Abstract
Anticoagulation clinics (ACs) have a greater impact on anticoagulation control than usual medical care (UMC). There is little evidence of the performance of AC in patients on warfarin living in low and middle-income countries. We sought to investigate the efficacy and safety of an AC in patients treated at a Brazilian public hospital. This was a randomized clinical trial that tested the efficacy of a recently implemented AC, compared to UMC, in outpatients with heart disease. The primary and secondary endpoints were time in the therapeutic range (TTR) and warfarin-related complications, respectively. Overall, 280 patients were enrolled and randomly assigned to Group A: one year at an AC (A1: first half-year; A2: second half-year); and Group B: first half-year receiving UMC (B1) and second half-year being assisted at the AC (B2). The mean age was 56.8 ± 13.1 years, and most patients were female (54.6%). Above 68% of patients had limited reading capability. A1 demonstrated greater TTR (62.4 ± 20.8%) than B1 (55.1 ± 28.5%) (p = 0.014). Group B improved TTR from 55.1 ± 28.5% (B1) to 62.2 ± 23.1% (B2) (p = 0.008). Despite the underpowered analysis of safety, A1 exhibited a lower incidence rate (IR) per patient-year (p-y) of total bleeding than B1 (incidence rate ratio (IRR): 0.78; p = 0.041) and a reduction in intra-group comparisons (both groups: IRR 0.58; p < 0.001). AC care helped increase TTR in a low-income setting showing favorable performance in a distinct population of those evaluated by previous studies. Extending AC care to similar populations may improve the outcomes of warfarin use.
1. Background
Warfarin is a vitamin K antagonist (VKA) that remains an important option for primary and secondary prophylaxis of thromboembolic events in patients with atrial fibrillation (AF) [1] or prosthetic heart valves [2]. In Latin America, warfarin is often indicated for the prevention of ischemic stroke in a subgroup of patients with chronic Trypanosoma cruzi infection (i.e., Chagas disease), as well as in the presence of other risk factors for cardioembolic events [3]. Warfarin response is subject to wide inter- and intra-individual variability that may be influenced by multiple factors, such as genetic polymorphisms [4], nutritional status [5], patient compliance [6], and a high propensity for drug–drug and drug–food interactions [7]. Frequent measurements of the International Normalized Ratio (INR) are required to guide dosage adjustments [8]. Maintaining INR in the therapeutic range in patients treated with warfarin is challenging in clinical practice, and deviations from pre-determined INR targets may lead to serious adverse events. Patients who are undertreated remain at increased risk of thromboembolism, and those receiving excessive oral anticoagulation (OA) are exposed to unnecessary bleeding risk [9,10].
Time in the therapeutic range (TTR) is a surrogate marker generally used as a quality measure of anticoagulation control and represents a well-accepted predictor of bleeding and thromboembolic events [11,12,13]. Major bleeding, stroke, and mortality rates have been reported as being significantly lower in patients with a TTR > 60% [14]. Numerous therapeutic strategies have been examined to improve the TTR obtained by different healthcare settings and to prevent warfarin-related adverse events [15]. Anticoagulation clinics (ACs) have been used as an effective intervention provided by specialists to perform frequent INR monitoring, patient education, and management of anticoagulation therapy [15,16,17,18,19]. Several studies have indicated that anticoagulation care performed by an AC is associated with a significantly higher TTR [17,20,21,22,23] and a significantly lower incidence of bleeding compared to usual medical care (UMC) [20,21,22]. The anticoagulation control performed by UMC involves non-standardized interventions to adjust out-of-range INR values and to educate patients on drug therapy [21,22]. Usual medical care lays is not supported. There is little evidence of the performance of AC in patients living in low and middle-income countries. Thus, we sought to investigate the efficacy and safety of an AC in patients treated at a Brazilian public hospital.
2. Methods
2.1. Design and Setting
This was a randomized, open-label, single-center clinical trial designed to compare two strategies to monitor warfarin therapy. The studied patients were treated at a public university hospital in Belo Horizonte, Southeast Brazil, which is a regional referral center of specialized care for the Brazilian Health System in the State of Minas Gerais. The study design is depicted in Figure 1. This trial was conducted according to the Declaration of Helsinki and complied with the Consolidated Standards of Reporting Trials (CONSORT). Informed consent was obtained from all participants, and access to their medical records was approved by the Ethics Committee of the Universidade Federal de Minas Gerais. The study protocol was registered at ClinicalTrials.gov (https://www.clinicaltrials.gov/study/NCT01006486?id=NCT01006486&rank=1, accessed on 8 July 2023).
Figure 1.
Study design.
