Geographic Prevalence Patterns and Modifiable Risk Factors for Monoclonal Gammopathy of Undetermined Significance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Study Selection
2.3. Data Extraction, Synthesis, and Quality Assessment
3. Results
3.1. Geographic Prevalence
| Reference | Geographic Location | Age | Setting | Diagnostic Method | Diagnostic Criteria | Sample Size | Prevalence % (95% CI) | Notes |
|---|---|---|---|---|---|---|---|---|
| Bertrand et al. [42] | United States | 39 to 89 | population-based | agarose gel | M protein < 3 g/dL | 13,030 | 9.0 (7.6–10.4) | Included only Black females |
| Landgren et al. [43] | Ghana | 50 to 74 | population-based | agarose gel | M protein < 3 g/dL | 917 | 5.84 (4.27–7.40) | Included only Black males |
| Thordardottir [44] | Iceland | 66 to 98 | Population-based | agarose gel | M protein < 3 g/dL | 5764 | 5% (4.9–5.2) | Excluded patients diagnosed with multiple myeloma via review of the national cancer registry data |
| Love et al. [45] | Iceland | 41 to >80 | population-based | agarose gel | M protein < 3 g/dL, FLC ratio < 100, no end-organ damage | 75,422 | All ages: 3.9 (3.8–4.0) Age > 50: 5.0 (4.9–5.2) | |
| Kyle et al. [46] | USA | >50 | population-based | agarose gel | M protein < 3 g/dL | 16,485 | 3.2 (3.0–3.5) | |
| Bowden et al. [49] | Japan | 63 to 95 | population-based | agarose gel | M protein < 3 g/dL | 146 | 2.7 (not reported) | Compared prevalence of MGUS in Japan to prevalence in the US in the same age group |
| Wu et al. [51] | China | 50 to 65 | population-based | agarose gel | M protein < 3 g/dL, bone marrow < 10%, no end-organ damage | 1000 | 0.8 (0.3–1.4) | |
| Landgren et al. [28] | USA | 10 to 49 | population-based | agarose gel | M protein < 3 g/dL | 12,372 | 0.34 (0.11–0.45) | |
| Onwah et al. [52] | Nigeria | 20 to 84 | population-based | agarose gel | M protein < 3 g/dL | 410 | 0.24 (0.01–1.38) | |
| Iwanaga et al. [48] | Japan | 42 to >80 | population-based | cellulose acetate | M protein < 3 g/dL | 52,781 | 2.1 (1.9–2.2) | |
| Saleun et al. [50] | France | 30 to >80 | population-based | cellulose acetate | M protein < 3 g/dL | 30,279 | 1.10 (not reported) | |
| Axelsson et al. [61] | Sweden | >70 | population-based | paper electrophoresis | M protein < 3 g/dL, bone marrow < 10%, no end-organ damage | 6995 | 0.9 (not reported) | |
| Park et al. [47] | Korea | 65 to 97 | population-based | not reported | M protein < 3 g/dL | 945 | 3.3 (2.0–4.6) | |
| Suan et al. [59] | Australia | >50 | population-based | capillary zone | M protein < 3 g/dL, no end-organ damage | 2993 | 4.6 (3.8–5.3)) | |
| Eisele et al. [60] | Germany | 47 to 75 | population-based | capillary zone | M protein < 3 g/dL, no end-organ damage | 4702 | 3.5 (3.0–4.1) | |
| Tamimi et al. [53] | Saudi Arabia | Not reported | hospital-based | agarose gel | M protein < 3 g/dL, no end-organ damage | 6624 | 6.3 (not reported) | |
| Chang et al. [56] | Taiwan | 58 to 85 | hospital-based | capillary zone | M protein < 3 g/dL, bone marrow < 10%, no end-organ damage | 327 | 8.19 (not reported) | |
| Cicero et al. [57] | South Africa | 35 to >85 | hospital-based | capillary zone | M protein < 3 g/dL | 386 | All ages: 8.03 (5.32–10.74)Age > 50: 8.11; 5.63–11.54 | Included only Black males |
| Veronicchi et al. [31] | Italy | <50 to >90 | hospital-based | capillary zone | M protein < 3 g/dL | 44,474 | 6.0 (5.7–6.3) | Prevalence reported for subjects > 50 years |
| Ma et al. [48] | China | >40 | hospital-based | capillary zone | M protein < 3 g/dL, bone marrow < 10%, no end-organ damage | 1797 | 2.73 (not reported) | |
