Preparation of Suaeda Tea Through Semi-Solid Fermentation Utilizing Kluyveromyces marxianus, Komagataeibacter europaeus, and Acetobacter schutzenbachii: Physicochemical Characteristics, Process Optimization, and Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock
2.1.1. The Suaeda
2.1.2. Preparation of Microbial Agent
2.2. Fermentation Methods of Suaeda Tea
2.3. Determination of Tea Polyphenol Content and Total Free Amino Acids in Tea
2.3.1. Tea Polyphenol Content
2.3.2. Total Free Amino Acids in Tea
2.4. Determination of DPPH Radical, •OH and Total Antioxidant Scavenging Capacity
2.4.1. DPPH Radical Scavenging Capacity
2.4.2. • OH Scavenging Capacity
2.4.3. Total Antioxidant Capacity
2.5. Sensory Evaluation Criteria
3. Results and Discussion
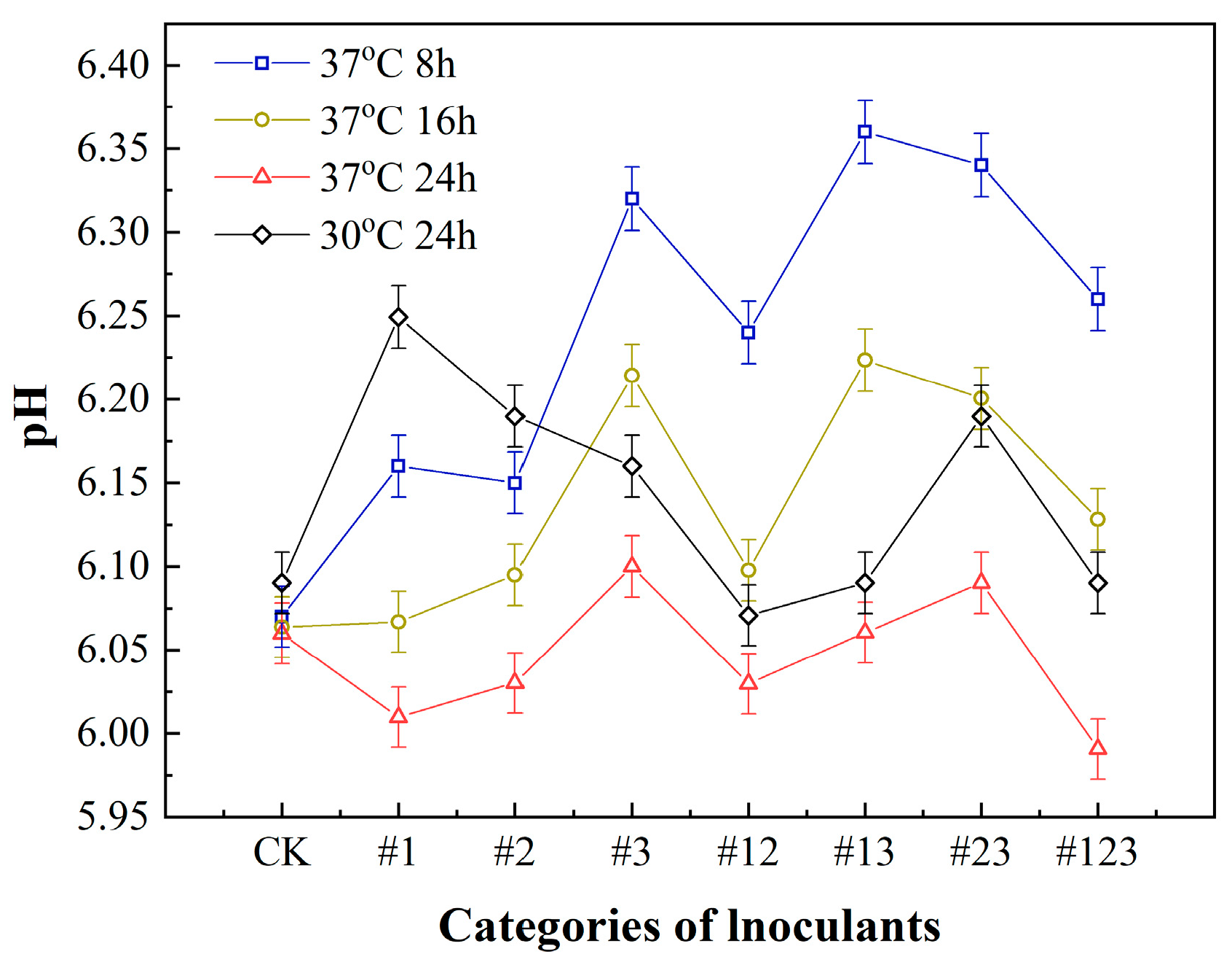
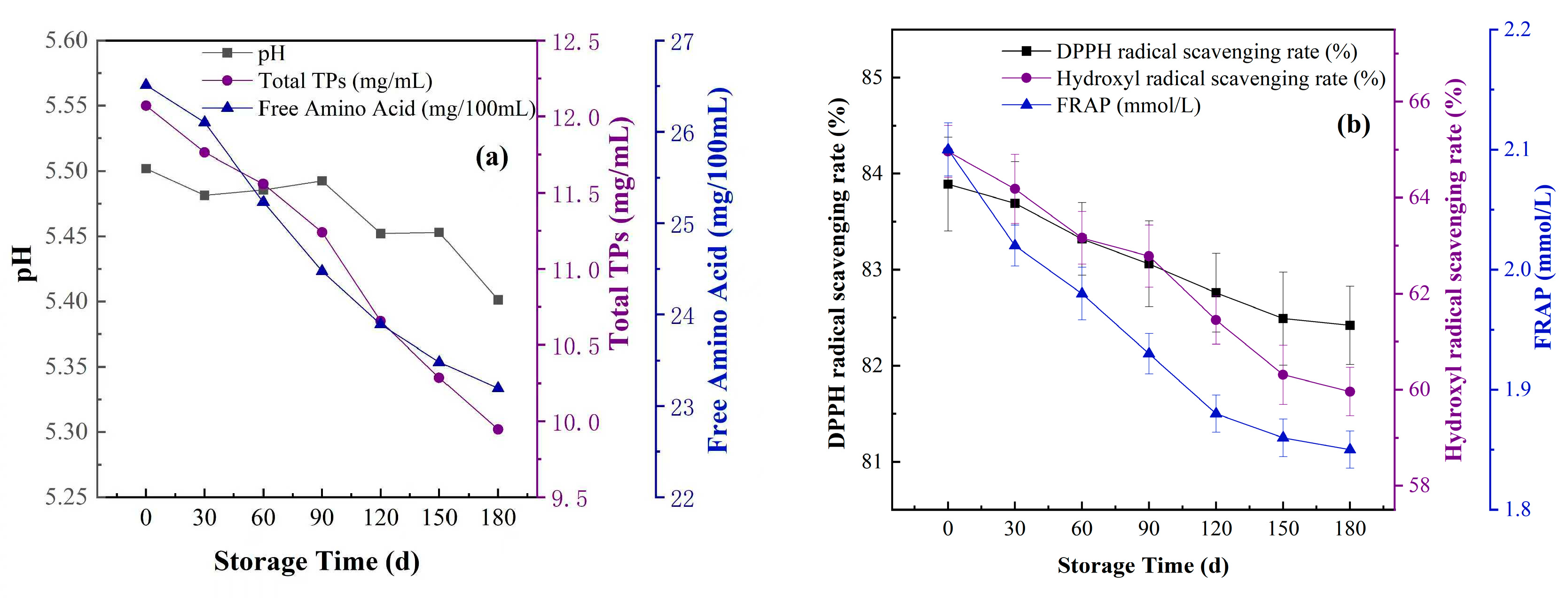
3.1. The pH of Fermented Suaeda Tea Inoculated with Different Microbial Agents
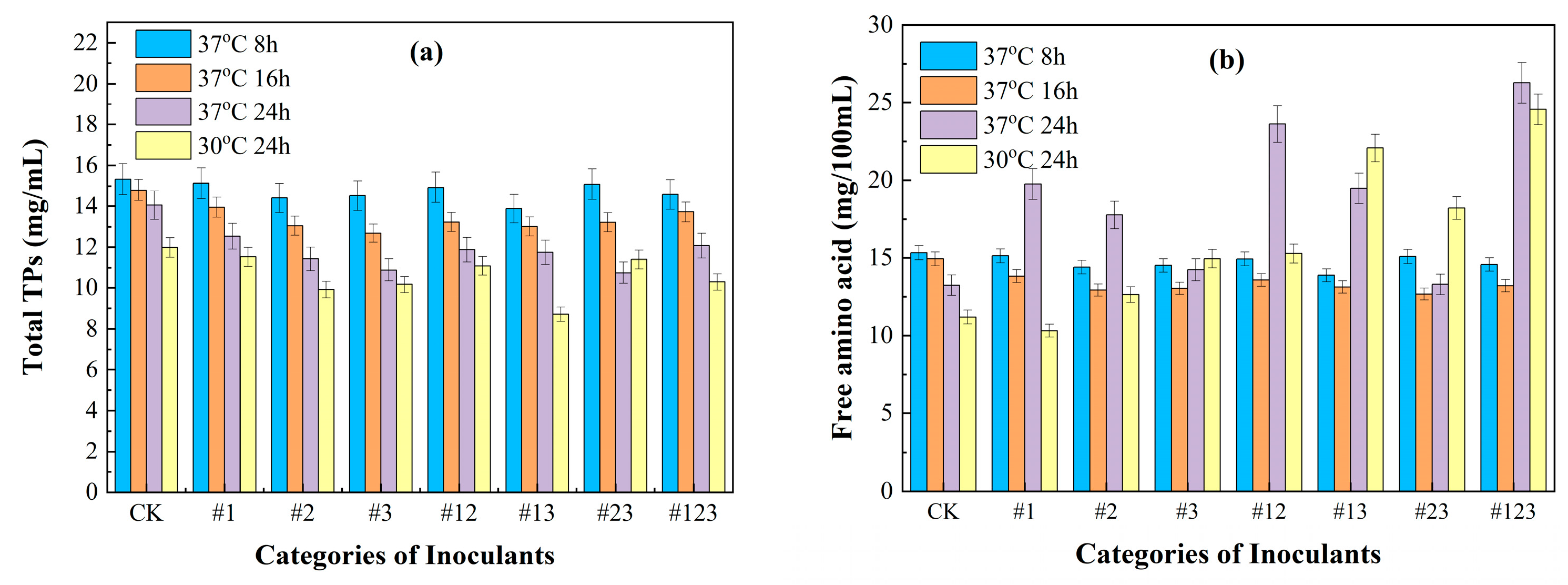
3.2. The Content of Total Tea Polyphenols and Free Amino Acid Content Inoculated with Different Microbial Agents
3.3. The Free Radical Scavenging Rate and Antioxidant Capacity of Suaeda Tea Inoculated with Different Microbial Agents
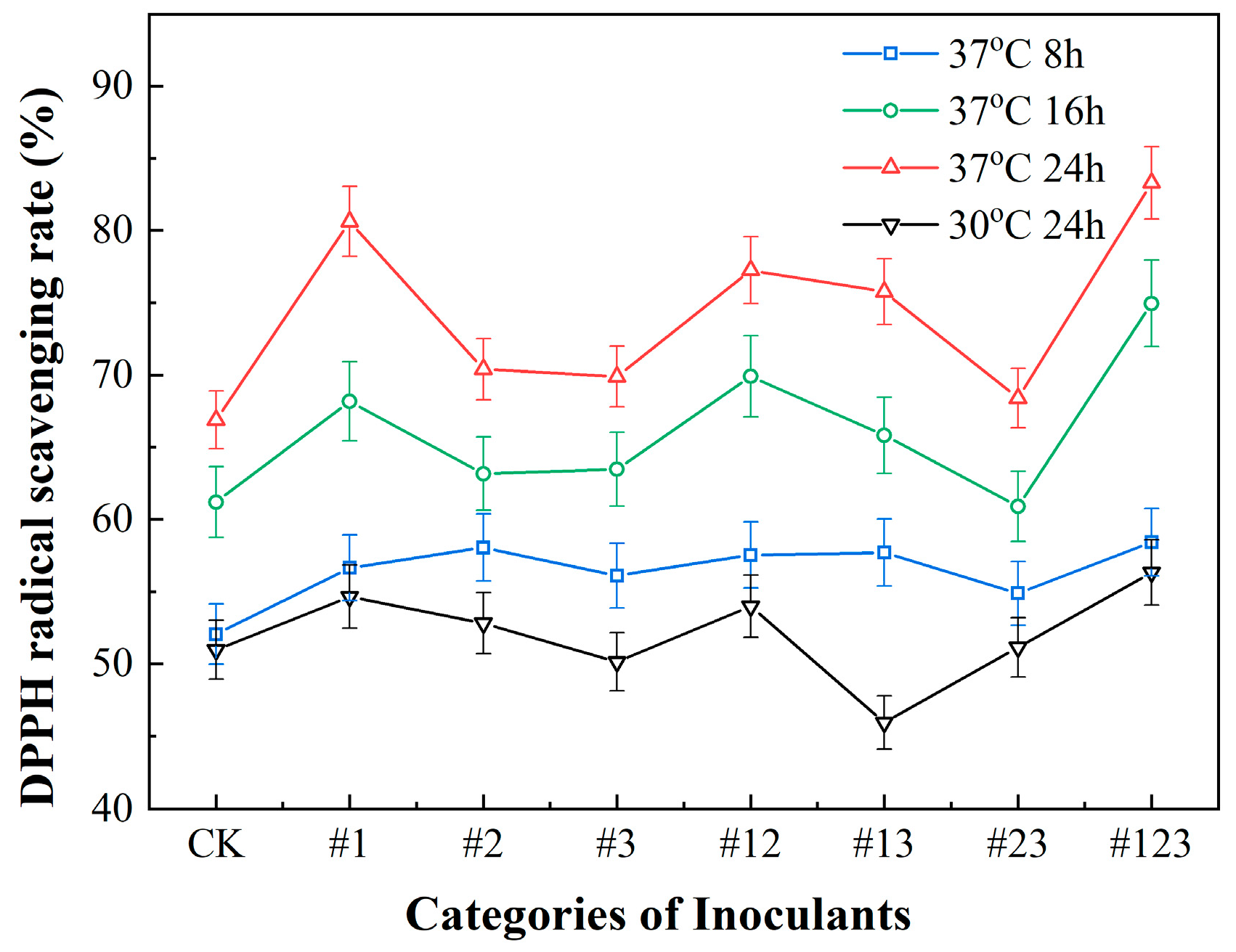
3.3.1. DPPH Radical Scavenging Capacity of Suaeda Tea Inoculated with Different Microbial Agents
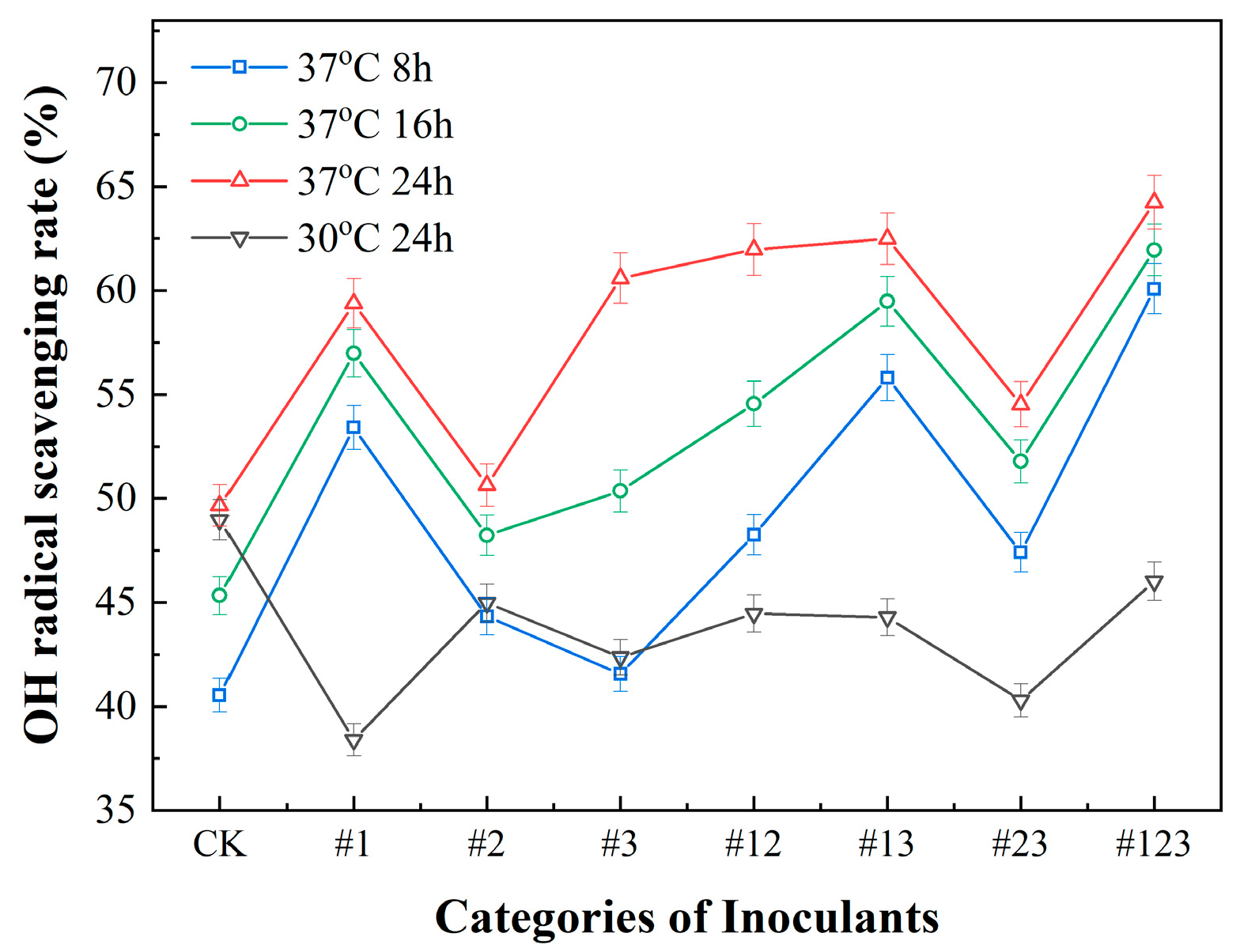
3.3.2. •OH Scavenging Capacity of Suaeda Tea Inoculated with Different Microbial Agents
3.3.3. FRAP Scavenging Capacity Inoculated with Different Microbial Agents
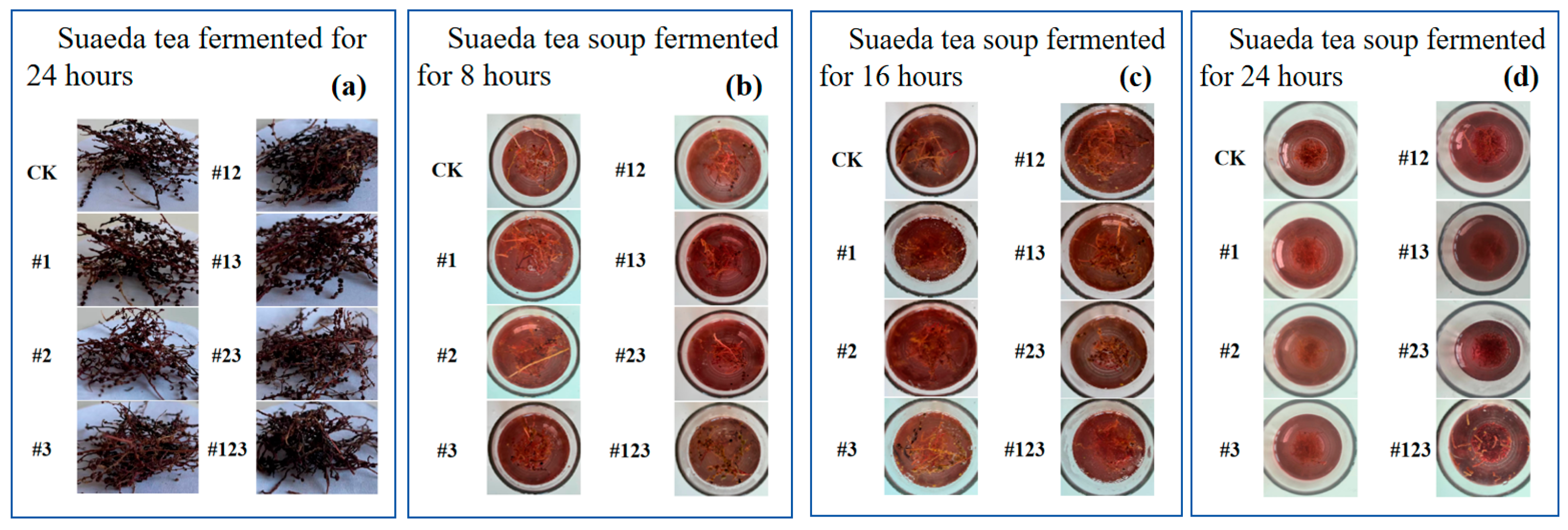
3.4. Sensory Evaluation
3.5. Variations in the Physical and Chemical Properties of Suaeda Tea During the Storage Period
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Hu, M.; Ma, H.; Jiang, L.; Zhao, Z.; Ma, J.; Wang, L. Differential responses of dimorphic seeds and seedlings to abiotic stresses in the halophyte Suaeda salsa. Front. Plant Sci. 2021, 12, 630338. [Google Scholar] [CrossRef]
