1. Introduction
Multiple myeloma (MM) is a clonal plasma cell malignancy that is the second most common hematologic cancer worldwide, with an annual incidence of ~160,000 new cases and ~100,000 deaths globally [
1]. MM is characterized by the uncontrolled proliferation of malignant plasma cells within the bone marrow, leading to end-organ damage, including osteolytic lesions, renal impairment, anemia, and hypercalcemia [
2]. Despite significant improvements over the past decade due to the introduction of proteasome inhibitors, immunomodulatory drugs, monoclonal antibodies, and autologous stem cell transplantation, MM remains largely incurable, with most patients eventually relapsing and developing drug resistance [
3,
4,
5].
Among the emerging regulators of MM biology, microRNAs (miRNAs) have attracted increasing attention. miRNAs are small, non-coding RNAs that post-transcriptionally regulate gene expression by binding to the 3′ untranslated regions of target mRNAs, leading to their degradation or translational repression [
6]. In MM, aberrant miRNA expression profiles have been implicated in proliferation, apoptosis resistance, angiogenesis, and interactions with the bone marrow niche, suggesting their potential as biomarkers and therapeutic targets [
7]. Moreover, emerging studies indicate that microRNAs in bone marrow stromal cells can significantly influence multiple myeloma progression by modulating cytokine secretion, adhesion, and immune response. These findings justify our investigation of miR-429 not only in malignant plasma cells but also within the stromal compartment [
8,
9].
miR-429, a member of the miR-200 family, has been reported to play dual, context-dependent roles in various cancers. In some solid tumors, including endometrial, prostate, and lung cancers, miR-429 acts as an oncogene by enhancing proliferation, invasion, and the epithelial–mesenchymal transition (EMT) [
10,
11,
12]. Conversely, in cancers such as renal cell carcinoma, gastric cancer, and osteosarcoma, miR-429 exerts a tumor-suppressive effect by inhibiting pathways related to proliferation, migration, and EMT [
13,
14,
15,
16,
17].
Recent studies suggest that miR-429 may have a tumor-suppressive role in MM, possibly via the modulation of the Bmi1/AKT pathway, which is known to be implicated in MM cell proliferation and survival [
18]. Nevertheless, the precise function of miR-429 in MM remains largely unexplored, and its potential as a therapeutic target has not been systematically evaluated. Given the urgent need for novel molecular strategies to overcome drug resistance and improve patient outcomes in MM, elucidating the role of miR-429 in MM cell proliferation and survival could offer new avenues for therapeutic intervention.
While the oncogenic or tumor-suppressive roles of miR-429 have been described in other malignancies, its expression pattern and functional relevance in MM, particularly in the context of the bone marrow microenvironment, remain insufficiently characterized. Our study seeks to provide preliminary functional insights into the role of miR-429 in MM cell behavior using both monoculture and stromal co-culture systems, which more closely mimic in vivo conditions.
Therefore, in this study, we aimed to investigate the expression of miR-429 in MM cell lines and primary patient-derived plasma cells compared to normal plasma cells and to evaluate the effects of miR-429 inhibition on MM cell proliferation and apoptosis, both in monoculture and in co-culture with bone marrow stromal cells. By delineating the functional impact of miR-429 in MM, our work provides insights into its potential utility as a biomarker and a target for innovative miRNA-based therapeutic strategies.
2. Materials and Methods
Reagents
Materials used in this study were as follows: Annexin V/PI kit (BMS500-FI-300, Invitrogen, Carlsbad, CA, USA); cell proliferation reagent MTT (M5655, Sigma-Aldrich, Saint-Louis, MO, USA); Hiperfect and reverse transcription reagent ( ID: 301704, Qiagen); fetal bovine serum (FBS, PAN-Biotech, Aidenbach, Germany); RPMI medium (PAN-Biotech, Aidenbach, Germany); and miScript inhibitor Negative control and miR-429 inhibitor (Qiagen).
Patient sample
Patients who were clinically diagnosed with MM were admitted to the affiliated hospital of Aziza Othmana (Tunis, Tunisia) between May 2019 and March 2020. Clinical data of the patients, presented in
Table 1 for patients P1 to P14, were collected in a dedicated electronic database. Normal plasma cells were isolated from bone marrow aspirates obtained from healthy donors recruited through the National Bone Marrow Transplantation Center (Centre National de Greffe de Moelle Osseuse, CNGMO), under the authority of the Ministry of Public Health (Tunisia). All samples were collected with informed consent and according to institutional ethical guidelines. Sample collection was approved by the Clinical Research Ethics Committee of RABTA Hospital.
Cell culture and plasma cell (PC) sorting
Bone marrow samples (2–4 mL) were diluted in phosphate-buffered saline (PBS; Sigma-Aldrich, St. Louis, MO, USA), and a Ficoll gradient was used to separate mononuclear cells. The mononuclear cell layer was carefully collected and washed twice with PBS, then centrifuged at 100× g for 10 min. PCs were isolated from the mononuclear fraction using CD138 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) by magnetic-activated cell sorting (MACS). A portion of these sorted plasma cells were used for RNA extraction to analyze miR expression, while the remaining cells were cultured as primary patient-derived plasma cells in RPMI 1640 with 10% FBS at 37 °C in a humidified incubator with 5% CO2. Specifically, multiple myeloma plasma cells from patient 7 (MM PC) were cultured under the same conditions as the myeloma cell lines.
In addition, myeloma cell lines (U266, RPMI 8226, and LP1) were cultured in RPMI medium containing 10% FBS under the same incubator conditions and seeded at a density of 2 × 105 cells/mL. Normal stromal HS-5 cells were maintained in RPMI medium supplemented with 10% FBS and seeded at a density of 105 cells per well in 24-well plates. HS-5 cells were passaged upon reaching 80–90% confluency; for detachment, 1 mL of 0.25% trypsin-EDTA was added to each T25 flask, and cells were incubated at 37 °C for 2–5 min until they were rounded up. Trypsinization was stopped by adding an equal volume of complete RPMI medium, and cells were centrifuged at 200× g for 5 min before reseeding.