| Han et al. [55] | China | 25 to 96 | hospital-based | capillary zone | M protein < 3 g/dL, bone marrow < 10%, no end-organ damage | 154,597 | All ages: 0.53 (0.49–0.57)Age ≥ 50: 1.11 (1.02–1.18) | |
| Gupta et al. [54] | North India | 40–88 | hospital-based | not reported | M protein < 3 g/dL, bone marrow < 10%, no end-organ damage, no amyloidosis attributed to plasma cell disorder | 3429 | 1.43 (not reported) |
3.2. Risk Factors for MGUS Development
3.2.1. Population-Specific Risk Factor Assessment
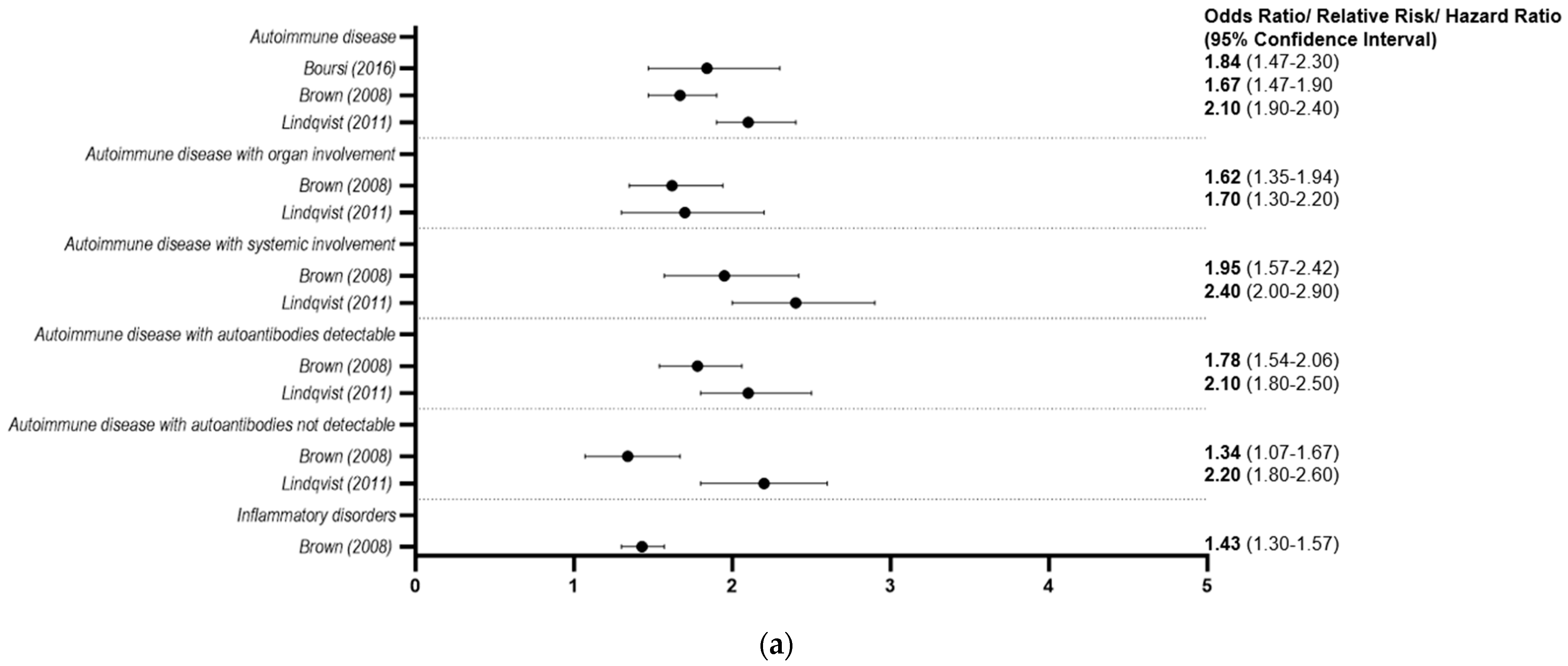
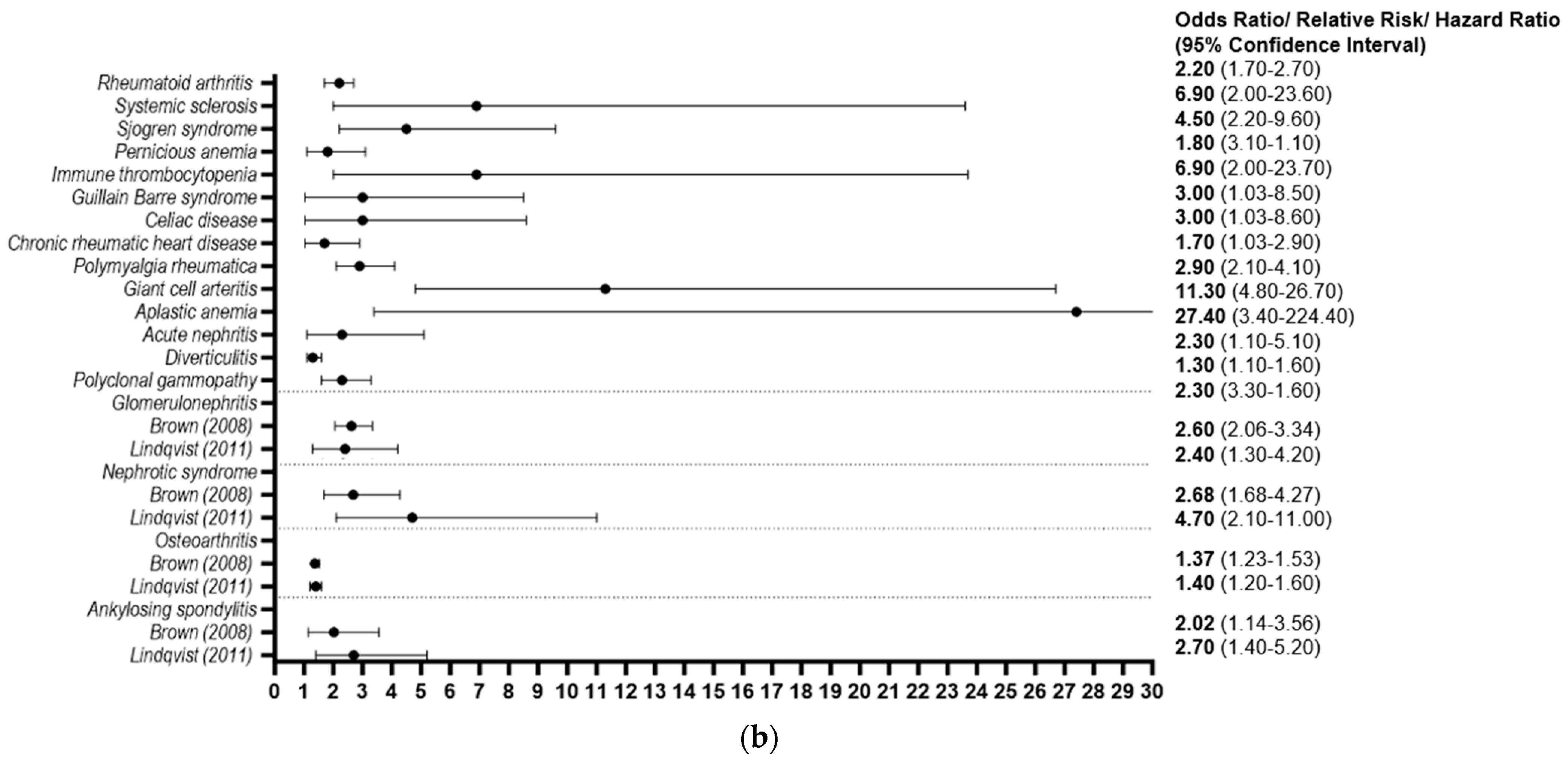
3.2.2. Autoimmune and Inflammatory Conditions
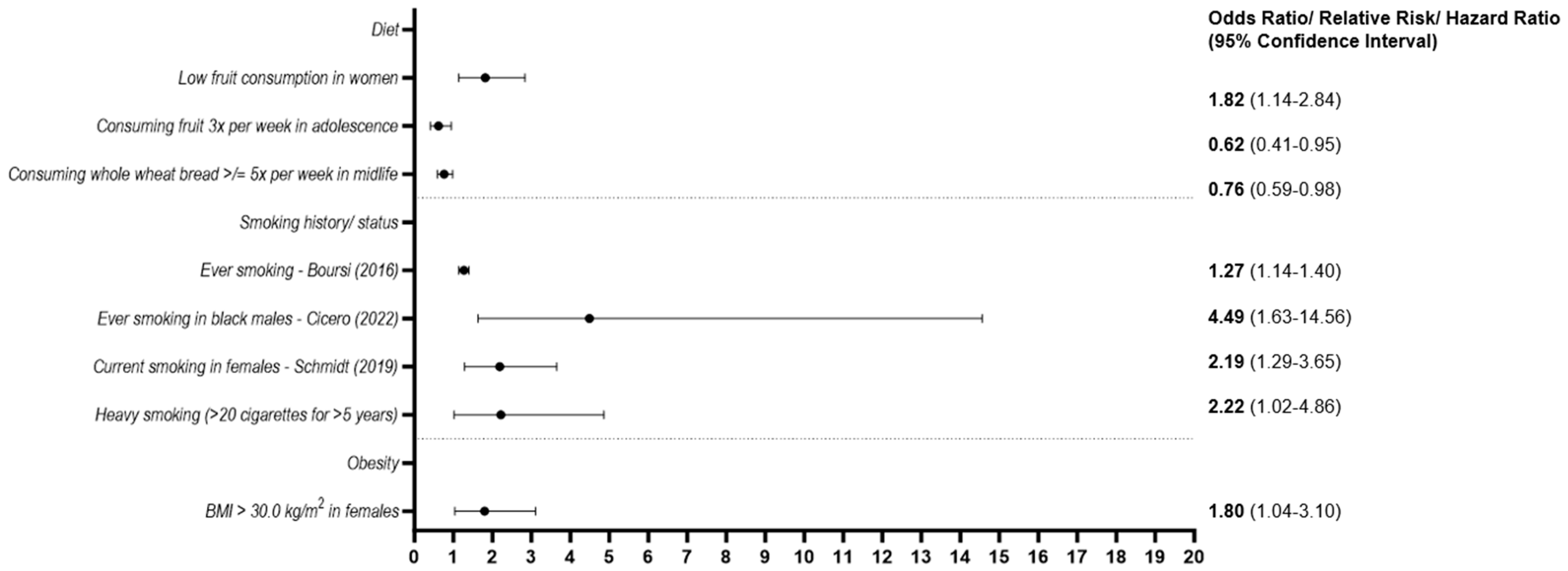
3.2.3. Environmental Exposures
3.2.4. Lifestyle
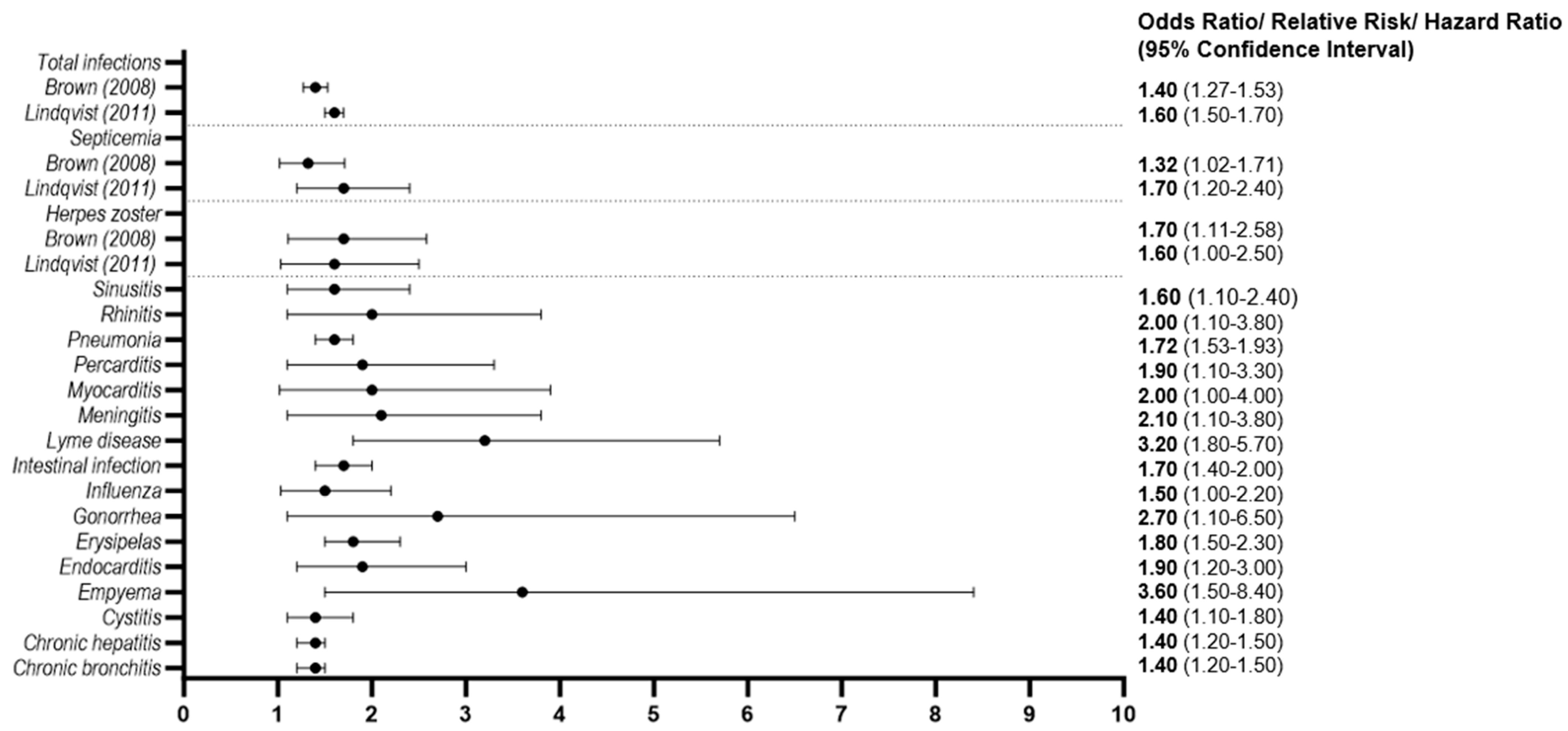
3.2.5. Infectious Diseases
3.2.6. Socioeconomic Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landgren, O.; Kyle, R.A.; Pfeiffer, R.M.; Katzmann, J.A.; Caporaso, N.E.; Hayes, R.B.; Dispenzieri, A.; Kumar, S.; Clark, R.J.; Baris, D.; et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: A prospective study. Blood 2009, 113, 5412–5417. [Google Scholar] [CrossRef]
- Weiss, B.M.; Abadie, J.; Verma, P.; Howard, R.S.; Kuehl, W.M. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood 2009, 113, 5418–5422. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Kumar, S. Multiple myeloma current treatment algorithms. Blood Cancer J. 2020, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Kyle, R.A.; Buadi, F.K. Advances in the Diagnosis, Classification, Risk Stratification, and Management of Monoclonal Gammopathy of Undetermined Significance: Implications for Recategorizing Disease Entities in the Presence of Evolving Scientific Evidence. Mayo Clin. Proc. 2010, 85, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Therneau, T.M.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R.; Plevak, M.F.; Melton, L.J. A Long-Term Study of Prognosis in Monoclonal Gammopathy of Undetermined Significance. N. Engl. J. Med. 2002, 346, 564–569. [Google Scholar] [CrossRef]
- Bida, J.P.; Kyle, R.A.; Therneau, T.M.; Melton, L.J., 3rd; Plevak, M.F.; Larson, D.R.; Dispenzieri, A.; Katzmann, J.A.; Rajkumar, S.V. Disease associations with monoclonal gammopathy of undetermined significance: A population-based study of 17,398 patients. Mayo Clin. Proc. 2009, 84, 685–693. [Google Scholar] [CrossRef]
- Schwartz, B.; Schou, M.; Ruberg, F.L.; Rucker, D.; Choi, J.; Siddiqi, O.; Monahan, K.; Køber, L.; Gislason, G.; Torp-Pedersen, C.; et al. Cardiovascular Morbidity in Monoclonal Gammopathy of Undetermined Significance. JACC CardioOncology 2022, 4, 313–322. [Google Scholar] [CrossRef]
- Kristinsson, S.Y.; Pfeiffer, R.M.; Björkholm, M.; Goldin, L.R.; Schulman, S.; Blimark, C.; Mellqvist, U.-H.; Wahlin, A.; Turesson, I.; Landgren, O. Arterial and venous thrombosis in monoclonal gammopathy of undetermined significance and multiple myeloma: A population-based study. Blood 2010, 115, 4991–4998. [Google Scholar] [CrossRef] [PubMed]
- Mouhieddine, T.H.; Weeks, L.D.; Ghobrial, I.M. Monoclonal gammopathy of undetermined significance. Blood 2019, 133, 2484–2494. [Google Scholar] [CrossRef]
- Belouni, R.; Allam, I.; Cherguelaine, K.; Berkani, L.; Belaid, B.; Berkouk, Y.; Nekkal, S.; Saidani, M.; Belhani, M.; Ghaffor, M.; et al. Epidemiological and immunochemical parameters of monoclonal plasma cell dyscrasias of 2121 cases in Algeria. Curr. Res. Transl. Med. 2020, 68, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Abell, K.; Chadwell, S.E.; Burrow, T.A.; Becker, A.P.P.; Bailey, L.; Steele, P.; Zhang, X.; Islas-Ohlmayer, M.; Bittencourt, R.; Schwartz, I.V.D.; et al. Outcomes of screening for gammopathies in children and adults with Gaucher disease type 1 in a cohort from Brazil and the United States. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 1052–1059. [Google Scholar] [CrossRef]
