Beyond TKIs: Advancing Therapeutic Frontiers with Immunotherapy, Targeted Agents, and Combination Strategies in Resistant Chronic Myeloid Leukemia
Abstract
1. Introduction
1.1. Background of Chronic Myeloid Leukemia (CML)
1.2. Historical Impact of Tyrosine Kinase Inhibitors (TKIs) on CML Treatment and Outcomes
1.3. Limitations of Current TKI Therapies
1.4. Objective and Scope of the Review
2. Search Databases and Inclusion/Exclusion Criteria
2.1. Inclusion Criteria
2.2. Exclusion Criteria
3. Results and Discussion
3.1. Mechanisms of Resistance in CML Patients
3.1.1. BCR-ABL-Dependent Mechanisms
- 1.
- Mutations:
- 2.
- Overexpression of the BCR-ABL Gene:
3.1.2. BCR-ABL-Independent Mechanisms
- 1.
- Activation of Alternative Signaling Pathways:
- 2.
- Epigenetic Modifications:
3.2. Emerging Therapies for Resistant Chronic Myeloid Leukemia
3.2.1. Clinical Trials
3.2.2. Real-World Evidence
3.2.3. Comparative Analysis of Emerging Therapies for Resistant Chronic Myeloid Leukemia (CML)
3.3. Challenges and Future Directions for Resistant CML Therapies
3.4. Clinical Implications for Practice in Resistant Chronic Myeloid Leukemia
3.4.1. Clinical Factors That Affect the Treatment Choices of Patients with Resistant Chronic Myeloid Leukemia
3.4.2. Criteria for Switching from TKIs to New Therapies
- Major Molecular Response (MMR): Achieving and maintaining MMR is a critical factor. Patients who do not achieve MMR within a specific timeframe may need to switch therapies. For instance, patients who do not achieve MMR within 12 months of treatment may experience shorter cytogenetic remission and poorer outcomes, suggesting the need for alternative therapies [71].
- Molecular Recurrence (MRec): Discontinuation of TKIs can be considered if patients achieve a deep molecular response, such as undetectable BCR-ABL1 levels. However, detectable BCR-ABL1 by real-time quantitative PCR (RQ-PCR) or droplet digital PCR (ddPCR) at the time of TKI discontinuation is associated with a higher risk of MRec, indicating that these patients might need to continue or switch therapies [72].
- Treatment-Free Remission (TFR): Successful TFR is another criterion. Patients who maintain TFR without molecular recurrence may not need to switch therapies, but those who relapse may require re-initiation or switching of TKIs.
- ⮚
- Health risks and Adverse Effects:
3.4.3. Managing Treatment-Related Toxicity and Maintaining Quality of Life
- ⮚
- Toxicity Management: Given the numerous difficulties associated with these leukemias, the treatment is likely to entail significant adverse effects; therefore, prioritizing the control of these toxicities is essential. This may involve dosage adjustments, temporarily halting the therapy, or considering alternative treatment options [77].
- ⮚
- Life Quality: Clinical decisions should be informed by the ongoing assessment of quality of life, aligning with treatment goals and the individual’s health status and life aspirations. Psychological therapy and additional supporting interventions should be provided [78].
3.5. Personalized Treatment Approaches
3.6. Limitations of the Review
3.6.1. Limitations Due to the Availability of Literature and Clinical Trials
3.6.2. Possible Publication Bias
3.6.3. Identifying Research Gaps
- Leukemic stem cells are enduring and can be eradicated; however, current medicines can only mature leukemic cells without eliminating leukemic stem cells. This gap results in the persistence of the disease and recurrence. Subsequent research should develop techniques that specifically target these stem cells.
- Understanding inhibition mechanisms related to drug resistance: Despite the availability of third-generation TKIs, resistance remains a significant concern. Increased emphasis is necessary on the source of resistance mechanisms, especially concerning recent mutations that existing medicines do not address.
- Significant impact and danger for the duration of emerging medicines: There is a lack of long-term data on the safety and efficacy of numerous novel TKI therapies and immunotherapies. Conducting longitudinal studies on their impact is essential.
- The ambit of combination therapy addresses challenges: Although combination therapy offers advantages, there is no unequivocally established ideal treatment strategy. It is essential to examine several drug combinations that may synergize effectively to identify the optimal procedures.
- Performance Predictors for Treatment: Identifying biomarkers that can forecast a patient’s treatment response signifies a transition towards a more personalized therapeutic approach. This necessitates extensive biomarker discovery and validation research.
3.7. Future Directions and Use of Available Resources and New Technologies
- AI in Diagnosis:
- 2.
- ML in Treatment personalization:
- 3.
- AI-based Monitoring Accessories:
- 4.
- Robotic—robotics:
- 5.
- Virtual Reality (VR) and Augmented Reality:
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chereda, B.; Melo, J.V. Natural course and biology of CML. Ann. Hematol. 2015, 94, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, H.R.; Rein, L.A. The evolving landscape of frontline therapy in chronic phase chronic myeloid leukemia (CML). Curr. Hematol. Malig. Rep. 2021, 16, 448–454. [Google Scholar] [CrossRef]