RNA isolation, reverse transcription, and quantitative real-time PCR
Total RNA was extracted from MM cells or primary plasma cells using Trizol reagent and was then quantified for the concentration. qRT-PCR was performed on a 7500 Fast Real-Time PCR System (Applied Biosystems). MicroRNA Reverse Transcription Kit and Universal Master Mix II were used to test miR-429 and its endogenous control U6 (Applied Biosystems, Foster City, CA, USA). Normalization for miR-429 was accomplished by comparison to U6. Fold change of target miR was calculated according to the 2−ΔΔCT method.
Inhibition of miR-429
MM PCs, MM cell lines, and HS-5 cells were seeded in 24- or 96-well plates and then transfected with miR-429 inhibitor or miScript inhibitor Negative control using Hiperfect transfection reagent (Qiagen) according to the manufacturer’s instructions [
19]. The miR-429 inhibitor used was a chemically modified antisense oligonucleotide (Qiagen) designed to specifically bind to mature miR-429 and inhibit its function. Its effectiveness and specificity have been validated in previous studies using similar models. The transfection efficiency was evaluated and validated by testing 50, 100, and 150 nM of inhibitors dosed by Q-RT-PCR after 24 and 48 h. miScript inhibitor Negative control was used as negative control.
MTT assay
Cell survival was evaluated by MTT assay in 96-well plates [
20,
21,
22,
23]. U266 cells were transfected then seeded in a 96-well plate at a density of 2 × 10
5 cells/well. A volume of 10 µL of 5 mg/mL MTT (Sigma) reagent was added to each well, and cells were further incubated for 4 h at 37 °C. Then medium was removed, and 100 mL of dimethyl sulfoxide (DMSO) was added to each well to dissolve the formazan. The optical density (OD) was evaluated at wavelength of 490 nm. Wells without cells (DMSO alone) were used as blank, and experiments were repeated at least 3 times [
24].
MM cancer cell co-culture with stromal cells
The U266, RPMI8226, and LP1 multiple myeloma cells and HS-5 cells were cultured in RPMI-1640 medium supplemented with 10% FBS and 1% penicillin-streptomycin at 37 °C in a 5% CO2 incubator. For the co-culture, 2 × 105 HS-5 cells were seeded onto 24-well plates with 500 µL RPMI-1640 medium. After 48 h, cells were transfected with miR-inhibitor, then MM cells were added. Cell growth was evaluated by Trypan blue exclusion cell count. Each sample was run at least in triplicate.
Cell Apoptosis Detection
Apoptosis was detected by flow cytometry using Annexin V/Propidium Iodide (An/PI) double staining. Briefly, U266 cells or MM PCs were transfected with miR-429 inhibitor. Then, U266 cells were incubated with An/PI as recommended in the kit’s instructions. An (+)/PI (-) was considered as early apoptotic cells, while An (+)/PI (+) was considered as late apoptotic cells [
25].
Statistical Analysis
Each experiment was repeated at least 3 times. Numerical data are presented as mean ± SD. The difference between means was analyzed with Student’s t test. All statistical analyses were performed using Excel. Differences were considered significant when p < 0.05.
3. Results
3.1. miR-429 Expression in Multiple Myeloma Cell Lines and Primary Patient Multiple Myeloma Cells