- Geedicke, I.; Oldeland, J.; Leishman, M.R. Urban stormwater run-off promotes compression of saltmarshes by freshwater plants and mangrove forests. Sci. Total Environ. 2018, 637, 137–144. [Google Scholar] [CrossRef]
- Jian, S.; Zhu, T.; Wang, J.; Yan, D. The current and future potential geographical distribution and evolution process of Catalpa bungei in China. Forests 2022, 13, 96. [Google Scholar] [CrossRef]
- Cao, C.; Su, F.; Song, F.; Yan, H.; Pang, Q. Distribution and disturbance dynamics of habitats suitable for Suaeda salsa. Ecol. Indic. 2022, 140, 108984. [Google Scholar] [CrossRef]
- Song, J.; Fan, H.; Zhao, Y.; Jia, Y.; Du, X.; Wang, B. Effect of salinity on germination, seedling emergence, seedling growth and ion accumulation of a euhalophyte Suaeda salsa in an intertidal zone and on saline inland. Aquat. Bot. 2008, 88, 331–337. [Google Scholar] [CrossRef]
- Song, J.; Wang, B. Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann. Bot. 2015, 115, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.M.; Zhang, Z.S.; Liu, Y.L.; Wei, A.C.; Ma, Y.X. Triacylglycerol composition and physicochemical property of Suaeda salsa seed oil. Cereals Oils 2018, 31, 29–33. [Google Scholar]
- Altay, V.; Ozturk, M. The genera Salsola and Suaeda (Amaranthaceae) and their value as fodder. In Handbook of Halophytes: From Molecules to Ecosystems Towards Biosaline Agriculture; Springer: Cham, Switzerland, 2020; pp. 1–12. [Google Scholar]
- Zhao, Y.; Yang, Y.; Song, Y.; Li, Q.; Song, J. Analysis of storage compounds and inorganic ions in dimorphic seeds of euhalophyte Suaeda salsa. Plant Physiol. Biochem. 2018, 130, 511–516. [Google Scholar] [CrossRef]
- Liu, X.; Liu, F.; Zhao, S.; Guo, B.; Ling, P.; Han, G.; Cui, Z. Purification of an acidic polysaccharide from Suaeda salsa plant and its anti-tumor activity by activating mitochondrial pathway in MCF-7 cells. Carbohydr. Polym. 2019, 215, 99–107. [Google Scholar] [CrossRef]
- Qiu, P.; Guan, F.; Feng, X.; Liu, F.; Liu, X.; Jin, T.; Lyu, H.; Huang, J.; Tong, H.; Chen, M. Suaeglaucin B, an Isoflavone from Suaeda glauca, and its Antioxidant Activity. Chem. Nat. Compd. 2021, 57, 16–19. [Google Scholar] [CrossRef]