- Babel, N.; Schwarzmann, F.; Pruss, A.; Volk, H.-D.; Reinke, P. Monoclonal gammopathy of undetermined significance (MGUS) is associated with an increased frequency of Epstein-Barr Virus (EBV) latently infected B lymphocytes in long-term renal transplant patients. Transplant. Proc. 2004, 36, 2679–2682. [Google Scholar] [CrossRef]
- Bigot-Corbel, E.; Gassin, M.; Corre, I.; Le Carrer, D.; Delaroche, O.; Hermouet, S. Hepatitis C virus (HCV) infection, monoclonal immunoglobulin specific for HCV core protein, and plasma-cell malignancy. Blood 2008, 112, 4357–4358. [Google Scholar] [CrossRef]
- Hermouet, S.; Corre, I.; Gassin, M.; Bigot-Corbel, E.; Sutton, C.A.; Casey, J.W. Hepatitis C virus, human herpesvirus 8, and the development of plasma-cell leukemia. N. Engl. J. Med. 2003, 348, 178–179. [Google Scholar] [CrossRef]
- Ali, Y.M.; Urowitz, M.B.; Ibanez, D.; Gladman, D.D. Monoclonal gammopathy in systemic lupus erythematosus. Lupus 2007, 16, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Amara, S.; Dezube, B.J.; Cooley, T.P.; Pantanowitz, L.; Aboulafia, D.M. HIV-associated monoclonal gammopathy: A retrospective analysis of 25 patients. Clin. Infect. Dis. 2006, 43, 1198–1205. [Google Scholar] [CrossRef]
- Arnulf, B.; Bengoufa, D.; Sarfati, E.; Toubert, M.E.; Meignin, V.; Brouet, J.C.; Fermand, J.P. Prevalence of monoclonal gammopathy in patients with primary hyperparathyroidism: A prospective study. Arch. Intern. Med. 2002, 162, 464–467. [Google Scholar] [CrossRef]
- Eder, L.; Thavaneswaran, A.; Pereira, D.; Sussman, G.; Gladman, D.D. Prevalence of Monoclonal Gammopathy Among Patients with Psoriatic Arthritis. J. Rheumatol. 2012, 39, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Garton, M.J.; Keir, G.; Dickie, A.; Steven, M.; Rennie, J.A. Prevalence and long-term significance of paraproteinaemia in rheumatoid arthritis. Rheumatol. Oxf. 2006, 45, 355–356. [Google Scholar] [CrossRef]
- Geller, H.I.; Singh, A.; Mirto, T.M.; Padera, R.; Mitchell, R.; Laubach, J.P.; Falk, R.H. Prevalence of Monoclonal Gammopathy in Wild-Type Transthyretin Amyloidosis. Mayo Clin. Proc. 2017, 92, 1800–1805. [Google Scholar] [CrossRef]
- Heer, M.; Joller-Jemelka, H.; Fontana, A.; Seefeld, U.; Schmid, M.; Ammann, R. Monoclonal gammopathy in chronic active hepatitis. Liver 1984, 4, 255–263. [Google Scholar] [CrossRef]
- Papadaki, H.A.; Eliopoulos, D.G.; Ponticoglou, C.; Eliopoulos, G.D. Increased frequency of monoclonal gammopathy of undetermined significance in patients with nonimmune chronic idiopathic neutropenia syndrome. Int. J. Hematol. 2001, 73, 339–345. [Google Scholar] [CrossRef]
- Phull, P.; Sanchorawala, V.; Connors, L.H.; Doros, G.; Ruberg, F.L.; Berk, J.L.; Sarosiek, S. Monoclonal gammopathy of undetermined significance in systemic transthyretin amyloidosis (ATTR). Amyloid 2018, 25, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, N.M.; El Deeb, M.; Nasr, A.S. Monoclonal gammopathy among patients with chronic hepatitis C virus infection. Am. J. Med. Sci. 2013, 345, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Wadhera, R.K.; Rajkumar, S.V. Prevalence of Monoclonal Gammopathy of Undetermined Significance: A Systematic Review. Mayo Clin. Proc. 2010, 85, 933–942. [Google Scholar] [CrossRef]