Fourteen patients newly diagnosed with multiple myeloma were included in this study. The cohort consisted of eight males and six females, with a median age of 57 years (range: 34–75 years). The most frequent presenting symptoms were bone pain (in eight patients) and anemia (in six patients), while some patients presented with hypercalcemia, pathological fractures, or compressive plasmacytomas. Bone marrow plasma cell infiltration varied widely, ranging from 4% to 70%. Chromosomal abnormalities such as t(4;14) and del(17p) were detected in certain cases, whereas others showed no detectable cytogenetic alterations. Responses to treatment ranged from very good partial responses to treatment failure, with one patient lost to follow-up (
Table 1).
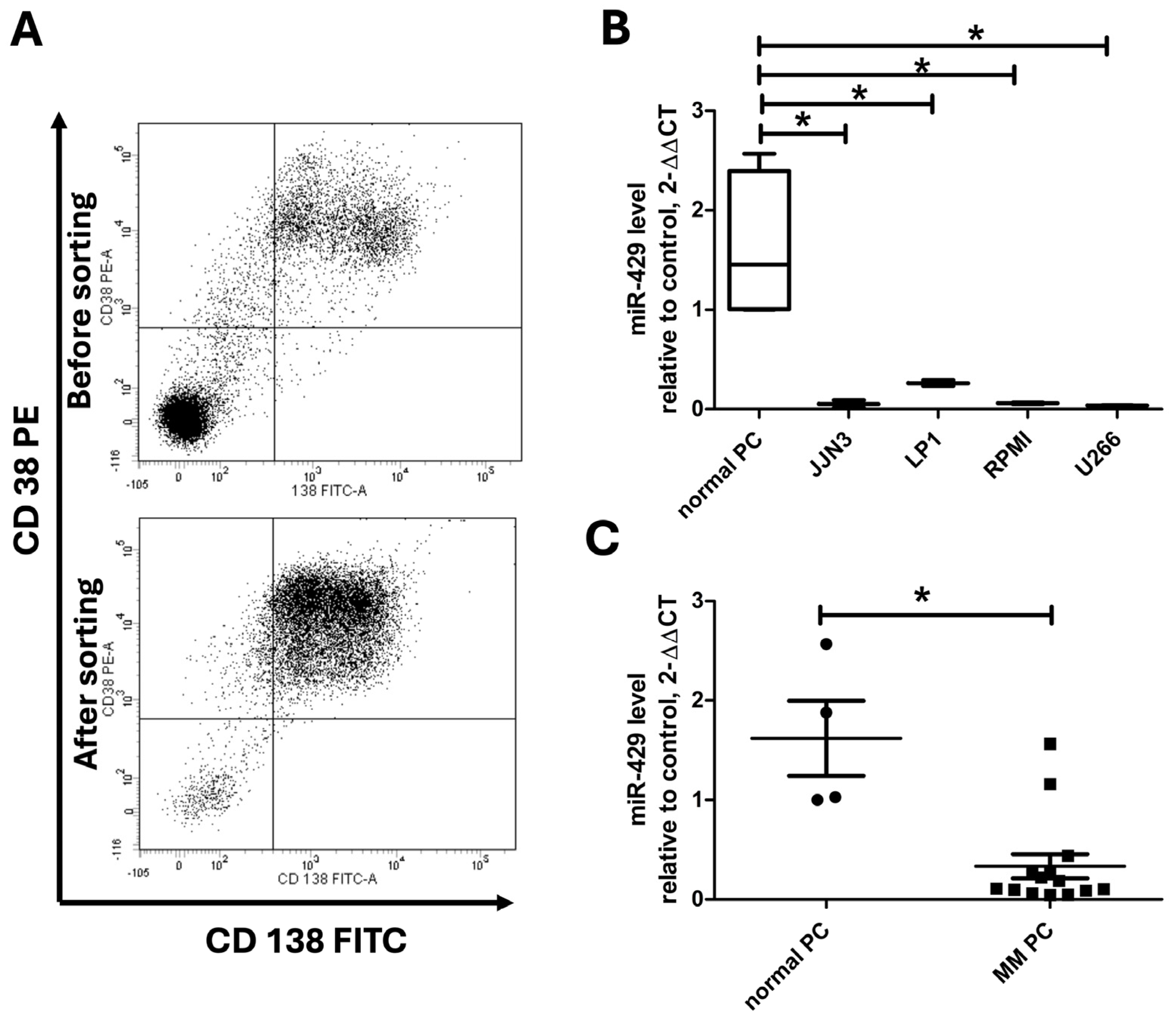
Once plasma cells were isolated from these patients, labeling with anti-CD138 and CD38 antibodies enabled the validation of the cell sorting by flow cytometry (
Figure 1A). Using RT-PCR, we assessed miR-429 expression levels in multiple myeloma cell lines, JJN3, LP1, RPMI-8226, and U266, in comparison with normal PCs (
Figure 1B). As shown in
Figure 1B, we observed variable miR-429 expression across these cell lines: normal plasma cells exhibited the highest expression, whereas all tested myeloma cell lines displayed markedly reduced levels of miR-429. Specifically, miR-429 levels (ΔCt values) were 0.05 ± 0.04 in JJN3, 0.26 ± 0.04 in LP1, 0.0524 ± 0.0065 in RPMI-8226, and 0.0321 ± 0.0040 in U266, with RPMI-8226 and U266 showing the lowest expression levels. These data confirm a significant and consistent downregulation of miR-429 across multiple myeloma cell models compared to normal plasma cells, further supporting its potential tumor-suppressor role in multiple myeloma. Consistent with these findings in cell lines, miR-429 expression was significantly downregulated in primary multiple myeloma plasma cells isolated from patients compared with normal plasma cells from healthy donors, as demonstrated in
Figure 1C. Specifically, the ΔCt values of miR-429 in patients’ primary plasma cells ranged from 0.0469 to 1.5645, with a median of approximately 0.27, whereas the values in normal donors were consistently higher, reflecting significantly greater miR-429 expression in healthy plasma cells. Although this trend was statistically significant, we observed a partial overlap between the two groups, which limits the interpretability of miR-429 as a diagnostic biomarker in this cohort. Nevertheless, these results indicate a consistent downregulation of miR-429 in both established myeloma cell lines and primary malignant plasma cells, supporting a potential tumor-suppressor role for miR-429 in multiple myeloma.
3.2. Pro-Proliferative Effects of miR-429 Inhibition on Multiple Myeloma Cells
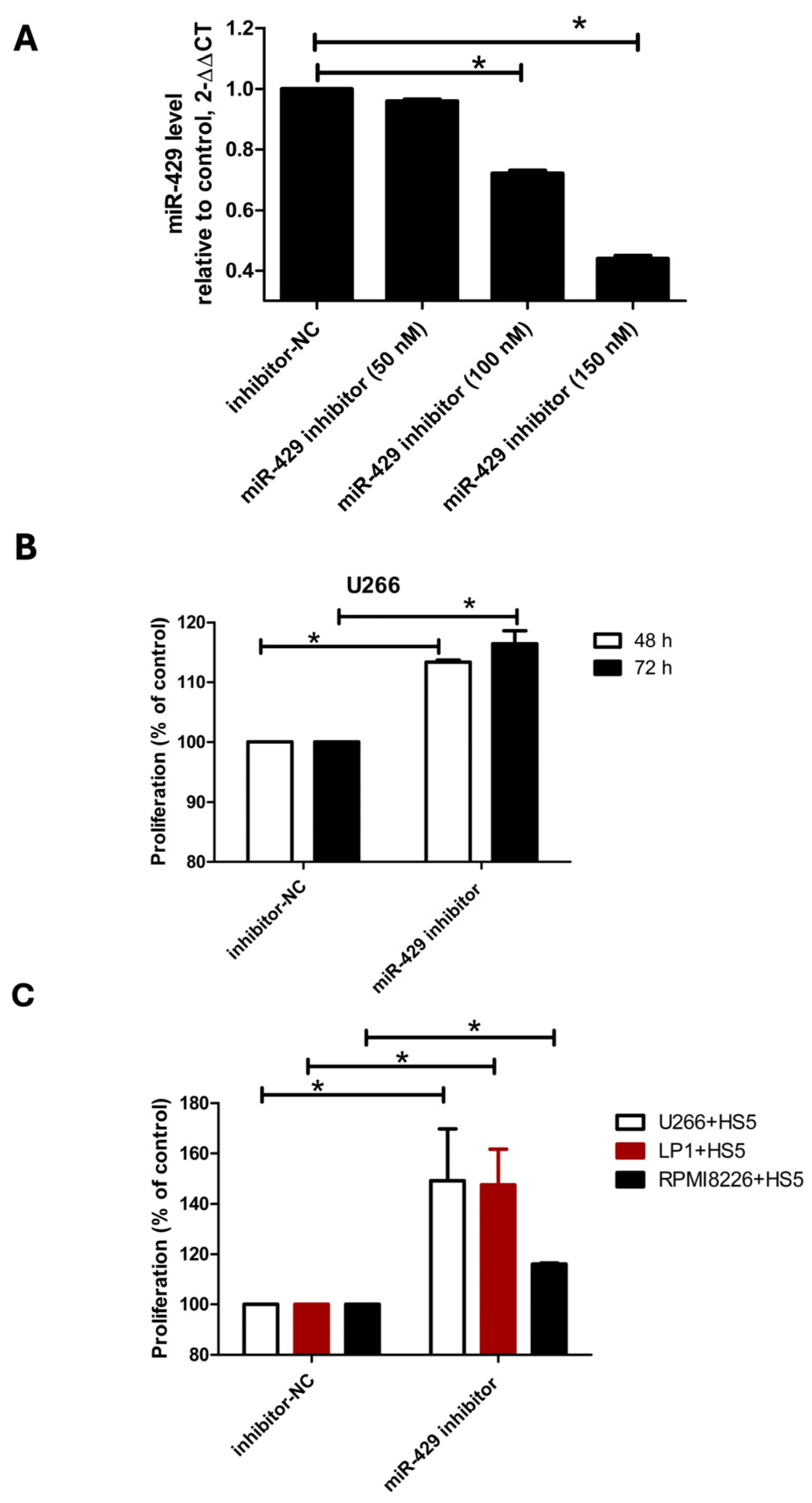
To investigate the role of miR-429 in multiple myeloma, we transfected U266 cells with increasing concentrations of miR-429 inhibitors (50, 100, and 150 nM) and assessed cell proliferation using the MTT assay. Transfection efficiency was confirmed by measuring miR-429 expression levels post-transfection: the cells treated with the 50 nM inhibitor exhibited a relative miR-429 level of 0.96 ± 0.0058, those with the 100 nM inhibitor showed a reduction to 0.72 ± 0.01, and those with the 150 nM inhibitor had an miR-429 expression level that had further decreased to 0.44 ± 0.01 (
