Abstract
The finding of lymphadenopathy is usually the consequence of a benign infection, although a neoplastic origin must always be excluded. Through a careful anamnesis, physical examination, and serological tests several differential diagnoses are frequently possible. Nevertheless, sometimes an excisional biopsy of superficial lymph nodes is required, which is the best means to reach a definitive diagnosis. More concerns arise when lymphadenopathy is only abdominal/retroperitoneal: percutaneous biopsy is often inconclusive and the excisional node biopsy becomes a surgical procedure, certainly indicated in case of malignancy but avoidable in case of inflammatory diseases. We present the case of a 30-year-old man with a deep iliac lymphadenopathy who was evaluated at the Hematological Unit of Sapienza University of Rome. The enlargement of an iliac lymph node is quite unusual for an infectious disease. Although symptoms such as pain, fever, and chills suggested it was the case, cat-scratch disease was not hypothesized. Radiological investigations did not exclude a malignant disease and a laparoscopic excisional biopsy was scheduled, but the slight improvement of his spontaneous symptoms suggested a careful follow-up. Given the lack of disappearance of lymphadenopathy, the lack of diagnosis, and an ipsilateral superficial (inguinal) lymph node with similar ultrasonographic and radiological features, the patient underwent biopsy, which disclosed a diagnosis of cat-scratch disease, avoiding more invasive surgical procedures.
1. Introduction
Lymphadenopathy refers to lymph nodes that are abnormal in size, consistency, or number. Infectious or neoplastic diseases may be the cause. Lymphadenomegaly may be localized superficially (often palpable) or may involve deep stations (unpalpable). Localization may suggest the primary infectious or neoplastic site []. Deep lymphadenopathy is usually diagnosed after medical checks are performed following related or unrelated symptoms, and often has a neoplastic origin including hematological malignancies []. In most cases, a careful history and physical examination are the focus of the evaluation, but further diagnostic investigations are often required, sometimes including lymph node excisional biopsy [].
Cat-scratch disease is a Bartonella henselae infection that usually presents itself with superficial lymphadenopathy. The diagnosis is usually obtained from taking a history of exposure to cats and from a serologic test (high levels of antibodies to Bartonella). It is the most common cause of superficial localized lymphadenitis in children and adolescents [,,], but it also should be hypothesized in patients with a fever of unknown origin [,], with persistent or chronic (≥3 weeks) lymphadenopathy, and is not to be confused with lymphoma.
2. Case Report
A 30-year-old man underwent a clinical evaluation for right iliac fossa pain, who suffered from fever, chills, and a loss of four kilograms in two weeks. A clinical suspicion of acute appendicitis emerged. Therefore, he undertook a course of therapy with Rifaximin and Ciprofloxacin, but symptoms persisted. Blood tests performed in an external laboratory five months before (as screening before blood donation) suggested no anomalies.
The patient underwent an abdominal ultrasound that did not show acute appendicitis, but a 40 × 28 mm deep hypoechogenic lymphadenomegaly with a homogeneous aspect and sharp margins, associated with perilesional and retrocaecal adipose tissue inhomogeneity (Figure 1). Two enlarged additional inguinal lymph nodes were detected of 18 and 20 mm with reduced hilum, suggesting that the patient was suffering from a lymphoproliferative disease.
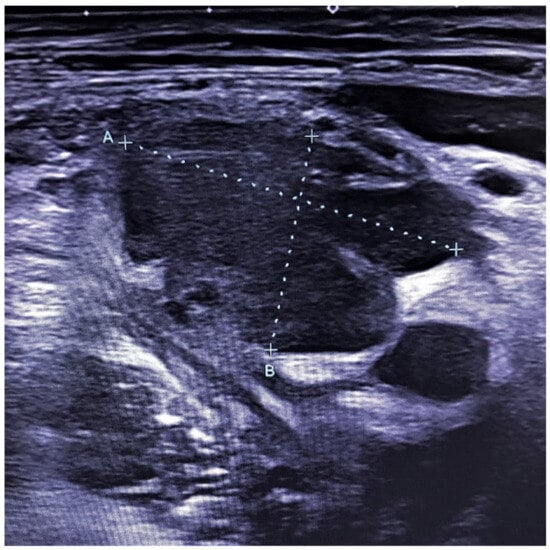
Figure 1.