2.2. Recruitment and Randomization
From September 2009 to August 2010, patients on warfarin scheduled for medical appointments at three hospital ambulatory clinics (Internal Medicine, Cardiology, and Infectious Diseases) were identified and evaluated for potential eligibility. Those satisfying the pre-defined criteria were included in a consecutive manner. The inclusion criteria were age ≥ 18 years, a defined heart disease diagnosis (e.g., Chagas cardiomyopathy), and at least one indication for long-term OA (e.g., thrombosis, mechanical heart valves, AF/flutter, stroke/transient ischemic attack). The exclusion criteria were as follows: currently taking phenprocoumon and treatment with warfarin for <30 days prior to eligibility assessment. Using a list of random numbers, we assigned each of the included patients to one of the two following arms: (i) Group A: patients treated for one year at AC (A1 in the first half-year and A2 in the second half-year); (ii) Group B: patients treated in the first half-year by UMC (B1), and in the second half-year at AC (B2). It is noteworthy that all participants were receiving UMC prior to recruitment, as the AC was implemented as a new hospital ambulatory clinic in the context of this study development.
2.3. Data Collection
Variables included age, sex, self-declared skin color, body mass index, marital status, education, occupation, and monthly income. Clinical information included heart disease etiology and clinical status, using the New York Heart Association criteria [24]; the number of chronic drug and herbal products and over-the-counter (OTC) drugs used continuously by patients [25]; indications for anticoagulation; targets for prothrombin time, expressed as INR; and comorbidities. Chronic medication use was defined as treatment initiated at least 30 days prior to the interview. Prescriptions and patient charts were also used as sources of information for data collection. Researchers were not blinded to the patient allocation group. All data were collected using report forms specially designed for this study.
2.4. Interventions
Specialized care was offered at AC to participants assigned to Group A and was provided by a multidisciplinary team of hematologists, pharmacists, and supervised undergraduate medical and pharmacy students. The procedures were based on an anticoagulation protocol for INR monitoring and warfarin dosing adjustments. During the initial visit, patients received specific information about indication(s) for OA, risks for adverse events, especially bleeding, proper drug regimens, and risks for drug–drug and drug–food interactions. At subsequent appointments, self-care behaviors were encouraged, and additional information on anticoagulation control was adapted to each patient’s level of comprehension, if necessary. Patients were also provided with educational materials and an anticoagulation card to be updated at each appointment with INR values and dosing adjustments [16,26,27]. B1 received UMC, which consisted of physicians and medical residents being responsible for INR monitoring, warfarin dosing adjustments, and patient education without the support of an anticoagulation protocol. During this phase, data about INR results and adverse events were collected by telephone or during medical appointments. After six months, patients allocated to Group B were transitioned to AC to receive more specialized care (B2), as described for Group A. The investigators specialized in anticoagulation and cared for patients in Group A, being also involved in running the implementation of the AC in the study hospital and training the staff to follow the AC protocol.
2.5. Outcomes
The primary outcome measured was TTR, and the secondary outcome was warfarin-related adverse events (bleeding and thromboembolism) occurring during a one-year follow-up period. INR results during the follow-up were used to calculate TTR by the Rosendaal method [28], which uses linear interpolation to assign an INR result to each day between two successive observed INR values. The TTR calculation did not include the INR value determined at the first appointment at AC for either group, and it was adjusted to fit within the patient INR-target range (2.00–3.00 or 2.50–3.50). INR was measured in the intervention group weekly or once within two weeks if out of range and once a month if within therapeutic range. In the control group, the frequency varied depending on the decision of assistant physicians. Any type of bleeding (e.g., epistaxis, hematoma, gastrointestinal, genitourinary, intracranial, and retroperitoneal hemorrhages) was recorded and evaluated according to its severity. Minor bleeding was defined as events not related to systemic complications and not requiring specific medical intervention. Major bleeding included intracranial and retroperitoneal hemorrhages, episodes involving a fall of at least 2.0 g/dL in hemoglobin or a need for at least two units of red blood cells, and any bleeding episode considered the patient’s primary cause of death. Venous and arterial thromboembolisms (thromboembolic/thrombotic events) were recorded when confirmed by imaging combined with an appropriate medical evaluation.
2.6. Sample Size
The sample size was calculated to detect a minimum difference of 10% in the absolute values of TTR between groups using Stata software (StataCorp. 2004. Stata Statistical Software: Release 8. College Station, TX, USA), with a statistical power of 90% and a 95% confidence level. Based on published data, variations from 3% to 26% in TTR were found in studies evaluating the performance of UMC and AC [15,20,21,22,29,30,31]. A calculated sample size of T. cruzi non-infected patients resulted in 84 subjects for each group. A target sample size with ~18% more participants was selected to ensure sufficient statistical power and to account for ‘drop-outs’ during the study. All T. cruzi-infected patients were considered for recruitment.
