Abstract
Argemone mexicana L. is considered a weed; however, it contains secondary metabolites that can control phytopathogenic fungi in vitro, with the potential to adapt its effectiveness in the field. In the present study, leaf extracts of A. mexicana (hexane and methanol) were prepared, and their chemical profiles were analyzed using gas chromatography–mass spectrometry (GC-MS). The in vitro antifungal activity of each extract was evaluated at different concentrations (500, 1000, 2000, 4000, and 8000 mg L−1) against phytopathogens such as Monilinia fructicola, Colletotrichum gloeosporioides, Fusarium oxysporum, and Sclerotinia sclerotiorum. Based on their chemical profiles, 14 compounds were identified in the hexanic extract, and 11 compounds were identified in the methanolic extract. These compounds included those with antifungal activity, such as Benzene; 1.3-bis(1.1-dimethylethyl)-; pentanoic acid; 5-hydroxy-, 2,4-di-1-butylphenyl esters; 1,2,4-Triazol-4-amine; and N-(2-thienylmethyl). The hexanic extract demonstrated fungistatic activity on the four fungi tested, while the methanolic extract exhibited fungicidal activity against C. gloeosporioides and F. oxysporum. The results of the Probit analysis showed variations in the sensitivity of phytopathogenic fungi to the treatments evaluated. In M. fructicola, the hexane extract presented an EC50 of 317,146 mg L−1 and an EC90 of 400,796 mg L−1. For C. gloeosporioides, the EC50 was 2676 mg L−1 and the EC90 was 888,177 mg L−1, while in F. oxysporum an EC50 of 34,274 mg L−1 and an EC90 of 1528 mg L−1 were estimated. In the case of S. sclerotiorum, an EC50 of 560 mg L−1 and an EC90 of 7776 mg L−1 were obtained. Finally, for the commercial fungicide Captan®, an EC50 of 1.19 mg L−1 and an EC90 of 1.67 mg L−1. These results suggest that extracts from A. mexicana could provide a natural alternative for the control of phytopathogenic fungi.
Key Contribution:
The article describes the phytochemical composition of Argemone mexicana L. extracts and their in vitro antimicrobial activity against phytopathogenic fungi that significantly reduce crop yield. In regenerative agriculture, plant extracts are an ecological alternative to chemical fungicides.
1. Introduction
Plant diseases caused by phytopathogenic fungi are responsible for major economic losses, such as lost crop yields, due to issues with product quality and safety. The most common phytopathogenic fungi include Monilinia fructicola (G. Wint.) Honey, Colletotrichum gloeosporioides (Penz.), Fusarium oxysporum Schltdl., and Sclerotinia sclerotiorum (Lib. de Bary) [1]. Monilinia fructicola is an ascomycetous fungus that causes brown rot in many stone fruits [2]. Most of the damage occurs postharvest, resulting in yield losses of up to 80% [3]. Colletotrichum gloeosporioides is the causative agent of anthracnose, a disease that affects a wide range of horticultural and fruit crops. Damage can occur at any stage of the production chain: field growth, storage, and postharvest marketing [4,5]. Anthracnose symptoms include cuticular and subcuticular lesions on the stem, leaves, and inflorescence, with the growth destructive of tissue (necrosed tissue) [6]. Fruit lesions on the epidermis begin as small circles that enlarge and darken. In advanced stages, the fungus produces acervuli containing numerous conidia ranging from white to salmon in color [7]. Fusarium oxysporum causes destructive vascular wilt and root rot [8]. This fungus penetrates through specialized hyphae and colonizes the cortex through intracellular and intercellular growth [9]. Sclerotinia sclerotiorum is a homothetic necrotrophic plant pathogen that is responsible for cotton soft rot [10]. This fungus infects more than 400 plant species worldwide, including important crops such as sunflower, oilseed rape, soybean, bean, pea, lentil, chickpea, potato, lettuce, carrot, cabbage, celery, pepper, and poppy seeds [11].
Plant protection against these phytopathogens is a primary concern for the agricultural sector. Since their initial appearance, some of these devastating diseases have been controlled using synthetic fungicides [12]. However, the inappropriate use of fungicides in agriculture has a negative impact on the environment and human health, resulting in the development of fungicide-resistant fungi [13]. New ecological alternatives are leading to a reduction in synthetic fungicides [14]. Extracts from wild plants are an excellent resource for obtaining new natural fungicides due to their content of secondary metabolites [15]. In addition, these extracts are considered environmentally friendly agents because of their natural origins and typically have limited field persistence and a shorter shelf life compared to synthetic fungicides. Overall, they have the potential to reduce the accumulation of persistent residues and environmental pressure. However, some naturally occurring compounds can also generate undesirable metabolites or residues, so their safety and stability must be assessed [16,17]. These biofungicides utilize several mechanisms of action against phytopathogens, including inhibition of germ tube elongation, delay of sporulation, DNA damage, inhibition of protein synthesis, and damage to hyphal and mycelial structures [18].
An ecological alternative for the control of diseases caused by phytopathogenic fungi is Argemone mexicana L., also known as chicalote, a species endemic to Mexico and widely distributed throughout the continent. It is generally considered a weed but contains alkaloids, terpenoids, flavonoids, phenolic compounds, long-chain aliphatic compounds, and some aromatic compounds that are thought to have antifungal activity [19]. For example, Singh et al. [20] evaluated the methanolic extract of chicalote for antifungal activity against Ustilago cynodontis (Pass.) Henn., Cercospora cajani Henn, and Sphaerotheca spp. In their study, fungicidal activity was attributed to compounds such as caffeic acid, ferulic acid, and tannic acid. Another study [21] assessed the fungicidal activity of an aqueous extract and ethanolic extract of A. mexicana against Botrytis cinerea and Cladosporium spp. Resistant activity was attributed to the compounds carvacrol, 7,9-Di-tert-butyl-1-oxaspiro[4.5]deca-6,9-diene-2,8-dione, allocryptopine, and oxyberberine. Carvacrol, in particular, has been shown to negatively affect the permeability of cell membranes and cause a marked decrease in the total lipid content of cells in phytopathogenic fungi, suggesting the destruction of cell membrane structures [22], while allocryptopine exhibits cytotoxic and antimicrobial effects that compromise the structural integrity of pathogenic fungi [23].
However, most studies reported to date have been limited to insufficient phytopathogen numbers and have not examined the effect of solvent polarity on the extraction of bioactive compounds from A. mexicana. Therefore, the present study provides novel evidence by evaluating the antifungal activity of A. mexicana extracts against the phytopathogenic fungi M. fructicola, C. gloeosporioides, F. oxysporum, and S. sclerotiorum. By using two solvents with different polarities, hexane (non-polar) and methanol (polar), we aimed to extract the greatest possible diversity of secondary metabolites [18]. The extracts were analyzed using gas chromatography coupled with mass spectrometry (GC-MS), an analytical tool used for the qualitative analysis of plant matrices due to its high sensitivity and selectivity for volatile and semi-volatile compounds [24]. We hypothesize that the polarity of the solvent modifies the chemical profile of the extracts and, consequently, their antifungal efficacy.
2. Materials and Methods
2.1. Plant Material
The leaves of A. mexicana L. were randomly collected at the vegetative development stage during winter in the region of Cuautepec de Hinojosa, Hidalgo, Mexico, at 20° 09′00″ N, 98′00″ W, and an altitude of 2200–2900 m above sea level. This region has a cold climate with abundant vegetation, natural resources, and semi-arid soils rich in organic matter and nutrients (Figure 1). The identification of species was carried out in the Botany Laboratory of the Institute of Biological Sciences of the Autonomous University of the State of Hidalgo. Leaves were stored at −70 °C (Thermo Scientific 703 Ultra-Low Freezer, Grand Island, NY, USA) and then preserved in a freeze-dryer (Model 79480; Labconco Corporation, Kansas City, MO, USA). The leaves were then ground in a blade grinder (GM 200, Grindomix, Glen Mills Inc., Clifton, NJ, USA) at 10,000 rpm for one minute, and the samples were stored for later use in a desiccator at 26 °C and 0% humidity.
Figure 1.
(A) Argemone mexicana L. plant material collection site; (B) Argemone mexicana L. plants in vegetative development, the red arrow indicates the species used for the experiment. Map data ©2025 Google.
2.2. Extraction of the Phytochemical Compounds
The plant extracts were obtained by mixing previously freeze-dried plant material with either hexane or methanol. Each solvent (500 mL) was mixed with 50 g of plant material, and the mixtures were soaked for 15 days. Then, the extracts were filtered twice through Whatman filter paper No. 1. The solvents of the extracts were removed under vacuum using a rotary evaporator (BUCHI model R-215, Equipar, Diclab™,Zapopan, Jalisco, Mexico) for four hours at a temperature of 40 °C and a pressure of 100 mbar for hexane and 60 mbar for methanol, as indicated by the device. We followed the methodology proposed by De Rodríguez [25] with some modifications, specifically in the amount of sample used. The extracts were stored in a desiccator at 26 °C and 0% humidity until use in the bioassays. This procedure was repeated throughout the experiment to ensure a sufficient supply of plant extracts for the various microbiological assays. On average, the extract yield was 44% for hexane and 68% for methanol (22 and 34 g of solids, respectively).
