Transcriptional Regulation by the NFAT Family in Acute Myeloid Leukaemia
Abstract
1. Introduction
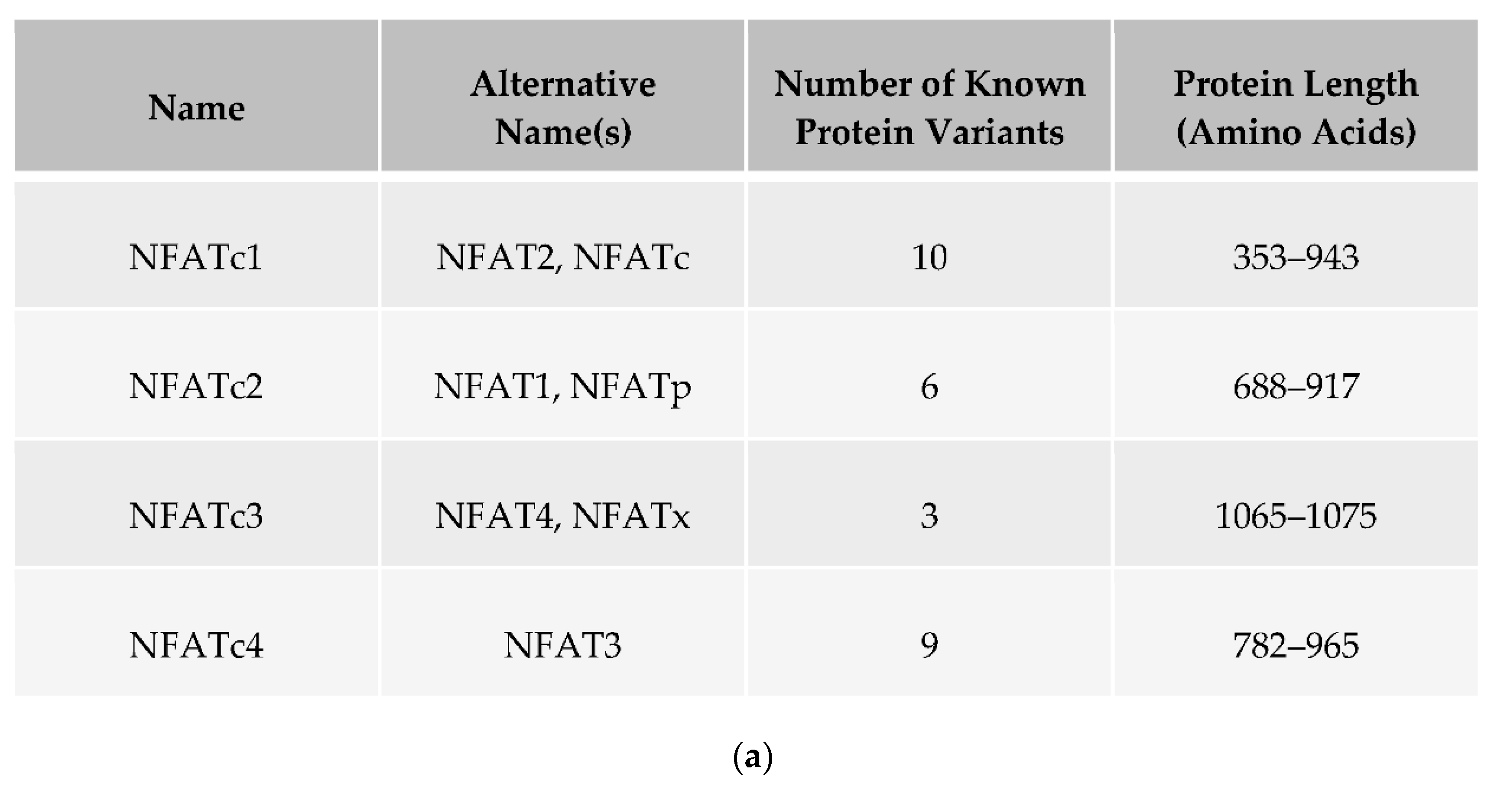
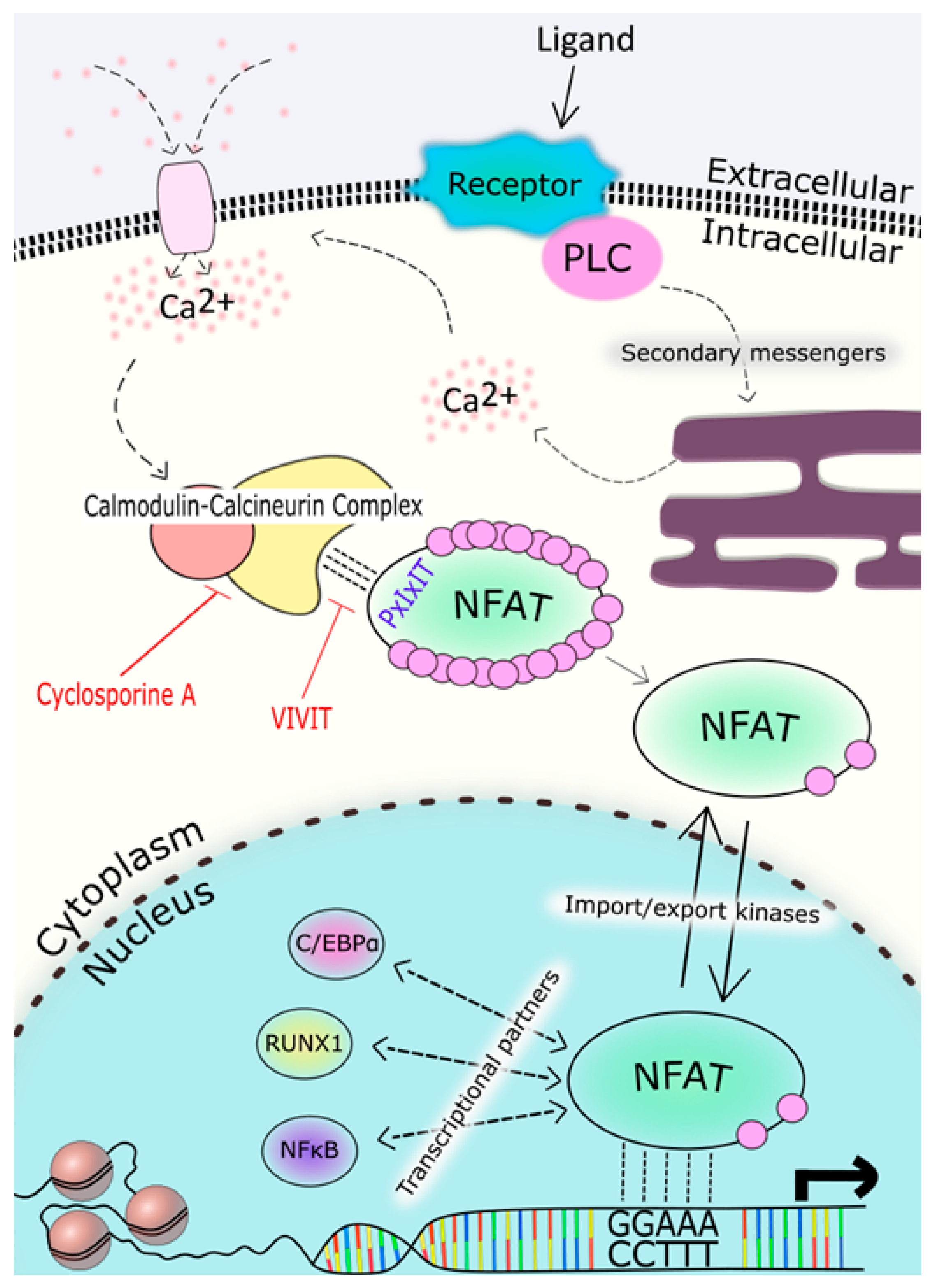
2. NFAT Proteins: Structure, Function and Regulation
3. NFAT Expression in the Myeloid Lineage
4. The Role of NFAT in Myeloid Cells
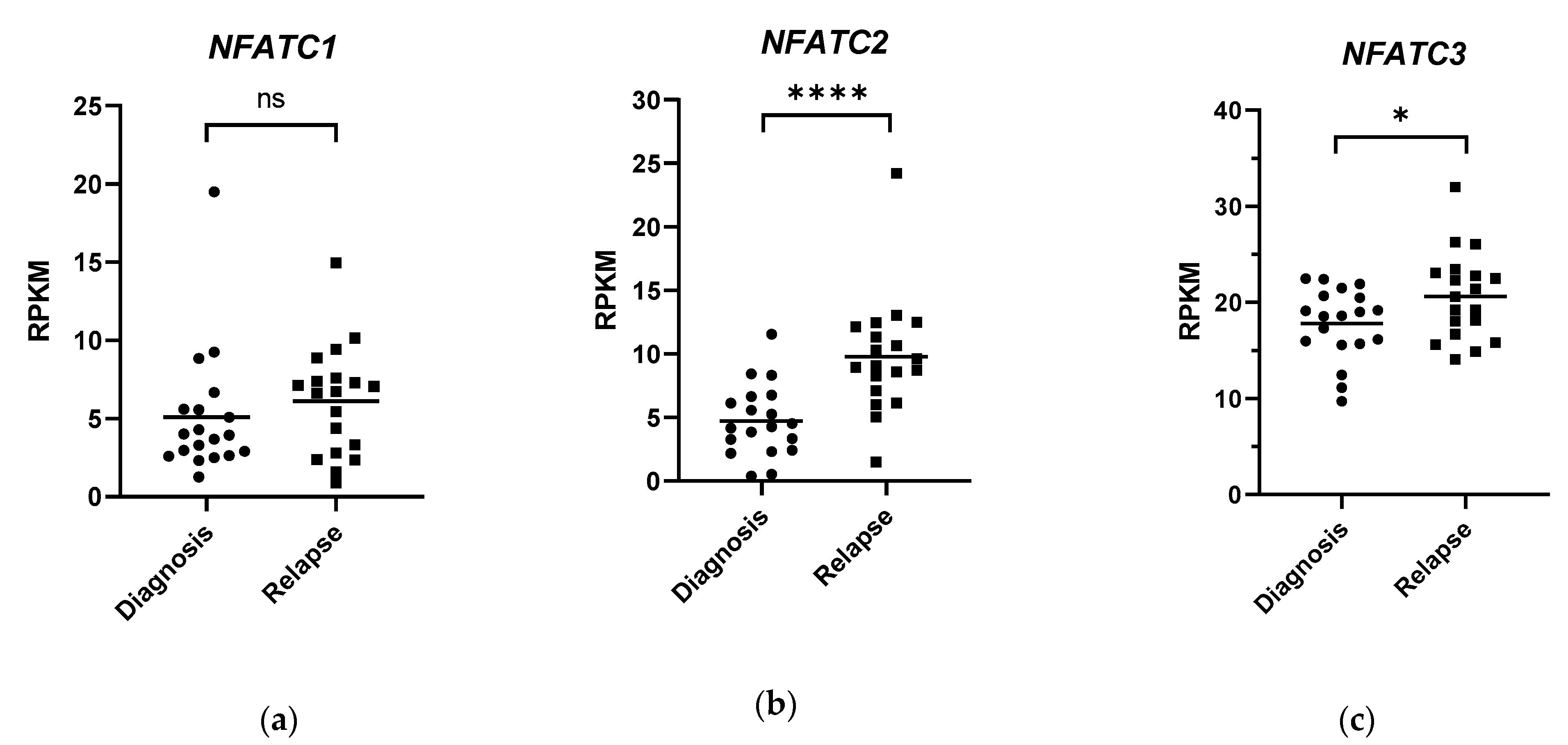
5. NFAT Signalling in AML
6. Therapeutic Targeting of NFAT Proteins
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. FAB Classification of AML
| FAB Classification | Description |
| M0 | Minimally differentiated AML |
| M1 | Myeloid leukaemia (without maturation) |
| M2 | Myeloid leukaemia (with maturation) |