2.7. Statistical Analysis
Baseline patient characteristics and data collected during follow-up were registered by double entry using EpiData software (version 3.1, EpiData Assoc, Odense, Denmark). Descriptive statistical methods for data evaluation included the calculation of proportions and measurements of central tendency and variability using the Statistical Package for the Social Sciences (SPSS for Windows, version 18.0, SPSS Inc., Chicago, IL, USA). Intra-group (A1 vs. A2; B1 vs. B2) and inter-group (A1 vs. B1) TTR and warfarin-related adverse events were compared between the first and the second half-year. The Kolmogorov–Smirnov test was used to evaluate normality for the distribution of TTR results. Intra-group comparisons of mean TTR were made using the paired t-test, and inter-group comparisons were made using the independent samples t-test. The incidence rate (IR) per patient-year (p-y) was calculated for thromboembolic events, for total hemorrhagic events, and for their stratification into minor and major hemorrhagic events. The calculations of IR and the incidence rate ratio (IRR) were performed with Stata software (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX, USA), using Poisson regression for dependent and independent groups, when indicated. The non-occurrence of adverse events made the calculations of IR and confidence level (CI) impossible for dependent samples. A 95% CI based on intention-to-treat analysis was considered.
3. Results
Two hundred eighty out of 386 outpatients screened for eligibility were included in the study: 129 patients were in Group A, and 151 were in Group B. Figure 2 depicts a CONSORT diagram.
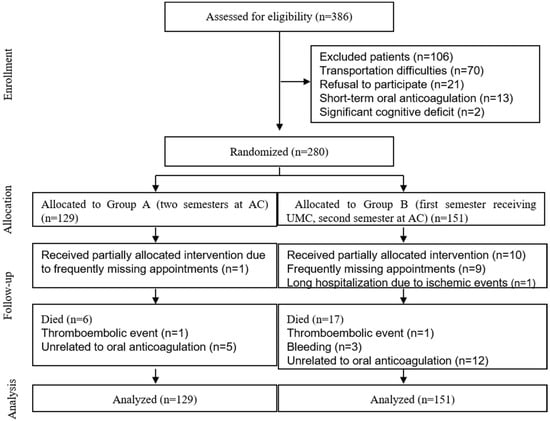
Figure 2.
CONSORT study algorithm of the randomized trial evaluating the effects of an anticoagulation clinic in Brazilian patients on warfarin. AC, anticoagulation clinic; UMC, usual medical care.
The baseline sociodemographic and clinical characteristics of the patients who underwent randomization are detailed in Table 1 and Table 2. The mean age was 56.8 ± 13.1 years, and the majority of patients were female (54.6%). The participants were characterized by their limited socioeconomic level, low reading capability (>68%), and low median monthly income (464 US dollars). AF/flutter was the primary indication for anticoagulation therapy (63.6%). The length of warfarin use was mostly higher than three years. Most patients were taking at least five drugs continuously. Systemic arterial hypertension (60.0%) and heart failure (60.0%) were the most frequent comorbidities.

Table 1.
Baseline sociodemographic data of 280 participants receiving warfarin therapy.

Table 2.
Baseline clinical characteristics of the 280 participants in warfarin use.
The median annual number of INR tests was nine for Group A; among Group B patients, this number increased from seven (B1) to 10 (B2) during the one-year follow-up period. The median interval between INR tests was approximately 22 days in both arms of the study. Patients in the AC experienced an improved TTR, as demonstrated by inter- and intra-group comparisons. Therefore, in the first half-year of follow-up, the comparison between independent groups showed that A1 reached a TTR of 62.4 ± 20.8%, whereas B1 exhibited a TTR of 55.1 ± 28.5% (p = 0.014). When intra-group comparison was performed, we observed an improvement in TTR values in Group B individuals from 55.1 ± 28.5% (B1) to 62.2 ± 23.1% (B2) (p = 0.008). Similar results were observed in the group that showed an improvement in its TTR from 62.4 ± 20.8% (A1) to 67.3 ± 21.1% (A2), although no significant difference was noted (p = 0.059). Descriptive data about INR measurements and the comparative evaluation of TTR are depicted in Table 3.

Table 3.
Inter- and intra-group comparisons between the first and the second half-year regarding INR measurements and time-in-therapeutic range in patients treated with warfarin.