2.3. Gas Chromatography-Mass Spectrometry Analysis (GC-MS)
Data were collected as previously described by Hernández-Soto et al. [26]. For analysis, 1 g of each dried extract was dissolved in 20 mL of either hexane or methanol. The resulting solutions were filtered through Whatman No. 1 filter paper to remove solid particles and ensure clarity, conforming to the methodology in [27]. This procedure was repeated four times with three replicates per treatment. Specifically, the chromatographic analysis was performed on an Agilent Technologies 7890A GC system, Santa Clara CA, USA connected to a 5975 GC/MSD in splitless scan mode. Metabolite separation of the extracts was performed on a DB17HT column (30 m × 0.25 mm 1D × 0.15 µL) and an ionization system with energy at 70 eV. Helium was the carrier gas with a constant flow of 3 mL/min. A sample volume of 1 µL was injected at a temperature of 270 °C. Data were analyzed using Agilent MassHunter (B.07) software and AMDIS32 V2.1. The mass spectra of the compounds were analyzed using the National Institute of Standards and Technology (NIST) database, considering only those compounds present in at least 70% of the runs and with a minimum similarity index of 800 (Match Factor). We also identified their biological plausibility and bibliographic support, according to the methodology proposed in [28].
2.4. In Vitro Evaluation of the Extracts Against Phytopathogenic Fungi
The strains used in this study were donated by the Agricultural and Environmental Chemistry Laboratory of the Autonomous University of the State of Hidalgo. They have the following Genbank accession numbers: Monilia fructicola: MN179292; Colletotrichum gloeosporiodes: MT850050; Fusarium oxysporum: MK605264; and Sclerotinia sclerotiorum: ON401416. The antifungal activity of A. mexicana leaf extracts on phytopathogenic fungi was evaluated using the agar dilution method with some modifications, specifically for the concentrations of the extract evaluated [29]. The various extracts were incorporated into the Potato Dextrose Agar (PDA) culture medium after sterilization at 121 °C for 15 min in an autoclave (Evar, EV-30, EQUIMLAB, Mexico City, Mexico) and cooling to below 50 °C. The extract concentrations used were 500, 1000, 2000, 4000, and 8000 mg L−1. To perform the agar dilution method, each extract was resuspended in its respective solvent (2 mL), and 0.1% (v/v) Tween 20 was added to facilitate homogenization in the medium. This was specifically necessary for the hexane extract because preliminary tests identified difficulties in incorporating the lipophilic extract into the hydrophilic culture medium. While the methanolic extract is miscible in water, the surfactant was used to achieve a homogeneous mixture as the concentration of the extract incorporated into the Potato Dextrose Agar medium increased. Six controls were also prepared, as described below: PDA only (control 1), which was prepared according to the manufacturer’s recommendations (39 g L−1 of PDA) diluted in distilled water; PDA + solvent (control 2) with 2 mL of solvent and the total volume of PDA medium used for three replicates per strain; PDA + Tween 20 (control 3), with 20 µL of Tween 20 per replicate incorporated into the PDA medium; PDA + Captan® commercial fungicide. To determine the effective concentration of the commercial fungicide Captan®, a stock solution (30 mL) was prepared from the manufacturer’s recommended dosage (3 kg ha−1), equivalent to 3 × 104 mg L−1, considering an application volume of 100 L ha−1. From this solution, 1:10, 1:100, and 1:1000 dilutions were prepared, corresponding to concentrations of 3.0, 0.3, and 0.03 mg L−1, respectively. To maintain the desired final concentration of the fungicide in the medium, a constant volume of 2 mL of each dilution was added to 18 mL of PDA medium, obtaining a total volume of 20 mL per Petri dish (90 mm). Since the final volume in each dish represents a dilution factor of 0.1 (2/20), the added solutions were prepared 10 times more concentrated than the target final concentration, thus ensuring that the effective concentration of Captan® in the medium exactly corresponded to the 1:10 (control 4), 1:100 (control 5), and 1:1000 (control 6) treatments. The medium was homogenized before solidification to ensure uniform distribution of fungicide. The homogenized agar was poured into Petri dishes with the extract and allowed to solidify. Petri dishes were inoculated by placing a 5 mm plug of each fungus in the center of each plate. The strains were grown in mycelial culture for 12 days. Assessments of the different concentrations were carried out in triplicate, and the inoculated Petri dishes were incubated at 23 ± 2 °C. The efficacy with which each extract treated the fungi was evaluated by measuring the inhibition of mycelial growth (in mm), the diameter of which was measured using a digital Vernier caliper (CALDI-6MP, Truper, Mexico). Measurements were taken every 24 h for 7 days. The percentage of inhibition of mycelial growth was determined using control 1 as a reference according to the method described in [30]:
where is the fungal growth (mm), is the fungal growth diameter of the corresponding negative control in each replicate; and % GI is the percentage of growth inhibition.
2.5. Statistical Analysis
Experimental trials on antifungal activity were conducted using a completely randomized experimental design. For each fungus, fourteen treatments (two solvents at five different concentrations) and four controls were evaluated. Three replicates were performed per treatment. A multivariate analysis of variance with repeated measures was performed alongside the Hotelling test (α = 0.05). The statistical procedures used Infostat 2020 software, and probit analyses calculated the EC50 and EC90 values of the extracts using the statistical software package SAS-PC (version 9.1.3) for Windows.
3. Results
3.1. GC-MS Analysis
The identification of metabolites in the obtained extracts allowed us to recognize various molecules with previously documented biological activity in the scientific literature. Some of these compounds have demonstrated antifungal, antioxidant, antibacterial, and other bioactive functions in different experimental models. The identified compounds are described below, as well as the activities attributed to them in previous studies.
3.1.1. Hexanic Extract
The results of the GC-MS analysis for the hexanic extract derived from leaves of A. mexicana are shown in Table 1 and Figure 2. In this extract, 14 compounds were identified, 56% of which were reported to have antibacterial, antifungal, and antiviral activity against pathogenic microorganisms. Only one compound that was previously reported in another study, 1,2,4-Triazol-4-amine, N-(2-thienylmethyl)-, showed antifungal activity against a phytopathogenic fungus. The compounds reported for the first time are N-Methyl-2-isopropoxycarbonylazetidine; Methanesulfinyl fluoride, trifluoro-; and dl-Methionine, N-[ (4-1) methylphenyl) sulfonyl]-.

Table 1.
Chemical compounds identified in the hexane extracts of Argemone mexicana L.
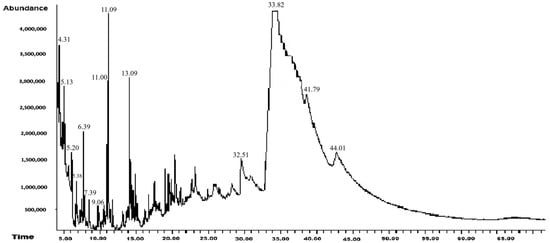
Figure 2.
Chromatogram of the hexane extract of Argemone mexicana L.
3.1.2. Methanolic Extract
The GC-MS results for the methanolic extract of A. mexicana L. are shown in Table 2 and Figure 3. In this extract, 11 compounds were identified, 50% of which exhibited antifungal activity. These were the most abundant compounds in the extract, with Benzene, 1.3-bis(1.1-dimethylethyl)-, being the most common. It is important to mention that antifungal activities of A. mexicana extracts against pathogenic fungi have been previously reported; however, this study is the first to identify antifungal activity against M. fructicola, C. gloesoporioides, F. oxysporum, and S. sclerotinia.

Table 2.
Chemical compounds identified in the methanolic extract of Argemone mexicana L.
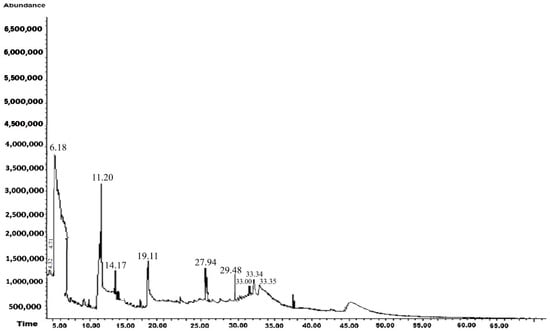
Figure 3.
Chromatogram of the methanol extract of Argemone mexicana L.
3.2. In Vitro Evaluation of the Extracts Against Phytopathogenic Fungi
A. mexicana extracts exhibited antifungal activity against the different phytopathogens, such as M. fructicola, considered in this study (Figure 4). The hexanic extract showed the strongest biological activity between 4000 and 8000 mg L−1 (Figure 4A). Between these concentrations, the hexanic extract inhibited more than 80% of radial growth on the first day (Figure 4C), and the inhibition remained above 70% throughout the experiment. The methanolic extract (Figure 4B) showed a growth inhibition of more than 60% from 2000 mg L−1 on the first day (Figure 4D). The Captan® 1:100 (control 6) treatment maintained antifungal activity >80% and from the fourth day onwards the biological activity remained >70% (Figure 4). Probit analysis showed the mean effective concentration required to inhibit the fungicidal activity by 50% (EC50) and 90% (EC90), respectively, at the end of the experiments (7 days). The hexanic extract had an EC50 of 317,146 mg L−1 in a range of 312, 879 to 321,090 mg L−1, and an EC90 of 400,796 mg L−1 in a range of 396,131 to 405,958 mg L−1 (Figure 4E). By contrast, the methanolic extract had an EC50 of 352 mg L−1 in a range of 225 to 510 mg L−1 (Figure 4F).