| M3 | Acute progranulocytic leukaemia |
| M4 | Myelomonocytic leukaemia |
| M5 | Monocytic leukaemia |
| M6 | Erythroid leukaemia |
| M7 | Megakaryocytic leukaemia |
| Table showing the description of each FAB category [43]. | |
Abbreviations
| APL | Acute Promyelocytic Leukaemia |
| AML | Acute Myeloid Leukaemia |
| CAMK | Calmodulin Kinase |
| CML | Chronic Myeloid Leukaemia |
| CNBR | Calcineurin-Binding Region |
| CsA | Cyclosporine A |
| DC | Dendritic Cell |
| FAB Classification | French–American–British Classification |
| FLT3 | Fms-related tyrosine kinase receptor 3 |
| FLT3ITD | FLT3 Internal Tandem Duplication |
| FLT3-L | FLT3 Ligand |
| G-CSF | Granulocyte Colony-Stimulating Factor |
| GMP | Granulocyte-Monocyte Progenitor |
| HSC | Hematopoietic Stem Cell |
| LSC | Leukemic Stem Cell |
| M-CSF | Macrophage Colony-Stimulating Factor |
| NFAT | Nuclear Factor of Activated T Cells |
| NHD | NFAT Homology Domain |
| Pgp | P-glycoprotein |
| PLC | Phospholipase C |
| PML | Promyelocytic Leukaemia |
| PRR | Pattern Recognition Receptor |
| SOCE | Store-Operated Calcium Entry |
| TAD | Transactivation Domain |
| TKI | Tyrosine Kinase Inhibitor |
References
- Network, H.M.R. Survival: Acute Myeloid Leukaemia. Available online: https://www.hmrn.org/statistics/survival (accessed on 16 January 2021).