- Wang, Q.; Qiu, P.; Guan, F.; Shan, Y.; Yin, M.; Feng, X.; Liu, F. A New isoflavane from Suaeda glauca. Chem. Nat. Compd. 2018, 54, 38–40. [Google Scholar] [CrossRef]
- An, R.-B.; Sohn, D.-H.; Jeong, G.-S.; Kim, Y.-C. In vitro hepatoprotective compounds from Suaeda glauca. Arch. Pharmacal Res. 2008, 31, 594–597. [Google Scholar] [CrossRef]
- Sun, H.; Zhong, R.; Liu, H.; Wang, M.; Sun, J.; Zhou, D. Meat quality, fatty acid composition of tissue and gastrointestinal content, and antioxidant status of lamb fed seed of a halophyte (Suaeda glauca). Meat Sci. 2015, 100, 10–16. [Google Scholar] [CrossRef]
- Mohammadi, M.A.; Xu, M.; Wang, Y.; Zhang, Z.; Wai, M.H.; Rizwan, H.M.; Chang, A.; Aslam, M.; Qin, Y.; Cheng, Y. A highly efficient organogenesis system based on 6-benzylaminopurine and indole-6-butyric acid in Suaeda glauca-a medicinal halophyte under varying photoperiods. Ind. Crops Prod. 2024, 216, 118672. [Google Scholar] [CrossRef]
- Ding, H.; Hong, Z.; Yang, Z.; Wang, M.; Wang, K.; Zhu, X. Progress of study on halophyte Suaeda salsa. Acta Agric. Jiangxi 2008, 20, 35–37. [Google Scholar]
- Mohammed, H.A. The valuable impacts of halophytic genus Suaeda; nutritional, chemical, and biological values. Med. Chem. 2020, 16, 1044–1057. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Chen, C.; Li, W. Chemical composition and anti-inflammation of methanol/chloroform extracts from seeds and seedlings of Suaeda salsa (L.) Pall. Chin. Tradit. Pat. Med. 2003, 25, 997–1002. [Google Scholar]
- Chen, H.-Y.; Yen, G.-C. Antioxidant activity and free radical-scavenging capacity of extracts from guava (Psidium guajava L.) leaves. Food Chem. 2007, 101, 686–694. [Google Scholar] [CrossRef]
- Wang, Q.-Z.; Qiu, P.; Liu, F.; Wang, B.; Guan, F.-Q.; Feng, X.; Xu, S. Suaeglaucin A, a new coumaronochromone from Suaeda glauca. J. Asian Nat. Prod. Res. 2018, 20, 1081–1087. [Google Scholar] [CrossRef]
- GONG, Y.; Liu, F.; Jin, H. Optimization of ethanol extraction of total flavonoids from Suaeda salsa and its antioxidant, hypoglycemic and hypolipidemic activities. Food Sci. 2016, 37, 1–7. [Google Scholar]
