Innovative Approaches to EMT-Related Biomarker Identification in Breast Cancer: Multi-Omics and Machine Learning Methods
Abstract
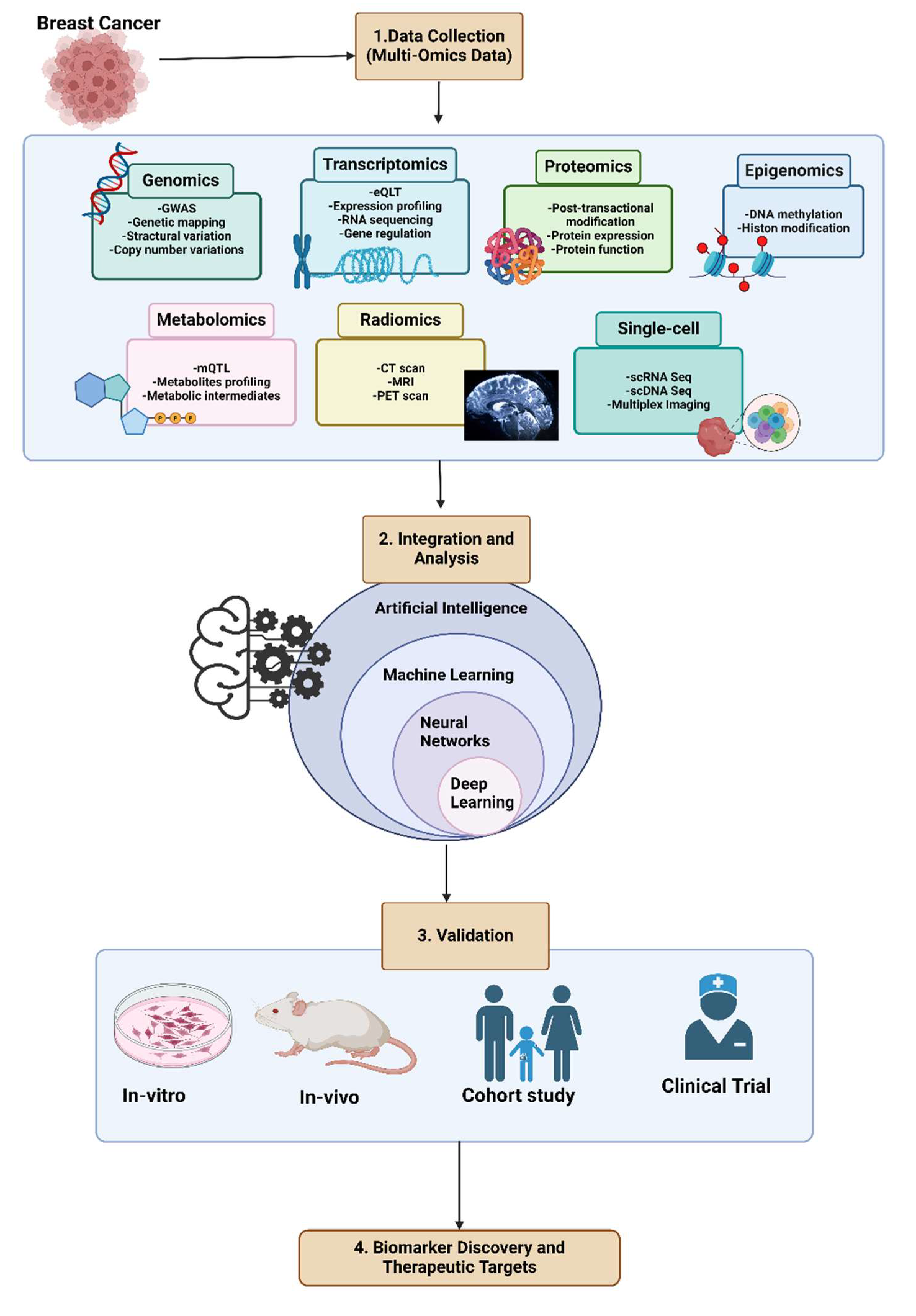
1. Introduction
2. Epithelial–Mesenchymal Transition (EMT)
3. Biomarkers and Analyzing Multi-Omics Data
4. Integrative Multi-Omics Analysis with Artificial Intelligence
5. Machine Learning: Revolutionizing Multiomics Data Interpretation
| Biomarker(s) | Role in Breast Cancer | Dataset(s) | ML Method(s) | Validation | Cohort Size | Clinical Context (Assay) | Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|
| BCHE, ATP7B, PPP4R4, TFF1, PTGFR, TTYH1, SERPINA6, CDKN2A, WIF1, ZNF521, MUC16, WNK4, COL2A1, S100A7, S100B, POU2AF1 | Prognostic (multi-gene EMT-associated panel) | GEO | XGBoost | Internal CV; Kaplan–Meier survival analysis | n = 623 TNBC and 527 non-TNBC samples (GEO cohorts) | Prognostic; RNA-seq, microarray | Higher expression is associated with better survival | [84] |
| E-cadherin (CDH1), Vimentin (VIM) | Prognostic (classical EMT markers) | ECM Select Array | Hierarchical clustering | Experimental (Spearman correlation, t-test) | Cell line/tissue assays | Prognostic; IHC, array-based | Worse prognosis due to EMT features | [78] |
| RBM47, ESRP1/2 | Prognostic (RNA splicing regulators of EMT) | GEO | Random Forest, Cox regression | Internal CV; Log-rank, Wilcoxon tests | Prognostic; RNA-seq, microarray | Worse prognosis in basal-like breast cancer | [85] | |
| RGS7, SPPL2C, KRT23 | Prognostic (linked to EMT signaling) | TCGA, DisGeNET, KEGG | XGBoost | Internal CV; Kaplan–Meier survival analysis | TCGA: n= 22 samples (metastasis to other organs) | Prognostic; RNA-seq | Worse prognosis | [86] |
| CDH2, FN1, CDH1, VIM | Prognostic & Predictive (epithelial–mesenchymal switch signature) | TCGA, GEO, METABRIC | Random Forest, Consensus Clustering | Internal CV (TCGA), External validation (METABRIC) | TCGA: n = 116 TNBC; GEO: 815 TNBC METABRIC: n = 313 (ER- and HER2-negative BC) | Prognostic/Predictive; RNA-seq, IHC | Response to immune checkpoint blockade (ICB) and better survival | [79] |
| miR-21, miR-148b, miR-144, miR-203a, miR-140 | Prognostic (EMT-related miRNA) | miRecords, miRTarBase, TarBase | Linear SVM | Internal CV; Fisher’s exact test | n = 66 (primary breast cancer) | Prognostic; qPCR, RNA-seq | miR-21, prognostic marker of worse outcome. miR-148b, miR-144, miR-203a, miR-140, predictive markers for targeted therapy | [81] |
| Tumor microenvironment-related gene (TRG) score | Prognostic & Predictive (EMT and immune infiltration) | TCGA, GEO, UCSC Xena | LASSO, OCLR, Cox regression | Internal CV; ROC, PCA; External validation in GEO | GEO: multiple cohorts | Prognostic/Predictive; RNA-seq | Low TME-related gene scores are associated with improved prognosis and better response to immunotherapy. | [88] |
| MFGE8 | Diagnostic & Prognostic (linked to EMT signaling) | TCGA, KM Plotter | SVM, Decision Tree, Random Forest | Experimental (qPCR, LC-MS/MS); Internal validation | TCGA: n = 140 TNBC and 737 non-TNBC | Diagnostic/Prognostic; qPCR, proteomics | MFGE8 overexpression is associated with poor prognosis | [89] |
| Fibronectin, FAK, MEK1 | Diagnostic (EMT-related adhesion/migration proteins) | RPPA, immunoblotting, EM | k-NN, Logistic Regression | Experimental (t-test, ROC) | Cell lines, patient tissue | Diagnostic; RPPA, IHC | Protein clusters distinguish sample types; some predict relapse and therapy response | [82] |