- Waxman, A.J.; Mink, P.J.; Devesa, S.S.; Anderson, W.F.; Weiss, B.M.; Kristinsson, S.Y.; McGlynn, K.A.; Landgren, O. Racial disparities in incidence and outcome in multiple myeloma: A population-based study. Blood 2010, 116, 5501–5506. [Google Scholar] [CrossRef] [PubMed]
- Landgren, O.; Graubard, B.I.; Kumar, S.; Kyle, R.A.; Katzmann, J.A.; Murata, K.; Costello, R.; Dispenzieri, A.; Caporaso, N.; Mailankody, S.; et al. Prevalence of myeloma precursor state monoclonal gammopathy of undetermined significance in 12372 individuals 10–49 years old: A population-based study from the National Health and Nutrition Examination Survey. Blood Cancer J. 2017, 7, e618. [Google Scholar] [CrossRef]
- Du, Z.; Weinhold, N.; Song, G.C.; Rand, K.A.; Van Den Berg, D.J.; Hwang, A.E.; Sheng, X.; Hom, V.; Ailawadhi, S.; Nooka, A.K.; et al. A meta-analysis of genome-wide association studies of multiple myeloma among men and women of African ancestry. Blood Adv. 2020, 4, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tyler, J.K. Epigenetics and aging. Sci. Adv. 2016, 2, e1600584. [Google Scholar] [CrossRef] [PubMed]
- Steves, C.J.; Spector, T.D.; Jackson, S.H.D. Ageing, genes, environment and epigenetics: What twin studies tell us now, and in the future. Age Ageing 2012, 41, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Bosseboeuf, A.; Feron, D.; Tallet, A.; Rossi, C.; Charlier, C.; Garderet, L.; Caillot, D.; Moreau, P.; Cardó-Vila, M.; Pasqualini, R.; et al. Monoclonal IgG in MGUS and multiple myeloma targets infectious pathogens. JCI Insight 2017, 2, e95367. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Kyle, R.A.; Plevak, M.F.; Murray, J.A.; Therneau, T.M. Helicobacter pylori infection and monoclonal gammopathy of undetermined significance. Br. J. Haematol. 2002, 119, 706–708. [Google Scholar] [CrossRef]
- Nair, S.; Branagan, A.R.; Liu, J.; Boddupalli, C.S.; Mistry, P.K.; Dhodapkar, M.V. Clonal Immunoglobulin against Lysolipids in the Origin of Myeloma. N. Engl. J. Med. 2016, 374, 555–561. [Google Scholar] [CrossRef]
- Rögnvaldsson, S.; Love, T.J.; Thorsteinsdottir, S.; Reed, E.R.; Óskarsson, J.Þ.; Pétursdóttir, Í.; Sigurðardóttir, G.Á.; Viðarsson, B.; Önundarson, P.T.; Agnarsson, B.A.; et al. Iceland screens, treats, or prevents multiple myeloma (iStopMM): A population-based screening study for monoclonal gammopathy of undetermined significance and randomized controlled trial of follow-up strategies. Blood Cancer J. 2021, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, i4086. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Obeid, J.S.; McGraw, C.A.; Minor, B.L.; Conde, J.G.; Pawluk, R.; Lin, M.; Wang, J.; Banks, S.R.; Hemphill, S.A.; Taylor, R.; et al. Procurement of shared data instruments for Research Electronic Data Capture (REDCap). J. Biomed. Inform. 2013, 46, 259–265. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Bertrand, K.A.; Zirpoli, G.; Niharika Pillalamarri, B.; Szalat, R.; Palmer, J.R.; Kataria, Y. Prevalence of monoclonal gammopathy of undetermined significance in US black women. Am. J. Hematol. 2022, 97, E341. [Google Scholar] [CrossRef] [PubMed]
- Landgren, O.; Katzmann, J.A.; Hsing, A.W.; Pfeiffer, R.M.; Kyle, R.A.; Yeboah, E.D.; Biritwum, R.B.; Tettey, Y.; Adjei, A.A.; Larson, D.R.; et al. Prevalence of monoclonal gammopathy of undetermined significance among men in Ghana. Mayo Clin. Proc. 2007, 82, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Thordardottir, M.; Lindqvist, E.K.; Lund, S.H.; Costello, R.; Burton, D.; Steingrimsdottir, L.; Korde, N.; Mailankody, S.; Eiriksdottir, G.; Launer, L.J.; et al. Dietary intake is associated with risk of multiple myeloma and its precursor disease. PLoS ONE 2018, 13, e0206047. [Google Scholar] [CrossRef]