- Hochhaus, A.; Wang, J.; Kim, D.D.H.; Mayer, J.; Goh, Y.-T.; le Coutre, P.; Takahashi, N.; Kim, I.; Etienne, G.; Andorsky, D.; et al. Asciminib in Newly Diagnosed Chronic Myeloid Leukemia. New Engl. J. Med. 2024, 391, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Towachiraporn, S.; Punnachet, T.; Hantrakun, N.; Piriyakhuntorn, P.; Rattanathammethee, T.; Hantrakool, S.; Chai-Adisaksopha, C.; Rattarittamrong, E.; Norasetthada, L.; Tantiworawit, A. Long-Term Outcomes with Sequential Tyrosine Kinase Inhibitors Treatment in Chronic Myeloid Leukemia Patients. Asian Pac. J. Cancer Prev. APJCP 2023, 24, 1513. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Gonçalves, A.C.; Rutella, S.; Almeida, A.M.; Rivas, J.D.L.; Trougakos, I.P.; Ribeiro, A.B.S. Resistance to tyrosine kinase inhibitors in chronic myeloid leukemia—From molecular mechanisms to clinical relevance. Cancers 2021, 13, 4820. [Google Scholar] [CrossRef]
- Sundaram, D.N.M.; Jiang, X.; Brandwein, J.M.; Valencia-Serna, J.; Remant, K.; Uludağ, H. Current outlook on drug resistance in chronic myeloid leukemia (CML) and potential therapeutic options. Drug Discov. Today 2019, 24, 1355–1369. [Google Scholar] [CrossRef]
- Massaro, F.; Molica, M.; Breccia, M. Ponatinib: A review of efficacy and safety. Curr. Cancer Drug Targets 2018, 18, 847–856. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Jabbour, E.; Deininger, M.; Abruzzese, E.; Apperley, J.; Cortes, J.; Chuah, C.; DeAngelo, D.J.; DiPersio, J.; Hochhaus, A.; et al. Ponatinib after failure of second-generation tyrosine kinase inhibitor in resistant chronic-phase chronic myeloid leukemia. Am. J. Hematol. 2022, 97, 1419–1426. [Google Scholar] [CrossRef]
- Abasian, Z.; Rostamzadeh, A.; Mohammadi, M.; Hosseini, M.; Rafieian-Kopaei, M. A review on role of medicinal plants in polycystic ovarian syndrome: Pathophysiology, neuroendocrine signaling, therapeutic status and future prospects. Middle East Fertil. Soc. J. 2018, 23, 255–262. [Google Scholar] [CrossRef]
- Cuellar, S.; Vozniak, M.; Rhodes, J.; Forcello, N.; Olszta, D. BCR-ABL1 tyrosine kinase inhibitors for the treatment of chronic myeloid leukemia. J. Oncol. Pharm. Pract. 2018, 24, 433–452. [Google Scholar] [CrossRef]
- Shyam Sunder, S.; Sharma, U.C.; Pokharel, S. Adverse effects of tyrosine kinase inhibitors in cancer therapy: Pathophysiology, mechanisms and clinical management. Signal Transduct. Target. Ther. 2023, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Sasaki, K.; Kim, D.-W.; Hughes, T.P.; Etienne, G.; Mauro, M.J.; Hochhaus, A.; Lang, F.; Heinrich, M.C.; Breccia, M.; et al. Asciminib monotherapy in patients with chronic-phase chronic myeloid leukemia with the T315I mutation after ≥1 prior tyrosine kinase inhibitor: 2-year follow-up results. Leukemia 2024, 38, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yeung, D.T.; Shanmuganathan, N.; Hughes, T.P. Asciminib: A new therapeutic option in chronic-phase CML with treatment failure. Blood J. Am. Soc. Hematol. 2022, 139, 3474–3479. [Google Scholar] [CrossRef]
- Tomassetti, S.; Lee, J.; Qing, X. A case of chronic myelogenous leukemia with the T315I mutation who progressed to myeloid blast phase and was successfully treated with asciminib. Clin. Case Rep. 2022, 10, e6478. [Google Scholar] [CrossRef]
- Miller, G.D.; Bruno, B.J.; Lim, C.S. Resistant mutations in CML and Ph+ ALL–role of ponatinib. Biol. Targets Ther. 2014, 8, 243–254. [Google Scholar]
- Chandran, R.K.; Geetha, N.; Sakthivel, K.M.; Aswathy, C.G.; Gopinath, P.; Raj, T.V.A.; Priya, G.; Nair, J.K.K.M.; Sreedharan, H. Genomic amplification of BCR-ABL1 fusion gene and its impact on the disease progression mechanism in patients with chronic myelogenous leukemia. Gene 2019, 686, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Valent, P. Targeting the JAK2-STAT5 pathway in CML. Blood J. Am. Soc. Hematol. 2014, 124, 1386–1388. [Google Scholar] [CrossRef]
- Awad, S.A.; Brück, O.; Shanmuganathan, N.; Jarvinen, T.; Lähteenmäki, H.; Klievink, J.; Ibrahim, H.; Kytölä, S.; Koskenvesa, P.; Hughes, T.P.; et al. Epigenetic modifier gene mutations in chronic myeloid leukemia (CML) at diagnosis are associated with risk of relapse upon treatment discontinuation. Blood Cancer J. 2022, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Machova Polakova, K.; Koblihova, J.; Stopka, T. Role of epigenetics in chronic myeloid leukemia. Curr. Hematol. Malig. Rep. 2013, 8, 28–36. [Google Scholar] [CrossRef]
- Lipton, J.H.; Chuah, C.; Guerci-Bresler, A.; Rosti, G.; Simpson, D.; Assouline, S.; Etienne, G.; Nicolini, F.E.; Le Coutre, P.; Clark, R.; et al. Epic: A phase 3 trial of ponatinib compared with imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CP-CML). Blood 2014, 124, 519. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, Z.; Qin, Y.; Li, W.; Xu, N.; Liu, B.; Zhang, Y.; Meng, L.; Zhu, H.; Du, X.; et al. Correction: Olverembatinib (HQP1351), a well-tolerated and effective tyrosine kinase inhibitor for patients with T315I-mutated chronic myeloid leukemia: Results of an open-label, multicenter phase 1/2 trial. J. Hematol. Oncol. 2023, 16. [Google Scholar] [CrossRef] [PubMed]