| DARS2, SLC2A1, ESRP1, TH, MAFF | Prognostic (EMT-related metabolic & splicing regulators) | TCGA, GEO, UCSC Xena | Cox regression, RSF | Internal CV; ROC, TIDE; External validation GEO | TCGA: n = 1113 (patients with overall survival (OS) time longer than 30 days); GEO: 327 | Prognostic; RNA-seq, bioinformatics | worse overall survival in patients with high lactate-hypoxia scores | [90] |
| GATA3, KRT6, ACTA2, CDH1 | Diagnostic & Prognostic (canonical EMT transcription factors) | TCGA, METABRIC | Neural Network (Cox-nnet) | Internal CV; Experimental (IMC imaging) | TCGA: n = 159 (TNBC), n = 599 (Luminal A); METABRIC: n = 299 (TNBC), 1369 (Luminal A) | Diagnostic/Prognostic; RNA-seq, IMC | KRT6 and ACTA2 over-expression and CDH1 under-expression show poor prognosis. | [93] |
| CDH1, PIK3CA, TP53, EFHD1 | Prognostic & Predictive (partly EMT-related) | TCGA, GDSC | Naïve Bayes, SMO, RF, k-NN | Internal CV; STRING/Cytoscape validation | TCGA: n = 1000 | Prognostic/Predictive; RNA-seq, microarray | Worse survival and potential treatment response | [92] |
| miR-222-3p | Diagnostic & Prognostic (EMT-associated miRNA) | TCGA, GEO, miRWalk | OCLR | Internal CV; ROC, Kaplan–Meier | TCGA: n= 1103; GEO: multiple | Diagnostic/Prognostic; qPCR, RNA-seq | Higher miR-222-3p expression indicates worse prognosis. | [80] |
| Quantitative EMT score (epithelial–mesenchymal traits) | Diagnostic (phenotypic EMT scoring) | DHM imaging | AdaBoost, SVM | Experimental (t-test, post hoc analysis) | Cell lines, tissue samples | Diagnostic; digital holographic microscopy | [72] | |
| Immune-radiomic models with EMT signatures | Predictive (therapy response prediction) | MRI-based radiomics | ML algorithms (unspecified) | External validation (MRI cohort) | n = 570 (breast MRI) | Predictive; MRI, radiomics | MRI-based model predicts risk of positive margins in BCS. | [83] |
6. EMT-Related Biomarkers: Predictive Indicators and Therapeutic Targets
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef]
- Trieu, P.D.Y.; Mello-Thoms, C.R.; Barron, M.L.; Lewis, S.J. Look how far we have come: BREAST cancer detection education on the international stage. Front. Oncol. 2022, 12, 1023714. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Makki, J. Diversity of Breast Carcinoma: Histological Subtypes and Clinical Relevance. Clin. Med. Insights Pathol. 2015, 8, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Aysola, K.; Desai, A.; Welch, C.; Xu, J.; Qin, Y.; Reddy, V.; Matthews, R.; Owens, C.; Okoli, J.; Beech, D.J.; et al. Triple Negative Breast Cancer—An Overview. Hered. Genet. 2013, 2013 (Suppl. S2), 001. [Google Scholar] [CrossRef]
- Exman, P.; Tolaney, S.M. HER2-positive metastatic breast cancer: A comprehensive review. Clin. Adv. Hematol. Oncol. 2021, 19, 40–50. [Google Scholar] [PubMed]
- Hacking, S.M.; Yakirevich, E.; Wang, Y. From Immunohistochemistry to New Digital Ecosystems: A State-of-the-Art Biomarker Review for Precision Breast Cancer Medicine. Cancers 2022, 14, 3469. [Google Scholar] [CrossRef]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef]
- Nassar, A.; Hoskin, T.L.; Stallings-Mann, M.L.; Degnim, A.C.; Radisky, D.C.; Frost, M.H.; Vierkant, R.A.; Hartmann, L.C.; Visscher, D.W. Ki-67 expression in sclerosing adenosis and adjacent normal breast terminal ductal lobular units: A nested case-control study from the Mayo Benign Breast Disease Cohort. Breast Cancer Res. Treat. 2015, 151, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Ogony, J.; Hoskin, T.L.; Stallings-Mann, M.; Winham, S.; Brahmbhatt, R.; Arshad, M.A.; Kannan, N.; Pena, A.; Allers, T.; Brown, A.; et al. Immune cells are increased in normal breast tissues of BRCA1/2 mutation carriers. Breast Cancer Res. Treat. 2023, 197, 277–285. [Google Scholar] [CrossRef]