With respect to warfarin-related adverse events, minor bleeding demonstrated an IR of 1.79 p-y for A1 and 2.27 p-y for B1, with a calculated IRR of 0.79 (CI 95%, 0.62–0.99; p = 0.046). There was a significant decline in minor bleeding risk between the first and the second half-year of follow-up among both Group B (IRR: 0.58; CI 95%, 0.45–0.75; p < 0.001) and Group A patients (IRR: 0.58; CI 95%, 0.43–0.79; p = 0.001). Regarding major bleedings, a low IR was noted in Group A (A1, 0.06 and A2, 0.03) and Group B (B1, 0.08 and B2, 0.06), with no significant IRR exhibited upon inter- and intra-group comparisons. For total bleeding (minor and major episodes analyzed together), the IR was 1.85 p-y for A1 and 2.36 p-y for B1, with a calculated IRR of 0.78 (CI 95%, 0.62–0.99; p = 0.041). The IR also decreased in the same groups in the second half-year of follow-up, and a significant IRR was noted upon comparisons of B2 and B1 (IRR: 0.58; CI 95%, 0.45–0.75; p < 0.001) or A2 and A1 (IRR: 0.58; CI 95%, 0.43–0.79; p < 0.001). Thromboembolism demonstrated an IR of 0.02 p-y for A1 and 0.12 p-y for B1, with a calculated IRR of 0.12 (CI 95%, 0.02–0.97; p = 0.047), but intra-group comparisons showed no significant differences (Table 4).

Table 4.
Comparison of groups between the first and the second half-year regarding warfarin-related adverse events.
The types and severity of warfarin-related adverse events observed in the first and second half-year of follow-up are described in Table 5. In general, hematoma, epistaxis, and gum bleeding were the most frequently reported minor bleeding episodes. All three fatal bleeding episodes (two episodes of hemorrhagic stroke and one of intra-abdominal bleeding) occurred in B1. Transient ischemic attack (TIA) was the most frequent thromboembolic event, but five of the six TIA identified were observed in a single patient. One episode of fatal thromboembolism was observed in each study group during the first half-year of follow-up, represented by mesenteric thrombosis in Group A1 and ischemic stroke in Group B1. The frequency of bleeding and thromboembolic episodes observed in the first and second half-year of follow-up for groups A and B is demonstrated in Figure 3.

Table 5.
Descriptive data of the types and severity of warfarin-related adverse events in the first and second half-year of follow-up.
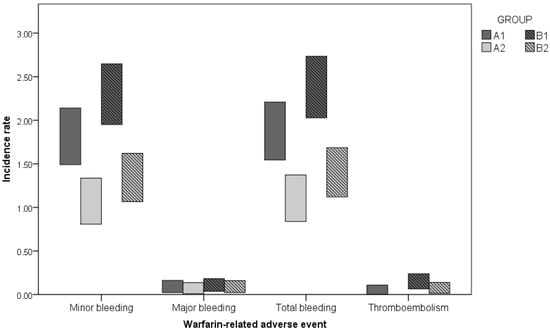
Figure 3.
Incidence rates for warfarin complications according to the group and period of follow-up. A1—first half-year at AC; A2—second half-year at AC; Group B: B1—first half-year receiving UMC; B2—second half-year at AC.
4. Discussion
In this clinical trial, we demonstrated that patient care provided by an AC significantly improved TTR in warfarin outpatients compared to UMC, even in a low-income population. The achievement of a TTR ≥ 62.2% met the criteria established for the efficacy of OA over isolated antiplatelet therapy [11]. Our results are consistent with previous findings indicating that AC achieved a TTR above 60.0% [29,30,31]. The benefit of AC care over UMC has been demonstrated by other comparative studies (TTR ranging from 63.0 to 82.0%) [17,20,21,22]. In a meta-analysis, Baker et al. [17] found that compared with AC, patients treated by UMC may spend 11% less time in the therapeutic INR range. In our study, intra-group comparisons showed that the care provided by the AC to Group A resulted in an increase in TTR from 62.5% in A1 to 67.3% in A2, which may reflect a positive carryover effect. Alternatively, this result may be explained by the time required to structure care and develop professional skills to manage OA. Besides, strategies have been implemented to improve patients’ behavior, particularly adherence and self-care. Our findings suggest that long-term care at an AC may result in a cumulative increase in TTR. For Group B, treated by UMC for the first half-year and at AC thereafter, a 7.1% elevation of TTR was observed after the intervention, which may minimize the risk of warfarin-related complications [13]. In a systematic review of retrospective studies, Wan et al. [32] found that a 6.9% improvement in TTR significantly reduced the incidence of major hemorrhage by one event per 100 patient-years of treatment.