Figure 2A). Based on these results, we selected the concentration of 150 nM for subsequent experiments to investigate the role of miR-429 in multiple myeloma. U266 cells were transfected with a 150 nM miR-429 inhibitor, and cell proliferation was assessed using the MTT assay. We observed a significant increase in U266 cell growth by 13% at 48 h and 16% at 72 h compared to controls (
Figure 2B). Although the increase in proliferation upon miR-429 inhibition was statistically significant, the magnitude of this effect was moderate. This suggests that miR-429 acts more as a modulatory factor than a dominant driver of proliferation.
Next, we examined the impact of miR-429 inhibition in the bone marrow microenvironment by transfecting HS-5 stromal cells with miR-429 inhibitors prior to co-culture with MM cell lines. Trypan blue exclusion assays revealed the proliferation of multiple myeloma cells had significantly increased after three days of co-culture with miR-429-inhibited HS-5 stromal cells. Specifically, LP1 cells co-cultured with HS-5 cells transfected with the miR-429 inhibitor showed a 24.6% increase in cell proliferation (124.6 ± 0.15% compared to the control), U266 cells exhibited an 8% increase (108% compared to the control), and RPMI-8226 cells displayed a 16% increase (116% compared to the control) compared to their respective controls (inhibitor-NC set at 100%) (
Figure 2C). These findings indicate that miR-429 inhibition, whether directly in multiple myeloma cells or indirectly via the stromal cell line HS-5, exerts a pronounced pro-proliferative effect on multiple myeloma cells without affecting apoptosis.
3.3. Apoptosis Analysis
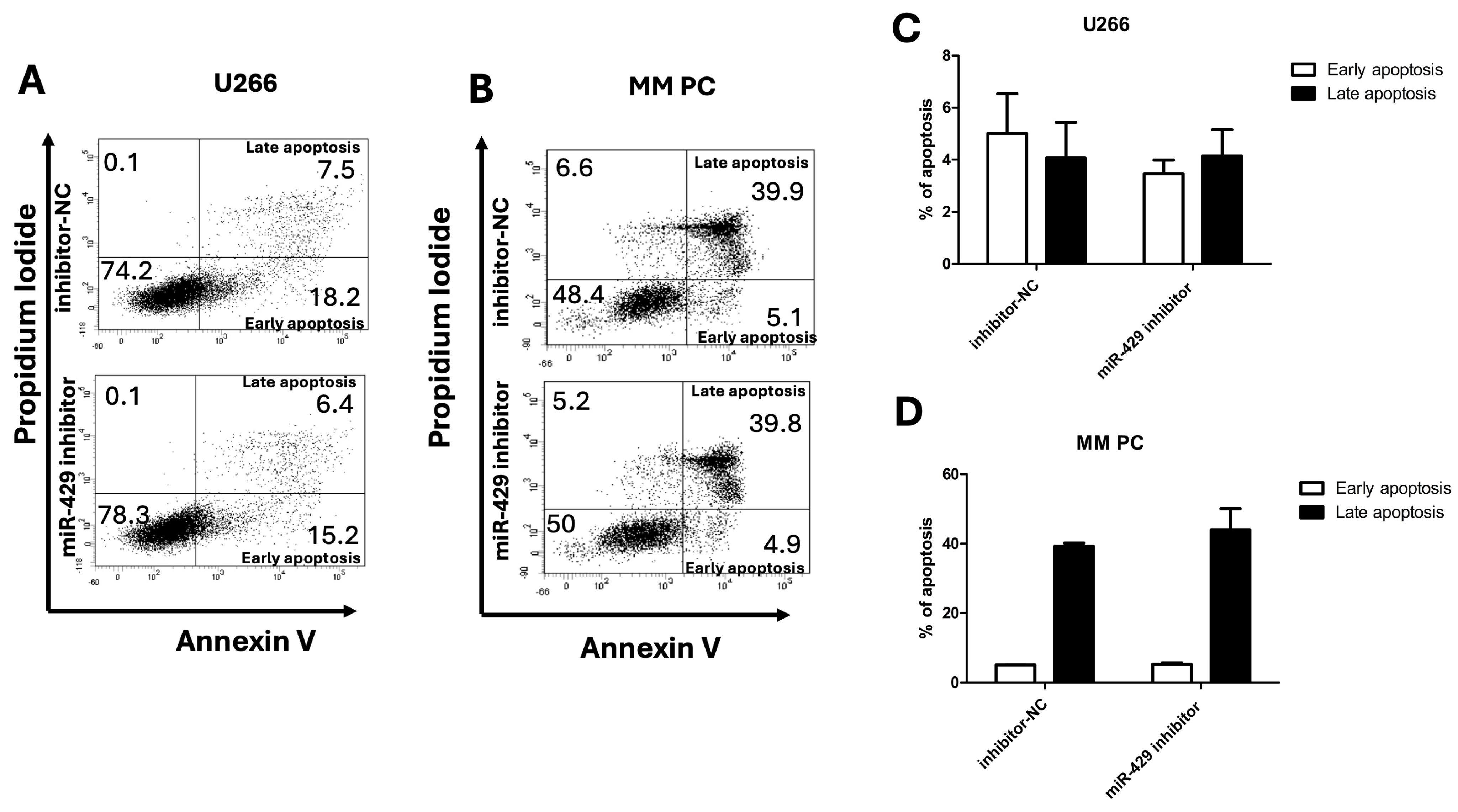
Apoptotic cell death was evaluated in the U266 myeloma cell line following transfection with either the negative control inhibitor (inhibitor-NC) or miR-429 inhibitors. In inhibitor-NC-treated cells, early apoptosis averaged 5.01 ± 6.77%, while late apoptosis was 4.06 ± 5.46%. In the miR-429 inhibitor group, early apoptosis reached 3.47 ± 0.69%, and late apoptosis was 4.15 ± 0.96% (
Figure 3A,C). A statistical analysis revealed no significant differences between the groups for either early or late apoptosis in U266 cells. Similarly, primary malignant plasma cells isolated from patient 7 (MM PC) were analyzed for apoptosis induction (
Figure 3B,D). In these cells, early apoptosis was comparable between inhibitor-NC (5.05 ± 0.07%) and miR-429 inhibitors (5.25 ± 0.49%). Late apoptosis showed a modest, non-significant increase in the miR-429 inhibitor group (44.05 ± 6.01%) compared to inhibitor-NC (39.25 ± 0.92%). Collectively, these results indicate that the inhibition of miR-429 does not significantly alter the induction of early or late apoptosis in either U266 myeloma cells or MM PCs.
4. Discussion
Numerous studies have demonstrated that the aberrant expression of miRNAs is associated with diverse biological processes, such as proliferation, migration, and invasion [