- Wang, X.; Collet, L.; Rediti, M.; Debien, V.; De Caluwe, A.; Venet, D.; Romano, E.; Rothe, F.; Sotiriou, C.; Buisseret, L. Predictive Biomarkers for Response to Immunotherapy in Triple Negative Breast Cancer: Promises and Challenges. J. Clin. Med. 2023, 12, 953. [Google Scholar] [CrossRef] [PubMed]
- Khalili-Tanha, G.; Sebzari, A.; Moodi, M.; Hajipoor, F.; Naseri, M. Mutations analysis of BRCA1 gene in patients with breast cancer in South Khorasan province, East Iran. Med. J. Islam. Repub. Iran 2019, 33, 105. [Google Scholar] [PubMed]
- Craene, B.D.; Berx, G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 2013, 13, 97–110. [Google Scholar] [CrossRef]
- Roche, J. The epithelial-to-mesenchymal transition in cancer. Cancers 2018, 10, 52. [Google Scholar] [CrossRef]
- Orrantia-Borunda, E.; Anchondo-Nunez, P.; Acuna-Aguilar, L.E.; Gomez-Valles, F.O.; Ramirez-Valdespino, C.A. Subtypes of Breast Cancer. In Breast Cancer; Mayrovitz, H.N., Ed.; Exon Publications: Brisbane, Australia, 2022. [Google Scholar]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef]
- Nistico, P.; Bissell, M.J.; Radisky, D.C. Epithelial-mesenchymal transition: General principles and pathological relevance with special emphasis on the role of matrix metalloproteinases. Cold Spring Harb. Perspect. Biol. 2012, 4, a011908. [Google Scholar] [CrossRef]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef]
- Huang, Y.H.; Hong, W.Q.; Wei, X.W. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef] [PubMed]
- Radisky, E.S.; Radisky, D.C. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. J. Mammary Gland Biol. Neoplasia 2010, 15, 201–212. [Google Scholar] [CrossRef]
- Smith, B.N.; Bhowmick, N.A. Role of EMT in Metastasis and Therapy Resistance. J. Clin. Med. 2016, 5, 17. [Google Scholar] [CrossRef]
- Diaz, V.M.; Vinas-Castells, R.; Garcia de Herreros, A. Regulation of the protein stability of EMT transcription factors. Cell Adh. Migr. 2014, 8, 418–428. [Google Scholar] [CrossRef]
- Kang, E.; Seo, J.; Yoon, H.; Cho, S. The Post-Translational Regulation of Epithelial-Mesenchymal Transition-Inducing Transcription Factors in Cancer Metastasis. Int. J. Mol. Sci. 2021, 22, 3591. [Google Scholar] [CrossRef]
- Mitschke, J.; Burk, U.C.; Reinheckel, T. The role of proteases in epithelial-to-mesenchymal cell transitions in cancer. Cancer Metastasis Rev. 2019, 38, 431–444. [Google Scholar] [CrossRef]
- Radisky, E.S. Extracellular proteolysis in cancer: Proteases, substrates, and mechanisms in tumor progression and metastasis. J. Biol. Chem. 2024, 300, 107347. [Google Scholar] [CrossRef]
- Shoari, A.; Khalili-Tanha, G.; Coban, M.A.; Radisky, E.S. Structure and computation-guided yeast surface display for the evolution of TIMP-based matrix metalloproteinase inhibitors. Front. Mol. Biosci. 2023, 10, 1321956. [Google Scholar] [CrossRef]