Regarding safety, this study was underpowered to evaluate severe clinical events due to the small sample size and the low incidence of major hemorrhage and thromboembolic events. Similar limitations were raised by Wilson et al., who investigated the same endpoints in a randomized trial comparing the performance between AC and UMC [21]. Although minor bleedings have low clinical relevance and lack clear definition, the improvement in TTR may decrease the incidence of these events and prevent patients from feeling uncomfortable with their occurrence. In the present study, when total bleeding is considered, the IRR for the first half-year of follow-up was 0.78 (0.62–0.98, p = 0.041), favoring AC over UMC, which is consistent with other comparative studies [20,22]. Nevertheless, further studies designed to evaluate clinical complications as primary endpoints are needed.
Patients assisted by the AC may have benefited from the pharmacotherapy review performed by pharmacists targeting global adherence to drug therapy. Frequent appointments and specific approaches to avoid irregular use of prescribed drugs are important to reduce warfarin-drug interactions with potential interference with INR management. Several authors have noted the relevance of multidisciplinary care in OA [29,31,33,34]. Interactions among different professionals—physicians, pharmacists, and nurses—may be synergistic and contribute to patient education and to better decision-making. Wiley et al. (2003) [33] pointed out that pharmacists contributed to INR control, greater patient knowledge of OA, and reduction in major bleeding and hospitalizations or emergency room visits due to warfarin complications. AC care may also result in high levels of patient satisfaction [20,34].
The strengths of our study include patient randomization and control for treatment selection effects, which represent the gold standard for evaluating the efficacy and safety of therapeutic interventions. The choice of a trial design in which both groups received care at an AC in the second phase of follow-up was supported by previous studies that have demonstrated the superiority of specialized anticoagulation services over UMC to improve the quality of care [15,17,20,21,22,23]. The design allowed for double comparisons between independent groups and within the same group both before and after the intervention, demonstrating a similar magnitude of absolute TTR augmentation (~5%). Information on INR values, bleeding and thromboembolic events, and overall health status was collected in detail. The procedures for data collection, patient education, and anticoagulation management were standardized and relied on well-trained researchers on data collection and OA care. Therefore, we could evaluate the intervention in a real-world setting, using the scientific method to consistently compare the results of AC and UMC, as well as to provide useful information for healthcare providers.
Some limitations of this study should be addressed. First, investigators and participants were not blinded to treatment conditions, as all groups were aware of the intervention. Consequently, patients randomized to UMC could be more attentive, taking their medications correctly and not forgetting to perform blood sampling, and physicians aware of the ongoing trial could be more vigilant about managing anticoagulation than they were before the study initiation, perhaps reducing the magnitude of the intervention effect. Second, excessive patient monitoring for signs of bleeding in the initial phase of AC implementation, as well as patient over-reporting of minor bleeding, may have caused an information bias and affected data collection on secondary outcomes. Finally, our study was underpowered to detect differences between groups in major bleeding and thromboembolic events, which would be the ideal endpoints to illustrate the impact of AC on the safety of warfarin therapy.
This study was carried out in an era of direct oral anticoagulants but included patients who have little or no chance to use these therapeutic agents due to contraindications (heart valve diseases) or low socioeconomic status. In addition, this study population is characterized by ethnic diversity and low education level, as illustrated by the 81% of patients with an elementary school education or less, as well as a high proportion of Chagas disease patients (30%), who generally present with a lower literacy level than the overall population [35]. The assistance provided by AC worked even in a distinct population of those evaluated by European and North American studies that have demonstrated the effectiveness and safety of AC care.
The implementation of AC may be feasible in healthcare organizations. However, the effect of the anticoagulation service did not improve anticoagulation care in a multi-site randomized trial performed in the United States. The effect was limited by the utilization of the service, the degree of physician adherence to anticoagulation protocols, and the ability of AC to identify and respond to out-of-range INR values promptly [36]. We could assume that our results are potentially generalizable to the Brazilian population that has its OA management performed primarily by general physicians. The use of resources widely accessible in the country, such as INR sampling, warfarin, pharmacists, and physicians, may facilitate the implementation of ACs. A prospective cohort study performed at an AC belonging to the Brazilian Public Health System demonstrated that non-valvular AF patients with a few years of formal education (4.37 ± 3.2 years) achieved a TTR > 62.0% during warfarin therapy [18]. Low-income countries have important budget concerns involving the population supply of nutrients, education, access to drinking water, and other programs for health promotion. The role of AC as a cost-effective strategy for OA management and improvement in patient care deserves further investigations in these populations.