- De Kouchkovsky, I.; Abdul-Hay, M. ‘Acute myeloid leukemia: A comprehensive review and 2016 update’. Blood Cancer J. 2016, 6, e441. [Google Scholar] [CrossRef]
- Kantarjian, H.; Kadia, T.; DiNardo, C.; Daver, N.; Borthakur, G.; Jabbour, E.; Garcia-Manero, G.; Konopleva, M.; Ravandi, F. Acute myeloid leukemia: Current progress and future directions. Blood Cancer J. 2021, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Wang, F.; Jahn, K.; Hu, T.; Tanaka, T.; Sasaki, Y.; Kuipers, J.; Loghavi, S.; Wang, S.A.; Yan, Y.; et al. Clonal evolution of acute myeloid leukemia revealed by high-throughput single-cell genomics. Nat. Commun. 2020, 11, 5327. [Google Scholar] [CrossRef]
- Tyner, J.W.; Tognon, C.E.; Bottomly, D.; Wilmot, B.; Kurtz, S.E.; Savage, S.L.; Long, N.; Schultz, A.R.; Traer, E.; Abel, M.; et al. Functional genomic landscape of acute myeloid leukaemia. Nature 2018, 562, 526–531. [Google Scholar] [CrossRef]
- Ishikawa, F.; Yoshida, S.; Saito, Y.; Hijikata, A.; Kitamura, H.; Tanaka, S.; Nakamura, R.; Tanaka, T.; Tomiyama, H.; Saito, N.; et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat. Biotechnol. 2007, 25, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Pollyea, D.A.; Jordan, C.T. Therapeutic targeting of acute myeloid leukemia stem cells. Blood 2017, 129, 1627. [Google Scholar] [CrossRef]
- Jordan, C.T. The leukemic stem cell. Best Prac. Res. Clin. Haematol. 2007, 20, 13–18. [Google Scholar] [CrossRef]
- Docking, T.R.; Parker, J.D.K.; Jädersten, M.; Duns, G.; Chang, L.; Jiang, J.; Pilsworth, J.A.; Swanson, L.A.; Chan, S.K.; Chiu, R.; et al. A clinical transcriptome approach to patient stratification and therapy selection in acute myeloid leukemia. Nat. Commun. 2021, 12, 2474. [Google Scholar] [CrossRef]
- Ng, S.W.K.; Mitchell, A.; Kennedy, J.A.; Chen, W.C.; McLeod, J.; Ibrahimova, N.; Arruda, A.; Popescu, A.; Gupta, V.; Schimmer, A.D.; et al. A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature 2016, 540, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Massett, M.E.; Monaghan, L.; Patterson, S.; Mannion, N.; Bunschoten, R.P.; Hoose, A.; Marmiroli, S.; Liskamp, R.M.J.; Jørgensen, H.G.; Vetrie, D.; et al. A KDM4A-PAF1-mediated epigenomic network is essential for acute myeloid leukemia cell self-renewal and survival. Cell Death Dis. 2021, 12, 573. [Google Scholar] [CrossRef]
- Gentles, A.J.; Plevritis, S.K.; Majeti, R.; Alizadeh, A.A. Association of a leukemic stem cell gene expression signature with clinical outcomes in acute myeloid leukemia. JAMA 2010, 304, 2706–2715. [Google Scholar] [CrossRef]
- Darnell, J.E. Transcription factors as targets for cancer therapy. Nat. Rev. Cancer 2002, 2, 740–749. [Google Scholar] [CrossRef]
- Takei, H.; Kobayashi, S.S. Targeting transcription factors in acute myeloid leukemia. Int. J. Hematol. 2019, 109, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Metzelder, S.K.; Michel, C.; von Bonin, M.; Rehberger, M.; Hessmann, E.; Inselmann, S.; Solovey, M.; Wang, Y.; Sohlbach, K.; Brendel, C.; et al. NFATc1 as a therapeutic target in FLT3-ITD-positive AML. Leukemia 2015, 29, 1470. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.A.; Phang, T.L.; Neviani, P.; Alvarez-Calderon, F.; Eide, C.A.; O’Hare, T.; Zaberezhnyy, V.; Williams, R.T.; Druker, B.J.; Perrotti, D.; et al. Wnt/Ca2+/NFAT signaling maintains survival of Ph+ leukemia cells upon inhibition of Bcr-Abl. Cancer Cell 2010, 18, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Fric, J.; Zelante, T.; Wong, A.Y.W.; Mertes, A.; Yu, H.-B.; Ricciardi-Castagnoli, P. NFAT control of innate immunity. Blood 2012, 120, 1380. [Google Scholar] [CrossRef]
- Qin, J.-J.; Nag, S.; Wang, W.; Zhou, J.; Zhang, W.-D.; Wang, H.; Zhang, R. NFAT as cancer target: Mission possible? Biochim. Et Biophys. Acta 2014, 1846, 297–311. [Google Scholar] [CrossRef]