Ultrasound of the deep right iliac lymphadenopathy (lymph node size: A 40 mm, B 28 mm).
A 64-layer total-body computed tomography scan was performed, after administration of positive oral contrast medium (Gastrografin), as well as before and after intravenous administration of an iodinated contrast agent. The exam showed an axillary lymph node (maximum diameter 5 × 15 mm) with inflammatory aspect, few mesenteric 6–10 mm lymph nodes with reactive nonspecific aspect and confirmed the presence of a 45 × 29 mm iliac hyperaemic lymphadenopathy associated with homolateral inguinal lymphadenopathies (maximum size 5 × 10 mm) (Figure 2).
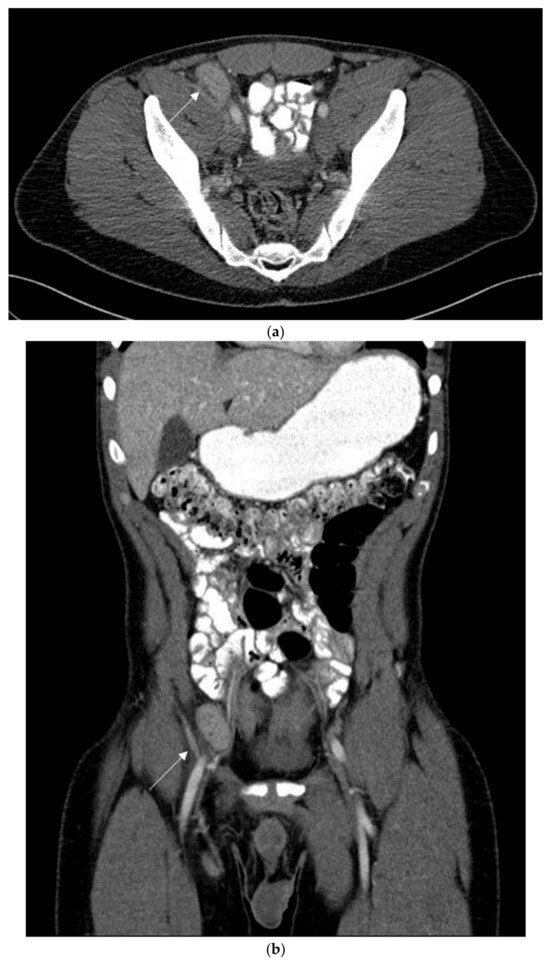
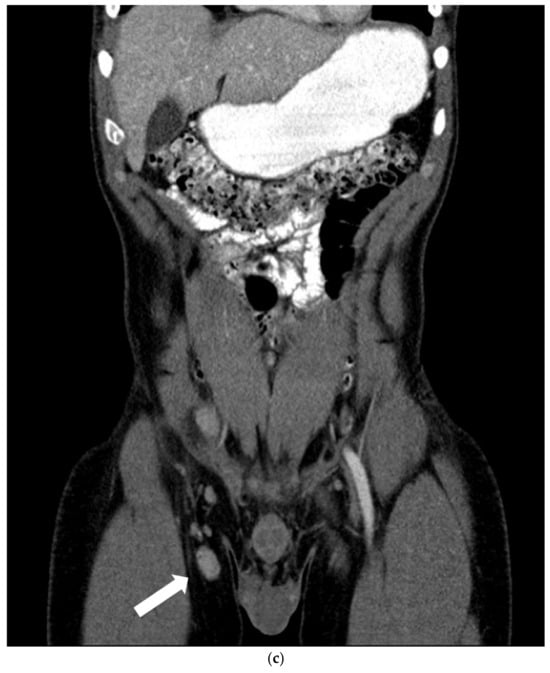
Figure 2.
Contrast-enhanced computed tomography with axial (a) and coronal (b,c) reconstructions in the venous phase. (a,b) The thin arrow shows, both on the axial and coronal image, the hyper vascular right iliac lymphadenopathy. (c) The coronal image shows several right hyper vascular inguinal lymphadenopathies. The larger in size was selected for biopsy (thick arrow).