In conclusion, the establishment of AC as supportive specialized care demonstrated improved TTR and may have the potential to reduce the frequency of warfarin-related complications in patients of low socioeconomic status. These findings may be applied to other healthcare settings in Latin America, where the population primarily consists of people of low education levels. The extension of AC care to candidate populations from those countries may help reduce hospitalizations and deaths in warfarin patients.
Author Contributions
Conceptualization, M.A.P.M., D.D.R., C.C.C., M.O.d.C.R. and A.L.P.R.; methodology, M.A.P.M., J.A.d.Q.O., D.D.R., C.C.C., M.O.d.C.R. and A.L.P.R.; software, J.A.d.Q.O., D.D.R. and C.C.C.; validation, M.A.P.M., J.A.d.Q.O., D.D.R. and A.L.P.R.; formal analysis, J.A.d.Q.O. and C.C.C.; investigation, M.A.P.M., J.A.d.Q.O., D.D.R., D.M.F.P., M.O.d.C.R. and A.L.P.R.; resources, M.A.P.M., D.D.R., M.O.d.C.R. and A.L.P.R.; data curation, M.A.P.M., J.A.d.Q.O. and D.D.R.; writing—original draft preparation, M.A.P.M. and J.A.d.Q.O.; writing—review and editing, M.A.P.M., J.A.d.Q.O., D.D.R., C.C.C., V.A.N., D.M.F.P., M.O.d.C.R. and A.L.P.R.; visualization, M.A.P.M., J.A.d.Q.O., D.D.R., C.C.C., V.A.N., D.M.F.P., M.O.d.C.R. and A.L.P.R.; supervision, C.C.C., M.O.d.C.R. and A.L.P.R.; project administration, M.A.P.M., J.A.d.Q.O., D.D.R., M.O.d.C.R. and A.L.P.R.; funding acquisition, M.A.P.M., M.O.d.C.R. and A.L.P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Coordination for the Improvement of Higher Education Personnel (CAPES) grant number [001].
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Universidade Federal de Minas Gerais under the code ETIC 376/09 (Nov/2009).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
This study was supported by the Programa de Pós-Graduação em Ciências da Saúde: Infectologia e Medicina Tropical da Universidade Federal de Minas Gerais and the Pró-Reitoria de Pesquisa da Universidade Federal de Minas Gerais. A.L.P.R., M.O.d.C.R., V.A.N., and C.C.C. are fellows of the National Council for Scientific and Technological Development. A.L.P.R. is a member of the National Institute of Science and Technology for Health Technology. A.L.P.R. is supported in part by CNPq (310790/2021-2 and 465518/2014-1) and by FAPEMIG (PPM-00428-17 and RED-00081-16).
Conflicts of Interest
The authors declare no conflict of interest.
References
- You, J.J.; Singer, D.E.; Howard, P.A.; Lane, D.A.; Eckman, M.H.; Fang, M.C.; Hylek, E.M.; Schulman, S.; Go, A.S.; Hughes, M.; et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e531S–e575S. [Google Scholar] [CrossRef]
- Whitlock, R.P.; Sun, J.C.; Fremes, S.E.; Rubens, F.D.; Teoh, K.H. Antithrombotic and Thrombolytic Therapy for Valvular Disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e576S–e600S. [Google Scholar] [CrossRef] [PubMed]
- Carod-Artal, F.J.; Gascon, J. Chagas disease and stroke. Lancet Neurol. 2010, 9, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Mourão, A.D.O.M.; Gomes, K.B.; Reis, E.A.; Souza, R.P.; Campos, E.I.D.F.; Ribeiro, D.D.; Rocha, M.O.D.C.; Martins, M.A.P. Algorithm for predicting low maintenance doses of warfarin using age and polymorphisms in genes CYP2C9 and VKORC1 in Brazilian subjects. Pharmacogenom. J. 2020, 20, 104–113. [Google Scholar] [CrossRef]
- Moustafa, F.; Dopeux, L.; Mulliez, A.; Boirie, Y.; Morand, C.; Gentes, E.; Farigon, N.; Richard, D.; Lebreton, A.; Teissandier, D.; et al. Severe undernutrition increases bleeding risk on vitamin-K antagonists. Clin. Nutr. 2021, 40, 2237–2243. [Google Scholar] [CrossRef]
- Vianna, M.S.; Praxedes, M.F.D.S.; de Araújo, V.E.; Ferreira, C.B.; de Sousa, W.J.F.N.; Viana, C.C.; Martins, M.A.P. Self-report instruments for assessing adherence to warfarin therapy: A systematic review. Eur. J. Clin. Pharmacol. 2021, 77, 1765–1781. [Google Scholar] [CrossRef]