- Mancini, M.; Toker, A. NFAT Proteins: Emerging Roles in Cancer Progression. Nat. Rev. Cancer 2009, 9, 810–820. [Google Scholar] [CrossRef]
- Mognol, G.P.; Carneiro, F.R.G.; Robbs, B.K.; Faget, D.V.; Viola, J.P.B. Cell cycle and apoptosis regulation by NFAT transcription factors: New roles for an old player. Cell Death Dis. 2016, 7, e2199. [Google Scholar] [CrossRef]
- Macián, F.; López-Rodríguez, C.; Rao, A. Partners in transcription: NFAT and AP-1. Oncogene 2001, 20, 2476–2489. [Google Scholar] [CrossRef]
- Macian, F. NFAT proteins: Key regulators of T-cell development and function. Nat. Rev. Immunol. 2005, 5, 472. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Luo, C.; Hogan, P.G. TRANSCRIPTION FACTORS OF THE NFAT FAMILY: Regulation and Function. Annu. Rev. Immunol. 1997, 15, 707–747. [Google Scholar] [CrossRef] [PubMed]
- Vihma, H.; Pruunsild, P.; Timmusk, T. Alternative splicing and expression of human and mouse NFAT genes. Genomics 2008, 92, 279–291. [Google Scholar] [CrossRef]
- Graef, I.A.; Gastier, J.M.; Francke, U.; Crabtree, G.R. Evolutionary relationships among Rel domains indicate functional diversification by recombination. Proc. Natl. Acad. Sci. USA 2001, 98, 5740. [Google Scholar] [CrossRef] [PubMed]
- Hogan, P.G.; Chen, L.; Nardone, J.; Rao, A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003, 17, 2205–2232. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, N.; Kaminuma, O. Isoform-Selective NFAT Inhibitor: Potential Usefulness and Development. Int. J. Mol. Sci. 2021, 22, 2725. [Google Scholar] [CrossRef] [PubMed]
- Gwack, Y.; Feske, S.; Srikanth, S.; Hogan, P.G.; Rao, A. Signalling to transcription: Store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium 2007, 42, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Villar, M.; Ortega-Pérez, I.; Were, F.; Cano, E.; Redondo, J.M.; Vázquez, J. Systematic characterization of phosphorylation sites in NFATc2 by linear ion trap mass spectrometry. Proteomics 2006, 6 (Suppl. 1), S16–S27. [Google Scholar] [CrossRef]
- Leung-Theung-Long, S.; Mondor, I.; Guiraud, M.; Lamare, C.; Nageleekar, V.; Paulet, P.-E.; Rincon, M.; Guerder, S. Impaired NFAT Transcriptional Activity in Antigen-Stimulated CD8 T Cells Linked to Defective Phosphorylation of NFAT Transactivation Domain. J. Immunol. 2009, 182, 6807. [Google Scholar] [CrossRef]
- Badran, B.M.; Wolinsky, S.M.; Burny, A.; Willard-Gallo, K.E. Identification of Three NFAT Binding Motifs in the 5′-Upstream Region of the Human CD3γ Gene That Differentially Bind NFATc1, NFATc2, and NF-κB p50. J. Biol. Chem. 2002, 277, 47136–47148. [Google Scholar] [CrossRef]
- Chen, L.; Glover, J.N.M.; Hogan, P.G.; Rao, A.; Harrison, S.C. Structure of the DNA-binding domains from NFAT, Fos and Jun bound specifically to DNA. Nature 1998, 392, 42–48. [Google Scholar] [CrossRef]
- Gabriel, C.H.; Gross, F.; Karl, M.; Stephanowitz, H.; Hennig, A.F.; Weber, M.; Gryzik, S.; Bachmann, I.; Hecklau, K.; Wienands, J.; et al. Identification of Novel Nuclear Factor of Activated T Cell (NFAT)-associated Proteins in T Cells. J. Biol. Chem. 2016, 291, 24172–24187. [Google Scholar] [CrossRef]
- Bierer, B.E.; Holländer, G.; Fruman, D.; Burakoff, S.J. Cyclosporin A and FK506: Molecular mechanisms of immunosuppression and probes for transplantation biology. Curr. Opin. Immunol. 1993, 5, 763–773. [Google Scholar] [CrossRef]
- Li, H.; Rao, A.; Hogan, P.G. Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol. 2011, 21, 91–103. [Google Scholar] [CrossRef]
- Aramburu, J.; Yaffe, M.B.; López-Rodrıíguez, C.; Cantley, L.C.; Hogan, P.G.; Rao, A. Affinity-Driven Peptide Selection of an NFAT Inhibitor More Selective Than Cyclosporin A. Science 1999, 285, 2129. [Google Scholar] [CrossRef]