- Love, T.J.; Rögnvaldsson, S.; Thorsteinsdottir, S.; Aspelund, T.; Reed, E.R.; Vidarsson, B.; Onundarson, P.T.; Agnarsson, B.A.; Sigurdardottir, M.; Thorsteinsdottir, I.; et al. Prevalence of MGUS Is High in the Istopmm Study but the Prevalence of IgA MGUS Does Not Increase with Age in the Way Other Immunoglobulin Subtypes Do. Blood 2022, 140, 256–258. [Google Scholar] [CrossRef]
- Kyle, R.A.; Therneau, T.M.; Rajkumar, S.V.; Larson, D.R.; Plevak, M.F.; Offord, J.R.; Dispenzieri, A.; Katzmann, J.A.; Melton, L.J., III. Prevalence of monoclonal gammopathy of undetermined significance. N. Engl. J. Med. 2006, 354, 1362–1369. [Google Scholar] [CrossRef]
- Park, H.K.; Lee, K.R.; Kim, Y.J.; Cho, H.I.; Kim, J.E.; Kim, K.W.; Kim, Y.J.; Lee, K.W.; Kim, J.H.; Bang, S.M.; et al. Prevalence of monoclonal gammopathy of undetermined significance in an elderly urban Korean population. Am. J. Hematol. 2011, 86, 752–755. [Google Scholar] [CrossRef]
- Iwanaga, M.; Tagawa, M.; Tsukasaki, K.; Kamihira, S.; Tomonaga, M. Prevalence of monoclonal gammopathy of undetermined significance: Study of 52,802 persons in Nagasaki City, Japan. Mayo Clin. Proc. 2007, 82, 1474–1479. [Google Scholar] [CrossRef]
- Bowden, M.; Crawford, J.; Cohen, H.J.; Noyama, O. A comparative study of monoclonal gammopathies and immunoglobulin levels in Japanese and United States elderly. J. Am. Geriatr. Soc. 1993, 41, 11–14. [Google Scholar] [CrossRef]
- Saleun, J.P.; Vicariot, M.; Deroff, P.; Morin, J.F. Monoclonal gammopathies in the adult population of Finistere, France. J. Clin. Pathol. 1982, 35, 63–68. [Google Scholar] [CrossRef]
- Wu, S.P.; Minter, A.; Costello, R.; Zingone, A.; Lee, C.K.; Au, W.Y.; Landgren, O. MGUS prevalence in an ethnically Chinese population in Hong Kong. Blood 2013, 121, 2363–2364. [Google Scholar] [CrossRef]
- Onwah, A.L.; Adeyemo, T.A.; Adediran, A.; Ajibola, S.O.; Akanmu, A.S. Prevalence and type of monoclonal gammopathy of undetermined significance in an apparently healthy Nigerian population: A cross sectional study. BMC Blood Disord. 2012, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, W.; Alaskar, A.; Alassiri, M.; Alsaeed, W.; Alarifi, S.A.; Alenzi, F.Q.; Jawdat, D. Monoclonal gammopathy in a tertiary referral hospital. Clin. Biochem. 2010, 43, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Dahiya, M.; Kumar, L.; Shekhar, V.; Sharma, A.; Ramakrishnan, L.; Sharma, O.D.; Begum, A. Prevalence of Monoclonal Gammopathy of Undetermined Significance in India-A Hospital-based Study. Clin. Lymphoma Myeloma Leuk. 2018, 18, e345–e350. [Google Scholar] [CrossRef]
- Han, J.H.; Wang, J.N.; Zhang, Y.L.; Cao, X.X.; Zhou, D.B.; Xu, T.D.; Su, W.; Li, J. Prevalence of monoclonal gammopathy of undetermined significance in a large population with annual medical check-ups in China. Blood Cancer J. 2020, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Su, M.J.; Lee, S.J.; Tsai, Y.H.; Kuo, L.Y.; Lin, I.H.; Huang, H.L.; Yen, T.H.; Chu, F.Y. The Immunotyping Distribution of Serum Monoclonal Paraprotein and Environmental Impact on Multiple Myeloma (MM) and Monoclonal Gammopathy of Uncertain Significance (MGUS) in Taiwan: A Medical Center-Based Experience. Asian Pac. J. Cancer Prev. 2016, 17, 395–399. [Google Scholar] [CrossRef]
- Cicero, K.I.; Joffe, M.; Patel, M.; Chiuzan, C.; Pentz, A.; Ruff, P.; Lentzsch, S.; Leng, S.; Jacobson, J.S.; Rebbeck, T.R.; et al. Prevalence of Monoclonal Gammopathy of Undetermined Significance in Black South African Men. Cancer Epidemiol. Biomark. Prev. 2022, 31, 2192–2198. [Google Scholar] [CrossRef]