An abdominal MRI was integrated into the examination. The MRI was performed with 3T equipment, using T1 weighted, T2 weighted, and DWI weighted sequences without intravenous contrast: the iliac and inguinal lymphadenopathies showed high signal at DWI weighted sequences and low ADC signal, features of hypercellular tissues, thus suggesting either an inflammatory or a neoplastic nature (Figure 3). The patient was then referred to a hematological clinical evaluation. The physical examination showed an inguinal palpable lymph node and abdominal pain in the right iliac fossa. Blood tests showed a slight increase of neutrophil granulocytes CRP, fibrinogen, alpha and gamma-globulines, suggesting that inflammation was present.
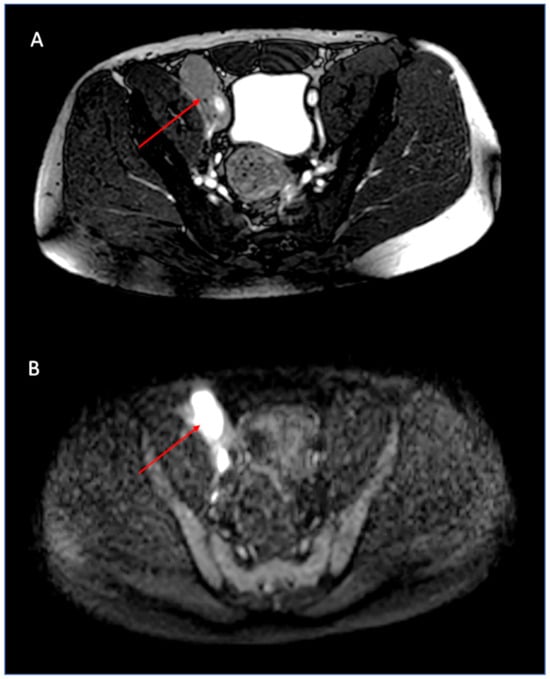
Figure 3.
Magnetic resonance imaging: the iliac lymphadenopathy (thin arrows) is clearly assessed on the axial balanced image (A). Diffusion-weighted imaging (DWI) shows a bright signal in the lymph node, suggesting hypercellularity or inflammation (B).
Given the slight spontaneous improvement of symptoms, and before performing the excisional biopsy of the large and suspected iliac lymph node, a fluorodeoxyglucose (FDG) PET-TC was scheduled and showed tracer accumulation at the level of lymphadenopathies (a standardized uptake value (SUV) of 12.4 for external iliac lymphadenopathies and 8.7 for right inguinal lymphadenopathies), confirming hypercellularity (Figure 4).
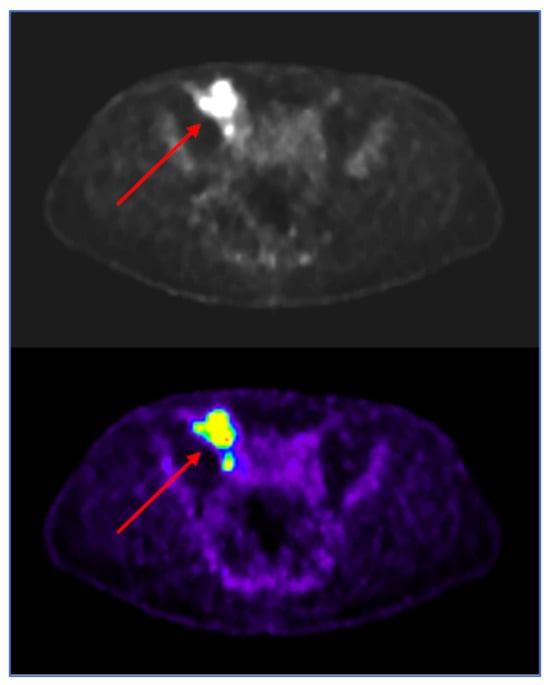
Figure 4.
Fluorodeoxyglucose positron emission tomography (FDG-PET): Hypermetabolic iliac lymph node (red arrows), with high SUV-max (12.4) suggestive of inflammation or hypercellular tissue, in perfect agreement with the MR DWI image (3B).
Considering the presence of inguinal lymph nodes with similar structure and SUV as the iliac lymph node seen on the PET scan, an inguinal lymph node excisional biopsy was then scheduled.