- Kiani, A.; Habermann, I.; Haase, M.; Feldmann, S.; Boxberger, S.; Sanchez-Fernandez, M.A.; Thiede, C.; Bornhäuser, M.; Ehninger, G. Expression and regulation of NFAT (nuclear factors of activated T cells) in human CD34+ cells: Down-regulation upon myeloid differentiation. J. Leukoc. Biol. 2004, 76, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.; Kuithan, H.; Kuithan, F.; Kyttälä, S.; Habermann, I.; Temme, A.; Bornhäuser, M.; Ehninger, G. Expression analysis of nuclear factor of activated T cells (NFAT) during myeloid differentiation of CD34+ cells: Regulation of Fas ligand gene expression in megakaryocytes. Exp. Hematol. 2007, 35, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Pabst, C.; Bergeron, A.; Lavallée, V.-P.; Yeh, J.; Gendron, P.; Norddahl, G.L.; Krosl, J.; Boivin, I.; Deneault, E.; Simard, J.; et al. GPR56 identifies primary human acute myeloid leukemia cells with high repopulating potential in vivo. Blood 2016, 127, 2018–2027. [Google Scholar] [CrossRef]
- Ley, T.J.; Miller, C.; Ding, L.; Raphael, B.J.; Mungall, A.J.; Robertson, A.; Hoadley, K.; Triche, T.J., Jr.; Laird, P.W.; Baty, J.D.; et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013, 368, 2059–2074. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Olsson, I.; Bergh, G.; Ehinger, M.; Gullberg, U. Cell differentiation in acute myeloid leukemia. Eur. J. Haematol. 1996, 57, 1–16. [Google Scholar] [CrossRef]
- Schiffer, C.A.; Stone, R.M. Morphologic Classification and Clinical and Laboratory Correlates. In Holland-Frei Cancer Medicine, 6th ed.; Kufe, D.W., Pollock, P.E., Weichselbaum, R.R., Bast, R.C., Jr., Gansler, T.S., Holland, J.F., Frei, E., III, Eds.; BC Decker: Hamilton, ON, USA, 2003. [Google Scholar]
- Fric, J.; Lim, C.X.F.; Mertes, A.; Lee, B.T.K.; Viganò, E.; Chen, J.; Zolezzi, F.; Poidinger, M.; Larbi, A.; Strobl, H.; et al. Calcium and calcineurin-NFAT signaling regulate granulocyte-monocyte progenitor cell cycle via Flt3-L. Stem Cells 2014, 32, 3232–3244. [Google Scholar] [CrossRef] [PubMed]
- Fric, J.; Lim, C.X.F.; Koh, E.G.L.; Hofmann, B.; Chen, J.; Tay, H.S.; Mohammad Isa, S.A.B.; Mortellaro, A.; Ruedl, C.; Ricciardi-Castagnoli, P. Calcineurin/NFAT signalling inhibits myeloid haematopoiesis. EMBO Mol. Med. 2012, 4, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.M.; Bincoletto, C.; Barros, C.C.; Ferreira, A.T.; Paredes-Gamero, E.J. PLCγ2 and PKC are important to myeloid lineage commitment triggered by M-SCF and G-CSF. J. Cell Biochem. 2014, 115, 42–51. [Google Scholar] [CrossRef]
- Bendickova, K.; Tidu, F.; Fric, J. Calcineurin-NFAT signalling in myeloid leucocytes: New prospects and pitfalls in immunosuppressive therapy. EMBO Mol. Med. 2017, 9, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Elloumi, H.Z.; Maharshak, N.; Rao, K.N.; Kobayashi, T.; Ryu, H.S.; Mühlbauer, M.; Li, F.; Jobin, C.; Plevy, S.E. A cell permeable peptide inhibitor of NFAT inhibits macrophage cytokine expression and ameliorates experimental colitis. PLoS ONE 2012, 7, e34172. [Google Scholar] [CrossRef]
- Yu, H.-B.; Yurieva, M.; Balachander, A.; Foo, I.; Leong, X.; Zelante, T.; Zolezzi, F.; Poidinger, M.; Ricciardi-Castagnoli, P. NFATc2 mediates epigenetic modification of dendritic cell cytokine and chemokine responses to dectin-1 stimulation. Nucleic Acids Res. 2014, 43, 836–847. [Google Scholar] [CrossRef]
- Monlish, D.A.; Bhatt, S.T.; Schuettpelz, L.G. The Role of Toll-Like Receptors in Hematopoietic Malignancies. Front. Immunol. 2016, 7, 390. [Google Scholar] [CrossRef]
- Rybka, J.; Butrym, A.; Wróbel, T.; Jaźwiec, B.; Stefanko, E.; Dobrzyńska, O.; Poręba, R.; Kuliczkowski, K. The expression of Toll-like receptors in patients with acute myeloid leukemia treated with induction chemotherapy. Leuk. Res. 2015, 39, 318–322. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A.; Chávez-Valencia, V. FLT3–ITD and its current role in acute myeloid leukaemia. Med. Oncol. 2017, 34, 114. [Google Scholar] [CrossRef]
- Chan, P.M. Differential signaling of Flt3 activating mutations in acute myeloid leukemia: A working model. Protein Cell 2011, 2, 108–115. [Google Scholar] [CrossRef]