- Martins, M.A.P.; Carlos, P.P.S.; Ribeiro, D.D.; Nobre, V.A.; César, C.C.; Rocha, M.O.C.; Ribeiro, A.L.P. Warfarin drug interactions: A comparative evaluation of the lists provided by five information sources. Eur. J. Clin. Pharmacol. 2011, 67, 1301–1308. [Google Scholar] [CrossRef]
- Ageno, W.; Gallus, A.S.; Wittkowsky, A.; Crowther, M.; Hylek, E.M.; Palareti, G. Oral Anticoagulant Therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e44S–e88S. [Google Scholar] [CrossRef] [PubMed]
- Oake, N.; Jennings, A.; Forster, A.J.; Fergusson, D.; Doucette, S.; van Walraven, C. Anticoagulation intensity and outcomes among patients prescribed oral anticoagulant therapy: A systematic review and meta-analysis. CMAJ 2008, 179, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Andreotti, F.; Fauchier, L.; Huber, K.; Hylek, E.; Knight, E.; Lane, D.; Levi, M.; Marín, F.; Palareti, G.; et al. Bleeding risk assessment and management in atrial fibrillation patients. Executive Summary of a Position Document from the European Heart Rhythm Association (EHRA), endorsed by the European Society of Cardiology (ESC) Working Group on Thrombosis. Thromb. Haemost. 2011, 106, 997–1011. [Google Scholar]
- Connolly, S.J.; Pogue, J.; Eikelboom, J.; Flaker, G.; Commerford, P.; Franzosi, M.G.; Healey, J.S.; Yusuf, S. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation 2008, 118, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- de Lima Silva, R.G.; Bertollo, C.M.; Ferreira, I.G.; Brant, L.C.; Martins, M.A.P. Assessment of oral anticoaguation control at two pharmacist managed clinics in Brazil. Int. J. Clin. Pharm. 2017, 39, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Nieuwlaat, R.; Connolly, B.J.; Hubers, L.M.; Cuddy, S.M.; Eikelboom, J.W.; Yusuf, S.; Connolly, S.J. Quality of individual INR control and the risk of stroke and bleeding events in atrial fibrillation patients: A nested case control analysis of the ACTIVE W study. Thromb. Res. 2012, 129, 715–719. [Google Scholar] [CrossRef]
- White, H.D.; Gruber, M.; Feyzi, J.; Kaatz, S.; Tse, H.F.; Husted, S.; Albers, G.W. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: Results from SPORTIF III and V. Arch. Intern Med. 2007, 167, 239–245. [Google Scholar] [CrossRef] [PubMed]
- van Walraven, C.; Jennings, A.; Oake, N.; Fergusson, D.; Forster, A.J. Effect of study setting on anticoagulation control: A systematic review and metaregression. Chest 2006, 129, 1155–1166. [Google Scholar] [CrossRef]
- Wofford, J.L.; Wells, M.D.; Singh, S. Best strategies for patient education about anticoagulation with warfarin: A systematic review. BMC Health Serv. Res. 2008, 8, 40. [Google Scholar] [CrossRef]
- Baker, W.L.; Cios, D.A.; Sander, S.D.; Coleman, C.I. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the United States. J. Manag. Care Pharm. 2009, 15, 244–252. [Google Scholar] [CrossRef]
- Costa, G.L.d.B.; Ferreira, D.C.; Valacio, R.A.; Moreira, M.d.C.V. Quality of management of oral anticoagulation as assessed by time in therapeutic INR range in elderly and younger patients with low mean years of formal education: A prospective cohort study. Age Ageing 2011, 40, 375–381. [Google Scholar] [CrossRef]
- Navgren, M.; Forsblad, J.; Wieloch, M. Bleeding complications related to warfarin treatment: A descriptive register study from the anticoagulation clinic at Helsingborg Hospital. J. Thromb. Thrombolysis 2014, 38, 98–104. [Google Scholar] [CrossRef]
- Chiquette, E.; Amato, M.G.; Bussey, H.I. Comparison of an anticoagulation clinic with usual medical care: Anticoagulation control, patient outcomes, and health care costs. Arch. Intern Med. 1998, 158, 1641–1647. [Google Scholar] [CrossRef]
- Wilson, S.J.-A.; Wells, P.S.; Kovacs, M.J.; Lewis, G.M.; Martin, J.; Burton, E.; Anderson, D.R. Comparing the quality of oral anticoagulant management by anticoagulation clinics and by family physicians: A randomized controlled trial. CMAJ 2003, 169, 293–298. [Google Scholar]