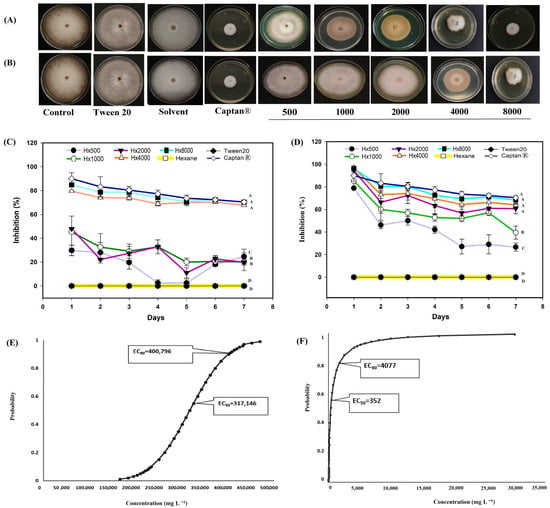
Figure 4.
Antifungal effect of Argemone mexicana L. extracts against Monilinia fructicola after 7 days: (A) hexanic extract; (B) methanolic extract; (C) fungal growth inhibition (%) of the hexanic extract; (D) fungal growth inhibition (%) of the methanolic extract; (E) Probit analysis of the hexanic extract; and (F) Probit analysis of the methanolic extract. Different letters between treatments indicate significant differences according to the Hotelling test (α = 0.05). N = 3 replicates per treatment. Controls: Negative control = PDA only; Tween 20 = PDA + 0.1% Tween 20 surfactant; Solvent = PDA + hexane or methanol; and Control 6: Captan® = PDA +1:1000 Captan. A. mexicana extracts contained PDA, surfactant, and solvent. Effective concentrations (EC50 and EC90) correspond to the fungicide concentrations required to inhibit 50% and 90% of mycelial growth, respectively, compared with the untreated control. These values represent the biological effect produced by the concentration of the extract estimated through dose–response regression analysis.
In C. gloeosporioides (Figure 5), the hexanic extract inhibited more than 70% growth (Figure 5A) on the six day at a concentration of 8000 mg L−1, while the other concentrations exhibited fungistatic activity and kept the percentage of inhibition below 40% throughout the evaluation (Figure 5C). The methanolic extract showed fungicidal activity for the 500 mg L−1 treatments (Figure 5B). The Captan® 1:100 (control 6) treatment showed an activity greater than 35% in the first days, from the third day onwards the antifungal activity remained above 50% (Figure 5). Probit analysis for C. gloeosporioides (Figure 5D) included only the hexanic extract, as no growth was observed with the methanolic extract. It is possible that the EC50 and EC90 of this extract are below the 500 mg L−1 treatment concentration. The EC50 for the hexanic extract was 2676 mg L−1, within a range of 1485 and 4409 mg L−1.
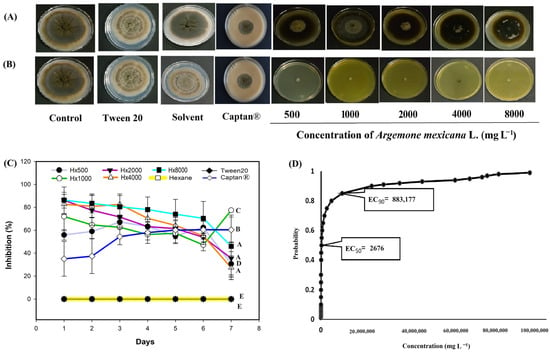
Figure 5.
Antifungal effect of Argemone mexicana L. extracts against Colletotrichum gloeosporioides after 7 days: (A) hexanic extract; (B) methanolic extract; (C) fungal growth inhibition (%) of the hexanic extract; and (D) Probit analysis of the hexanic extract. Different letters between treatments indicate significant differences according to the Hotelling test (α = 0.05). N = 3 replicates per treatment. Controls: Negative control = PDA only; Tween 20 = PDA + 0.1% Tween 20 surfactant; Solvent = PDA + hexane or methanol; and Control 6: Captan® = PDA +1:1000 Captan. A. mexicana extracts contained PDA, surfactant, and solvent. Effective concentrations (EC50 and EC90) corresponded to the fungicide concentrations required to inhibit 50% and 90% of mycelial growth, respectively, compared with the untreated control. These values represent the biological effect produced by the concentration of the extract estimated through dose–response regression analysis.
For F. oxysporum (Figure 6), the hexanic extract only showed >60% inhibition at 8000 mg L−1 and during the first two days of evaluation; by day 3, its activity declined, and inhibition at all doses remained below 10% for the rest of the 7-day period (Figure 6C). In contrast, the methanolic extract produced >60% inhibition at all tested concentrations (1000–8000 mg L−1) during the first three days (Figure 6D), and at 8000 mg L−1, it exhibited fungicidal activity (Figure 6A). Captan® 1:100 treatment inhibited the growth of F. oxysporum by over 70% during 5 days of evaluation; at the end of the evaluation, the antifungal activity remained above 50% (Figure 6). Probit analysis for F. oxysporum revealed that the hexanic extract of A. mexicana (Figure 6E) had an EC50 of 34,274 mg L−1, within the range of 31,717 and 36,444 mg L−1. The EC50 of the methanolic extract (Figure 6F) was 52 mg L−1, ranging between 1.40 and 320 mg L−1. Since the methanolic extract had the lowest EC50 concentration, it could be considered a viable option for the control of F. oxysporum.
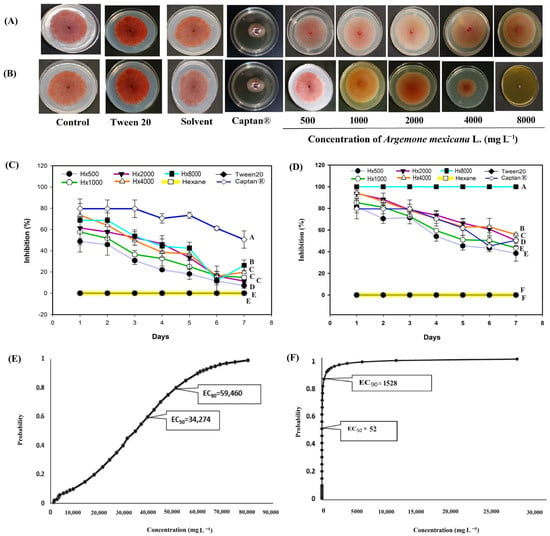
Figure 6.
Antifungal effect of Argemone mexicana L. extracts against Fusarium oxysporum after 7 days: (A) hexanic extract; (B) methanolic extract; (C) fungal growth inhibition (%) of hexanic extract; (D) fungal growth inhibition (%) of the methanolic extract; (E) Probit analysis of the hexanic extract; and (F) Probit analysis of the methanolic extract. Different letters between treatments indicate significant differences according to the Hotelling test (α = 0.05). N = 3 replicates per treatment. Controls: Negative control = PDA only; Tween 20 = PDA + 0.1% Tween 20 surfactant; Solvent = PDA + hexane or methanol; and Control 6: Captan® = PDA +1:1000 Captan. A. mexicana extracts contained PDA, surfactant, and solvent. Effective concentrations (EC50 and EC90) correspond to the fungicide concentrations required to inhibit 50% and 90% of mycelial growth, respectively, compared with the untreated control. These values represent the biological effect produced by the extract concentration estimated through dose–response regression analysis.
For S. sclerotiorum (Figure 7), the hexanic extract was effective at concentrations of 4000 and 8000 mg L−1 (Figure 7A,B). At 8000 mg L−1, biological activity remained above 60% (Figure 7C), while at concentrations of 4000 and 8000 mg L−1, the methanolic extract inhibited growth by more than 90% throughout the evaluation (Figure 7D). In this phytopathogenic fungus the commercial fungicide Captan® presented fungistatic activity with the different concentration gradients 1:10 (control 4) 1:100 (control 5) and 1:1000 (control 6) unlike the rest of the phytopathogenic fungi considered in this experiment, where controls 4 and 5 presented fungicidal activity. For example in S. sclerotiorum controls, number 4 and 5 completely inhibited growth throughout the first three days of evaluation, control number 4 (1:10) maintained its efficiency above 80% the rest of the evaluation, while control 5 (1:100) remained above 70% throughout the experiment (Figure 7C,D); With control number 6 (1:1000) the first day presented an inhibition of 100% and throughout the evaluation it had a decline, however the biological efficiency remained above 50% (Figure 7A,B). Probit analysis for S. sclerotiorum revealed that the hexane extract had an EC50 of 560 mg L−1, with a range between 469 and 655 mg L−1, and an EC90 of 7776 within a range of 6600 and 9384 mg L−1 (Figure 7E). The methanolic extract had an EC50 of 53 mg L−1, and an EC90 of 909 mg L−1 (Figure 7F).
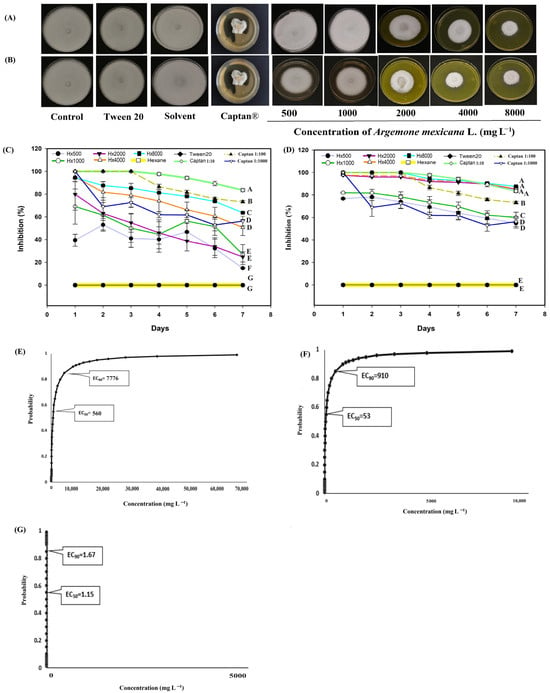
Figure 7.