- Rea, D.; Mauro, M.J.; Boquimpani, C.; Minami, Y.; Lomaia, E.; Voloshin, S.; Turkina, A.; Kim, D.W.; Apperley, J.F.; Abdo, A.; et al. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood J. Am. Soc. Hematol. 2021, 138, 2031–2041. [Google Scholar]
- Zou, J.-Y.; Huang, S.-M.; Zhoub, H.-X.; Xu, M.-Z.; Sun, A.-N.; Wu, D.-P.; Xue, S.-L.; Zhang, T.-T. Combination of venetoclax with BCR-ABL tyrosine kinase inhibitor as a therapeutic strategy for Philadelphia chromosome-positive leukemias. Hematology 2023, 28, 2237790. [Google Scholar] [CrossRef]
- Maiti, A.; Franquiz, M.J.; Ravandi, F.; Cortes, J.E.; Jabbour, E.J.; Sasaki, K.; Marx, K.; Daver, N.G.; Kadia, T.M.; Konopleva, M.Y.; et al. Venetoclax and BCR-ABL tyrosine kinase inhibitor combinations: Outcome in patients with philadelphia chromosome-positive advanced myeloid leukemias. Acta Haematol. 2021, 143, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Beagle, B.R.; Nguyen, D.M.; Mallya, S.; Tang, S.S.; Lu, M.; Zeng, Z.; Konopleva, M.; Vo, T.T.; Fruman, D.A. mTOR kinase inhibitors synergize with histone deacetylase inhibitors to kill B-cell acute lymphoblastic leukemia cells. Oncotarget 2014, 6, 2088. [Google Scholar] [CrossRef]
- Mitchell, R.; Hopcroft, L.E.; Baquero, P.; Allan, E.K.; Hewit, K.; James, D.; Hamilton, G.; Mukhopadhyay, A.; O’prey, J.; Hair, A.; et al. Targeting BCR-ABL-independent TKI resistance in chronic myeloid leukemia by mTOR and autophagy inhibition. JNCI J. Natl. Cancer Inst. 2018, 110, 467–478. [Google Scholar] [CrossRef]
- Hofmann, S.; Schubert, M.-L.; Wang, L.; He, B.; Neuber, B.; Dreger, P.; Müller-Tidow, C.; Schmitt, M. Chimeric antigen receptor (CAR) T cell therapy in acute myeloid leukemia (AML). J. Clin. Med. 2019, 8, 200. [Google Scholar] [CrossRef]
- Fiorenza, S.; Turtle, C.J. CAR-T cell therapy for acute myeloid leukemia: Preclinical rationale, current clinical progress, and barriers to success. BioDrugs 2021, 35, 281–302. [Google Scholar] [CrossRef]
- Xu, X.; Huang, S.; Xiao, X.; Sun, Q.; Liang, X.; Chen, S.; Zhao, Z.; Huo, Z.; Tu, S.; Li, Y. Challenges and clinical strategies of CAR T-cell therapy for acute lymphoblastic leukemia: Overview and developments. Front. Immunol. 2021, 11, 569117. [Google Scholar] [CrossRef]
- Champlin, R.; Jabbour, E.; Kebriaei, P.; Anderlini, P.; Andersson, B.; de Lima, M. Allogeneic stem cell transplantation for chronic myeloid leukemia resistant to tyrosine kinase inhibitors. Clin. Lymphoma Myeloma Leuk. 2011, 11, S96–S100. [Google Scholar] [CrossRef]
- Radich, J. Stem Cell Transplant for Chronic Myeloid Leukemia in the Imatinib Era. In Seminars in Hematology; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Wang, Z.-Q.; Zhang, Z.-C.; Wu, Y.-Y.; Pi, Y.-N.; Lou, S.-H.; Liu, T.-B.; Lou, G.; Yang, C. Bromodomain and extraterminal (BET) proteins: Biological functions, diseases, and targeted therapy. Signal Transduct. Target. Ther. 2023, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.; Choucair, K.; Ashraf, M.; Hammouda, D.M.; Alloghbi, A.; Khan, T.; Senzer, N.; Nemunaitis, J. Bromodomain and extra-terminal motif inhibitors: A review of preclinical and clinical advances in cancer therapy. Future Sci. OA 2019, 5, FSO372. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Apperley Elza Lomaia, J.; Moiraghi, B.; Undurraga Sutton, M.; Pavlovsky, C.; Chuah, C.; Sacha, T.; Lipton, J.H.; Schiffer, C.A.; McCloskey, J.; et al. Ponatinib dose-ranging study in chronic-phase chronic myeloid leukemia: A randomized, open-label phase 2 clinical trial. Blood J. Am. Soc. Hematol. 2021, 138, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Sanford, D.; Kantarjian, H.; Skinner, J.; Jabbour, E.; Cortes Sanford, J. Phase II trial of ponatinib in patients with chronic myeloid leukemia resistant to one previous tyrosine kinase inhibitor. Haematologica 2015, 100, e494. [Google Scholar] [CrossRef]
- Hochhaus, A.; Réa, D.; Boquimpani, C.; Minami, Y.; Cortes, J.E.; Hughes, T.P.; Apperley, J.F.; Lomaia, E.; Voloshin, S.; Turkina, A. Asciminib vs bosutinib in chronic-phase chronic myeloid leukemia previously treated with at least two tyrosine kinase inhibitors: Longer-term follow-up of ASCEMBL. Leukemia 2023, 37, 617–626. [Google Scholar] [CrossRef]
- Réa, D.; Mauro, M.J.; Boquimpani, C.; Minami, Y.; Lomaia, E.; Voloshin, S.; Turkina, A.; Kim, D.W.; Apperley, J.F.; Abdo, A.; et al. CML-395 Efficacy and Safety Results From ASCEMBL, a Phase III Study of Asciminib vs. Bosutinib in Patients with Chronic Myeloid Leukemia in Chronic Phase (CML-CP) After ≥2 Prior Tyrosine Kinase Inhibitors (TKIs): Week 96 Update. Clin. Lymphoma Myeloma Leuk. 2022, 22, S295–S296. [Google Scholar] [CrossRef]
- Daver, N.; Perl, A.E.; Maly, J.; Levis, M.; Ritchie, E.; Litzow, M.; McCloskey, J.; Smith, C.C.; Schiller, G.; Bradley, T.; et al. Venetoclax in combination with gilteritinib in patients with relapsed/refractory acute myeloid leukemia: A phase 1b study. Blood 2019, 134, 3910. [Google Scholar]
- Lap, C.J.; Nassereddine, S.; Liu, M.L.; Nava, V.E.; Aggarwal, A. Combined ruxolitinib and venetoclax treatment in a patient with a BCR-JAK2 rearranged myeloid neoplasm. Case Rep. Hematol. 2021, 2021, 2348977. [Google Scholar] [CrossRef]