- Solovey, M.; Wang, Y.; Michel, C.; Metzeler, K.H.; Herold, T.; Göthert, J.R.; Ellenrieder, V.; Hessmann, E.; Gattenlöhner, S.; Neubauer, A.; et al. Nuclear factor of activated T-cells, NFATC1, governs FLT3(ITD)-driven hematopoietic stem cell transformation and a poor prognosis in AML. J. Hematol. Oncol. 2019, 12, 72. [Google Scholar] [CrossRef]
- Kelly, L.M.; Liu, Q.; Kutok, J.L.; Williams, I.R.; Boulton, C.L.; Gilliland, D.G. FLT3 internal tandem duplication mutations associated with human acute myeloid leukemias induce myeloproliferative disease in a murine bone marrow transplant model. Blood 2002, 99, 310. [Google Scholar] [CrossRef]
- Rocnik, J.L.; Okabe, R.; Yu, J.-C.; Lee, B.H.; Giese, N.; Schenkein, D.P.; Gilliland, D.G. Roles of tyrosine 589 and 591 in STAT5 activation and transformation mediated by FLT3-ITD. Blood 2006, 108, 1339–1345. [Google Scholar] [CrossRef]
- Levis, M.; Small, D. FLT3: ITDoes matter in leukemia. Leukemia 2003, 17, 1738. [Google Scholar] [CrossRef]
- Fathi, A.T.; Chen, Y.-B. Treatment of FLT3-ITD acute myeloid leukemia. Am. J. Blood Res. 2011, 1, 175–189. [Google Scholar]
- Valent, P. Imatinib-resistant chronic myeloid leukemia (CML): Current concepts on pathogenesis and new emerging pharmacologic approaches. Biologics 2007, 1, 433–448. [Google Scholar] [PubMed]
- Sung, P.J.; Sugita, M.; Koblish, H.; Perl, A.E.; Carroll, M. Hematopoietic cytokines mediate resistance to targeted therapy in FLT3-ITD acute myeloid leukemia. Blood Adv. 2019, 3, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.C.S.; Radich, J. Predicting Chemotherapy Resistance in AML. Curr. Hematol. Malig. Rep. 2017, 12, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Garrett-Bakelman, F.E.; Chung, S.S.; Sanders, M.A.; Hricik, T.; Rapaport, F.; Patel, J.; Dillon, R.; Vijay, P.; Brown, A.L.; et al. Distinct evolution and dynamics of epigenetic and genetic heterogeneity in acute myeloid leukemia. Nat. Med. 2016, 22, 792–799. [Google Scholar] [CrossRef]
- van Gils, N.; Denkers, F.; Smit, L. Escape From Treatment; the Different Faces of Leukemic Stem Cells and Therapy Resistance in Acute Myeloid Leukemia. Front. Oncol. 2021, 11, 1454. [Google Scholar] [CrossRef] [PubMed]
- Behrens, K.; Maul, K.; Tekin, N.; Kriebitzsch, N.; Indenbirken, D.; Prassolov, V.; Müller, U.; Serve, H.; Cammenga, J.; Stocking, C. RUNX1 cooperates with FLT3-ITD to induce leukemia. J. Exp. Med. 2017, 214, 737–752. [Google Scholar] [CrossRef]
- Sood, R.; Kamikubo, Y.; Liu, P. Role of RUNX1 in hematological malignancies. Blood 2017, 129, 2070–2082. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Kubota, H.; Sakuramoto, N.; Hada, A.; Horiuchi, A.; Sasaki, A.; Takeda, K.; Takeda, M.; Matsuo, H.; Sugiyama, H.; et al. RUNX-NFAT Axis As a Novel Therapeutic Target for AML and T Cell Immunity. Blood 2020, 136, 25–26. [Google Scholar] [CrossRef]
- Lo, Y.H.; Wu, C.C.; Shih, H.M.; Lai, M.Z. Selective activation of NFAT by promyelocytic leukemia protein. Oncogene 2008, 27, 3821. [Google Scholar] [CrossRef]
- Mu, Z.M.; Chin, K.V.; Liu, J.H.; Lozano, G.; Chang, K.S. PML, a growth suppressor disrupted in acute promyelocytic leukemia. Mol. Cell Biol. 1994, 14, 6858–6867. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takahashi, S. Identification of Flt3 internal tandem duplications downstream targets by high-throughput immunoblotting protein array system. Am. J. Hematol. 2006, 81, 717–719. [Google Scholar] [CrossRef]
- Ptasinska, A.; Pickin, A.; Assi, S.A.; Chin, P.S.; Ames, L.; Avellino, R.; Gröschel, S.; Delwel, R.; Cockerill, P.N.; Osborne, C.S.; et al. RUNX1-ETO Depletion in t(8;21) AML Leads to C/EBPα- and AP-1-Mediated Alterations in Enhancer-Promoter Interaction. Cell Rep. 2019, 28, 3022–3031.e3027. [Google Scholar] [CrossRef]