- He, Y.; Qu, C.; Zhao, H.; Wang, P.; Zheng, Z.; Miao, J. Conjugated linoleic acid from Suaeda salsa improves the intestinal health in T2DM mice by regulating colonic barrier function, intestinal glycolipid transporters and intestinal flora. Food Biosci. 2024, 58, 103805. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Xu, L.; Wang, Z. Effect of Suaeda seed oil on blood-fat and immunologic function of mouse. Occup. Health 2008, 24, 1529–1530. [Google Scholar]
- Karak, T.; Bhagat, R. Trace elements in tea leaves, made tea and tea infusion: A review. Food Res. Int. 2010, 43, 2234–2252. [Google Scholar] [CrossRef]
- Yang, C.S.; Jin, H.; Guan, F.; Chen, Y.-K.; Wang, H. Cancer preventive activities of tea polyphenols. J. Food Drug Anal. 2012, 20, 37. [Google Scholar] [CrossRef]
- Pan, H.; Gao, Y.; Tu, Y. Mechanisms of body weight reduction by black tea polyphenols. Molecules 2016, 21, 1659. [Google Scholar] [CrossRef]
- Rothenberg, D.O.N.; Zhou, C.; Zhang, L. A review on the weight-loss effects of oxidized tea polyphenols. Molecules 2018, 23, 1176. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ye, Y.; Kang, J.; Yao, X.; Zhang, Y.; Jiang, W.; Gao, M.; Dai, Y.; Xin, Y.; Wang, Q. l-Theanine prevents alcoholic liver injury through enhancing the antioxidant capability of hepatocytes. Food Chem. Toxicol. 2012, 50, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Brennan, M.; Li, S.; Zhao, H.; Lange, K.W.; Brennan, C. How does the tea L-theanine buffer stress and anxiety. Food Sci. Hum. Wellness 2022, 11, 467–475. [Google Scholar] [CrossRef]
- Shojaei-Zarghani, S.; Rafraf, M.; Yari-Khosroushahi, A. Theanine and cancer: A systematic review of the literature. Phytother. Res. 2021, 35, 4782–4794. [Google Scholar] [CrossRef]
- Chen, S.; Kang, J.; Zhu, H.; Wang, K.; Han, Z.; Wang, L.; Liu, J.; Wu, Y.; He, P.; Tu, Y. L-Theanine and Immunity: A Review. Molecules 2023, 28, 3846. [Google Scholar] [CrossRef]