Antifungal effect of Argemone mexicana L. extracts against Sclerotinia sclerotiorum after 7 days: (A) hexanic extract; (B) methanolic extract; (C) fungal growth inhibition (%) of the hexanic extract; (D) fungal growth inhibition (%) of the methanolic extract; (E) Probit analysis of the hexanic extract; and (F) Probit analysis of the methanolic extract. (G) Probit analysis of the Captan®. Different letters between treatments indicate significant differences according to the Hotelling test (α = 0.05). N = 3 replicates per treatment. Controls: Negative control = PDA only; Tween 20 = PDA + 0.1% Tween 20 surfactant; Solvent = PDA + hexane or methanol; and Control 6: Captan® = PDA +1:1000 Captan. A. mexicana extracts contained PDA, surfactant, and solvent. Effective concentrations (EC50 and EC90) correspond to the fungicide concentrations required to inhibit 50% and 90% of mycelial growth, respectively, compared with the untreated control. These values represent the biological effect produced by the extract concentration estimated through dose–response regression analysis.
Regarding the results of the commercial fungicide Captan®, the EC50 was 1.19 mg L−1 and the EC90 was 1.67 mg L−1 (Figure 7G). In this probit analysis, as in the methanolic extract, fiducial limits were not obtained because the effective concentration intervals were very narrow. This indicates that the range of concentrations tested was within the zone of maximum sensitivity of the fungus and therefore, the reported values should be interpreted as point estimates within the experimental range. In M. fructicola, C. gloesporioides and F. oxysporum, it was not possible to calculate the EC50 and EC90 because the gradient interval tested (1:10 and 1:100) inhibited 100% growth, making it impossible to fit a Probit model. However, with the 1:1000 concentration, the observed and calculated inhibition is reported (Figure 4, Figure 5 and Figure 6). Although it was impossible to fit a Probit model for all species, these results clearly reflect the biological behavior of the Captan® fungicide, providing a more accurate picture of its efficacy and a more robust comparison with A. mexicana extracts. Although the results indicate that plant extracts have no commercial development value against the four plant pathogens, the plant can be used as green manure to reduce the fungal source of the four pathogens.
4. Discussion
The present study demonstrated that Argemone mexicana L. extracts exhibit in vitro antifungal activity against the phytopathogenic fungi such as Monilinia fructicola, Colletotrichum gloeosporioides, Fusarium oxysporum, and Sclerotinia sclerotiorum. These results confirm our hypothesis that solvent polarity modifies the extract chemical profile and consequent antifungal activity. The methanolic extract was more effective than the hexanic extract, showing complete inhibition of C. gloeosporioides and F. oxysporum at concentrations ≥4000 mg L−1. These findings are consistent with previous reports stating that polar solvents extract a wider diversity of bioactive compounds [18]. The fungistatic behavior of the hexanic extract may be due to its high content of non-polar hydrocarbons and terpenoids that integrate into the fungal membrane but lack reactive functional groups to cause irreversible damage [58]. In contrast, the methanolic extract contained polar phenolic and alkaloid-type compounds capable of hydrogen bonding and oxidative disruption, which likely accounts for its fungicidal effect. The mechanisms of action of plant extracts against phytopathogenic fungi are described below. Although the results are preliminary, they provide valuable evidence that A. mexicana contains bioactive secondary metabolites with antifungal potential.
Regarding the behavior of some concentrations of hexane extract against Monilinia fructicola (Figure 4C), in Colletotrichum gloesporioides (Figure 5C) Fusarium oxysporum (Figure 6C) and Sclerotinia sclerotiorum (Figure 7C), the inhibition pattern showed a decrease at some points, followed by a notable increase in inhibition and finally showing a slight decrease towards the end of the experiment. This reduction in antifungal efficacy could be related to a delay in the diffusion or solubilization of non-polar compounds present in the hexane extract, which require time to reach the fungal colony and exert their inhibitory action [58,59]. Furthermore, phytopathogenic fungi produce extracellular enzymes and exopolysaccharides that can interact with lipophilic metabolites, temporarily reducing their bioavailability and allowing limited initial mycelial growth [60]. Once these compounds reach an effective concentration in the medium, the inhibition increases significantly, reflecting the accumulated fungistatic activity of the extract [61]. The decrease observed in most bioassays (Figure 4C, Figure 5C, Figure 6C and Figure 7C) after the sixth day could be due to the degradation or volatilization of the active compounds, which is a common phenomenon in extracts rich in terpenoids and hydrocarbons [62].
In Figure 5D, Probit regression analysis yielded EC50 = 2,676 mg L−1 and EC90 = 883,177 mg L−1, the latter of which is much higher than the maximum concentration tested (8000 mg L−1). This behavior is explained because the dose–response curve presents an asymptotic trend without reaching 100% inhibition within the experimental range [63]. Therefore, the high EC90 value should be interpreted as a form of statistical extrapolation and not a biologically applicable parameter. At concentrations above the solubility limit, efficacy did not increase, since the low solubility and volatility of hexane compounds limit their availability in the culture medium [64,65]. Thus, although the Probit model mathematically predicts complete inhibition at extrapolated concentrations, such conditions are not viable or reproducible in practice.
Beyond these concentration–response dynamics, understanding the biochemical basis of the observed effects provides further insight into the antifungal action of A. mexicana extracts. Plants’ secondary metabolites are essential components of their immune system, mediating defense responses through complex and dynamic interactions with pathogens. These compounds have evolved over millions of years as part of plants’ adaptation to various biotic stresses, resulting in a chemically diverse repertoire [66]. In order to investigate the mechanisms of action against phytopathogenic fungi, these metabolites were categorized into the following groups: terpenes, phenolic compounds, and nitrogenous compounds [67]. The antifungal activity observed in this study against M. fructicola, C. gloeosporioides, F. oxysporum, and S. sclerotiorum (Figure 4, Figure 5, Figure 6 and Figure 7) are attributed to compounds present in hexanic (Table 1 and Figure 2) and methanolic (Table 2 and Figure 3) extracts of A. mexicana, including 3,7,11,15-Tetramethyl-2-hexadecen-1-ol; 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione and 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione (terpenes); Pentanoic acid, 5-hydroxy-, 2,4-di-t-butylphenyl esters and phenol, 2,2′-methylenebis[6-(1,1-dimethylethyl)-4-ethyl- (phenolic compounds); 2-Propanamine, N-methyl-N-nitroso- and 1,2,4-Triazol-4-amine, N-(2-thienylmethyl)-(nitrogenous compounds) [36,41,47,48,49,50,51,52,53,54,55,56].
Mitochondria are essential organelles responsible for energy production, redox balance, and the regulation of apoptosis by maintaining mitochondrial membrane potential [68,69]. In this study, GC-MS analysis of Argemone mexicana extracts revealed the presence of the terpene 3,7,11,15-Tetramethyl-2-hexadecen-1-ol; terpenes are hydrocarbons with hydrophobic properties that allow them to integrate into the lipid bilayer of the cell membrane, increasing membrane permeability. This results in a loss of ions and other molecules essential for cell viability and function [70]. Furthermore, terpene-induced disruption of the plasma membrane has been shown to affect mitochondrial membrane potential, triggering imbalances in proton transport, the disruption of ATP synthesis, and the accumulation of reactive oxygen species, which contribute to mitochondrial damage and cell death [71,72]. These mechanisms are consistent with the antifungal activity observed in this investigation against M. fructicola, C. gloeosporioides, F. oxysporum, and S. sclerotiorum, since the inhibition of mycelial growth suggests that A. mexicana causes damage in the target fungi. Because mitochondrial disruption could potentially affect non-target organisms, future formulations must evaluate selectivity and potential phytotoxicity. Strategies such as nanoencapsulation or emulsified formulations could minimize environmental persistence while maintaining efficacy [64].
In general, terpenes are among the most studied natural antifungals used in botanical products; for example, thymol and carvacrol have an in vitro EC50 of =21.3 mg L−1 and =9.1 mg L−1 against Botrytis cinerea, respectively, and values of = 21–50 mg L−1 against F. oxysporum, depending on the isolate and bioassay conditions. In comparison, for methanolic extracts of A. mexicana (52 mg L−1), their EC50 was of the same order of magnitude but generally higher than pure monoterpenes [61,73,74,75].
Polar solvents, such as methanol, are known to extract phenolic compounds, such as flavonoids, tannins, saponins, unsaturated sterols, triterpenes, and nitrogen-containing compounds, with greater affinity than hexane [18]. For example, pentanoic acid, 5-hydroxy-, 2,4-di-t-butylphenyl esters, and phenol, 2,2′-methylenebis[6-(1,1-dimethylethyl)-4-ethyl are phenolic compounds known to act against pathogens by targeting three sites: the cell wall, the cell membrane, and the mitochondria [76]. Phenolic compounds, including flavonoids, tend to penetrate the hydrophobic core or the lipid bilayer interface, altering its fluidity, permeability, and integrity. These modifications cause the loss of ions (K+, H+), metabolites, and essential compounds, and disrupt membrane potential and the sodium–potassium pump. Additionally, chelation of divalent cations induces intracellular hyperacidification, triggering alterations in plasma membranes [77,78]. Regarding the inhibition of the mitochondrial electron transport chain (ETC), this leads to a decrease in membrane potential. This inhibition generally occurs in the ETC by inhibiting proton pumps, which reduces ATP synthesis and, therefore, cell death. Finally, the mechanism of action on cell walls suggests that flavonoids inhibit the synthesis of β-glucans and chitin, causing their deformation [79]. Similarly, commercially used chemical fungicides also act on critical cellular sites, albeit through more specific and targeted mechanisms. For example, triazoles such as tebuconazole or propiconazole interfere with ergosterol biosynthesis by inhibiting the enzyme 14α-demethylase (CYP51), which is essential for fungal membrane formation. Synthetic fungicides act on specific enzyme targets, while extracts containing phenolic compounds exert a multisite effect that limits the emergence of resistance [80].