- Paz-Priel, I.; Friedman, A. C/EBPα dysregulation in AML and ALL. Crit. Rev. Oncog. 2011, 16, 93–102. [Google Scholar] [CrossRef]
- Tedesco, D.; Haragsim, L. Cyclosporine: A review. J. Transpl. 2012, 2012, 230386. [Google Scholar] [CrossRef]
- Klintmalm, G. A review of FK506: A new immunosuppressant agent for the prevention and rescue of graft rejection. Transplant. Rev. 1994, 8, 53–63. [Google Scholar] [CrossRef]
- Spiekerkoetter, E.; Sung, Y.K.; Sudheendra, D.; Scott, V.; Del Rosario, P.; Bill, M.; Haddad, F.; Long-Boyle, J.; Hedlin, H.; Zamanian, R.T. Randomised placebo-controlled safety and tolerability trial of FK506 (tacrolimus) for pulmonary arterial hypertension. Eur. Respir. J. 2017, 50, 1602449. [Google Scholar] [CrossRef]
- List, A.F.; Kopecky, K.J.; Willman, C.L.; Head, D.R.; Persons, D.L.; Slovak, M.L.; Dorr, R.; Karanes, C.; Hynes, H.E.; Doroshow, J.H.; et al. Benefit of cyclosporine modulation of drug resistance in patients with poor-risk acute myeloid leukemia: A Southwest Oncology Group study. Blood 2001, 98, 3212–3220. [Google Scholar] [CrossRef]
- Noguchi, H.; Matsushita, M.; Okitsu, T.; Moriwaki, A.; Tomizawa, K.; Kang, S.; Li, S.-T.; Kobayashi, N.; Matsumoto, S.; Tanaka, K.; et al. A new cell-permeable peptide allows successful allogeneic islet transplantation in mice. Nat. Med. 2004, 10, 305–309. [Google Scholar] [CrossRef]
- Yu, H.; Van Berkel, T.J.C.; Biessen, E.A.L. Therapeutic Potential of VIVIT, a Selective Peptide Inhibitor of Nuclear Factor of Activated T cells, in Cardiovascular Disorders. Cardiovasc. Drug Rev. 2007, 25, 175–187. [Google Scholar] [CrossRef]
- Román, J.; de Arriba, A.F.; Barrón, S.; Michelena, P.; Giral, M.; Merlos, M.; Bailón, E.; Comalada, M.; Gálvez, J.; Zarzuelo, A.; et al. UR-1505, a new salicylate, blocks T cell activation through nuclear factor of activated T cells. Mol. Pharm. 2007, 72, 269–279. [Google Scholar] [CrossRef]
- Vives, R.; Pontes, C.; Sarasa, M.; Millier, A. Safety and Activity of UR-1505 in Atopic Dermatitis: A Randomized, Double-blind Phase II Exploratory Trial. Clin. Ther. 2015, 37, 1955–1965. [Google Scholar] [CrossRef]
- Ramadass, V.; Vaiyapuri, T.; Tergaonkar, V. Small Molecule NF-κB Pathway Inhibitors in Clinic. Int. J. Mol. Sci. 2020, 21, 5164. [Google Scholar] [CrossRef]
- Vago, L.; Gojo, I. Immune escape and immunotherapy of acute myeloid leukemia. J. Clin. Investig. 2020, 130, 1552–1564. [Google Scholar] [CrossRef]
- Buggins, A.G.S.; Milojkovic, D.; Arno, M.J.; Lea, N.C.; Mufti, G.J.; Thomas, N.S.B.; Hirst, W.J.R. Microenvironment Produced by Acute Myeloid Leukemia Cells Prevents T Cell Activation and Proliferation by Inhibition of NF-κB, c-Myc, and pRb Pathways. J. Immunol. 2001, 167, 6021–6030. [Google Scholar] [CrossRef]
- van Galen, P.; Hovestadt, V.; Wadsworth Ii, M.H.; Hughes, T.K.; Griffin, G.K.; Battaglia, S.; Verga, J.A.; Stephansky, J.; Pastika, T.J.; Lombardi Story, J.; et al. Single-Cell RNA-Seq Reveals AML Hierarchies Relevant to Disease Progression and Immunity. Cell 2019, 176, 1265–1281.e1224. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patterson, S.D.; Huang, X.; Jørgensen, H.G.; Michie, A.M. Transcriptional Regulation by the NFAT Family in Acute Myeloid Leukaemia. Hemato 2021, 2, 556-571. https://doi.org/10.3390/hemato2030035
Patterson SD, Huang X, Jørgensen HG, Michie AM. Transcriptional Regulation by the NFAT Family in Acute Myeloid Leukaemia. Hemato. 2021; 2(3):556-571. https://doi.org/10.3390/hemato2030035
Chicago/Turabian StylePatterson, Shaun D., Xu Huang, Heather G. Jørgensen, and Alison M. Michie. 2021. "Transcriptional Regulation by the NFAT Family in Acute Myeloid Leukaemia" Hemato 2, no. 3: 556-571. https://doi.org/10.3390/hemato2030035
APA StylePatterson, S. D., Huang, X., Jørgensen, H. G., & Michie, A. M. (2021). Transcriptional Regulation by the NFAT Family in Acute Myeloid Leukaemia. Hemato, 2(3), 556-571. https://doi.org/10.3390/hemato2030035