- Vernocchi, A.; Longhi, E.; Lippi, G.; Gelsumini, S. Increased Monoclonal Components: Prevalence in an Italian Population of 44 474 Outpatients Detected by Capillary Electrophoresis. J. Med. Biochem. 2016, 35, 50–54. [Google Scholar] [CrossRef]
- Suan, D.; Hughan, M.; Bates, S.; Rochtchina, E.; Empson, M.; Mitchell, P.; Fulcher, D.A. Prevalence of paraproteinaemia in older Australians. Intern. Med. J. 2012, 42, 165–169. [Google Scholar] [CrossRef]
- Eisele, L.; Dürig, J.; Hüttmann, A.; Dührsen, U.; Assert, R.; Bokhof, B.; Erbel, R.; Mann, K.; Jöckel, K.H.; Moebus, S. Prevalence and progression of monoclonal gammopathy of undetermined significance and light-chain MGUS in Germany. Ann. Hematol. 2012, 91, 243–248. [Google Scholar] [CrossRef]
- Axelsson, U.; Bachmann, R.; Hällén, J. Frequency of Pathological Proteins (M-components) in 6,995 Sera from an Adult Population. Acta Med. Scand. 2009, 179, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Boursi, B.; Weiss, B.M.; Haynes, K.; Mamtani, R.; Yang, Y.X. Reappraisal of risk factors for monoclonal gammopathy of undetermined significance. Am. J. Hematol. 2016, 91, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.M.; Gridley, G.; Check, D.; Landgren, O. Risk of multiple myeloma and monoclonal gammopathy of undetermined significance among white and black male United States veterans with prior autoimmune, infectious, inflammatory, and allergic disorders. Blood 2008, 111, 3388–3394. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, E.K.; Goldin, L.R.; Landgren, O.; Blimark, C.; Mellqvist, U.H.; Turesson, I.; Wahlin, A.; Bjorkholm, M.; Kristinsson, S.Y. Personal and family history of immune-related conditions increase the risk of plasma cell disorders: A population-based study. Blood 2011, 118, 6284–6291. [Google Scholar] [CrossRef]
- Orban, E.; Arendt, M.; Hennig, F.; Lucht, S.; Eisele, L.; Jakobs, H.; Durig, J.; Hoffmann, B.; Jockel, K.H.; Moebus, S. Is long-term particulate matter and nitrogen dioxide air pollution associated with incident monoclonal gammopathy of undetermined significance (MGUS)? An analysis of the Heinz Nixdorf Recall study. Environ. Int. 2017, 108, 237–245. [Google Scholar] [CrossRef]
- Iwanaga, M.; Tagawa, M.; Tsukasaki, K.; Matsuo, T.; Yokota, K.; Miyazaki, Y.; Fukushima, T.; Hata, T.; Imaizumi, Y.; Imanishi, D.; et al. Relationship between monoclonal gammopathy of undetermined significance and radiation exposure in Nagasaki atomic bomb survivors. Blood 2009, 113, 1639–1650. [Google Scholar] [CrossRef]
- Fujimura, K.; Sugiyama, A.; Akita, T.; Ohisa, M.; Nagashima, S.; Katayama, K.; Maeda, R.; Tanaka, J. Screening for M-proteinemia consisting of monoclonal gammopathy of undetermined significance and multiple myeloma for 30 years among atomic bomb survivors in Hiroshima. Int. J. Hematol. 2021, 113, 576–585. [Google Scholar] [CrossRef]
- Pasqualetti, P.; Collacciani, A.; Casale, R. Risk of monoclonal gammopathy of undetermined significance: A case-referent study. Am. J. Hematol. 1996, 52, 217–220. [Google Scholar] [CrossRef]
- Landgren, O.; Shim, Y.K.; Michalek, J.; Costello, R.; Burton, D.; Ketchum, N.; Calvo, K.R.; Caporaso, N.; Raveche, E.; Middleton, D.; et al. Agent Orange Exposure and Monoclonal Gammopathy of Undetermined Significance: An Operation Ranch Hand Veteran Cohort Study. JAMA Oncol. 2015, 1, 1061–1068. [Google Scholar] [CrossRef]
- Landgren, O.; Kyle, R.A.; Hoppin, J.A.; Beane Freeman, L.E.; Cerhan, J.R.; Katzmann, J.A.; Rajkumar, S.V.; Alavanja, M.C. Pesticide exposure and risk of monoclonal gammopathy of undetermined significance in the Agricultural Health Study. Blood 2009, 113, 6386–6391. [Google Scholar] [CrossRef]