- Gombert, A.K.; Madeira, J.V., Jr.; Cerdán, M.-E.; González-Siso, M.-I. Kluyveromyces marxianus as a host for heterologous protein synthesis. Appl. Microbiol. Biotechnol. 2016, 100, 6193–6208. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Luo, J.; Shi, Y.; Chen, Q. Unlocking the potential of Komagataeibacter europaeus FMES: A novel source of bacterial cellulose from Monascus vinegar biofilm. Carbohydr. Polym. Technol. Appl. 2025, 10, 100841. [Google Scholar] [CrossRef]
- Román-Camacho, J.J.; Mauricio, J.C.; Santos-Dueñas, I.M.; García-Martínez, T.; García-García, I. Unraveling the role of acetic acid bacteria comparing two acetification profiles from natural raw materials: A quantitative approach in Komagataeibacter europaeus. Front. Microbiol. 2022, 13, 840119. [Google Scholar] [CrossRef] [PubMed]
- Mitina, I.; Grajdieru, C.; Sturza, R.; Mitin, V.; Rubtov, S.; Balanuta, A.; Behta, E.; Deaghileva, A.; Inci, F.; Hacıosmanoğlu, N. Molecular Detection of Acetobacter aceti and Acetobacter pasteurianus at Different Stages of Wine Production. Foods 2025, 14, 132. [Google Scholar] [CrossRef]
- Li, Y.-N.; Peng, M.-Y.; Lu, Z.-M.; Dong, Y.-L.; Chai, L.-J.; Shi, J.-S.; Zhang, X.-J.; Xu, Z.-H. Lactiplantibacillus plantarum and Komagataeibacter europaeus enhance energy metabolism, acetic acid and aromatic amino acids catabolism flux in cider vinegar fermentation. LWT 2024, 198, 115968. [Google Scholar] [CrossRef]
- Valera, M.J.; Mas, A.; Streit, W.R.; Mateo, E. GqqA, a novel protein in Komagataeibacter europaeus involved in bacterial quorum quenching and cellulose formation. Microb. Cell Factories 2016, 15, 88. [Google Scholar] [CrossRef]
- Spohner, S.C.; Schaum, V.; Quitmann, H.; Czermak, P. Kluyveromyces lactis: An emerging tool in biotechnology. J. Biotechnol. 2016, 222, 104–116. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, K.; Bian, L.; Li, G.; Zhang, C. High-yield bacterial cellulose production from rice bran using a genetically characterized Komagataeibacter europaeus strain. Int. J. Biol. Macromol. 2025, 310, 143201. [Google Scholar] [CrossRef]
