Exposure to Sulfur Hexafluoride Influences Viability in Cell Transplant Suspensions
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Origin, and Ethical Assessment
2.2. Human Mesenchymal Stem Cell Isolation, Culture, and Expansion
2.3. Transgenic Porcine Fibroblast Isolation, Culture, and Expansion
2.4. Contrast Agent
2.5. Real-Time Cytotoxicity Assay
2.6. Statistics and Correlation Analysis
3. Results
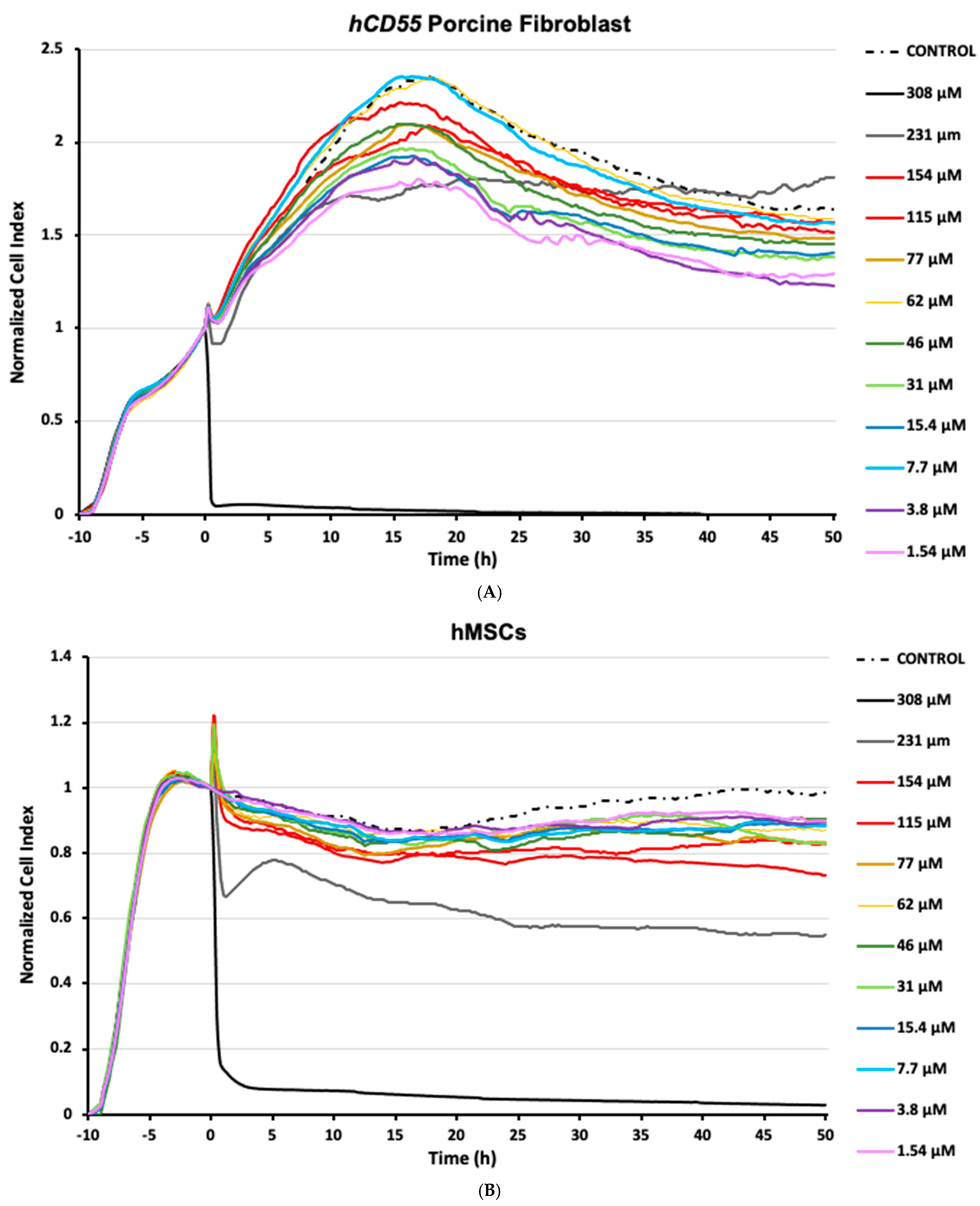
3.1. Human Mesenchymal Stem Cells Exhibit Growth Inhibition Responses to SF6
3.2. Human Mesenchymal Stem Cell Responses Exhibit Dose-Dependent Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porter, T.R.; Xie, F. Myocardial perfusion imaging with contrast ultrasound. JACC Cardiovasc. Imaging 2010, 3, 176–187. [Google Scholar] [CrossRef]
- Soliman, O.I.; De Jong, N.; Van Der Zwaan, H.B.; Galema, T.W.; Vletter, W.B.; Van Dalen, B.M.; Schinkel, A.F.L.; Cate, F.T.; Geleijnse, M.L. Contrast echocardiography: Mechanism of action, safety and clinical applications. Minerva Cardioangiol. 2010, 58, 343–355. [Google Scholar]
- Numata, K.; Luo, W.; Morimoto, M.; Kondo, M.; Kunishi, Y.; Sasaki, T.; Nozaki, A.; Tanaka, K. Contrast enhanced ultrasound of hepatocellular carcinoma. World J. Radiol. 2010, 2, 68–82. [Google Scholar] [CrossRef]
- Piscaglia, F.; Lencioni, R.; Sagrini, E.; Pina, C.D.; Cioni, D.; Vidili, G.; Bolondi, L. Characterization of focal liver lesions with contrast-enhanced ultrasound. Ultrasound Med. Biol. 2010, 36, 531–550. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Burns, P.N. Microbubble-enhanced US in body imaging: What role? Radiology 2010, 257, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Reid, C.N.; McHale, A.P. Enhancing ultrasound-mediated cell membrane permeabilisation (sonoporation) using a high frequency pulse regime and implications for ultrasound-aided cancer chemotherapy. Cancer Lett. 2008, 266, 156–162. [Google Scholar] [PubMed]