The nitrogen-containing compounds 2-Propanamine, N-methyl-N-nitroso- and 1,2,4-Triazol-4-amine, N-(2-thienylmethyl)- can interfere with the translocation of protons across fungal cell membranes [81]. These compounds act as uncouplers by disrupting the proton gradient required for oxidative phosphorylation and altering the permeability of the mitochondrial membrane, preventing the coupling of the electron transport chain with ATP synthesis [82]. The disruption of adenosine diphosphate (ADP) to ATP phosphorylation directly affects the primary energy metabolism of the fungus and leads to a significant decrease in cellular energy production [83]. In addition, nitrogen compounds inhibit the synthesis of DNA, RNA, proteins, and polysaccharides in phytopathogenic fungi. These compounds bind to enzymes and other important components involved in DNA replication and RNA transcription, preventing the production of nucleic acids [84]. These mechanisms may help explain the antifungal activity observed in this study against M. fructicola (Figure 4) and C. gloeosporioides (Figure 5), as well as F. oxysporum (Figure 6) and S. sclerotiorum (Figure 7). For instance, fluytosine (5-fluorocytosine) acts as an analog of cytosine, and when converted to 5-fluorouracil inside the fungal cell, it inhibits thymidylate synthase, blocking DNA and RNA synthesis [85]. Similarly, toyocamycin, a nucleoside analog of adenosine, is misincorporated into RNA and disrupts ribosomal RNA processing, affecting both transcription and translation [86]. Lactimidomycin directly interferes with fungal ribosomes by preventing peptide elongation during protein synthesis [87]. Furthermore, neothramycin, a nitrogen-containing antibiotic, binds to the minor groove of DNA (specifically to guanine residues), thereby blocking the action of DNA and RNA polymerases [88]. Finally, α-amanitin, a type of nitrogen-containing cyclic peptide, selectively inhibits RNA polymerase II via a bridge, thereby halting transcription in the fungal cell [89]. These compounds represent specific molecular targets that compromise fungal cell viability by interfering with its fundamental biosynthetic processes. In particular, these reasons could explain the antifungal activity of Argemone extracts against the phytopathogenic fungi investigated in this study (Figure 4, Figure 5, Figure 6 and Figure 7).
Nitrogen compounds can also interfere with ribosomes and other elements of the translation apparatus and inhibit protein synthesis. This inhibition leads to the disruption of fungal growth and reproduction processes, as proteins and polysaccharides are important structural and functional components in fungal cells [90]. This explains the growth inhibition observed in this study (Figure 4, Figure 5, Figure 6 and Figure 7). While these mechanisms are supported by correlations with previous literature, their confirmation requires genetic or histological studies, including the silencing of genes involved in ergosterol biosynthesis, visualization of mitochondrial damage, and targeted editing using CRISPR–Cas9.
According to the findings of Mitidieri et al. [91], commercial lemon essential oil (Citrus limon L.) inhibited the growth of M. fructicola by more than 80%. Álvarez-García [92] found that extracts of Melaleuca alternifolia Cheel inhibited the growth of M. fructicola by more than 40% after three days (Maiden and Betche). These results are consistent with the present study (Figure 4C). De Rodríguez [25] tested the hexanic extract of Lippia graveolens Kunth against C. gloeosporioides and reported fungistatic activity from 2000 mg L−1, which is four times higher than the minimum concentration tested in this study. Feng et al. [93] found that extracts of Camptotheca acuminata Decne inhibited more than 50% of growth for Colletotrichum at some evaluation points, consistent with the findings of the present study (Figure 5C). Hernández-Soto et al. [30] evaluated the methanolic extract of a plant from the same family, Argemone ochroleuca, against C. gloeosporioides. They reported growth inhibition of 77% at a concentration of 5000 mg L−1. In the present study, 100% inhibition was found at a concentration of 500 mg L−1, which is ten times lower than that used in [30] (Figure 5B). Jeewon et al. [94] reported fungicidal activity of Syzygium aromaticum (L.) Merr & L.M.Perry methanolic extracts on F. oxysporum at a concentration of 9000 mg L−1 over a period of seven days, which is 1.12 times higher than the highest concentration considered in the present study. Kordali et al. [95] investigated the activity of hexanic extracts from Achillea gypsicola L. and Achillea biebersteinii L. against F. oxysporum and reported less than 30% growth inhibition throughout the study, similar to the effect reported in the present study (Figure 6C). Saha et al. [96] reported an EC50 between 642 and 4915 mg L−1 for the hexanic extract of Tagetes minuta L. leaves, which is also similar to the values reported in this study (Figure 6E). Pansera et al. [97] evaluated the hexanic extracts of Boswellia sacra Flueck. against S. sclerotiorum and reported an inhibition of 80%. Tortelli et al. [98] evaluated the extract of Diaporthe infecunda R.R.Gomes, Glienke & Crous against the same pathogen, S. sclerotiorum, and reported an inhibition of 83%. Both reports are in agreement with the results of the present study (Figure 7D). Onaran et al. [99] tested the methanolic extract of Rosa canina L. at a concentration of 1,000 mg L−1 and found an inhibition of 40%, which is 0.5 times lower than the values obtained in the present study with the same concentration. Finally, for probit analysis, the literature was reviewed and EC50 values for Captan® against Fusarium oxysporum of 19.14 mg L−1 [100] with intervals between 2.79 and 35.75 mg L−1, and 978 mg L−1 against Colletotrichum capsica [101] were found, without detailed fiducial intervals, the reported concentrations are 16 and 800 times, respectively, above those reported in this research, which suggests a greater sensitivity of the strains studied in this work to Captan® under controlled in vitro conditions.
Differences in fungal susceptibility can be explained by variations in cell wall composition and antioxidant systems. For example, F. oxysporum and S. sclerotiorum possess thicker chitin–glucan layers and greater tolerance to oxidative stress than M. fructicola, reducing their sensitivity to oxidative and membrane-disrupting compounds [102,103,104]. Future research should scale up these results to greenhouse and field trials, considering in vivo efficacy, phytotoxicity, profitability, and effects on non-target organisms (such as pollinators and soil microflora). Although these results justify continued research, the evidence of the antifungal potential of methanolic extracts of A. mexicana is preliminary.
5. Conclusions
Hexane and methanol extracts of Argemone mexicana L. leaves inhibited the mycelial growth of Monilinia fructicola and Colletotrichum gloeosporioides, two pathogens associated with postharvest diseases, as well as Fusarium oxysporum and Sclerotinia sclerotiorum fungi, which infect the roots of agriculturally important plants. To our knowledge, this study is the first to report evidence for the in vitro antifungal activity of A. mexicana against phytopathogenic fungi. The phytochemical analysis also reports the detection of compounds 2-propenoic acid, 2-methyl-, 1,2-ethanediyl ester; 1,1,3,3-tetramethyl-1,3-disilaphenalane; and cyclohexasiloxane, dodecamethyl- in A. mexicana extracts. Although these findings are preliminary and limited to laboratory conditions, they suggest that the compounds present in A. mexicana may have potential as natural antifungal agents. Further studies, including in vivo efficacy assays, phytotoxicity tests, and environmental safety assessments, are recommended before considering their application in biofungicide formulations or agricultural disease control strategies.