- Scheau, C.; Badarau, I.A.; Costache, R.; Caruntu, C.; Mihai, G.L.; Didilescu, A.C.; Constantin, C.; Neagu, M. The Role of Matrix Metalloproteinases in the Epithelial-Mesenchymal Transition of Hepatocellular Carcinoma. Anal. Cell. Pathol. 2019, 2019, 9423907. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Luo, W. Matrix metalloproteinase 2 contributes to aggressive phenotype, epithelial-mesenchymal transition and poor outcome in nasopharyngeal carcinoma. Onco Targets Ther. 2019, 12, 5701–5711. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; He, J.; Wang, F.; Wang, X.; Yang, F.; Zhao, C.Y.; Feng, C.L.; Li, T.J. Role of MMP-9 in epithelial-mesenchymal transition of thyroid cancer. World J. Surg. Oncol. 2020, 18, 181. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Lochter, A.; Sympson, C.J.; Huey, B.; Rougler, J.P.; Gray, J.W.; Pinkel, D.; Bissell, M.J.; Werb, Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell 1999, 98, 137–146. [Google Scholar] [CrossRef]
- Lin, Y.X.; Liu, J.J.; Huang, Y.Q.; Liu, D.L.; Zhang, G.W.; Kan, H.P. microRNA-489 Plays an Anti-Metastatic Role in Human Hepatocellular Carcinoma by Targeting Matrix Metalloproteinase-7. Transl. Oncol. 2017, 10, 211–220. [Google Scholar] [CrossRef]
- Liu, M.; Qi, Y.; Zhao, L.; Chen, D.; Zhou, Y.; Zhou, H.; Lv, Y.; Zhang, L.; Jin, S.; Li, S.; et al. Matrix metalloproteinase-14 induces epithelial-to-mesenchymal transition in synovial sarcoma. Hum. Pathol. 2018, 80, 201–209. [Google Scholar] [CrossRef]
- Liu, F.; Gu, L.N.; Shan, B.E.; Geng, C.Z.; Sang, M.X. Biomarkers for EMT and MET in breast cancer: An update. Oncol. Lett. 2016, 12, 4869–4876. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dey, M.K.; Devireddy, R.; Gartia, M.R. Biomarkers in Cancer Detection, Diagnosis, and Prognosis. Sensors 2023, 24, 37. [Google Scholar] [CrossRef]
- Davis, K.D.; Aghaeepour, N.; Ahn, A.H.; Angst, M.S.; Borsook, D.; Brenton, A.; Burczynski, M.E.; Crean, C.; Edwards, R.; Gaudilliere, B.; et al. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: Challenges and opportunities. Nat. Rev. Neurol. 2020, 16, 381–400. [Google Scholar] [CrossRef]
- Hassan, M.; Awan, F.M.; Naz, A.; deAndres-Galiana, E.J.; Alvarez, O.; Cernea, A.; Fernandez-Brillet, L.; Fernandez-Martinez, J.L.; Kloczkowski, A. Innovations in Genomics and Big Data Analytics for Personalized Medicine and Health Care: A Review. Int. J. Mol. Sci. 2022, 23, 4645. [Google Scholar] [CrossRef] [PubMed]
- Malagoli Tagliazucchi, G.; Wiecek, A.J.; Withnell, E.; Secrier, M. Genomic and microenvironmental heterogeneity shaping epithelial-to-mesenchymal trajectories in cancer. Nat. Commun. 2023, 14, 789. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Pan, X.; Zhang, S.; Zhang, Y.H.; Chen, L.; Wan, S.; Huang, T.; Cai, Y.D. Identification of Gene Signatures and Expression Patterns During Epithelial-to-Mesenchymal Transition from Single-Cell Expression Atlas. Front. Genet. 2020, 11, 605012. [Google Scholar] [CrossRef] [PubMed]
- Neagu, M.; Constantin, C.; Bostan, M.; Caruntu, C.; Ignat, S.R.; Dinescu, S.; Costache, M. Proteomic Technology “Lens” for Epithelial-Mesenchymal Transition Process Identification in Oncology. Anal. Cell. Pathol. 2019, 2019, 3565970. [Google Scholar] [CrossRef]
- Matadamas-Guzman, M.; Zazueta, C.; Rojas, E.; Resendis-Antonio, O. Analysis of Epithelial-Mesenchymal Transition Metabolism Identifies Possible Cancer Biomarkers Useful in Diverse Genetic Backgrounds. Front. Oncol. 2020, 10, 1309. [Google Scholar] [CrossRef]
- Orsini, A.; Diquigiovanni, C.; Bonora, E. Omics Technologies Improving Breast Cancer Research and Diagnostics. Int. J. Mol. Sci. 2023, 24, 12690. [Google Scholar] [CrossRef]