- Lin, C.Y.; Tseng, H.C.; Shiu, H.R.; Wu, M.-F.; Chou, C.-Y.; Lin, W.-L. Ultrasound sonication with microbubbles disrupts blood vessels and enhances tumor treatments of anticancer nanodrug. Int. J. Nanomed. 2012, 7, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Xiong, Y.; Li, K.; He, M.; Deng, Y.; Chen, L.; Zou, M.; Chen, W.; Wang, Z.; He, J.; et al. Clinical utility of a microbubble-enhancing contrast (“SonoVue”) in treatment of uterine fibroids with high intensity focused ultrasound: A retrospective study. Eur. J. Radiol. 2012, 81, 3832–3838. [Google Scholar] [CrossRef]
- van den Oord, S.C.; ten Kate, G.L.; Akkus, Z.; Renaud, G.; Sijbrands, E.J.; Ten Cate, F.J.; van der Lugt, A.; Bosch, J.G.; de Jong, N.; van der Steen, A.F.W.; et al. Assessment of subclinical atherosclerosis using contrast-enhanced ultrasound. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 56–61. [Google Scholar] [CrossRef]
- Abellaneda, J.M.; Ramis, G.; Martínez-Alarcón, L.; Majado, M.; Quereda, J.; Herrero-Medrano, J.; Mendonça, L.; García-Nicolás, O.; Reus, M.; Insausti, C.; et al. Generation of human-to-pig chimerism to induce tolerance through transcutaneous in utero injection of cord blood-derived mononuclear cells or human bone marrow mesenchymals cells in a preclinical program of liver xenotransplantation: Preliminary results. Transplant. Proc. 2012, 44, 1574–1578. [Google Scholar] [CrossRef]
- Boletín Oficial del Estado (BOE). Real Decreto 324/2000, de 3 de Marzo, by Establishing Basic Rules Management of Pig Farms. Available online: http://www.boe.es/boe/dias/2000/03/08/pdfs/A09505-09512.pdf (accessed on 14 March 2024).