Author Contributions
Conceptualization, A.d.J.C.-G., A.M.-N., A.D.H.-F. and I.H.-S. Data curation, A.S., A.J.-M., A.D.H.-F. and I.H.-S., Formal analysis, A.J.-M., A.S. and I.H.-S. Investigation, A.M.-N., A.d.J.C.-G., J.M.P.-E. and I.H.-S.; Methodology, A.H.-P., J.M.P.-E., A.D.H.-F., A.S. and I.H.-S.; Resources, A.M.-N., A.H.-P., J.M.P.-E., A.D.H.-F. and A.d.J.C.-G.; Software A.S., A.J.-M. and I.H.-S.; Supervision, A.J.-M. and A.D.H.-F.; Validation, A.J.-M. and A.D.H.-F.; Visualization, A.H.-P. and I.H.-S.; Writing—original draft, A.D.H.-F. and I.H.-S.; Writing—review and editing, A.d.J.C.-G. and I.H.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to acknowledge the Universidad Autónoma del Estado de Hidalgo (UAEH), the Centro de Bioingenierias del Instituto Tecnológico y de Estudios Superiores de Monterrey ITESM for allowing us to conduct the study in its facilities.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sharma, I. Phytopathogenic fungi and their biocontrol applications. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology; Academic Press: Cambridge, MA, USA, 2021; Volume 1, pp. 155–188. [Google Scholar] [CrossRef]
- Di Liberto, M.G.; Stegmayer, M.I.; Fernández, L.N.; Quiroga, A.D.; Svetaz, L.A.; Derita, M.G. Control of Brown Rot Produced by Monilinia fructicola in Peaches Using a Full-Spectrum Extract of Zuccagnia punctata Cav. Horticulturae 2023, 9, 1141. [Google Scholar] [CrossRef]
- Kolytaitė, A.; Vaitiekūnaitė, D.; Antanynienė, R.; Baniulis, D.; Frercks, B. Monilinia fructigena suppressing and plant growth promoting endophytic Pseudomonas spp. bacteria isolated from plum. Microorganisms 2022, 10, 2402. [Google Scholar] [CrossRef]
- Peralta-Ruiz, Y.; Rossi, C.; Grande-Tovar, C.D.; Chaves-López, C. Green management of postharvest anthracnose caused by Colletotrichum gloeosporioides. J. Fungi 2023, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Gomdola, D.; Jeewon, R.; McKenzie, E.H.C.; Jayawardena, R.S.; Al-Otibi, F.; Tang, X.; Wang, Y.; Hyde, K.D.; Fu, L. Phylogenetic diversity of Colletotrichum species (Sordariomycetes, Glomerellales, Glomerellaceae) associated with plant diseases in Thailand. MycoKeys 2025, 119, 137–195. [Google Scholar] [CrossRef] [PubMed]
- Bhunjun, C.S.; Jayawardena, R.S.; Wei, D.P.; Huanraluek, N.; Abeywickrama, P.D.; Jeewon, R.; Monkai, J.; Hyde, K.D. Multigene phylogenetic characterisation of Colletotrichum artocarpicola sp. nov. from Artocarpus heterophyllus in northern Thailand. Phytotaxa 2019, 418, 273–286. [Google Scholar] [CrossRef]
- Rodríguez-Velázquez, N.D.; la Cruz, I.G.-D.; López-Guillen, G.; Chávez-Ramírez, B.; Santos, P.E.-D.L. Isolation and biological control of Colletotrichum sp. causing anthracnosis in Theobroma cacao L. in Chiapas, Mexico. J. Fungi 2025, 11, 312. [Google Scholar] [CrossRef]
- Crous, P.; Sandoval-Denis, M.; Costa, M.; Groenewald, J.; van Iperen, A.; Starink-Willemse, M.; Hernández-Restrepo, M.; Kandemir, H.; Ulaszewski, B.; de Boer, W.; et al. Fusarium and allied fusarioid taxa (FUSA). 1. Fungal Syst. Evol. 2022, 9, 161–200. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, C.; Akbar, S.; Wu, S.; Yue, Y.; Wang, G.; Zhou, Y.; Powell, C.A.; Yao, W.; Xu, J.; et al. Characterization and genome analysis of Fusarium oxysporum provides insights into the pathogenic mechanisms of the Pokkah Boeng disease in China. Microorganisms 2025, 13, 573. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Li, W.; Tran, L.S.P.; Mostofa, M.G. Sclerotinia sclerotiorum (Lib.) de Bary: Insights into the pathogenomic features of a global pathogen. Cells 2023, 12, 1063. [Google Scholar] [CrossRef]
- Macioszek, V.K.; Marciniak, P.; Kononowicz, A.K. Impact of Sclerotinia sclerotiorum infection on lettuce (Lactuca sativa L.) survival and phenolics content—A case study in a horticulture farm in Poland. Pathogens 2023, 12, 1416. [Google Scholar] [CrossRef]
- Pandit, M.A.; Kumar, J.; Gulati, S.; Bhandari, N.; Mehta, P.; Katyal, R.; Rawat, C.D.; Mishra, V.; Kaur, J. Major biological control strategies for plant pathogens. Pathogens 2022, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Seepe, H.A.; Nxumalo, W.; Amoo, S.O. Natural products from medicinal plants against phytopathogenic Fusarium species: Current research endeavours, challenges and prospects. Molecules 2021, 26, 6539. [Google Scholar] [CrossRef]
- Hyde, K.D.; Xu, J.; Rapior, S.; Jeewon, R.; Lumyong, S.; Niego, A.G.T.; Abeywickrama, P.D.; Aluthmuhandiram, J.V.S.; Brahamanage, R.S.; Brooks, S.; et al. The amazing potential of fungi, 50 ways we can exploit fungi industrially. Fungal Divers. 2019, 97, 1–136. [Google Scholar] [CrossRef]
- Soto, I.H.; Méndez, J.P.; Navarrete, A.M.; Montiel, R.G.C.; Alvarado, R.J.; Fuentes, A.D.H. Actividad biológica in vitro del extracto acuoso de Argemone mexicana L. en un hongo fitopatógeno: Sclerotinia sclerotiorum. Boletín Cienc. Agropecu. Del ICAP 2020, 6, 12–14. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of botanical pesticides in agriculture as an alternative to synthetic pesticides. Agriculture 2022, 12, 600. [Google Scholar] [CrossRef]
- Beier, R.C. Natural pesticides and bioactive components in foods. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Ed.; Springer: New York, NY, USA, 1990; Volume 113. [Google Scholar] [CrossRef]
- Cenobio-Galindo, A.D.J.; Hernández-Fuentes, A.D.; González-Lemus, U.; Zaldívar-Ortega, A.K.; González-Montiel, L.; Madariaga-Navarrete, A.; Hernández-Soto, I. Biofungicides based on plant extracts: On the road to organic farming. Int. J. Mol. Sci. 2024, 25, 6879. [Google Scholar] [CrossRef]
- Soto, I.H.; Maldonado, A.J.; Montiel, R.G.C.; Álvarez, G.A.; Hernández-Fuentes, A.D. Argemone mexicana contiene metabolitos secundarios que controlan hongos fitopatógenos. Boletín Cienc. Agropecu. Del ICAP 2022, 8, 6–10. [Google Scholar] [CrossRef]
- Singh, S.; Singh, T.D.; Singh, V.P.; Pandey, V.B. Quaternary alkaloids of Argemone mexicana. Pharm. Biol. 2010, 48, 158–160. [Google Scholar] [CrossRef]
- Yaulema, C.S.C.; Molina, W.E.M.; Vilema, W.P.T.; Astudillo, L.J.P.; D’Armas, H. Composición química y actividad antifúngica del látex de Argemone mexicana (Cardo Santo). FACSALUD-UNEMI 2023, 7, 19–36. [Google Scholar] [CrossRef]
- Duan, W.-Y.; Zhu, X.-M.; Zhang, S.-B.; Lv, Y.-Y.; Zhai, H.-C.; Wei, S.; Ma, P.-A.; Hu, Y.-S. Antifungal effects of carvacrol, the main volatile compound in Origanum vulgare L. essential oil, against Aspergillus flavus in postharvest wheat. Int. J. Food Microbiol. 2024, 410, 110514. [Google Scholar] [CrossRef]
- Thawabteh, A.M.; Ghanem, A.W.; AbuMadi, S.; Thaher, D.; Jaghama, W.; Karaman, R.; Scrano, L.; Bufo, S.A. Antibacterial activity and antifungal activity of monomeric alkaloids. Toxins 2024, 16, 489. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Ao, C.; Yu, J.; Zhao, Y.; Huang, H. GC-MS combined with proteomic analysis of volatile compounds and formation mechanisms in green teas with different aroma types. Foods 2024, 13, 848. [Google Scholar] [CrossRef]
- De Rodríguez, D.J.; García, R.R.; Castillo, F.H.; González, C.A.; Galindo, A.S.; Quintanilla, J.V.; Zuccolotto, L.M. In vitro antifungal activity of extracts of Mexican Chihuahuan Desert plants against postharvest fruit fungi. Ind. Crops Prod. 2011, 34, 960–966. [Google Scholar] [CrossRef]
- Hernández-Soto, I.; González-García, Y.; Juárez-Maldonado, A.; Hernández-Fuentes, A.D. Impact of Argemone mexicana L. on tomato plants infected with Phytophthora infestans. PeerJ 2024, 12, e16666. [Google Scholar] [CrossRef]
- Ullah, R.; Bakht, J.; Shafi, M.; Shah, M.R. GC-MS profile of bioactive compounds from medicinally important Periploca hydaspidis. Pak. J. Pharm. Sci. 2018, 31, 1967–1973. [Google Scholar]
- Rajeswari, N.R.; RamaLakshmi, S.; Muthuchelian, K. GC-MS analysis of bioactive components from the ethanolic leaf extract of Canthium dicoccum (Gaertn.) Teijsm & Binn. J. Chem. Pharm. Res. 2011, 3, 792–798. [Google Scholar]
- Vásquez-Covarrubias, D.A.; Belmont, R.M.; Pérez, A.J.; Flores Moctezuma, H.E. Essential oils and aqueous extracts for the in vitro management of Fusarium oxysporum f. sp. lycopersici and F. solani. Rev. Mex. Fitopatol. 2013, 31, 170–180. [Google Scholar]