26]. In multiple myeloma (MM), dysregulation of miRNAs has been implicated in various physiological processes, and these molecules have emerged as potential therapeutic targets for disease management [
7]. Accumulating evidence indicates that miR-429 dysregulation contributes to the epithelial–mesenchymal transition, tumor progression, invasion, metastasis, apoptosis, and drug resistance across different cancer types [
27].
Our data indicate that miR-429 is consistently downregulated in MM cell lines and primary malignant plasma cells. Functional inhibition led to a moderate increase in proliferation, both in monoculture and in co-culture with stromal cells, without affecting spontaneous apoptosis. These observations support a potential tumor-suppressive role for miR-429 in MM biology. Although miR-429 is significantly downregulated in MM cells compared to healthy plasma cells, residual expression remains detectable in these cells. Our inhibition experiments targeted this remaining pool of miR-429. The observed enhancement in proliferation upon inhibition suggests that even low levels of miR-429 retain functional activity and constrain tumor cell growth to some extent. Further suppression of this residual expression removes that constraint, resulting in increased proliferation. This reinforces the notion that miR-429 exerts a biologically meaningful tumor-suppressive effect, even at a low level of abundance. Notably, the inhibitory experiments targeted the residual pool of miR-429 still present in MM cells. The fact that further downregulation enhanced proliferation suggests that even low levels of this miRNA may retain biologically meaningful tumor-suppressive activity.
This observation is consistent with previous studies reporting the tumor-suppressive functions of miR-429 in various solid tumors. For instance, miR-429 has been shown to inhibit proliferation, invasion, or metastasis in renal cell carcinoma [
28], gastric cancer [
29], osteosarcoma [
30], and esophageal carcinoma [
15].
Conversely, in cancers like endometrial [
31], prostate [
32], and lung carcinomas [
12,
33], miR-429 has been implicated as an oncogene promoting proliferation and migration. These contrasting roles highlight the context-dependent nature of miR-429, whose function varies across cancer types and cellular environments.
For MM specifically, only limited previous data are available. A recent study suggested that miR-429 may suppress invasion and migration by targeting the Bmi1/AKT pathway, but its role in MM cell proliferation has not been directly explored [
18]. While the study by Yang et al. (2022) proposed that miR-429 exerts tumor-suppressive effects through the regulation of Bmi1 and AKT signaling, we did not investigate this axis in our experimental model. Therefore, we refer to this mechanism as a plausible hypothesis, which will require further experimental validation using target gene analysis and pathway-specific assays. Our study confirms the proliferative effect of miR-429 inhibition in U266 cells and further demonstrates that this effect extends to a co-culture model, in which modulation of stromal cells also promotes myeloma cell growth.