- Unalan-Altintop, T.; Jansson, K.; Özenci, V. Performance of Short-Term Culture and Direct MALDI-TOF MS for Identification of Candida Species From Blood Cultures. Apmis 2025, 133, e70063. [Google Scholar] [CrossRef]
- Ha-Tran, D.M.; Lai, R.Y.; Nguyen, T.T.M.; Huang, E.; Huang, C.C. Construction of engineered RuBisCO Kluyveromyces marxianus for a dual microbial bioethanol production system. PLoS ONE 2021, 16, e0247135. [Google Scholar] [CrossRef]
- Peng, M.-y.; Huang, T.; Zhang, X.-j.; Chai, L.-j.; Lu, Z.-m.; Shi, J.-s.; Xu, Z.-h. Comparative genomics reveals the functional differences between Acetobacter pasteurianus and Komagataeibacter europaeus in vinegar pei of Zhenjiang aromatic vinegar. Acta Microbiol. Sin. 2023, 63, 638–655. [Google Scholar]
- Yin, J.; Wang, J.; Zhao, L.; Cui, Z.; Yao, S.; Li, G.; Yuan, J. Compost tea: Preparation, utilization mechanisms, and agricultural applications potential–A comprehensive review. Environ. Technol. Innov. 2025, 38, 104137. [Google Scholar] [CrossRef]
- Soares, I.F.; de Lima, M.A.; da Silva, R.A.; Colussi, R. Effects of processing on the preparation of kombucha: A systematic review. Int. J. Food Sci. Technol. 2023, 58, 5648–5656. [Google Scholar] [CrossRef]
- Zhou, F.; Lu, B.; Chen, X.; Jia, Z.; Tao, F.; Peng, J. Interaction of major tea polyphenols with bovine milk proteins and its effect on in vitro bioaccessibility of tea polyphenols. Food Chem. 2025, 475, 143341. [Google Scholar] [CrossRef]
- Liyanaarachchi, G.V.V.; Mahanama, K.R.; Somasiri, S.; Punyasiri, N.; Gunawardhana, K.V.T.; Kottawa-Arachchi, J.D. Impact of parboiling and cultivars on the free and total amino acid composition of rice (Oryza sativa L.). J. Food Process. Preserv. 2021, 45, e15763. [Google Scholar] [CrossRef]
- Jabeen, S.; Alam, S.; Saleem, M.; Ahmad, W.; Bibi, R.; Hamid, F.S.; Shah, H.U. Withering timings affect the total free amino acids and mineral contents of tea leaves during black tea manufacturing. Arab. J. Chem. 2019, 12, 2411–2417. [Google Scholar] [CrossRef]