- Hernández-Soto, I.H.; Prieto-Méndez, J.; Aquino-Torres, E.; Pacheco-Trejo, J. Evaluation of the effect of the methanolic extract of Argemone ochroleuca for environmentally friendly control of Colletotrichum gloeosporioides, Fusarium oxysporum and Rhizoctonia solani. Ciência Técnica Vitivinícola 2018, 33, 65–74. [Google Scholar]
- Kobylina, T.; Novikov, A.; Sadyrova, G.; Kyrbassova, E.; Nazarbekova, S.; Imanova, E.; Parmanbekova, M.; Tynybekov, B. The volatile compounds composition of different parts of wild Kazakhstan Sedum ewersii Ledeb. Separations 2024, 11, 208. [Google Scholar] [CrossRef]
- Ilya, E.; Kulikova, L.; Van der Eycken, E.V.; Voskressensky, L. Recent advances in phthalan and coumaran chemistry. ChemistryOpen 2018, 7, 914–929. [Google Scholar] [CrossRef]
- Buttery, R.G.; Takeoka, G.R.; Krammer, G.E.; Ling, L.C. Identification of 2,5-dimethyl-4-hydroxy-3(2H)-furanone (furaneol) and 5-methyl-4-hydroxy-3(2H)-furanone in fresh and processed tomato. LWT Food Sci. Technol. 1994, 27, 592–594. [Google Scholar] [CrossRef]
- Bindu, T.K.; Udayan, P.S. GC-MS analysis of bioactive compounds in methanolic extract of tubers of Pueraria tuberosa. Int. J. Environ. Agric. Biotechnol. 2018, 3, 1493–1498. [Google Scholar] [CrossRef][Green Version]
- Rizvi, S.N.R.; Afzal, S.; Khan, K.-U.; Aati, H.Y.; Rao, H.; Ghalloo, B.A.; Shahzad, M.N.; Khan, D.A.; Esatbeyoglu, T.; Korma, S.A. Chemical characterisation, antidiabetic, antibacterial, and in silico studies for different extracts of Haloxylon stocksii (Boiss.) Benth: A promising halophyte. Molecules 2023, 28, 3847. [Google Scholar] [CrossRef] [PubMed]
- Naga Lakshmi, M.; Pavithra, S.; Ponmozhi, V.; Jaganathan, J.; Karthik, L.; Shree Devi, M.S. Anti-bacterial activity of selected medicinal plants. Malaya J. Biosci. 2015, 2, 153–159. [Google Scholar]
- Ahmad, S.; Zafar, R.; Khan, I.H.; Javaid, A.; Iqbal, M. Control of khapra beetle by leaf extract of Melia azedarach and identification of possible insecticidal compounds through GC-MS analysis. Pak. J. Weed Sci. Res. 2022, 28, 419–426. [Google Scholar]
- Mansour, S.A.A.; Roff, M.; Noor, M.R.M.; Saad, K.A.; Abuzid, I.; Kermani, N. Identification of semiochemicals released by brinjal, tomato, okra and chilli plants infested with whitefly, B. tabaci. Libyan J. Basic Sci. 2015, 2, 25–40. [Google Scholar]
- Akachukwu, D.; Uchegbu, R. Phytochemical, antimicrobial and free radical scavenging activity of Ficus capensis Thunb leaves. J. Complement. Altern. Med. Res. 2016, 1, 29851. [Google Scholar] [CrossRef]
- Minh, T.N.; Xuan, T.D.; Van, T.M.; Andriana, Y.; Viet, T.D.; Khanh, T.D.; Tran, H.D. Phytochemical analysis and potential biological activities of essential oil from rice leaf. Molecules 2019, 24, 546. [Google Scholar] [CrossRef]
- Marzouk, T.; Chaouachi, M.; Sharma, A.; Jallouli, S.; Mhamdi, R.; Kaushik, N.; Djébali, N. Biocontrol of Rhizoctonia solani using volatile organic compounds of solanaceae seed-borne endophytic bacteria. Postharvest Biol. Technol. 2021, 181, 111655. [Google Scholar] [CrossRef]
- Marengo, A.; Maciel, L.S.; Cagliero, C.; Rubiolo, P.; Herodes, K. Free amino acids and biogenic amines profiling and variation in wild and sub-endemic Cardueae species from Sardinia and Corse. Plants 2023, 12, 319. [Google Scholar] [CrossRef]
- Shettima, A.Y.; Karumi, Y.; Sodipo, O.A.; Usman, H.; Tijjani, M.A. Gas chromatography–mass spectrometry (GC-MS) analysis of bioactive components of ethyl acetate root extract of Guiera senegalensis J.F. Gmel. J. Appl. Pharm. Sci. 2013, 3, 146–150. [Google Scholar]
- Gayathri, M.; Ramasamy, M.; Santhiya, N. GC-MS analysis of bioactive compounds and antipathogenic activity in freshwater ampullariidae Pila virens. J. Environ. Life Sci. 2017, 2, 80–84. [Google Scholar]
- Jayashree, G.V.; Rachitha, P.; Krupashree, K.; Kumar, K.H.; Khanum, F. Chemical composition of Asparagus racemosus root by GC–MS analysis. Science, Technol. Arts Res. J. 2015, 4, 124–126. [Google Scholar] [CrossRef]
- Prabhu, V.; Devi, K.V.; Priya, M.K. GC-MS analysis of bioactive compounds present in the petroleum ether, chloroform and methanol extract of Ixora coccinea’s flower and in vitro cytotoxic activity of. Int. J. Res. Anal. Rev. 2018, 5, 801–807. [Google Scholar]
- Mohan, T.; Pandiyan, J.; Chandru, G.; Jayakumar, S.; Elumalai, K.; Vijayakumar, S.; Subasri, K.S.; Krishnappa, K.; Annathurai, M.; Paramanandham, J.P. Phyto-constituents of Manihot esculenta Crantz. (Euphorbiaceae): A novel bio-weapon against human threats ecto-parasitic vectors and lesser environmental risk. Indian J. Sci. Technol. 2023, 16, 4401–4409. [Google Scholar] [CrossRef]
- Khan, A.; More, K.C.; Mali, M.H.; Deore, S.V.; Patil, M.B. Phytochemical screening and gas chromatography–mass spectrometry analysis on Ischaemum pilosum (Klein ex Willd.). Plant Sci. Today 2023, 10, 88–96. [Google Scholar] [CrossRef]
- Omoregie, G.O.; Ovuakporie-Uvo, O.; Idu, M. Phyto-composition and antimicrobial activities of the ethanol seed extracts of Buchholzia coriacea. Afr. J. Pharmacol. Ther. 2018, 7, 1–6. [Google Scholar]
- Kale, M.V. GC-MS analysis of phytocomponents on whole plant extracts Adiantum capillus-veneris L.—A potential folklore medicinal plant. Res. J. Life Sci. Bioinform. Pharm. Chem. Sci. 2015, 2, 116–121. [Google Scholar] [CrossRef]
- Bhardwaj, R. GC-MS analysis and antimicrobial activity of alkaloids of Tecomella undulata. J. Med. Plant Stud. 2018, 6, 68–72. [Google Scholar]
- Shahla, F.; Muthuswami, R. GC-MS analysis of ethanolic leaf extract of Achyranthes aspera Linn. J. Pharmacogn. Phytochem. 2020, 9, 1207–1210. [Google Scholar]
- Afrin, N.S.; Hossain, M.A.; Saha, K. Phytochemical screening of plant extracts and GC-MS analysis of n-hexane soluble part of crude chloroform extract of Cuscuta reflexa (Roxb.). J. Pharmacogn. Phytochem. 2019, 8, 560–564. [Google Scholar]
- Chetia, J.; Saikia, L.R. Phytochemical analysis of Leucas aspera (Willd.) Link. from Dibrugarh. J. Sci. Res. 2020, 64, 96–98. [Google Scholar] [CrossRef]
- Nyaberi, M.O.; Onyango, C.A.; Mathooko, F.M.; Maina, J.M.; Makobe, M. Profiling active phytochemical compounds of Ziziphus abyssinica herb responsible for antioxidant and antimicrobial activity. J. Anim. Plant Sci. 2017, 34, 5413–5424. [Google Scholar]
- Shakiba, Y.; Rezatofighi, S.E.; Nejad, S.M.S.; Ardakani, M.R. Inhibition of foot-and-mouth disease virus replication by hydro-alcoholic and aqueous-acetic acid extracts of Alhagi maurorum: Antiviral activity of Alhagi maurorum against FMDV. Iran. J. Pharm. Sci. 2018, 14, 85–96. [Google Scholar] [CrossRef]
- Lu, J.; Li, N.; Li, S.; Liu, W.; Li, M.; Zhang, M.; Chen, H. Biochemical composition, antioxidant activity and antiproliferative effects of different processed garlic products. Molecules 2023, 28, 804. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Gupta, P.; Pruthi, P.A.; Pruthi, V. Role of exopolysaccharides in biofilm formation. In Introduction to Biofilm Engineering; American Chemical Society: Washington, DC, USA, 2019; pp. 17–57. [Google Scholar] [CrossRef]
- Marei, G.I.K.; Abdel Rasoul, M.A.; Abdelgaleil, S.A.M. Comparative antifungal activities and biochemical effects of monoterpenes on plant pathogenic fungi. Pestic. Biochem. Physiol. 2012, 103, 56–61. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Sakuma, M. Probit analysis of preference data. Appl. Entomol. Zool. 1998, 33, 339–347. [Google Scholar] [CrossRef]
- Campos, E.V.; Proença, P.L.; Oliveira, J.L.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Use of botanical insecticides for sustainable agriculture: Future perspectives. Ecol. Indic. 2019, 105, 483–495. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides in the twenty-first century—Fulfilling their promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef]
- Xu, S.; Gaquerel, E. Evolution of plant specialized metabolites: Beyond ecological drivers. Trends Plant Sci. 2025, 30, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A comprehensive review on the biological, agricultural and pharmaceutical properties of secondary metabolites based-plant origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef]