- Pullarkat, V.A.; Lacayo, N.J.; Jabbour, E.; Rubnitz, J.E.; Bajel, A.; Laetsch, T.W.; Leonard, J.; Colace, S.I.; Khaw, S.L.; Fleming, S.A.; et al. Venetoclax and navitoclax in combination with chemotherapy in patients with relapsed or refractory acute lymphoblastic leukemia and lymphoblastic lymphoma. Cancer Discov. 2021, 11, 1440–1453. [Google Scholar] [CrossRef]
- Eghtedar, A.; Verstovsek, S.; Estrov, Z.; Burger, J.; Cortes, J.; Bivins, C.; Faderl, S.; Ferrajoli, A.; Borthakur, G.; George, S.; et al. Phase 2 study of the JAK kinase inhibitor ruxolitinib in patients with refractory leukemias, including postmyeloproliferative neoplasm acute myeloid leukemia. Blood J. Am. Soc. Hematol. 2012, 119, 4614–4618. [Google Scholar] [CrossRef]
- Nicolini, F.E.; Khoury, H.J.; Akard, L.; Rea, D.; Kantarjian, H.; Baccarani, M.; Leonoudakis, J.; Craig, A.; Benichou, A.C.; Cortes, J. Omacetaxine mepesuccinate for patients with accelerated phase chronic myeloid leukemia with resistance or intolerance to two or more tyrosine kinase inhibitors. Haematologica 2013, 98, e78. [Google Scholar] [CrossRef]
- Gandhi, V.; Plunkett, W.; Cortes, J.E. Omacetaxine: A protein translation inhibitor for treatment of chronic myelogenous leukemia. Clin. Cancer Res. 2014, 20, 1735–1740. [Google Scholar] [CrossRef]
- Zhao, J.; Song, Y.; Liu, D. Clinical trials of dual-target CAR T cells, donor-derived CAR T cells, and universal CAR T cells for acute lymphoid leukemia. J. Hematol. Oncol. 2019, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Das, D.K. Olverembatinib Demonstrates Promising Results in T315I-Mutant CML. Oncol. Times 2023, 45, 1–15. [Google Scholar]
- Scalzulli, E.; Carmosino, I.; Costa, A.; Bisegna, M.L.; Martelli, M.; Breccia, M. Management of Chronic Myeloid Leukemia Patients in Later Lines: The Role of Ponatinib and New Compounds. Clin. Lymphoma Myeloma Leuk. 2023, 23, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.G.; Issa, G.C.; Jabbour, E.; Yilmaz, M. Ponatinib for the treatment of adult patients with resistant or intolerant chronic-phase chronic myeloid leukemia. Expert Opin. Pharmacother. 2022, 23, 751–758. [Google Scholar] [CrossRef]
- Cortes, J.E.; Hochhaus, A.; Takahashi, N.; Larson, R.A.; Issa, G.C.; Bombaci, F.; Ramscar, N.; Ifrah, S.; Hughes, T.P. Asciminib monotherapy for newly diagnosed chronic myeloid leukemia in chronic phase: The ASC4FIRST phase III trial. Future Oncol. 2022, 18, 4161–4170. [Google Scholar] [CrossRef]
- Mauro, M.; Minami, Y.; Hochhaus, A.; Lomaia, E.; Voloshin, S.; Turkina, A.; Kim, D.-W.; Apperley, J.F.; Cortes, J.; Andre, N.R.; et al. Sustained efficacy and safety with asciminib (ASC) after almost 4 years of median follow-up from ascembl, a phase 3 study of ASC Vs bosutinib (BOS) in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) after ≥2 prior tyrosine kinase inhibitors (TKIs): An end of study treatment (EOS Tx) update, including results from switch population. Blood 2023, 142, 4536. [Google Scholar]
- Roberts, A.W.; Ma, S.; Kipps, T.J.; Coutre, S.E.; Davids, M.S.; Eichhorst, B.; Hallek, M.; Byrd, J.C.; Humphrey, K.; Zhou, L.; et al. Efficacy of venetoclax in relapsed chronic lymphocytic leukemia is influenced by disease and response variables. Blood J. Am. Soc. Hematol. 2019, 134, 111–122. [Google Scholar] [CrossRef]
- Rogers, K.A.; Huang, Y.; Ruppert, A.S.; Abruzzo, L.V.; Andersen, B.L.; Awan, F.T.; Bhat, S.A.; Dean, A.; Lucas, M.; Banks, C.; et al. Phase II study of combination obinutuzumab, ibrutinib, and venetoclax in treatment-naïve and relapsed or refractory chronic lymphocytic leukemia. J. Clin. Oncol. 2020, 38, 3626–3637. [Google Scholar] [CrossRef] [PubMed]
- Rambaldi, A.; Biagi, E.; Bonini, C.; Biondi, A.; Introna, M. Cell-based strategies to manage leukemia relapse: Efficacy and feasibility of immunotherapy approaches. Leukemia 2015, 29, 1–10. [Google Scholar] [CrossRef]
- Khair, D.O.; Bax, H.J.; Mele, S.; Crescioli, S.; Pellizzari, G.; Khiabany, A.; Nakamura, M.; Harris, R.J.; French, E.; Hoffmann, R.M.; et al. Combining immune checkpoint inhibitors: Established and emerging targets and strategies to improve outcomes in melanoma. Front. Immunol. 2019, 10, 453. [Google Scholar] [CrossRef]
- Petrazzuolo, A.; Maiuri, M.C.; Zitvogel, L.; Kroemer, G.; Kepp, O. Trial Watch: Combination of tyrosine kinase inhibitors (TKIs) and immunotherapy. Oncoimmunology 2022, 11, 2077898. [Google Scholar] [CrossRef] [PubMed]
- Odenike, O.; Onida, F.; Padron, E. Myelodysplastic syndromes and myelodysplastic/myeloproliferative neoplasms: An update on risk stratification, molecular genetics, and therapeutic approaches including allogeneic hematopoietic stem cell transplantation. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, e398–e412. [Google Scholar] [CrossRef] [PubMed]
- Yassine, F.; Reljic, T.; Moustafa, M.A.; Iqbal, M.; Murthy, H.S.; Kumar, A.; Kharfan-Dabaja, M.A. Efficacy of allogeneic hematopoietic cell transplantation in patients with chronic phase CML resistant or intolerant to tyrosine kinase inhibitors. Hematol. Oncol. Stem Cell Ther. 2022, 15, 36–43. [Google Scholar] [CrossRef]