- Chen, X.; Liang, L.; Han, C. Borate suppresses the scavenging activity of gallic acid and plant polyphenol extracts on DPPH radical: A potential interference to DPPH assay. LWT 2020, 131, 109769. [Google Scholar] [CrossRef]
- Khan, M.; Rehman, M.U.; Alam, A.; Ashraf, M.; Salar, U.; Khan, K.M.; Alasmari, A.F.; Alasmari, F. Exploring the DPPH radical scavenging potential in 5-Arylidene-N, N-diethylthiobarbiturates. Russ. J. Bio. Chem. 2024, 50, 1928–1934. [Google Scholar] [CrossRef]
- Duan, Y.; Han, P.; Zhang, R.; Ren, P.; Shi, W.; Wong, N.H.; Sunarso, J.; Liu, S.; Yu, J. A CuO-modified ultrafiltration ceramic membrane inducing micro-nano bubble collapse to generate hydroxyl radicals for enhancing pollutant removal. Appl. Surf. Sci. 2024, 651, 159271. [Google Scholar] [CrossRef]
- Ye, H.; Zhou, C.; Sun, Y.; Zhang, X.; Liu, J.; Hu, Q.; Zeng, X. Antioxidant activities in vitro of ethanol extract from brown seaweed Sargassum pallidum. Eur. Food Res. Technol. 2009, 230, 101–109. [Google Scholar] [CrossRef]
- Dutta, S.; Ray, S. Comparative assessment of total phenolic content and in vitro antioxidant activities of bark and leaf methanolic extracts of Manilkara hexandra (Roxb.) Dubard. J. King Saud Univ.-Sci. 2020, 32, 643–647. [Google Scholar] [CrossRef]
- Yang, W.; Li, S.; Li, N.; Wang, J.; Long, H.; Gong, P. Study on optimization of preparation technology of astragalus membranaceus fermented tea and its antioxidant activity. J. Shaanxi Univ. Sci. Technol. 2025, 43, 89–96. [Google Scholar] [CrossRef]
- Hu, Y.; Piao, C.; Chen, Y.; Zhou, Y.; Wang, D.; Yu, H.; Xu, B. Soybean residue (okara) fermentation with the yeast Kluyveromyces marxianus. Food Biosci. 2019, 31, 100439. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Dong, S.; Ou, C.; Lu, J.; Ye, J. Anticarcinogenic potentials of tea catechins. Front. Nutr. 2022, 9, 1060783. [Google Scholar] [CrossRef]

| Scoring Grade | Evaluation Criteria | Scoring Grade |
|---|---|---|
| Soup color (30 points) | Bright color, clear and transparent, no tea residue | 25–30 |
| Bright color, low brightness, no tea residue | 15–24 | |
| Turbid soup, with tea residue, poor color | <14 | |