- Hofmann, J.N.; Beane Freeman, L.E.; Murata, K.; Andreotti, G.; Shearer, J.J.; Thoren, K.; Ramanathan, L.; Parks, C.G.; Koutros, S.; Lerro, C.C.; et al. Lifetime Pesticide Use and Monoclonal Gammopathy of Undetermined Significance in a Prospective Cohort of Male Farmers. Env. Health Perspect. 2021, 129, 17003. [Google Scholar] [CrossRef]
- Pasqualetti, P.; Festuccia, V.; Acitelli, P.; Collacciani, A.; Giusti, A.; Casale, R. Tobacco smoking and risk of haematological malignancies in adults: A case-control study. Br. J. Haematol. 1997, 97, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Debold, E.; Frank, M.; Arendt, M.; Dragano, N.; Durig, J.; Duhrsen, U.; Moebus, S.; Erbel, R.; Jockel, K.H.; et al. Socioeconomic Position is Positively Associated with Monoclonal Gammopathy of Undetermined Significance in a Population-based Cohort Study. Ann. Hematol. 2019, 98, 2761–2767. [Google Scholar] [CrossRef] [PubMed]
- Landgren, O.; Rajkumar, S.V.; Pfeiffer, R.M.; Kyle, R.A.; Katzmann, J.A.; Dispenzieri, A.; Cai, Q.; Goldin, L.R.; Caporaso, N.E.; Fraumeni, J.F.; et al. Obesity is associated with an increased risk of monoclonal gammopathy of undetermined significance among black and white women. Blood 2010, 116, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Thordardottir, M.; Lindqvist, E.K.; Lund, S.H.; Costello, R.; Burton, D.; Korde, N.; Mailankody, S.; Eiriksdottir, G.; Launer, L.J.; Gudnason, V.; et al. Obesity and risk of monoclonal gammopathy of undetermined significance and progression to multiple myeloma: A population-based study. Blood Adv. 2017, 1, 2186–2192. [Google Scholar] [CrossRef]
- Castaneda-Avila, M.A.; Ulbricht, C.M.; Epstein, M.M. Risk factors for monoclonal gammopathy of undetermined significance: A systematic review. Ann. Hematol. 2021, 100, 855–863. [Google Scholar] [CrossRef]
- Chang, S.-H.; Luo, S.; Thomas, T.S.; O’Brian, K.K.; Colditz, G.A.; Carlsson, N.P.; Carson, K.R. Obesity and the Transformation of Monoclonal Gammopathy of Undetermined Significance to Multiple Myeloma: A Population-Based Cohort Study. J. Natl. Cancer Inst. 2017, 109, djw264. [Google Scholar] [CrossRef]
- Parikh, R.; Tariq, S.M.; Marinac, C.R.; Shah, U.A. A comprehensive review of the impact of obesity on plasma cell disorders. Leukemia 2022, 36, 301–314. [Google Scholar] [CrossRef]
- Sanoja-Flores, L.; Flores-Montero, J.; Garcés, J.J.; Paiva, B.; Puig, N.; García-Mateo, A.; García-Sánchez, O.; Corral-Mateos, A.; Burgos, L.; Blanco, E.; et al. Next generation flow for minimally-invasive blood characterization of MGUS and multiple myeloma at diagnosis based on circulating tumor plasma cells (CTPC). Blood Cancer J. 2018, 8, 117. [Google Scholar] [CrossRef]
- Fonseca, R.; Barlogie, B.; Bataille, R.; Bastard, C.; Bergsagel, P.L.; Chesi, M.; Davies, F.E.; Drach, J.; Greipp, P.R.; Kirsch, I.R.; et al. Genetics and cytogenetics of multiple myeloma: A workshop report. Cancer Res. 2004, 64, 1546–1558. [Google Scholar] [CrossRef]
- Shimanovsky, A.; Alvarez Argote, J.; Murali, S.; Dasanu, C.A. Autoimmune manifestations in patients with multiple myeloma and monoclonal gammopathy of undetermined significance. BBA Clin. 2016, 6, 12–18. [Google Scholar] [CrossRef]
- Fonseca, R.; Bailey, R.J.; Ahmann, G.J.; Rajkumar, S.V.; Hoyer, J.D.; Lust, J.A.; Kyle, R.A.; Gertz, M.A.; Greipp, P.R.; Dewald, G.W. Genomic abnormalities in monoclonal gammopathy of undetermined significance. Blood 2002, 100, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, A.; Vilariño-García, T.; Fernández-Riejos, P.; Martín-González, J.; Segura-Egea, J.J.; Sánchez-Margalet, V. Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor. Rev. 2017, 35, 71–84. [Google Scholar] [CrossRef]