- Eicher, T.; Kinnebrew, G.; Patt, A.; Spencer, K.; Ying, K.; Ma, Q.; Machiraju, R.; Mathe, A.E.A. Metabolomics and Multi-Omics Integration: A Survey of Computational Methods and Resources. Metabolites 2020, 10, 202. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Agamah, F.E.; Bayjanov, J.R.; Niehues, A.; Njoku, K.F.; Skelton, M.; Mazandu, G.K.; Ederveen, T.H.A.; Mulder, N.; Chimusa, E.R.; t Hoen, P.A.C. Computational approaches for network-based integrative multi-omics analysis. Front. Mol. Biosci. 2022, 9, 967205. [Google Scholar] [CrossRef]
- Reimand, J.; Isserlin, R.; Voisin, V.; Kucera, M.; Tannus-Lopes, C.; Rostamianfar, A.; Wadi, L.; Meyer, M.; Wong, J.; Xu, C.; et al. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 2019, 14, 482–517. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Pan, D.; Wang, X.; Xu, Y.; Yan, J.; Wang, L.; Yang, X.; Yang, M.; Liu, G.P. Applications of multi-omics analysis in human diseases. MedComm (2020) 2023, 4, e315. [Google Scholar] [CrossRef]
- Mirza, B.; Wang, W.; Wang, J.; Choi, H.; Chung, N.C.; Ping, P. Machine Learning and Integrative Analysis of Biomedical Big Data. Genes 2019, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Miotto, R.; Wang, F.; Wang, S.; Jiang, X.; Dudley, J.T. Deep learning for healthcare: Review, opportunities and challenges. Brief. Bioinform. 2018, 19, 1236–1246. [Google Scholar] [CrossRef]
- Gao, F.; Huang, K.; Xing, Y. Artificial Intelligence in Omics. Genom. Proteom. Bioinform. 2022, 20, 811–813. [Google Scholar] [CrossRef]
- Tsimenidis, S.; Vrochidou, E.; Papakostas, G.A. Omics Data and Data Representations for Deep Learning-Based Predictive Modeling. Int. J. Mol. Sci. 2022, 23, 12272. [Google Scholar] [CrossRef]
- Sharma, A.; Lysenko, A.; Jia, S.R.; Boroevich, K.A.; Tsunoda, T. Advances in AI and machine learning for predictive medicine. J. Hum. Genet. 2024, 69, 487–497. [Google Scholar] [CrossRef]
- Das, T.; Andrieux, G.; Ahmed, M.; Chakraborty, S. Integration of online omics-data resources for cancer research. Front. Genet. 2020, 11, 578345. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, K.; Czerwińska, P.; Wiznerowicz, M. Review The Cancer Genome Atlas (TCGA): An immeasurable source of knowledge. Contemp. Oncol. Współczesna Onkol. 2015, 2015, 68–77. [Google Scholar] [CrossRef]
- Barrett, T.; Suzek, T.O.; Troup, D.B.; Wilhite, S.E.; Ngau, W.-C.; Ledoux, P.; Rudnev, D.; Lash, A.E.; Fujibuchi, W.; Edgar, R. NCBI GEO: Mining millions of expression profiles—Database and tools. Nucleic Acids Res. 2005, 33, D562–D566. [Google Scholar] [CrossRef] [PubMed]
- Jézéquel, P.; Gouraud, W.; Ben Azzouz, F.; Guérin-Charbonnel, C.; Juin, P.P.; Lasla, H.; Campone, M. bc-GenExMiner 4.5: New mining module computes breast cancer differential gene expression analyses. Database 2021, 2021, baab007. [Google Scholar] [CrossRef]
- Curtis, C.; Shah, S.P.; Chin, S.-F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The genomic and transcriptomic architecture of 2000 breast tumours reveals novel subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef]
- Subramanian, I.; Verma, S.; Kumar, S.; Jere, A.; Anamika, K. Multi-omics data integration, interpretation, and its application. Bioinform. Biol. Insights 2020, 14, 1177932219899051. [Google Scholar] [CrossRef]
- Torres-Martos, Á.; Bustos-Aibar, M.; Ramírez-Mena, A.; Cámara-Sánchez, S.; Anguita-Ruiz, A.; Alcalá, R.; Aguilera, C.M.; Alcalá-Fdez, J. Omics data preprocessing for machine learning: A case study in childhood obesity. Genes 2023, 14, 248. [Google Scholar] [CrossRef]
- Zebari, R.; Abdulazeez, A.; Zeebaree, D.; Zebari, D.; Saeed, J. A comprehensive review of dimensionality reduction techniques for feature selection and feature extraction. J. Appl. Sci. Technol. Trends 2020, 1, 56–70. [Google Scholar] [CrossRef]