Interestingly, the inhibition of miR-429 in stromal HS-5 cells promoted MM cell growth in co-culture, emphasizing the crucial role of the bone marrow microenvironment in MM progression. Although we did not directly assess stromal cytokine secretion in this study, it is plausible that miR-429 inhibition alters the stromal secretome in a way that promotes MM cell proliferation. Given the established contribution of bone marrow-derived cytokines, chemokines, and extracellular vesicles to MM progression and drug resistance, future studies should aim to characterize the molecular changes induced by miR-429 downregulation in stromal cells. This perspective is supported by recent reports showing that miRNA dysregulation in stromal cells can influence MM cell behavior and contribute to disease progression or treatment resistance [
34,
35].
In contrast to previous studies that showed miR-429 overexpression induces apoptosis in multiple myeloma cells by suppressing the Bmi1/AKT pathway [
18], our results did not detect any significant effect of miR-429 inhibition on spontaneous apoptosis. This suggests that the pro-apoptotic role of miR-429 may depend on whether it is overexpressed or inhibited or it could be influenced by specific experimental conditions.
Collectively, our findings suggest that miR-429 may act primarily as a regulator of proliferation with limited impact on spontaneous apoptosis in MM. While these observations support a potential tumor-suppressive role for miR-429, further mechanistic and in vivo studies are needed to substantiate its relevance. Thus, miR-429 could represent a candidate biomarker or therapeutic target, warranting additional investigations to evaluate whether restoring its expression might contribute to limiting myeloma cell growth.
This study has several limitations. First, the mechanistic basis of miR-429’s effects remains to be clarified. Although pathways such as Bmi1/AKT, NF-κB, and c-Myc have been implicated in related contexts, we did not experimentally assess target genes or signaling cascades. Taken together, our findings provide preliminary functional evidence supporting the role of miR-429 in MM cell proliferation and microenvironmental interactions. However, the exploratory nature of this study limits mechanistic interpretations. Future investigations will be needed to elucidate the downstream molecular targets and signaling pathways modulated by miR-429 and to determine whether its effects involve known regulators such as AKT, NF-κB, or MYC.
Second, our data are based on a limited number of patient samples and healthy donors, and we were unable to include patients with MGUS or smoldering MM to evaluate expression across disease stages. Although median miR-429 expression was lower in MM patient samples compared to healthy plasma cells, we observed a partial overlap between groups. This could reflect biological heterogeneity among patients or donors, including age-related differences in miRNA expression. Unfortunately, we did not have access to samples from individuals with MGUS or smoldering MM, which would have allowed for the assessment of miR-429 expression along the disease continuum. Future studies including these patient subsets will be critical to determine whether miR-429 downregulation is an early or late event in myeloma pathogenesis.
5. Conclusions
In summary, our study is the first to validate the expression levels of miR-429 directly in primary plasma cells isolated from multiple myeloma patients, demonstrating a consistent downregulation compared to normal plasma cells. Our results suggest that miR-429 may exert a suppressive effect on MM cell proliferation. Further mechanistic and in vivo studies are needed to fully characterize its role and clinical relevance in multiple myeloma. Moreover, further in-depth mechanistic studies are essential to fully elucidate the molecular pathways through which miR-429 exerts its effects on myeloma progression and to explore its potential for clinical application.
Author Contributions
Conceptualization, M.A., I.L. and F.B.A.-F.; Methodology, M.A., I.L., E.B., R.K. and F.B.A.-F.; Validation, M.A., I.L., B.S. and F.B.A.-F.; Investigation, M.A., I.L., B.S. and F.B.A.-F.; Resources, E.B. and R.K.; Writing—Original Draft Preparation, M.A.; Data curation, E.B. and R.K., Writing—Review and Editing, M.A., I.L., E.B., R.K. and F.B.A.-F.; Supervision, M.A. and F.B.A.-F.; Project Administration, M.A., B.S. and F.B.A.-F.; Funding Acquisition, F.B.A.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the local ethics committee of our institution (Clinical Research Ethics Committee of La Rabta Hospital, Tunis) (protocol code: CEBM.EPS.HR/05/2019; date of approval: 27 May 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available in the main text.