- Stelljes, M.; Middeke, J.M.; Bug, G.; Wagner-Drouet, E.M.; Müller, L.P.; Schmid, C.; Krause, S.W.; Bethge, W.; Jost, E.; Platzbecker, U.; et al. Remission induction versus immediate allogeneic haematopoietic stem cell transplantation for patients with relapsed or poor responsive acute myeloid leukaemia (ASAP): A randomised, open-label, phase 3, non-inferiority trial. Lancet Haematol. 2024, 11, e324–e335. [Google Scholar] [CrossRef]
- Jermakowicz, A.M.; Kurimchak, A.M.; Johnson, K.J.; Bourgain-Guglielmetti, F.; Kaeppeli, S.; Affer, M.; Pradhyumnan, H.; Suter, R.K.; Walters, W.; Cepero, M.; et al. RAPID resistance to BET inhibitors is mediated by FGFR1 in glioblastoma. Sci. Rep. 2024, 14, 9284. [Google Scholar] [CrossRef]
- Osman, A.E.; Deininger, M.W. Chronic Myeloid Leukemia: Modern therapies, current challenges and future directions. Blood Rev. 2021, 49, 100825. [Google Scholar] [CrossRef]
- Seiwerth, R.S.; Mrsić, M.; Nemet, D.; Bogdanić, V.; Mikulić, M.; Sertić, D.; Grković, L.; Cecuk, E.; Bojanić, I.; Batinić, D.; et al. Treatment of acute leukemia with allogeneic stem cell transplantation. Acta Medica Croat. Cas. Hravatske Akad. Med. Znanosti. 2009, 63, 205–208. [Google Scholar]
- Majhail, N.S.; Farnia, S.H.; Carpenter, P.A.; Champlin, R.E.; Crawford, S.; Marks, D.I.; Omel, J.L.; Orchard, P.J.; Palmer, J.; Saber, W.; et al. Indications for autologous and allogeneic hematopoietic cell transplantation: Guidelines from the American Society for Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2015, 21, 1863–1869. [Google Scholar] [CrossRef] [PubMed]
- Maziarz, R.T.; Devine, S.; Garrison, L.P.; Agodoa, I.; Badaracco, J.; Gitlin, M.; Perales, M.A. Estimating the lifetime medical cost burden of an allogeneic hematopoietic cell transplantation patient. Transplant. Cell. Ther. 2023, 29, 637-e1. [Google Scholar] [CrossRef] [PubMed]
- Pidala, J.; Anasetti, C.; Jim, H. Quality of life after allogeneic hematopoietic cell transplantation. Blood J. Am. Soc. Hematol. 2009, 114, 7–19. [Google Scholar] [CrossRef]
- Sun, J.; Hu, R.; Han, M.; Tan, Y.; Xie, M.; Gao, S.; Hu, J.F. Mechanisms underlying therapeutic resistance of tyrosine kinase inhibitors in chronic myeloid leukemia. Int. J. Biol. Sci. 2024, 20, 175. [Google Scholar] [CrossRef]
- Javidi-Sharifi, N.; Hobbs, G. Future directions in chronic phase CML treatment. Curr. Hematol. Malig. Rep. 2021, 16, 1–9. [Google Scholar] [CrossRef]
- Jabbour, E.; Cortes, J.; Kantarjian, H. Long-term outcomes in the second-line treatment of chronic myeloid leukemia: A review of tyrosine kinase inhibitors. Cancer 2011, 117, 897–906. [Google Scholar] [CrossRef]
- Flis, S.; Chojnacki, T. Chronic myelogenous leukemia, a still unsolved problem: Pitfalls and new therapeutic possibilities. Drug Des. Dev. Ther. 2019, 13, 825–843. [Google Scholar] [CrossRef] [PubMed]
- Silbermann, M.; Pitsillides, B.; Al-Alfi, N.; Omran, S.; Al-Jabri, K.; Elshamy, K.; Ghrayeb, I.; Livneh, J.; Daher, M.; Charalambous, H.; et al. Multidisciplinary care team for cancer patients and its implementation in several Middle Eastern countries. Ann. Oncol. 2013, 24, vii41–vii47. [Google Scholar] [CrossRef]
- Trivedi, D.; Landsman-Blumberg, P.; Darkow, T.; Smith, D.; McMorrow, D.; Mullins, C.D. Adherence and persistence among chronic myeloid leukemia patients during second-line tyrosine kinase inhibitor treatment. J. Manag. Care Pharm. 2014, 20, 1006–1015. [Google Scholar] [CrossRef]
- Lustberg, M.B.; Kuderer, N.M.; Desai, A.; Bergerot, C.; Lyman, G.H. Mitigating long-term and delayed adverse events associated with cancer treatment: Implications for survivorship. Nat. Rev. Clin. Oncol. 2023, 20, 527–542. [Google Scholar] [CrossRef]
- Fava, C.; Kantarjian, H.; Cortes, J. Molecular resistance: An early indicator for treatment change? Clin. Lymphoma Myeloma Leukemia. 2012, 12, 79–87. [Google Scholar] [CrossRef]
- Atallah, E.; Schiffer, C.A.; Radich, J.P.; Weinfurt, K.P.; Zhang, M.J.; Pinilla-Ibarz, J.; Kota, V.; Larson, R.A.; Moore, J.O.; Mauro, M.J.; et al. Assessment of outcomes after stopping tyrosine kinase inhibitors among patients with chronic myeloid leukemia: A nonrandomized clinical trial. JAMA Oncol. 2021, 7, 42–50. [Google Scholar] [CrossRef]
- Gustafson, D.; Fish, J.E.; Lipton, J.H.; Aghel, N. Mechanisms of cardiovascular toxicity of BCR-ABL1 tyrosine kinase inhibitors in chronic myelogenous leukemia. Curr. Hematol. Malig. Rep. 2020, 15, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Karakulak, U.N.; Aladag, E.; Hekimsoy, V.; Sahiner, M.L.; Kaya, E.B.; Ozer, N.; Aksu, S.; Demiroglu, H.; Goker, H.; Buyukasik, Y.; et al. Four-dimensional echocardiographic evaluation of left ventricular systolic functions in patients with chronic myeloid leukaemia receiving tyrosine kinase inhibitors. Cardiovasc. Toxicol. 2021, 21, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Rodia, R.; Pani, F.; Caocci, G.; La Nasa, G.; Simula, M.P.; Mulas, O.; Velluzzi, F.; Loviselli, A.; Mariotti, S.; Boi, F. Thyroid autoimmunity and hypothyroidism are associated with deep molecular response in patients with chronic myeloid leukemia on tyrosine kinase inhibitors. J. Endocrinol. Investig. 2022, 45, 291–300. [Google Scholar] [CrossRef]