- Tang, J.; Alelyani, S.; Liu, H. Feature selection for classification: A review. In Data Classification: Algorithms and Applications; CRC Press: Boca Raton, FL, USA, 2014; pp. 37–64. [Google Scholar]
- Koch, I.; Naito, K. Dimension selection for feature selection and dimension reduction with principal and independent component analysis. Neural Comput. 2007, 19, 513–545. [Google Scholar] [CrossRef]
- Haury, A.-C.; Gestraud, P.; Vert, J.-P. The influence of feature selection methods on accuracy, stability and interpretability of molecular signatures. PLoS ONE 2011, 6, e28210. [Google Scholar] [CrossRef]
- Bhavsar, H.; Ganatra, A. A comparative study of training algorithms for supervised machine learning. Int. J. Soft Comput. Eng. (IJSCE) 2012, 2, 2231–2307. [Google Scholar]
- Sambo, F.; Trifoglio, E.; Di Camillo, B.; Toffolo, G.M.; Cobelli, C. Bag of Naïve Bayes: Biomarker selection and classification from genome-wide SNP data. BMC Bioinform. 2012, 13, S2. [Google Scholar] [CrossRef] [PubMed]
- Vijayarani, S.; Muthulakshmi, M. Comparative analysis of bayes and lazy classification algorithms. Int. J. Adv. Res. Comput. Commun. Eng. 2013, 2, 3118–3124. [Google Scholar]
- Lam, V.K.; Nguyen, T.; Bui, V.; Chung, B.M.; Chang, L.-C.; Nehmetallah, G.; Raub, C.B. Quantitative scoring of epithelial and mesenchymal qualities of cancer cells using machine learning and quantitative phase imaging. J. Biomed. Opt. 2020, 25, 026002. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Biljecki, F. Unsupervised machine learning in urban studies: A systematic review of applications. Cities 2022, 129, 103925. [Google Scholar] [CrossRef]
- Lopez, C.; Tucker, S.; Salameh, T.; Tucker, C. An unsupervised machine learning method for discovering patient clusters based on genetic signatures. J. Biomed. Inform. 2018, 85, 30–39. [Google Scholar] [CrossRef]
- Myllyaho, L.; Raatikainen, M.; Männistö, T.; Mikkonen, T.; Nurminen, J.K. Systematic literature review of validation methods for AI systems. J. Syst. Softw. 2021, 181, 111050. [Google Scholar] [CrossRef]
- Ho, S.Y.; Phua, K.; Wong, L.; Goh, W.W.B. Extensions of the external validation for checking learned model interpretability and generalizability. Patterns 2020, 1, 100129. [Google Scholar] [CrossRef]
- Ganji, M.; Bakhshi, S.; Shoari, A.; Ahangari Cohan, R. Discovery of potential FGFR3 inhibitors via QSAR, pharmacophore modeling, virtual screening and molecular docking studies against bladder cancer. J. Transl. Med. 2023, 21, 111. [Google Scholar] [CrossRef]
- Rozova, V.S.; Anwer, A.G.; Guller, A.E.; Es, H.A.; Khabir, Z.; Sokolova, A.I.; Gavrilov, M.U.; Goldys, E.M.; Warkiani, M.E.; Thiery, J.P.; et al. Machine learning reveals mesenchymal breast carcinoma cell adaptation in response to matrix stiffness. PLoS Comput. Biol. 2021, 17, e1009193. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, M.; De Wilde, R.L.; Feng, R.; Su, M.; Torres-de la Roche, L.A.; Shi, W. A machine learning model to predict the triple negative breast cancer immune subtype. Front. Immunol. 2021, 12, 749459. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, Q.; Chen, C.; Chen, Z.; Zheng, R.; She, C.; Zhang, R.; Wu, J. Identification and comprehensive analysis of epithelial–mesenchymal transition related target genes of miR-222-3p in breast cancer. Front. Oncol. 2023, 13, 1189635. [Google Scholar] [CrossRef]