| Taste (40 points) | Mellow taste, strong tea flavor | 30–40 |
| Moderate acidity, strong tea flavor | 20–29 | |
| Moderate acidity, weak tea flavor | <19 | |
| Aroma (30 points) | Rich and pleasant aroma | 25–30 |
| Aroma with a hint of water vapor | 15–24 | |
| Aroma with odor | <14 |
| Sample | #1 | #2 | #3 | #12 | #13 | #23 | #123 |
|---|---|---|---|---|---|---|---|
| 37 °C 8 h | 55 | 63 | 69 | 62 | 75 | 63 | 62 |
| 37 °C 16 h | 67 | 71 | 62 | 63 | 78 | 70 | 65 |
| 37 °C 24 h | 58 | 86 | 72 | 64 | 81 | 75 | 71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, A.; Dong, X.; Dai, X.; Gao, Y.; Ning, Y.; Fan, X.; Liu, H. Preparation of Suaeda Tea Through Semi-Solid Fermentation Utilizing Kluyveromyces marxianus, Komagataeibacter europaeus, and Acetobacter schutzenbachii: Physicochemical Characteristics, Process Optimization, and Antioxidant Activity. BioTech 2025, 14, 83. https://doi.org/10.3390/biotech14040083
Dong A, Dong X, Dai X, Gao Y, Ning Y, Fan X, Liu H. Preparation of Suaeda Tea Through Semi-Solid Fermentation Utilizing Kluyveromyces marxianus, Komagataeibacter europaeus, and Acetobacter schutzenbachii: Physicochemical Characteristics, Process Optimization, and Antioxidant Activity. BioTech. 2025; 14(4):83. https://doi.org/10.3390/biotech14040083
Chicago/Turabian StyleDong, Aoqi, Xiaoying Dong, Xinying Dai, Yanru Gao, Yuewen Ning, Xiya Fan, and Haiyan Liu. 2025. "Preparation of Suaeda Tea Through Semi-Solid Fermentation Utilizing Kluyveromyces marxianus, Komagataeibacter europaeus, and Acetobacter schutzenbachii: Physicochemical Characteristics, Process Optimization, and Antioxidant Activity" BioTech 14, no. 4: 83. https://doi.org/10.3390/biotech14040083
APA StyleDong, A., Dong, X., Dai, X., Gao, Y., Ning, Y., Fan, X., & Liu, H. (2025). Preparation of Suaeda Tea Through Semi-Solid Fermentation Utilizing Kluyveromyces marxianus, Komagataeibacter europaeus, and Acetobacter schutzenbachii: Physicochemical Characteristics, Process Optimization, and Antioxidant Activity. BioTech, 14(4), 83. https://doi.org/10.3390/biotech14040083