- Kim, T.D.; Schwarz, M.; Nogai, H.; Grille, P.; Westermann, J.; Plöckinger, U.; Braun, D.; Schweizer, U.; Arnold, R.; Dörken, B.; et al. Thyroid dysfunction caused by second-generation tyrosine kinase inhibitors in Philadelphia chromosome-positive chronic myeloid leukemia. Thyroid 2010, 20, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Roshanaei, M.; Khan, M.R.; Sylvester, N.N. Enhancing Cybersecurity through AI and ML: Strategies, Challenges, and Future Directions. J. Inf. Secur. 2024, 15, 320–339. [Google Scholar] [CrossRef]
- Alsadie, D. A Comprehensive Review of AI Techniques for Resource Management in Fog Computing: Trends, Challenges and Future Directions. IEEE Access 2024, 12, 118007–118059. [Google Scholar] [CrossRef]
- Mojtahedi, H.; Yazdanpanah, N.; Rezaei, N. Chronic myeloid leukemia stem cells: Targeting therapeutic implications. Stem Cell Res. Ther. 2021, 12, 603. [Google Scholar] [CrossRef]
- Malik, V.; Mittal, R.; Rana, A. BiCNN-CML: Hybrid Deep Learning Approach for Chronic Myeloid Leukemia. In Proceedings of the 2022 5th International Conference on Contemporary Computing and Informatics (IC3I), Uttar Pradesh, India, 14 December 2022; IEEE: Piscataway, NJ, USA; pp. 1771–1777. [Google Scholar]
- Bernardi, S.; Vallati, M.; Gatta, R. Artificial Intelligence-Based Management of Adult Chronic Myeloid Leukemia: Where Are We and Where Are We Going? Cancers 2024, 16, 848. [Google Scholar] [CrossRef]
- Ghane, N.; Vard, A.; Talebi, A.; Nematollahy, P. Classification of effective cells in diagnosis of chronic myeloid leukemia (CML) using semi-automatic image processing of microscopic images. J. Isfahan Med. School. 2016, 34, 1304–1310. [Google Scholar]
- Ko, B.S.; Wang, Y.F.; Li, J.L.; Li, C.C.; Weng, P.F.; Hsu, S.C.; Hou, H.A.; Huang, H.H.; Yao, M.; Lin, C.T.; et al. Clinically validated machine learning algorithm for detecting residual diseases with multicolor flow cytometry analysis in acute myeloid leukemia and myelodysplastic syndrome. EBioMedicine 2018, 37, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Efthimiou, O.; Hoogland, J.; Debray, T.P.; Seo, M.; Furukawa, T.A.; Egger, M.; White, I.R. Measuring the performance of prediction models to personalize treatment choice. Stat. Med. 2023, 42, 1188–1206. [Google Scholar] [CrossRef]
- Berchialla, P.; Lanera, C.; Sciannameo, V.; Gregori, D.; Baldi, I. Prediction of treatment outcome in clinical trials under a personalized medicine perspective. Sci. Rep. 2022, 12, 4115. [Google Scholar] [CrossRef]
- Agarwal, S. Machine Learning Based Personalized Treatment Plans for Chronic Conditions. In Proceedings of the 2024 2nd International Conference on Intelligent Data Communication Technologies and Internet of Things (IDCIoT), Bengaluru, India, 4 January 2024; IEEE: Piscataway, NJ, USA; pp. 1127–1132. [Google Scholar]
- Patel, P.M.; Green, M.; Tram, J.; Wang, E.; Murphy, M.Z.; Abd-Elsayed, A.; Chakravarthy, K. Beyond the Pain Management Clinic: The Role of AI-Integrated Remote Patient Monitoring in Chronic Disease Management—A Narrative Review. J. Pain Res. 2024, 4223–4237. [Google Scholar] [CrossRef] [PubMed]
- Kanakaprabha, S.; Kumar, G.G.; Reddy, B.P.; Raju, Y.R.; Rai, P.C. Wearable Devices and Health Monitoring: Big Data and AI for Remote Patient Care. Intell. Data Anal. Bioinform. Biomed. Syst. 2024, 291–311. [Google Scholar] [CrossRef]
- More, D.; Khan, N.; Tekade, R.K.; Sengupta, P. An update on current trend in sample preparation automation in bioanalysis: Strategies, challenges and future direction. Crit. Rev. Anal. Chem. 2024, 1–25. [Google Scholar] [CrossRef]
- Medina, D.A.; Maciel, E.V.; Lanças, F.M. Modern automated sample preparation for the determination of organic compounds: A review on robotic and on-flow systems. TrAC Trends Anal. Chem. 2023, 166, 117171. [Google Scholar] [CrossRef]
- Sandu, M.O.; Gruescu, C.M.; Lovasz, E.C.; Ciupe, V. Synthesis of an Automation System for the PCR (Polymerase Chain Reaction) Samples Preparation Process. In Proceedings of the IFToMM International Symposium on Science of Mechanisms and Machines (SYROM), Iasi, Romania, 17 November 2022; Springer International Publishing: Cham, Switzerland, 2022; pp. 389–396. [Google Scholar]
- Hutapea, S.A.; Tumilaar, K.Y.; Nugroho, A.; Maulana, F.I. MycoAR: Augmented Reality Mobile Application for Mycology Education. In Proceedings of the 2024 International Conference on Information Management and Technology (ICIMTech), Bali, Indonesia, 28 August 2024; IEEE: Piscataway, NJ, USA; pp. 783–788. [Google Scholar]
- Syamsuar, D. The Implementation of Artificial Intelligence for Online Review: A Systematic Literature Review. In Proceedings of the 2024 International Conference on Information Management and Technology (ICIMTech), Bali, Indonesia, 28 August 2024; IEEE: Piscataway, NJ, USA; pp. 588–593. [Google Scholar]
- Ryan, G.; Murphy, J.; Higgins, M.; McAuliffe, F.; Mangina, E. Work-in-Progress—Development of a Virtual Reality Learning Environment: VR Baby. In Proceedings of the 2020 6th International Conference of the Immersive Learning Research Network (iLRN), San Luis Obispo, CA, USA, 21 January 2020; IEEE: Piscataway, NJ, USA; pp. 312–315. [Google Scholar]
- Goddard, T.D.; Brilliant, A.A.; Skillman, T.L.; Vergenz, S.; Tyrwhitt-Drake, J.; Meng, E.C.; Ferrin, T.E. Molecular visualization on the holodeck. J. Mol. Biology. 2018, 430, 3982–3996. [Google Scholar] [CrossRef]