- Mendoza, H.; Perlin, M.H.; Schirawski, J. Mitochondrial inheritance in phytopathogenic fungi—Everything is known, or is it? Int. J. Mol. Sci. 2020, 21, 3883. [Google Scholar] [CrossRef]
- Navarro-Espíndola, R.; Suaste-Olmos, F.; Peraza-Reyes, L. Dynamic regulation of peroxisomes and mitochondria during fungal development. J. Fungi 2020, 6, 302. [Google Scholar] [CrossRef]
- Agus, H.H. Terpene toxicity and oxidative stress. In Toxicology; Academic Press: Cambridge, MA, USA, 2021; Volume 6, pp. 33–42. [Google Scholar] [CrossRef]
- Chen, Q.; Ruan, D.; Shi, J.; Du, D.; Bian, C. The multifaceted roles of natural products in mitochondrial dysfunction. Front. Pharmacol. 2023, 14, 1093038. [Google Scholar] [CrossRef]
- Gbadebo, O.S.; Oke, E.D.; Ajibuwa, F.A. Anticancer properties of beta-caryophyllene and d-limonene terpenes: A review. Asian Pac. J. Trop. Biomed. 2025, 15, 129–140. [Google Scholar] [CrossRef]
- Hou, H.; Zhang, X.; Zhao, T.; Zhou, L. Effects of Origanum vulgare essential oil and its two main components, carvacrol and thymol, on the plant pathogen Botrytis cinerea. PeerJ 2020, 8, e9626. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhang, J.; Sun, C.; Chen, X.; Wang, Y.; Lu, H.; Chen, J.; Shi, Z.; Zhang, L.; Yang, L.; et al. Thymol induces cell death of Fusarium oxysporum f. sp. niveum. Agronomy 2023, 13, 189. [Google Scholar] [CrossRef]
- Lu, X.; Hu, K.; Ou, M.; Zhang, X.; Zhan, X.; Liao, X.; Li, M.; Li, R. Synergistic antifungal activity and mechanism of carvacrol/citral against Fusarium oxysporum. Pestic. Biochem. Physiol. 2025, in press. [Google Scholar] [CrossRef]
- Castaño-Duque, L.; Lebar, M.D.; Mack, B.M.; Lohmar, J.M.; Carter-Wientjes, C. Investigating the impact of flavonoids on Aspergillus flavus: Insights into cell wall damage and biofilms. J. Fungi 2024, 10, 665. [Google Scholar] [CrossRef]
- Lobiuc, A.; Pavăl, N.E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.C.; Amăriucăi-Mantu, D.; Stoleru, V. Future antimicrobials: Natural and functionalized phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef] [PubMed]
- Al Aboody, M.S.; Mickymaray, S. Anti-fungal efficacy and mechanisms of flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef]
- Ribera, A.E.; Zuñiga, G. Induced plant secondary metabolites for phytopathogenic fungi control: A review. J. Soil Sci. Plant Nutr. 2012, 12, 893–911. [Google Scholar] [CrossRef]
- Hollomon, D.W. Fungicide resistance: 40 years on and still a major problem. In Fungicide Resistance in Plant Pathogens; Ishii, H., Hollomon, D., Eds.; Springer: Tokyo, Japan, 2015. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, T.; Liang, Z.; Hao, J.; Liu, P.; Liu, X. Dynamic changes in plant secondary metabolites induced by Botrytis cinerea infection. Metabolites 2023, 13, 654. [Google Scholar] [CrossRef]
- Xu, D.; Xue, M.; Shen, Z.; Jia, X.; Hou, X.; Lai, D.; Zhou, L. Phytotoxic secondary metabolites from fungi. Toxins 2021, 13, 261. [Google Scholar] [CrossRef]
- Priyashantha, A.K.H.; Dai, D.-Q.; Bhat, D.J.; Stephenson, S.L.; Promputtha, I.; Kaushik, P.; Tibpromma, S.; Karunarathna, S.C. Plant–fungi interactions: Where it goes? Biology 2023, 12, 809. [Google Scholar] [CrossRef]
- Spitzer, M.; Robbins, N.; Wright, G.D. Combinatorial strategies for combating invasive fungal infections. Virulence 2017, 8, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Ojima, Y.; Yokota, N.; Tanibata, Y.; Nerome, S.; Azuma, M. Concentrative nucleoside transporter, CNT, results in selective toxicity of toyocamycin against Candida albicans. Microbiol. Spectr. 2022, 10, e0113822. [Google Scholar] [CrossRef]
- Schneider-Poetsch, T.; Ju, J.; E Eyler, D.; Dang, Y.; Bhat, S.; Merrick, W.C.; Green, R.; Shen, B.; Liu, J.O. Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin. Nat. Chem. Biol. 2010, 6, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Garreau de Loubresse, N.; Prokhorova, I.; Holtkamp, W.; Rodnina, M.V.; Yusupova, G.; Yusupov, M. Structural basis for the inhibition of the eukaryotic ribosome. Nature 2014, 513, 517–522. [Google Scholar] [CrossRef]
- Burgess-Beusse, B.; Farrell, C.; Gaszner, M.; Litt, M.; Mutskov, V.; Recillas-Targa, F.; Simpson, M.; West, A.; Felsenfeld, G. The insulation of genes from external enhancers and silencing chromatin. Proc. Natl. Acad. Sci. 2002, 99, 16433–16437. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, L.; Lu, A.; Xue, W. Synthesis and biological activity of novel oxazinyl flavonoids as antiviral and anti-phytopathogenic fungus agents. Molecules 2022, 27, 6875. [Google Scholar] [CrossRef]
- Mitidieri, M.S.; Barbieri, M.O.; Brambilla, M.V.; Piris, E. In vitro effect of lemon essential oil and garlic extract on Monilinia fructicola growth. Agrociencia Urug. 2021, 26, e403. [Google Scholar] [CrossRef]
- Álvarez-García, S.; Moumni, M.; Romanazzi, G. Antifungal activity of volatile organic compounds from essential oils against the postharvest pathogens Botrytis cinerea, Monilinia fructicola, Monilinia fructigena, and Monilinia laxa. Front. Plant Sci. 2023, 14, 1274770. [Google Scholar] [CrossRef]
- FeFeng, G.; Zhang, X.-S.; Zhang, Z.-K.; Ye, H.-C.; Liu, Y.-Q.; Yang, G.-Z.; Chen, C.; Chen, M.; Yan, C.; Wang, L.-Y.; et al. Fungicidal activities of camptothecin semisynthetic derivatives against Colletotrichum gloeosporioides in vitro and in mango fruit. Postharvest Biol. Technol. 2019, 147, 139–147. [Google Scholar] [CrossRef]
- Jeewon, R.; Pudaruth, S.B.; Bhoyroo, V.; Aullybux, A.A.; Rajeshkumar, K.C.; Alrefaei, A.F. Antioxidant and antifungal properties of cinnamon, cloves, Melia azedarach L. and Ocimum gratissimum L. extracts against Fusarium oxysporum isolated from Infected Vegetables in Mauritius. Pathogens 2024, 13, 436. [Google Scholar] [CrossRef] [PubMed]
- Kordali, S.; Cakir, A.; Akcin, T.A.; Mete, E.; Akcin, A.; Aydin, T.; Kilic, H. Antifungal and herbicidal properties of essential oils and n-hexane extracts of Achillea gypsicola Hub-Mor. and Achillea biebersteinii Afan. (Asteraceae). Ind. Crops Prod. 2009, 29, 562–570. [Google Scholar] [CrossRef]
- Saha, S.; Walia, S.; Kundu, A.; Kumar, B.; Joshi, D. Antifungal acetylenic thiophenes from Tagetes minuta: Potential biopesticide. J. Appl. Bot. Food Qual. 2013, 85, 207. [Google Scholar]
- Pansera, M.R.; Pauletti, M.; Fedrigo, C.P.; Sartori, V.C.; Ribeiro, R.T.d.S. Utilization of essential oil and vegetable extracts of Salvia officinalis L. in the control of rot sclerotinia in lettuce. Appl. Res. Agrotechnol. 2013, 6, 83–88. [Google Scholar]
- Tortelli, B.; Cappellaro, S.; Nava, F.F.d.M.; Tonial, F.; Huzar-Novakowiski, J.; Milanesi, P.M.; Chiomento, J.L.T. Bioactivity of an extract from the endophyte Diaporthe infecunda on soybean seeds inoculated with Colletotrichum truncatum and Sclerotinia sclerotiorum. Eur. J. Plant Pathol. 2023, 166, 509–520. [Google Scholar] [CrossRef]
- Onaran, A.; Yanar, Y. In vivo and in vitro antifungal activities of five plant extracts against various plant pathogens. Egypt. J. Biol. Pest Control. 2016, 26, 405. [Google Scholar]
- Mihajlović, M.; Rekanović, E.; Hrustić, J.; Grahovac, M.; Tanović, B. In vitro and in vivo toxicity of fungicides and biofungicides. Pestic. Fitomedicina 2021, 36, 23–33. [Google Scholar] [CrossRef]
- Chandini, A.; Kumar, R.; Sinha, B. Testing the efficacy of different fungicides against Colletotrichum capsici under in vitro. Asian J. Agric. Biol. Res. 2022, 2, 101–107. [Google Scholar]
- Zhang, Y.Z.; Li, Y.; Pi, Y.; Cheng, Y. FgCWM1 encodes a cell wall mannoprotein contributing to cell wall integrity and pathogenicity in Fusarium graminearum. Toxins 2019, 11, 628. [Google Scholar] [CrossRef]
- Yoshimi, A.; Miyazawa, K.; Kawauchi, M.; Abe, K. Cell wall integrity and its industrial applications in filamentous fungi. J. Fungi 2022, 8, 435. [Google Scholar] [CrossRef]
- Jiao, W.; Li, M.; Lei, T.; Liu, X.; Zhang, J.; Hu, J.; Zhang, X.; Liu, J.; Shi, S.; Pan, H.; et al. The APSES transcription factor SsStuA regulating cell wall integrity is essential for sclerotia formation and pathogenicity in Sclerotinia sclerotiorum. J. Fungi 2024, 10, 238. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).