- Triantafyllou, A.; Dovrolis, N.; Zografos, E.; Theodoropoulos, C.; Zografos, G.C.; Michalopoulos, N.V.; Gazouli, M. Circulating miRNA expression profiling in breast cancer molecular subtypes: Applying machine learning analysis in bioinformatics. Cancer Diagn. Progn. 2022, 2, 739. [Google Scholar] [CrossRef]
- Vinik, Y.; Ortega, F.G.; Mills, G.B.; Lu, Y.; Jurkowicz, M.; Halperin, S.; Aharoni, M.; Gutman, M.; Lev, S. Proteomic analysis of circulating extracellular vesicles identifies potential markers of breast cancer progression, recurrence, and response. Sci. Adv. 2020, 6, eaba5714. [Google Scholar] [CrossRef]
- Ma, J.; Chen, K.; Li, S.; Zhu, L.; Yu, Y.; Li, J.; Ma, J.; Ouyang, J.; Wu, Z.; Tan, Y.; et al. MRI-based radiomic models to predict surgical margin status and infer tumor immune microenvironment in breast cancer patients with breast-conserving surgery: A multicenter validation study. Eur. Radiol. 2024, 34, 1774–1789. [Google Scholar] [CrossRef]
- Thalor, A.; Joon, H.K.; Singh, G.; Roy, S.; Gupta, D. Machine learning assisted analysis of breast cancer gene expression profiles reveals novel potential prognostic biomarkers for triple-negative breast cancer. Comput. Struct. Biotechnol. J. 2022, 20, 1618–1631. [Google Scholar] [CrossRef]
- Villemin, J.-P.; Lorenzi, C.; Cabrillac, M.-S.; Oldfield, A.; Ritchie, W.; Luco, R.F. A cell-to-patient machine learning transfer approach uncovers novel basal-like breast cancer prognostic markers amongst alternative splice variants. BMC Biol. 2021, 19, 70. [Google Scholar] [CrossRef]
- Jung, J.; Yoo, S. Identification of Breast Cancer Metastasis Markers from Gene Expression Profiles Using Machine Learning Approaches. Genes 2023, 14, 1820. [Google Scholar] [CrossRef]
- Wang, H.; Tan, Z.; Hu, H.; Liu, H.; Wu, T.; Zheng, C.; Wang, X.; Luo, Z.; Wang, J.; Liu, S.; et al. microRNA-21 promotes breast cancer proliferation and metastasis by targeting LZTFL1. BMC Cancer 2019, 19, 738. [Google Scholar] [CrossRef]
- Gou, Q.; Liu, Z.; Xie, Y.; Deng, Y.; Ma, J.; Li, J.; Zheng, H. Systematic evaluation of tumor microenvironment and construction of a machine learning model to predict prognosis and immunotherapy efficacy in triple-negative breast cancer based on data mining and sequencing validation. Front. Pharmacol. 2022, 13, 995555. [Google Scholar] [CrossRef]
- Kothari, C.; Osseni, M.A.; Agbo, L.; Ouellette, G.; Déraspe, M.; Laviolette, F.; Corbeil, J.; Lambert, J.-P.; Diorio, C.; Durocher, F. Machine learning analysis identifies genes differentiating triple negative breast cancers. Sci. Rep. 2020, 10, 10464. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qiao, H.; Wu, F.; Sun, S.; Feng, C.; Li, C.; Yan, W.; Lv, W.; Wu, H.; Liu, M.; et al. A novel hypoxia-and lactate metabolism-related signature to predict prognosis and immunotherapy responses for breast cancer by integrating machine learning and bioinformatic analyses. Front. Immunol. 2022, 13, 998140. [Google Scholar] [CrossRef] [PubMed]
- Font-Clos, F.; Zapperi, S.; La Porta, C.A. Classification of triple-negative breast cancers through a Boolean network model of the epithelial-mesenchymal transition. Cell Syst. 2021, 12, 457–462.e4. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.; Kalakoti, Y.; Sundar, D. Deep learning assisted multi-omics integration for survival and drug-response prediction in breast cancer. BMC Genom. 2021, 22, 214. [Google Scholar] [CrossRef]
- Yadav, S.; Zhou, S.; He, B.; Du, Y.; Garmire, L.X. Deep learning and transfer learning identify breast cancer survival subtypes from single-cell imaging data. Commun. Med. 2023, 3, 187. [Google Scholar] [CrossRef]