- Nichol, M.B.; Knight, T.K.; Dow, T.; Wygant, G.; Borok, G.; Hauch, O.; O’Connor, R. Quality of anticoagulation monitoring in nonvalvular atrial fibrillation patients: Comparison of anticoagulation clinic versus usual care. Ann. Pharmacother. 2008, 42, 62–70. [Google Scholar] [CrossRef]
- Young, S.; Bishop, L.; Twells, L.; Dillon, C.; Hawboldt, J.; O’Shea, P. Comparison of pharmacist managed anticoagulation with usual medical care in a family medicine clinic. BMC Fam. Pract. 2011, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Nomenclature and Criteria for Diagnosis of Ischemic Heart Disease. Report of the Joint International Society and Federation of Cardiology/World Health Organization Task Force on Standardization of Clinical Nomenclature. Circulation 1979, 59, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.A.P.; Ribeiro, D.D.; Nobre, V.A.; Pereira, F.R.; César, C.C.; Rocha, M.O.C.; Ribeiro, A.L.P. Agreement among four drug information sources for the occurrence of warfarin drug interactions in Brazilian heart disease patients with a high prevalence of Trypanosoma cruzi infection. Eur. J. Clin. Pharmacol. 2013, 69, 919–928. [Google Scholar] [CrossRef]
- Garcia, D.A.; Witt, D.M.; Hylek, E.; Wittkowsky, A.K.; Nutescu, E.A.; Jacobson, A.; Moll, S.; Merli, G.J.; Crowther, M.; Earl, L.; et al. Delivery of optimized anticoagulant therapy: Consensus statement from the Anticoagulation Forum. Ann. Pharmacother. 2008, 42, 979–988. [Google Scholar] [CrossRef]
- Ansell, J.; Hirsh, J.; Hylek, E.; Jacobson, A.; Crowther, M.; Palareti, G. Pharmacology and Management of the Vitamin K Antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008, 133, 160S–198S. [Google Scholar] [CrossRef]
- Rosendaal, F.R.; Cannegieter, S.C.; van der Meer, F.J.M.; Briët, E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb. Haemost. 1993, 69, 236–239. [Google Scholar] [CrossRef]
- Abdelhafiz, A.H.; Wheeldon, N.M. Results of an open-label, prospective study of anticoagulant therapy for atrial fibrillation in an outpatient anticoagulation clinic. Clin. Ther. 2004, 26, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Holm, T.; Deutch, S.; Lassen, J.F.; Jastrup, B.; Husted, S.E.; Heickendorff, L. Prospective evaluation of the quality of oral anticoagulation management in an outpatient clinic and in general practices. Thromb. Res. 2002, 105, 103–108. [Google Scholar] [CrossRef]
- Menzin, J.; Boulanger, L.; Hauch, O.; Friedman, M.; Marple, C.B.; Wygant, G.; Hurley, J.S.; Pezzella, S.; Kaatz, S. Quality of anticoagulation control and costs of monitoring warfarin therapy among patients with atrial fibrillation in clinic settings: A multi-site managed-care study. Ann. Pharmacother. 2005, 39, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Heneghan, C.; Perera, R.; Roberts, N.; Hollowell, J.; Glasziou, P.; Bankhead, C.; Xu, Y. Anticoagulation control and prediction of adverse events in patients with atrial fibrillation: A systematic review. Circ. Cardiovasc. Qual. Outcomes 2008, 1, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Willey, M.L.; Chagan, L.; Sisca, T.S.; Chapple, K.J.; Callahan, A.K.; Crain, J.L.; Kitenko, L.E.; Martin, T.; Spedden, K.D. A pharmacist-managed anticoagulation clinic: Six-year assessment of patient outcomes. Am. J. Health Syst. Pharm. 2003, 60, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.W.H.; Wong, R.S.M.; Lau, W.-H.; Chan, T.Y.K.; Cheng, G.; You, J.H.S. Management of Chinese patients on warfarin therapy in two models of anticoagulation service—A prospective randomized trial. Br. J. Clin. Pharmacol. 2006, 62, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.C. Globalization, inequity and Chagas disease. Cad. Saude Publica 2007, 23 (Suppl. S1), S13–S22. [Google Scholar] [CrossRef]
- Matchar, D.; Samsa, G.P.; Cohen, S.J.; Oddone, E.Z.; Jurgelski, A.E. Improving the Quality of Anticoagulation of Patients with Atrial Fibrillation in Managed Care Organizations: Results of the Managing Anticoagulation Services Trial. Am. J. Med. 2002, 113, 42–51. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).