condition can be diagnosed on the basis of clinical presentation alone on most occasions, and a biopsy is required only for confirmation of the diagnosis, in cases of unusual features or in the presence of risk factors.
Author Contributions
A.N.B., I.B., M.N.I. and D.M.C. were responsible for the reviewing and editing of the manuscript, with A.N.B. writing the original manuscript draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from two of the subjects involved in the study. One patient’s consent was waived due to the lack of follow-up.
Acknowledgments
The Robert N. Levenson and Grace B. Dunlevy Oral Pathology Research Fund.
Conflicts of Interest
The authors declare no conflict of interest. The acknowledged funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bezerra, K.T.; Leite, T.C.; Roza, A.L.O.C.; Araújo, R.; Israel, M.S.; Canedo, N.H.S.; Agostini, M.; Benevenuto de Andrade, B.A.; Romañach, M.J. White sponge nevus: A condition not always clinically suspected. J. Cutan Pathol. 2020, 47, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Neville, B.W.; Damm, D.D.; Allen, C.M.; Chi, A.C. Oral and Maxillofacial Pathology, 4th ed.; Elsevier: St. Louis, MO, USA, 2016; 912p. [Google Scholar]
- Cannon, A.B. White Sponge Nevus of the Mucosa (Naevus spongiosus albus mucosae). Arch. Dermatol. Syphilol. 1935, 31, 365–370. [Google Scholar] [CrossRef]
- Martelli, H.; Pereira, S.M.; Rocha, T.M.; Nogueira dos Santos, P.L.; Batista de Paula, A.M.; Bonan, P.R. White sponge nevus: Report of a three-generation family. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 103, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Jorgenson, R.J.; Levin, S. White sponge nevus. Arch. Dermatol. 1981, 117, 73–76. [Google Scholar] [CrossRef]
- Rugg, E.L.; McLean, W.H.; Allison, W.E.; Lunny, D.P.; Macleod, R.I.; Felix, D.H.; Lane, E.B.; Munro, C.S. A mutation in the mucosal keratin K4 is associated with oral white sponge nevus. Nat. Genet. 1995, 11, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Terrinoni, A.; Candi, E.; Oddi, S.; Gobello, T.; Camaione, D.B.; Mazzanti, C.; Zambruno, G.; Knight, R.; Melino, G. A glutamine insertion in the 1A alpha helical domain of the keratin 4 gene in a familial case of white sponge nevus. J. Investig. Dermatol. 2000, 114, 388–391. [Google Scholar] [CrossRef]
- Lucchese, A.; Favia, G. White sponge naevus with minimal clinical and histological changes: Report of three cases. J. Oral Pathol. Med. 2006, 35, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Aghbali, A.; Pouralibaba, F.; Eslami, H.; Pakdel, F.; Jamali, Z. White sponge nevus: A case report. J. Dent. Res. Dent. Clin. Dent. Prospect. 2009, 3, 70–72. [Google Scholar] [CrossRef]
- Kürklü, E.; Öztürk, Ş.; Cassidy, A.J.; Ak, G.; Koray, M.; Çefle, K.; Palandüz, Ş.; Güllüoğlu, M.G.; Tanyeri, H.; McLean, W.H. Clinical features and molecular genetic analysis in a Turkish family with oral white sponge nevus. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e144–e150. [Google Scholar] [CrossRef]
- Sanjeeta, N.; Nandini, D.B.; Premlata, T.; Banerjee, S. White sponge nevus: Report of three cases in a single family. J. Oral Maxillofac. Pathol. 2016, 20, 300–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Songu, M.; Adibelli, H.; Diniz, G. White sponge nevus: Clinical suspicion and diagnosis. Pediatr. Dermatol. 2012, 29, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, Y.; Zhang, J.; Yokoo, S.; Umeda, M.; Komori, T. Constitutional mutation of keratin 13 gene in familial white sponge nevus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2003, 96, 561–565. [Google Scholar] [CrossRef]
- Woo, S.-B. Oral Pathology, A Comprehensive Atlas and Text, 1st ed.; Elsevier: Philadelphia, PA, USA, 2012; p. 442. [Google Scholar]
- Wood, N.; Goaz, P. Differential Diagnosis of Oral and Maxillofacial Lesions; Elsevier: Amsterdam, The Netherlands, 1997; p. 656. [Google Scholar]
- Regezi, J.A.; Sciubba, J.J.; Jordan, R.C. Oral Pathology Clinical Pathologic Correlations; Elsevier Health Sciences: Edinburgh, UK; London, UK; Oxford, UK, 2017; p. 496. [Google Scholar]
- Elfatoiki, F.Z.; Capatas, S.; Skali, H.D.; Hali, F.; Attar, H.; Chiheb, S. Oral White Sponge Nevus: An Exceptional Differential Diagnosis in Childhood. Case Rep. Dermatol. Med. 2020, 2020, 9296768. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).