Histology showed lymph node architectural effacement by well-defined, non-confluent granulomas, characterized by a central area of necrosis with neutrophils infiltration surrounded by palisading epithelioid mononuclear cells (Figure 5). There were not crescentic histocytes, clusters of plasmacytoid dendritic cells, or immunoblasts, suggestive of Kikuchi’s Lymphadenitis (immunostaining for CD123, a marker of plasmacytoid dendritic cells, was negative). Plasma cells were scanty. Follicle hyperplasia was also present.
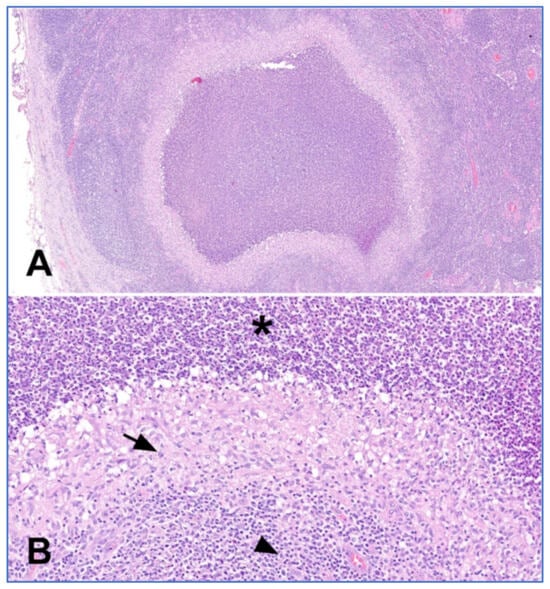
Figure 5.
Histologic features of cat-scratch disease. (A) Suppurative granuloma characterized by a central area of necrosis surrounded by palisading histiocytes and fibroblasts (haematoxylin-eosin, magnification 4×). (B) Higher magnification showing polymorphonuclear leukocytes (asterisk), palisading histiocytes (arrow) and lymphocytes (arrowhead) (haematoxylin-eosin, magnification 20×).
The evidence of suppurative granulomas was consistent with an infectious pathogenesis and cat-scratch disease was suspected. The Warthin–Starry stain, performed to highlight the presence of bacilli, gave negative results.
Cat-scratch disease had not been previously hypothesized. We then performed retrospectively specific serological Bartonella exams: CRP and IgM titer were negative (IgM 0.67, positive if >1.1 antic. index), but IgG titer was positive (IgG 2.81; positive if >1.1 antic. index), as a result of previous infection. Moreover, the patient’s history confirmed his exposure to cats.
Accordingly, the patient started a two-week trimethoprim/sulfamethoxazole course of therapy, and an abdominal ultrasound showed an iliac lymphadenopathy reduction.
3. Discussion and Conclusions
This case report shows an unusual localization of Bartonella infection. In fact, the most common lymph nodes involved are the cervical and axillary lymph nodes, and only a single node is usually involved. It is difficult to hypothesize cat-scratch disease when abdominal/retroperitoneal enlarged lymph nodes are observed, and instrumental diagnostic exams are lacking in specificity to differentiate between malignant and infectious disease, as reported by others [].
Cat-scratch disease should be always considered and history of exposure to domestic animals (e.g., cats) should be assessed. However, even if cat-scratch disease is hypothesized, it is not always easy to gain a definitive diagnosis clinically. Bartonella species are difficult to culture, and culture is not routinely recommended. Serology is the best initial test to assess Bartonella infection, and it can be performed by indirect fluorescent assay or enzyme-linked immunosorbent assay []. Immunoglobulin G titer less than 1:64 suggest the patient does not have a current Bartonella infection. Titers between 1:64 and 1:256 represent possible infection; repeated testing should be performed in these patients in 10 to 14 days. Titers higher than 1:256 strongly suggest an active or recent infection []. A positive immunoglobulin M test suggests acute disease, but the production of immunoglobulin M is brief. Polymerase chain reaction (PCR) can detect different Bartonella species; specificity is very high, but the sensitivity is lower than serology [].
In our patient, the retrospective (post-operative) evaluation of Bartonella IgM/IgG titers and PCR revealed only an increasing of IgG titer and the exposure to cats was confirmed.