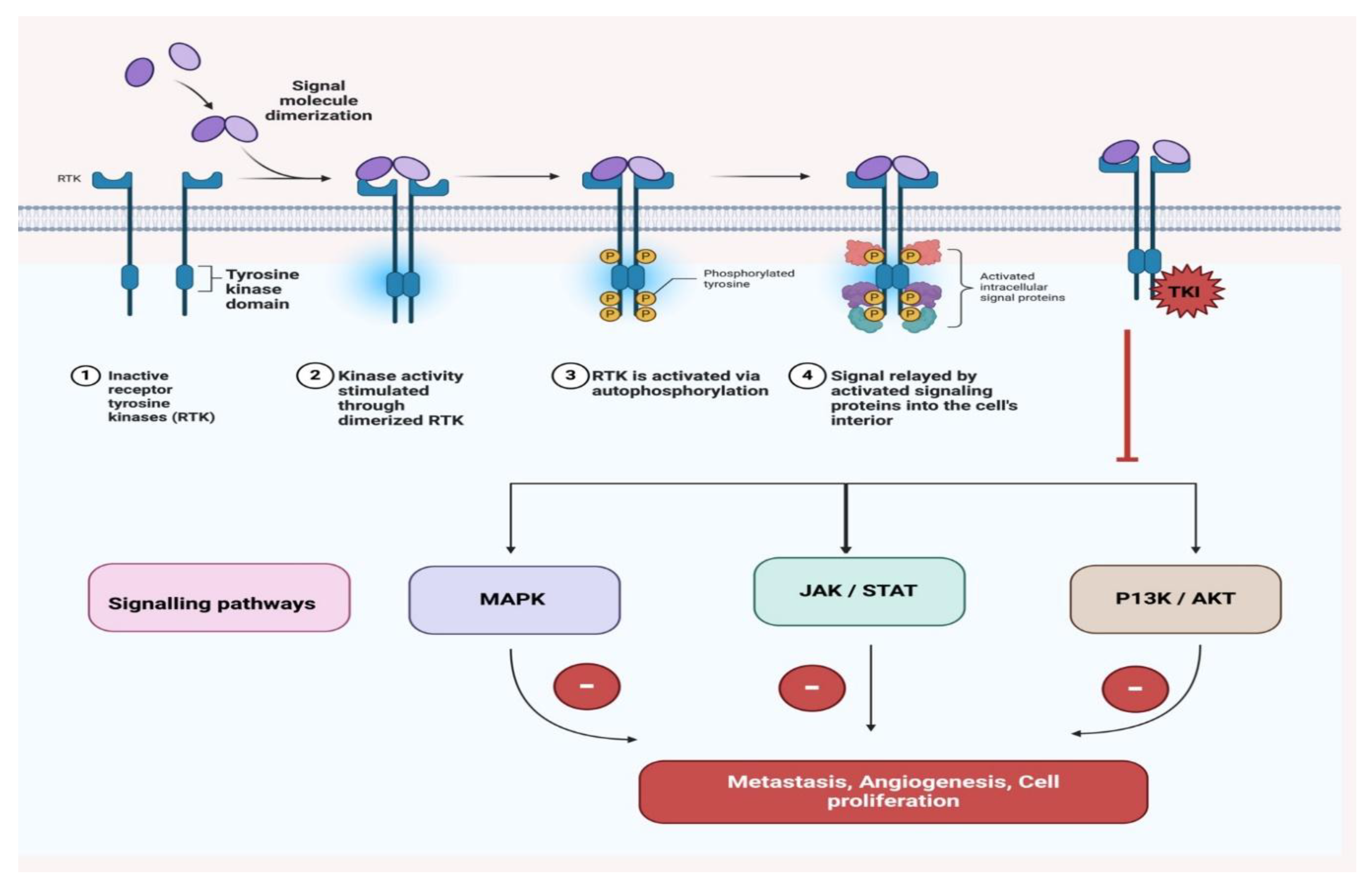
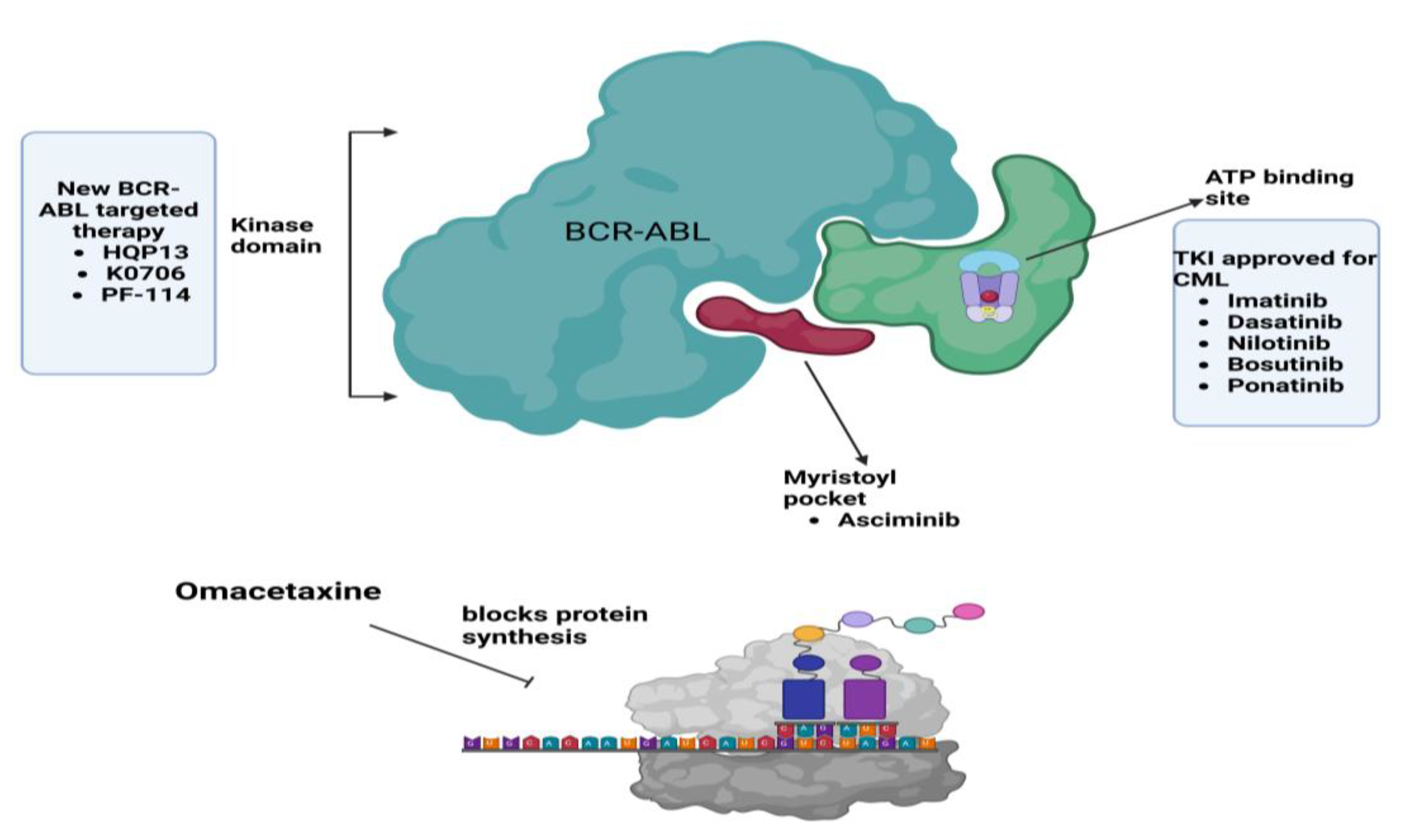
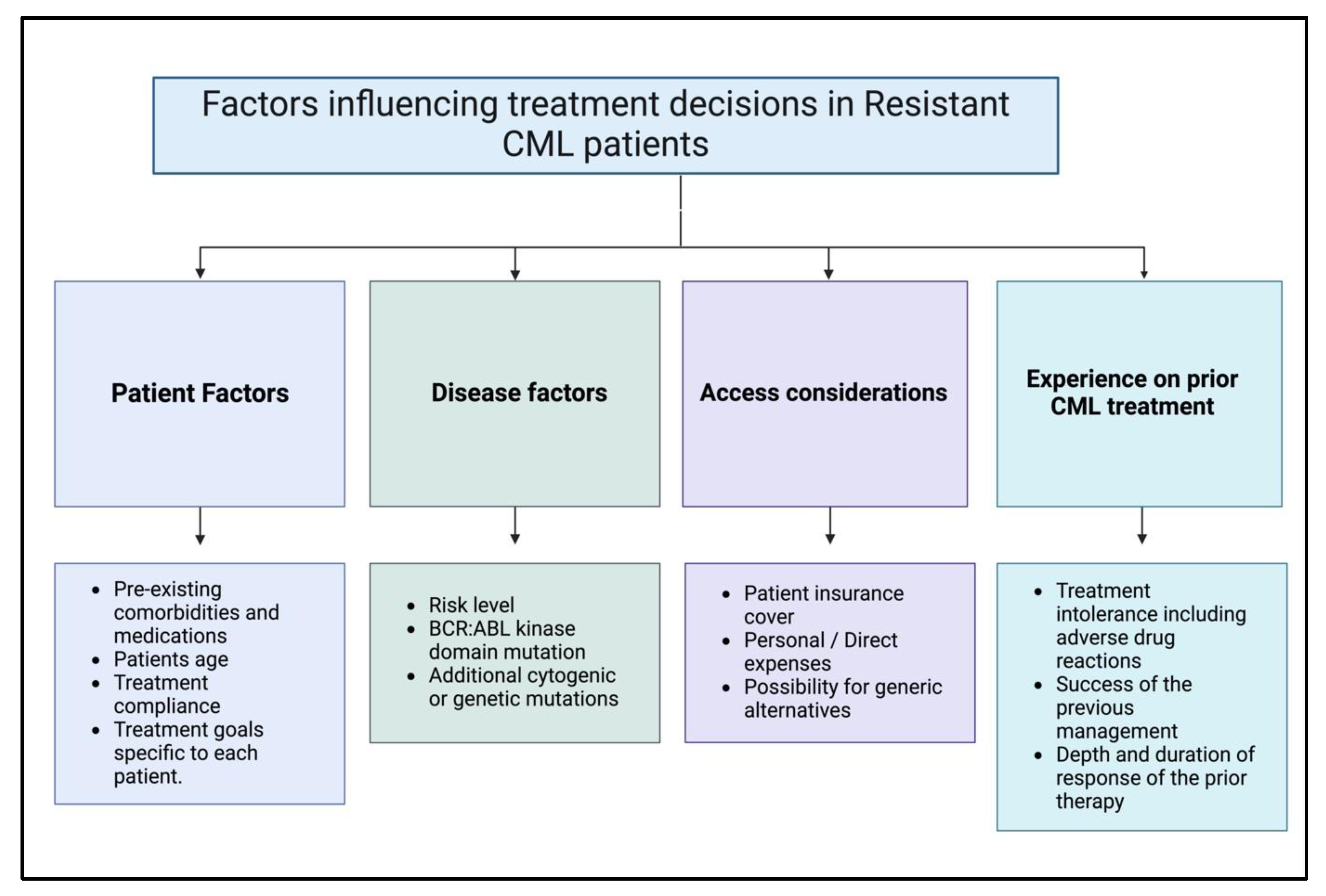
| Therapy/Strategy | Description and Mechanism | Clinical Trial Phase/Findings | References |
|---|---|---|---|
| Ponatinib and Olverembatinib (HQP1351) | TKI targets BCR-ABL mutations, including T315I. Known for broad-spectrum efficacy. | Phase III trials showed efficacy in T315I-positive patients but with significant vascular risks. Olverembatinib is a new third-generation TKI, and the drug is approved in China for the treatment of TKI-resistant CML. | [20,21] |
| Asciminib | First-in-class STAMP inhibitor targeting the myristoyl pocket of ABL1. | Phase III results demonstrate a superior safety profile and efficacy compared to older TKIs. | [22] |
| Combination: TKIs + Venetoclax | Combines BCR-ABL inhibition with apoptosis induction via BCL-2 inhibition. | Early clinical trials indicate potent efficacy in reducing residual disease. | [23,24] |
| Combination: TKIs + mTOR Inhibitors | Aims to inhibit the mTOR pathway alongside BCR-ABL for a synergistic effect. | Phase II studies show promise in overcoming resistance, particularly in refractory patients. | [25,26] |
| CAR-T Cell Therapy | Uses genetically modified T-cells to target and kill CML cells. | Phase I/II trials exploring efficacy; some challenges with toxicity observed. | [27,28,29] |
| Allogeneic HSCT | Curative approach involving stem cell transplantation from a donor. | Remains a standard for patients with advanced or TKI-resistant CML, with evolving protocols to reduce GVHD. | [30,31,32] |
| BET Inhibitors | Targets bromodomain and extra-terminal motif (BET) proteins to disrupt CML cell growth. | Preclinical and early clinical data suggest potential in combination with TKIs to overcome resistance. | [33,34] |
| Therapy/Trial Name | Study Phase | Key Findings | Limitations/Gaps | Real-World Data/Registry Insights | References |
|---|---|---|---|---|---|
| Ponatinib (OPTIC Trial) | Phase II/III | Demonstrated robust efficacy in T315I mutation carriers; dose adjustment is critical to balance efficacy and cardiovascular risks. | Cardiovascular adverse effects remain a concern; limited data on long-term survival beyond 5 years. | Registry data show improved molecular response with early intervention; discontinuations due to toxicity were reported in ~30% of cases. | [35,36] |
| Asciminib (ASCEMBL Trial) | Phase III | Superior to bosutinib with better tolerability; achieved higher major molecular response (MMR) rates at 24 weeks. | Limited data on use in the blast phase; long-term safety is still under investigation. | Real-world outcomes demonstrate sustained response in patients with T315I mutation; lower discontinuation rates compared to ponatinib. | [3,22,37,38] |
| TKI + Venetoclax Combination | Phase II (Ongoing) | Early-phase trials indicate a synergistic effect in reducing minimal residual disease. | Limited data on safety profile in larger populations; optimal dosing strategies are yet to be established. | Case reports highlight partial response in patients with molecular relapse. | [39,40,41] |
| JAK Inhibitor + TKI (Ruxolitinib) | Phase II (Ongoing) | Promising molecular response in TKI-refractory cases by blocking JAK-STAT pathways. | No phase III data available; safety concerns with dual inhibition. | Registry data suggest high variability in response rates; adherence challenges reported. | [41,42] |
| Omacetaxine (Protien synthesis inhibitor) | Phase II/III (Ongoing) | Phase II: Omacetaxine was effective in treating CML patients resistant or intolerant to two or more TKIs, especially in those with the T315I mutation. Phase III: Sustained use of Omacetaxine resulted in ongoing responses in patients with minimal residual disease, establishing its utility in salvage therapy. Ongoing Research: Studies are exploring the use of Omacetaxine in combination with newer agents like ponatinib to improve outcomes and overcome resistance. | Limited patient population and short follow-up period restrict broader conclusions. Potential side effects include myelosuppression and injection-site reactions. Lack of long-term safety data. The administration route (subcutaneous injection) may impact patient compliance. Early results are promising but incomplete. Need for extensive data to validate findings. | Registry data indicate a modest uptake of Omacetaxine, mainly in heavily pre-treated populations or those with specific mutations like T315I. Reports from treatment registries suggest variable response rates, with some patients achieving significant molecular responses. Emerging clinical practice is increasingly incorporating Omacetaxine in combination regimens, especially in refractory cases. | [43,44] |
| CAR-T Cell Therapy | Phase I/II (Ongoing) | Effective in eliminating leukemic stem cells; potential for durable remission. | Toxicity and manufacturing scalability remain major hurdles. | Limited case reports show positive response; early termination in some cases due to immune-related adverse events. | [27,45] |
| Therapy/Strategy | Efficacy | Safety | Cost | Quality of Life Impact | References |
|---|---|---|---|---|---|