Acknowledgments
We are grateful to the research platform of Medical Sciences and Technology of the Faculty of Medicine of Tunis (University of Tunis El Manar).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MM | Multiple myeloma |
| miRNAs | microRNAs |
| EMT | Epithelial–mesenchymal transition |
| Bmi1 | B lymphoma Mo-MLV insertion region 1 homolog |
| AKT | Protein kinase B pathway |
| PBS | Phosphate-buffered saline |
| PC | Plasma cell |
| MACS | Magnetic-activated cell sorting |
| FBS | Fetal bovine serum |
| RPMI | Roswell Park Memorial Institute medium |
| HS-5 | Human stromal cell line |
| RT-PCR | Reverse transcription polymerase chain reaction |
| DMSO | Dimethyl Sulfoxide |
| OD | Optical Density |
| An/PI | Annexin V/propidium iodide |
| VTD | Bortezomib (Velcade) plus thalidomide and dexamethasone |
| PVD | Bortezomib (Velcade) plus pegylated liposomal doxorubicin and dexamethasone |
| t(4;14) | Translocation between chromosome 4 and 14 |
| del(17p) | Deletion of the short arm of chromosome 17 |
References
- Mafra, A.; Laversanne, M.; Marcos-Gragera, R.; Chaves, H.V.S.; Mcshane, C.; Bray, F.; Znaor, A. The Global Multiple Myeloma Incidence and Mortality Burden in 2022 and Predictions for 2045. JNCI J. Natl. Cancer Inst. 2025, 117, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Rehmat, M.; Raza, A.; Kalpina, F.; Ahmad, M.; Alamgir, E.; Ikram, M.; Zeeshan, E. Renal Failure-Related Mortality in Multiple Myeloma: United States Trends From 1999 to 2020. Clin. Lymphoma Myeloma Leuk. 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.; Diels, J.; Ghilotti, F.; Mendes, J.; Van Hoorenbeeck, S.; Lee, S.; Schecter, J.M.; Lendvai, N.; Patel, N.; Triguero, A.; et al. Survival Outcomes with Cilta-Cel Versus Conventional Treatment Regimens for Patients with Lenalidomide-Refractory Multiple Myeloma Using Inverse Probability of Treatment Weighting. Adv. Ther. 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Song, Y.; Zhou, K.; Lin, Q. Emerging Therapeutic Agents in Multiple Myeloma: Highlights from the 2024 ASH Annual Meeting. Biomark. Res. 2025, 13, 90. [Google Scholar] [CrossRef]
- Amoozgar, B.; Bangolo, A.; Habibi, M.; Cho, C.; Goy, A. From Molecular Precision to Clinical Practice: A Comprehensive Review of Bispecific and Trispecific Antibodies in Hematologic Malignancies. Int. J. Mol. Sci. 2025, 26, 5319. [Google Scholar] [CrossRef]
- Papadimitriou, M.-A.; Soureas, K.; Papanota, A.-M.; Tsiakanikas, P.; Adamopoulos, P.G.; Ntanasis-Stathopoulos, I.; Malandrakis, P.; Gavriatopoulou, M.; Sideris, D.C.; Kastritis, E.; et al. miRNA-Seq Identification and Clinical Validation of CD138+ and Circulating miR-25 in Treatment Response of Multiple Myeloma. J. Transl. Med. 2023, 21, 245. [Google Scholar] [CrossRef]
- Lu, M.-Q.; He, Y.-Q.; Wu, Y.; Zhou, H.-X.; Jian, Y.; Gao, W.; Bao, L.; Chen, W.-M. Identification of Aberrantly Expressed lncRNAs and ceRNA Networks in Multiple Myeloma: A Combined High-Throughput Sequencing and Microarray Analysis. Front. Oncol. 2023, 13, 1160342. [Google Scholar] [CrossRef]
- Agafonova, A.; Prinzi, C.; Trovato Salinaro, A.; Ledda, C.; Cosentino, A.; Cambria, M.T.; Anfuso, C.D.; Lupo, G. Breaking Barriers: The Role of the Bone Marrow Microenvironment in Multiple Myeloma Progression. Int. J. Mol. Sci. 2025, 26, 7301. [Google Scholar] [CrossRef]
- Kamrani, S.; Naseramini, R.; Khani, P.; Razavi, Z.S.; Afkhami, H.; Atashzar, M.R.; Nasri, F.; Alavimanesh, S.; Saeidi, F.; Ronaghi, H. Mesenchymal Stromal Cells in Bone Marrow Niche of Patients with Multiple Myeloma: A Double-Edged Sword. Cancer Cell Int. 2025, 25, 117. [Google Scholar] [CrossRef]
- Wu, Y.; Xiao, Y.; Ding, X.; Zhuo, Y.; Ren, P.; Zhou, C.; Zhou, J. A miR-200b/200c/429-Binding Site Polymorphism in the 3′ Untranslated Region of the AP-2α Gene Is Associated with Cisplatin Resistance. PLoS ONE 2011, 6, e29043. [Google Scholar] [CrossRef]
- Li, J.; Du, L.; Yang, Y.; Wang, C.; Liu, H.; Wang, L.; Zhang, X.; Li, W.; Zheng, G.; Dong, Z. MiR-429 Is an Independent Prognostic Factor in Colorectal Cancer and Exerts Its Anti-Apoptotic Function by Targeting SOX2. Cancer Lett. 2013, 329, 84–90. [Google Scholar] [CrossRef]
- Lang, Y.; Xu, S.; Ma, J.; Wu, J.; Jin, S.; Cao, S.; Yu, Y. MicroRNA-429 Induces Tumorigenesis of Human Non-Small Cell Lung Cancer Cells and Targets Multiple Tumor Suppressor Genes. Biochem. Biophys. Res. Commun. 2014, 450, 154–159. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Wu, S.; Shi, X.; Li, L.; Zhao, J.; Xu, H. Tumor-Suppressing Effects of miR-429 on Human Osteosarcoma. Cell Biochem. Biophys. 2014, 70, 215–224. [Google Scholar] [CrossRef]
- Samantarrai, D.; Mallick, B. miR-429 Inhibits Metastasis by Targeting KIAA0101 in Soft Tissue Sarcoma. Exp. Cell Res. 2017, 357, 33–39. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Zang, W.; Ma, Y.; Wang, N.; Li, P.; Wang, T.; Zhao, G. MiR-429 up-Regulation Induces Apoptosis and Suppresses Invasion by Targeting Bcl-2 and SP-1 in Esophageal Carcinoma. Cell. Oncol. 2013, 36, 385–394. [Google Scholar] [CrossRef]