However, lymph node biopsy is appropriate in patients in whom diagnosis is uncertain or whose lymph nodes fail to reduce. From a histological point of view, suppurative necrotizing granulomatous lymphadenitis is characteristic of although not specific for cat-scratch disease. A variety of infectious organisms including mycobacteria, fungi, and bacteria (i.e., Chlamydia trachomatis) can show similar findings. Histochemical stains such as Ziehl–Neelsen, Grocott and Warthin–Starry can help to identify bacilli. However, their sensitivity and specificity are low, and clinical–pathological correlations and laboratory confirmation are mandatory. Moreover, the lack of plasma cells infiltrate excluded Chlamydia infection in the present case.
We believe that this case report would be beneficial for clinical practices, inviting them to consider cat-scratch disease in patients with fever, chills, and lymphadenopathy, even if intra-abdominal but limited to few lymph nodes stations, to avoid useless invasive procedures. In this case, the presence of an inguinal lymph node with the same features of the iliac lymph node, allowed to surgeons avoid more invasive procedures like a laparoscopic retroperitoneal excisional biopsy.
Author Contributions
Conceptualization, S.T. and V.F.; methodology, S.T. and M.B.; validation, R.C.; formal analysis, S.T. and R.C.; investigation, F.M., C.G., F.A. and L.B.; resources, M.Z. and P.S.; data curation, F.M. and C.G.; writing—original draft preparation, V.F. and S.T.; writing—review and editing, R.C.; supervision, S.T. and R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and Ethical review and approval were not applicable for this study, due to it is a single case report, no protocol was used.
Informed Consent Statement
Informed consent was obtained from the subject.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy issues.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the Institutional Review Board Statement. This change does not affect the scientific content of the article.
References
- Klotz, S.A.; Ianas, V.; Elliott, S.P. Cat-scratch Disease. Am. Fam. Physician 2011, 83, 152–155. [Google Scholar] [PubMed]
- Avvisati, G. Ematologia di Mandelli; Piccin: Padova, Italy, 2019. [Google Scholar]
- Ferrer, R. Lymphadenopathy: Differential diagnosis and evaluation. Am. Fam. Physician 1998, 58, 1313–1320. [Google Scholar] [PubMed]
- Margileth, A.M. Recent Advances in Diagnosis and Treatment of Cat Scratch Disease. Curr. Infect. Dis. Rep. 2000, 2, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Barr, Y.R.; Qiu, S. A 16-Year-Old Adolescent Boy with Unilateral Cervical Lymphadenopathy Suspicious for Malignancy. Arch. Pathol. Lab. Med. 2005, 129, 1065–1066. [Google Scholar] [CrossRef] [PubMed]
- Landes, M.; Maor, Y.; Mercer, D.; Habot-Wilner, Z.; Bilavsky, E.; Chazan, B.; Cohen, R.; Glikman, D.; Strahilevitz, J.; Katzir, M.; et al. Cat Scratch Disease Presenting as Fever of Unknown Origin Is a Unique Clinical Syndrome. Clin. Infect. Dis. 2020, 11, 2818–2824. [Google Scholar] [CrossRef] [PubMed]
- Spach, D.H.; Kaplan, S.L. Microbiology, Epidemiology, Clinical Manifestations, and Diagnosis of Cat Scratch Disease. Available online: http://www.uptodate.com (accessed on 20 September 2010).
- Imperiale, A.; Blondet, C.; Ben-Sellem, D.; Forestier, E.; Mohseni, M.; Piemont, Y.; Ojeda, M.; Christmann, D.; Constantinesco, A.; Hansmann, Y. Unusual Abdominal Localization of Cat Scratch Disease Mimicking Malignancy on F-18 FDG PET/CT Examination. Clin. Nucl. Med. 2008, 33, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Bergmans, A.M.; Peeters, M.F.; Schellekens, J.F.; Vos, M.C.; Sabbe, L.J.; Ossewaarde, J.M.; Verbakel, H.; Hooft, H.J.; Schouls, L.M. Pitfalls and fallacies of cat scratch disease serology: Evaluation of Bartonella henselae-based indirect fluorescence assay and enzyme-linked immunoassay. J. Clin. Microbiol. 1997, 35, 1931–1937. [Google Scholar] [CrossRef] [PubMed]
- Sander, A.; Posselt, M.; Oberle, K.; Bredt, W. Seroprevalence of antibodies to Bartonella henselae in patients with cat scratch disease and in healthy controls: Evaluation and comparison of two commercial serological tests. Clin. Diagn. Lab. Immunol. 1998, 5, 486–490. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).