| Ponatinib and Olverembatinib | High efficacy with the T315I mutation; Phase III success for ponatinib | Significant vascular risks; dose-related toxicity for ponatinib | High; specific data for olverembatinib pending | Cardiovascular side effects may significantly impact | [46,47,48] |
| Asciminib | Superior efficacy compared to older TKIs in Phase III | Better safety profile than other TKIs | Likely high | Potentially better due to fewer side effects | [22,49,50] |
| TKIs + Venetoclax | Potent efficacy in reducing residual disease in early trials | Increased risk of infections and immunosuppression | Increased due to combination therapy | Dependent on disease control vs. increased toxicity | [51,52] |
| TKIs + mTOR Inhibitors | Promising in overcoming resistance, especially in refractory patients | Potential added toxicities involving mTOR pathway inhibition | Increased due to combination therapy | Similar to TKIs + Venetoclax | |
| CAR-T Cell Therapy | Effective in eliminating leukemic stem cells; potential for durable remission | High toxicity; significant immune-related adverse events | Very high | Severe side effects may negatively impact despite the potential for remission | [45,53] |
| Immune Checkpoint Inhibitors | Early trials suggest potential benefits in combination with TKIs | Concerns about immune-related toxicity | High | Dependent on effective management of immune-related side effects | [54,55,56,57,58,59] |
| Allogeneic HSCT | Allogeneic HSCT is highly effective in achieving disease-free survival (DFS) and overall survival (OS) in patients with acute leukemia and other hematologic malignancies. The efficacy is often measured by the rates of DFS and OS, with studies showing significant long-term survival benefits | The major safety concern with allogeneic HSCT is graft-versus-host disease (GVHD), which can be acute or chronic and significantly impacts patient outcomes. Acute GVHD (aGVHD) and chronic GVHD (cGVHD) are associated with increased morbidity and mortality, and managing these complications is crucial for improving transplant outcomes | The lifetime cost of allogeneic HSCT is substantial, often exceeding USD 1,000,000 per patient. The majority of these costs are attributed to the treatment of chronic GVHD and the initial transplant procedure | Quality of life (QOL) post-transplant is a significant concern, with many patients experiencing moderate impairments that can persist for years. While some patients report a return to pre-transplant QOL levels within the first year, others face long-term challenges, including chronic health issues and psychological impacts Behavioral and rehabilitative interventions are being explored to improve QOL outcomes for long-term survivors | [60,61,62] |
| BET Inhibitors | Early data suggest potential efficacy in overcoming TKI resistance | Safety profile still under investigation | Expected to be high | Impact on quality of life uncertain until further data are available | [63] |
| Key Challenge | Details | References |
|---|---|---|
| Overcoming the Heterogeneity in Resistance Mechanisms | - Personalized medicine: Tailoring treatment based on molecular profiling to optimize patient outcomes. - Patient stratification strategies: Identifying subgroups to match with appropriate therapies and improve efficacy. | [64,65] |
| Long-Term Toxicities and Side Effects of New Therapies | - Cardiovascular risks: Ponatinib linked with vascular events; dose management essential. - Immunosuppression: Risks with dual therapies or CAR-T approaches. - Secondary malignancies: Potential long-term effects of TKIs. | [66,67] |
| Economic and Accessibility Issues | - Cost-effectiveness: The high cost of novel agents like ponatinib and asciminib limits use. - Access in resource-limited settings: Limited availability and high prices restrict treatment options. | [68] |
| Future Research Avenues | - Head-to-head trials: Comparisons between new therapies and standard TKIs to identify the most effective treatments. - Development of biomarkers: New biomarkers to predict therapy response and tailor treatments. | [69,70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangraze, I.; El-Tanani, M.; Wali, A.F.; Rizzo, M. Beyond TKIs: Advancing Therapeutic Frontiers with Immunotherapy, Targeted Agents, and Combination Strategies in Resistant Chronic Myeloid Leukemia. Hemato 2025, 6, 6. https://doi.org/10.3390/hemato6010006
Rangraze I, El-Tanani M, Wali AF, Rizzo M. Beyond TKIs: Advancing Therapeutic Frontiers with Immunotherapy, Targeted Agents, and Combination Strategies in Resistant Chronic Myeloid Leukemia. Hemato. 2025; 6(1):6. https://doi.org/10.3390/hemato6010006
Chicago/Turabian StyleRangraze, Imran, Mohamed El-Tanani, Adil Farooq Wali, and Manfredi Rizzo. 2025. "Beyond TKIs: Advancing Therapeutic Frontiers with Immunotherapy, Targeted Agents, and Combination Strategies in Resistant Chronic Myeloid Leukemia" Hemato 6, no. 1: 6. https://doi.org/10.3390/hemato6010006
APA StyleRangraze, I., El-Tanani, M., Wali, A. F., & Rizzo, M. (2025). Beyond TKIs: Advancing Therapeutic Frontiers with Immunotherapy, Targeted Agents, and Combination Strategies in Resistant Chronic Myeloid Leukemia. Hemato, 6(1), 6. https://doi.org/10.3390/hemato6010006