- Quereda, J.J.; Martínez-Alarcón, L.; Mendoça, L.; Majado, M.; Herrero-Medrano, J.; Pallarés, F.; Ríos, A.; Ramírez, P.; Muñoz, A.; Ramis, G. Validation of xCELLigence Real-Time Cell Analyzer to assess compatibility in xenotransplantation with pig-to-baboon model. Transplant. Proc. 2010, 42, 3239–3243. [Google Scholar] [PubMed]
- Ramis, G.; Martinez-Alarcon, L.; Majado, M.; Quereda, J.J.; Mendonça, L.; Herrero-Medrano, J.; Abellaneda, J.; Coelho, K.; López-Navas, A.; Ríos, A.; et al. Donor-Graft Compatibility Tests in Pig-to-Primate Xenotransplantation Model: Serum Ver-sus Plasma in Real-Time Cell Analyzer Trials. Transplant. Proc. 2011, 43, 249–253. [Google Scholar] [CrossRef]
- Ramis, G.; Martínez-Alarcón, L.; Quereda, J.J.; Mendonça, L.; Majado, M.J.; Gomez-Coelho, K.; Mrowiec, A.; Herrero-Medrano, J.M.; Abellaneda, J.M.; Pallares, F.J.; et al. Optimization of cytotoxicity assay by real-time, impedance-based cell analysis. Biomed. Microdevices 2013, 15, 985–995. [Google Scholar]
- Martínez-Alarcón, L.; Liarte, S.; Quereda, J.J.; Sáez-Acosta, A.; Torre-Minguela, C.d.; Mendonça, L.; Abellaneda, J.M.; Majado, M.J.; Ríos, A.; Ramírez, P.; et al. Profiling Human CD55 Transgene Performance Assist in Selecting Best Suited Specimens and Tissues for Swine Organ Xenotransplantation. Biology 2021, 10, 747. [Google Scholar] [CrossRef]
- Domenech, E.; Berná-Serna, J.d.D.; Polo, L.; Reus, M.; Berná-Mestre, J.d.D.; Canteras, M. Effect of SonoVue on the Synovial Membrane in Rabbit Knees. J. Ultrasound Med. 2011, 30, 1241–1246. [Google Scholar] [CrossRef]
- De Marchi, A.; Pozza, S.; Sutera, R.; del Prever, E.M.B.; Petraz, M.; Sena, C.; Linari, A.; Faletti, C. Study of neurinomas with ultrasound contrast media: Review of a case series to identify characteristic imaging patterns. Radiol. Med. 2011, 116, 634–643. [Google Scholar] [CrossRef][Green Version]
- Li, C.; Wang, W.; Ding, H.; Huang, B.; Cao, J.; Mao, F.; Ji, Z. Value of contrast-enhanced sonography in the diagnosis of peripheral intrahepatic cholangio-carcinoma. J. Ultrasound Med. 2011, 39, 447–453. [Google Scholar][Green Version]
- Dayton, P.A.; Zhao, S.; Bloch, S.H.; Schumann, P.; Penrose, K.; Matsunaga, T.O.; Zutshi, R.; Doinikov, A.; Ferrara, K.W. Application of ultrasound to selectively localize nanodroplets for targeted imaging and therapy. Mol. Imaging 2006, 5, 160–174. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Zhang, Y.; Wang, X.; Chen, J. Delivery of TFPI-2 using SonoVue and adenovirus results in the suppression of thrombosis and arterial re-stenosis. Exp. Biol. Med. 2010, 235, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Brunner, K.T.; Mauel, J.; Cerottini, J.C.; Chapuis, B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology 1968, 14, 181–196. [Google Scholar] [PubMed]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Ishiyama, M.; Tominaga, H.; Shiga, M.; Sasamoto, K.; Ohkura, Y.; Ueno, K. A combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol. Pharm. Bull. 1996, 19, 1518–1520. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, K.; De Baetselier, P.; Verschueren, H.; Geldhof, A.B. Morphometric analysis of cytolysis in cultured cell monolayers: A simple and versatile method for the evaluation of the lytic activity and the fate of LAK cells. J. Immunol. Methods 2003, 277, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Atienzar, F.A.; Tilmant, K.; Gerets, H.H.; Toussaint, G.; Speeckaert, S.; Hanon, E.; Depelchin, O.; Dhalluin, S. The use of Real-time Cell Analyzer technology in drug discovery: Defining optimal cell culture conditions and assay reproducibility with different adherent cellular models. J. Biomol. Screen. 2011, 16, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, D.L.; Shaw, S.S.W.; Shangaris, P.; Loukogeorgakis, S.; Guillot, P.V.; De Coppi, P.; David, A.L. In utero therapy for congenital disorders using amniotic fluid stem cells. Front. Pharmacol. 2014, 5, 270. [Google Scholar] [CrossRef]
- Derderian, S.C.; Jeanty, C.; Walters, M.C.; Vichinsky, E.; MacKenzie, T.C. In utero hematopoietic cell transplantation for hemoglobinopathies. Front. Pharmacol. 2015, 5, 278. [Google Scholar] [CrossRef]
- Chan, J.K.Y.; Götherström, C. Prenatal transplantation of mesenchymal stem cells to treat osteogenesis imperfect. Front. Pharmacol. 2014, 5, 223. [Google Scholar] [CrossRef]

| Effect vs. SF6 [µM] | 1.54 | 3.8 | 7.7 | 15.4 | 31 | 46 | 62 | 77 | 115 | 154 | 231 | 308 |
| Sharp initial CI decrease | x | x | x | |||||||||
| Early deviation from sham | x | x | x | |||||||||
| Late deviation from sham | x | x | x | x | x | x | ||||||
| Cumulative (p < 0.001) | x | x | x | x | x | x | x | x | x | x | x | x |
| Maximum CI decrease (%) | 14.48 | 14.58 | 16.62 | 16.68 | 17.44 | 19.3 | 20.09 | 21.76 | 25.13 | 35.77 | 45.55 | 84.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Alarcón, L.; Liarte, S.; Abellaneda, J.M.; Quereda, J.J.; Mendonça, L.; Muñoz, A.; Ramírez, P.; Ramis, G. Exposure to Sulfur Hexafluoride Influences Viability in Cell Transplant Suspensions. BioTech 2025, 14, 86. https://doi.org/10.3390/biotech14040086
Martínez-Alarcón L, Liarte S, Abellaneda JM, Quereda JJ, Mendonça L, Muñoz A, Ramírez P, Ramis G. Exposure to Sulfur Hexafluoride Influences Viability in Cell Transplant Suspensions. BioTech. 2025; 14(4):86. https://doi.org/10.3390/biotech14040086
Chicago/Turabian StyleMartínez-Alarcón, Laura, Sergio Liarte, Juana M. Abellaneda, Juan J. Quereda, Livia Mendonça, Antonio Muñoz, Pablo Ramírez, and Guillermo Ramis. 2025. "Exposure to Sulfur Hexafluoride Influences Viability in Cell Transplant Suspensions" BioTech 14, no. 4: 86. https://doi.org/10.3390/biotech14040086
APA StyleMartínez-Alarcón, L., Liarte, S., Abellaneda, J. M., Quereda, J. J., Mendonça, L., Muñoz, A., Ramírez, P., & Ramis, G. (2025). Exposure to Sulfur Hexafluoride Influences Viability in Cell Transplant Suspensions. BioTech, 14(4), 86. https://doi.org/10.3390/biotech14040086