- Hosseini, B.; Berthon, B.S.; Saedisomeolia, A.; Starkey, M.R.; Collison, A.; Wark, P.A.B.; Wood, L.G. Effects of fruit and vegetable consumption on inflammatory biomarkers and immune cell populations: A systematic literature review and meta-analysis. Am. J. Clin. Nutr. 2018, 108, 136–155. [Google Scholar] [CrossRef]
- Martín de Diego, C.E.; Gómez de Terreros Sánchez, F.J.; Caro de Miguel, M.C.; Medina Font, J.; Matesanz Ruiz, C.; Gómez de Terreros Caro, J. C-reactive protein value related to a smoking history and composition of nicotine and tobacco tar. An. Med. Interna Madr. Spain 1984 2006, 23, 3–10. [Google Scholar] [CrossRef]
- Diplock, A.T.; Charleux, J.L.; Crozier-Willi, G.; Kok, F.J.; Rice-Evans, C.; Roberfroid, M.; Stahl, W.; Viña-Ribes, J. Functional food science and defence against reactive oxidative species. Br. J. Nutr. 1998, 80 (Suppl. S1), S77–S112. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Møller, P.; Danielsen, P.H.; Karottki, D.G.; Jantzen, K.; Roursgaard, M.; Klingberg, H.; Jensen, D.M.; Christophersen, D.V.; Hemmingsen, J.G.; Cao, Y.; et al. Oxidative stress and inflammation generated DNA damage by exposure to air pollution particles. Mutat. Res. Mutat. Res. 2014, 762, 133–166. [Google Scholar] [CrossRef] [PubMed]
- Miyata, R.; Van Eeden, S.F. The innate and adaptive immune response induced by alveolar macrophages exposed to ambient particulate matter. Toxicol. Appl. Pharmacol. 2011, 257, 209–226. [Google Scholar] [CrossRef]
- Viehmann, A.; Hertel, S.; Fuks, K.; Eisele, L.; Moebus, S.; Möhlenkamp, S.; Nonnemacher, M.; Jakobs, H.; Erbel, R.; Jöckel, K.-H.; et al. Long-term residential exposure to urban air pollution, and repeated measures of systemic blood markers of inflammation and coagulation. Occup. Environ. Med. 2015, 72, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Hennig, F.; Fuks, K.; Moebus, S.; Weinmayr, G.; Memmesheimer, M.; Jakobs, H.; Bröcker-Preuss, M.; Führer-Sakel, D.; Möhlenkamp, S.; Erbel, R.; et al. Association between Source-Specific Particulate Matter Air Pollution and hs-CRP: Local Traffic and Industrial Emissions. Environ. Health Perspect. 2014, 122, 703–710. [Google Scholar] [CrossRef] [PubMed]
- El-Khoury, H.; Lee, D.J.; Alberge, J.-B.; Redd, R.; Cea-Curry, C.J.; Perry, J.; Barr, H.; Murphy, C.; Sakrikar, D.; Barnidge, D.; et al. Prevalence of monoclonal gammopathies and clinical outcomes in a high-risk US population screened by mass spectrometry: A multicentre cohort study. Lancet Haematol. 2022, 9, e340–e349. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, K.P.; Steuer, R.; Edwards, C.V. Geographic Prevalence Patterns and Modifiable Risk Factors for Monoclonal Gammopathy of Undetermined Significance. Hemato 2023, 4, 331-349. https://doi.org/10.3390/hemato4040027
Verma KP, Steuer R, Edwards CV. Geographic Prevalence Patterns and Modifiable Risk Factors for Monoclonal Gammopathy of Undetermined Significance. Hemato. 2023; 4(4):331-349. https://doi.org/10.3390/hemato4040027
Chicago/Turabian StyleVerma, Karina P., Rebecca Steuer, and Camille V. Edwards. 2023. "Geographic Prevalence Patterns and Modifiable Risk Factors for Monoclonal Gammopathy of Undetermined Significance" Hemato 4, no. 4: 331-349. https://doi.org/10.3390/hemato4040027
APA StyleVerma, K. P., Steuer, R., & Edwards, C. V. (2023). Geographic Prevalence Patterns and Modifiable Risk Factors for Monoclonal Gammopathy of Undetermined Significance. Hemato, 4(4), 331-349. https://doi.org/10.3390/hemato4040027