- Qiu, M.; Liang, Z.; Chen, L.; Tan, G.; Wang, K.; Liu, L.; Liu, J.; Chen, H. MicroRNA-429 Suppresses Cell Proliferation, Epithelial-Mesenchymal Transition, and Metastasis by Direct Targeting of BMI1 and E2F3 in Renal Cell Carcinoma. Urol. Oncol. 2015, 33, e9–e18. [Google Scholar] [CrossRef]
- Liu, D.; Xia, P.; Diao, D.; Cheng, Y.; Zhang, H.; Yuan, D.; Huang, C.; Dang, C. MiRNA-429 Suppresses the Growth of Gastric Cancer Cells in Vitro. J. Biomed. Res. 2012, 26, 389–393. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Z.; Wang, M.; Wu, Z.; Sun, Z.; Liu, M.; Li, G. MicroRNA-429 Regulates Invasion and Migration of Multiple Myeloma Cells via Bmi1/AKT Pathway. J. Biomater. Tissue Eng. 2022, 12, 2419–2426. [Google Scholar] [CrossRef]
- Lee, K.-J.; Singh, N.; Bizuneh, M.; Kim, N.-H.; Kim, H.S.; Kim, Y.; Lee, J.-J.; Kim, J.H.; Kim, J.; Jeong, S.Y.; et al. miR-429 Suppresses Endometrial Cancer Progression and Drug Resistance via DDX53. J. Pers. Med. 2023, 13, 1302. [Google Scholar] [CrossRef]
- Limam, I.; Ben Aissa-Fennira, F.; Essid, R.; Chahbi, A.; Kefi, S.; Mkadmini, K.; Elkahoui, S.; Abdelkarim, M. Hydromethanolic Root and Aerial Part Extracts from Echium Arenarium Guss Suppress Proliferation and Induce Apoptosis of Multiple Myeloma Cells through Mitochondrial Pathway. Environ. Toxicol. 2021, 36, 874–886. [Google Scholar] [CrossRef]
- Limam, I.; Abdelkarim, M.; Essid, R.; Chahbi, A.; Fathallah, M.; Elkahoui, S.; Ben Aissa-Fennira, F. Olea europaea L. cv. Chetoui Leaf and Stem Hydromethanolic Extracts Suppress Proliferation and Promote Apoptosis via Caspase Signaling on Human Multiple Myeloma Cells. Eur. J. Integr. Med. 2020, 37, 101145. [Google Scholar] [CrossRef]
- Limam, I.; Ghali, R.; Abdelkarim, M.; Ouni, A.; Araoud, M.; Abdelkarim, M.; Hedhili, A.; Ben-Aissa Fennira, F. Tunisian Artemisia campestris L.: A Potential Therapeutic Agent against Myeloma—Phytochemical and Pharmacological Insights. Plant Methods 2024, 20, 59. [Google Scholar] [CrossRef]
- Kharrat, R.; Lakhal, F.B.; Souia, H.; Limam, I.; Naji, H.B.; Abdelkarim, M. Anticancer Effects of Artemisia Campestris Extract on Acute Myeloid Leukemia Cells: An Ex Vivo Study. Med. Oncol. 2024, 41, 206. [Google Scholar] [CrossRef] [PubMed]
- Abdelkarim, M.; Ben Younes, K.; Limam, I.; Guermazi, R.; ElGaaied, A.B.A.; Aissa-Fennira, F.B. 3,6-Dichloro-1,2,4,5-Tetrazine Assayed at High Doses in the Metastatic Breast Cancer Cell Line MDA-MB-231 Reduces Cell Numbers and Induces Apoptosis. Curr. Bioact. Compd. 2020, 16, 546–550. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutellingsperger, C. A Novel Assay for Apoptosis Flow Cytometric Detection of Phosphatidylserine Expression on Early Apoptotic Cells Using Fluorescein Labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Klicka, K.; Grzywa, T.M.; Mielniczuk, A.; Klinke, A.; Włodarski, P.K. The Role of miR-200 Family in the Regulation of Hallmarks of Cancer. Front. Oncol. 2022, 12, 965231. [Google Scholar] [CrossRef]
- Guo, C.M.; Liu, S.Q.; Sun, M.Z. miR-429 as Biomarker for Diagnosis, Treatment and Prognosis of Cancers and Its Potential Action Mechanisms: A Systematic Literature Review. Neoplasma 2020, 67, 215–228. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Li, Q.; Guo, C.; Sun, W.; Zhao, D.; Jiang, S.; Hao, L.; Tian, Y.; Liu, S.; et al. miR-429-CRKL Axis Regulates Clear Cell Renal Cell Carcinoma Malignant Progression through SOS1/MEK/ERK/MMP2/MMP9 Pathway. Biomed. Pharmacother. 2020, 127, 110215. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, B.; Lu, M.; Zheng, M.; Chen, H.; Ding, J.; Xu, A.-M.; Xu, Y. miR-429 Functions as a Tumor Suppressor by Targeting FSCN1 in Gastric Cancer Cells. OncoTargets Ther. 2016, 9, 1123–1133. [Google Scholar] [CrossRef]
- Deng, Y.; Luan, F.; Zeng, L.; Zhang, Y.; Ma, K. MiR-429 Suppresses the Progression and Metastasis of Osteosarcoma by Targeting ZEB1. EXCLI J. 2017, 16, 618–627. [Google Scholar] [CrossRef]
- Yang, J.; Barkley, J.E.; Bhattarai, B.; Firouzi, K.; Monk, B.J.; Coonrod, D.V.; Zenhausern, F. Identification of Endometrial Cancer-Specific microRNA Biomarkers in Endometrial Fluid. Int. J. Mol. Sci. 2023, 24, 8683. [Google Scholar] [CrossRef]
- Ouyang, Y.; Gao, P.; Zhu, B.; Chen, X.; Lin, F.; Wang, X.; Wei, J.; Zhang, H. Downregulation of microRNA-429 Inhibits Cell Proliferation by Targeting p27Kip1 in Human Prostate Cancer Cells. Mol. Med. Rep. 2015, 11, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; He, J.; Chen, D.; Zhang, B.; Xu, L.; Ma, H.; Liu, X.; Zhang, Y.; Le, H. Expression of miR-29c, miR-93, and miR-429 as Potential Biomarkers for Detection of Early Stage Non-Small Lung Cancer. PLoS ONE 2014, 9, e87780. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yang, X.; Liu, M.; Zhang, Z.; Xing, E. Roles of miRNA Dysregulation in the Pathogenesis of Multiple Myeloma. Cancer Gene Ther. 2021, 28, 1256–1268. [Google Scholar] [CrossRef]
- Alipoor, S.D.; Chang, H. Exosomal miRNAs in the Tumor Microenvironment of Multiple Myeloma. Cells 2023, 12, 1030. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).