- Zhong, W.; Sun, T. Epithelial-mesenchymal transition (EMT) as a therapeutic target in cancer, Volume II. Front. Oncol. 2023, 13, 1218855. [Google Scholar] [CrossRef]
- Joosse, S.A.; Pantel, K. Biologic challenges in the detection of circulating tumor cells. Cancer Res. 2013, 73, 8–11. [Google Scholar] [CrossRef]
- De Las Rivas, J.; Brozovic, A.; Izraely, S.; Casas-Pais, A.; Witz, I.P.; Figueroa, A. Cancer drug resistance induced by EMT: Novel therapeutic strategies. Arch. Toxicol. 2021, 95, 2279–2297. [Google Scholar] [CrossRef]
- Akbar, M.W.; Isbilen, M.; Belder, N.; Canli, S.D.; Kucukkaraduman, B.; Turk, C.; Sahin, O.; Gure, A.O. A stemness and EMT based gene expression signature identifies phenotypic plasticity and is a predictive but not prognostic biomarker for breast cancer. J. Cancer 2020, 11, 949. [Google Scholar] [CrossRef] [PubMed]
- Imani, S.; Wei, C.; Cheng, J.; Khan, M.A.; Fu, S.; Yang, L.; Tania, M.; Zhang, X.; Xiao, X.; Zhang, X.; et al. MicroRNA-34a targets epithelial to mesenchymal transition-inducing transcription factors (EMT-TFs) and inhibits breast cancer cell migration and invasion. Oncotarget 2017, 8, 21362. [Google Scholar] [CrossRef]
- Addison, J.B.; Voronkova, M.A.; Fugett, J.H.; Lin, C.-C.; Linville, N.C.; Trinh, B.; Livengood, R.H.; Smolkin, M.B.; Schaller, M.D.; Ruppert, J.M.; et al. Functional hierarchy and cooperation of EMT master transcription factors in breast cancer metastasis. Mol. Cancer Res. 2021, 19, 784–798. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, S.; Kim, S.-J.; Shao, F.; Ho, J.W.K.; Wong, K.U.; Miao, Z.; Hao, D.; Zhao, M.; Xu, J.; et al. Cisplatin prevents breast cancer metastasis through blocking early EMT and retards cancer growth together with paclitaxel. Theranostics 2021, 11, 2442. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Yu, H.; Li, D.; Jin, G.; Dai, S.; Gong, P.; Kong, C.; Wang, X. The miR-5694/AF9/Snail axis provides metastatic advantages and a therapeutic target in basal-like breast cancer. Mol. Ther. 2021, 29, 1239–1257. [Google Scholar] [CrossRef]
- Yang, X.; Shang, P.; Yu, B.; Jin, Q.; Liao, J.; Wang, L.; Ji, J.; Guo, X. Combination therapy with miR34a and doxorubicin synergistically inhibits Dox-resistant breast cancer progression via down-regulation of Snail through suppressing Notch/NF-κB and RAS/RAF/MEK/ERK signaling pathway. Acta Pharm. Sin. B 2021, 11, 2819–2834. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.R.; Li, S.; Kennedy, M.; Payne, S.; Kilibarda, K.; Groth, J.; Bowie, M.; Parilla-Castellar, E.; de Ridder, G.; Marcom, P.K.; et al. Chemotherapy enriches for an invasive triple-negative breast tumor cell subpopulation expressing a precursor form of N-cadherin on the cell surface. Oncotarget 2016, 7, 84030. [Google Scholar] [CrossRef]
- Senthebane, D.A. The Role of the Tumour Microenvironment Components in Cancer Cell Behaviour and Drug Response. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 2022. [Google Scholar]
- Ahmad, A.; Aboukameel, A.; Kong, D.; Wang, Z.; Sethi, S.; Chen, W.; Sarkar, F.H.; Raz, A. Phosphoglucose isomerase/autocrine motility factor mediates epithelial-mesenchymal transition regulated by miR-200 in breast cancer cells. Cancer Res. 2011, 71, 3400–3409. [Google Scholar] [CrossRef]
- Zhu, D.; Zha, X.; Hu, M.; Tao, A.; Zhou, H.; Zhou, X.; Sun, Y. High expression of TIMP-1 in human breast cancer tissues is a predictive of resistance to paclitaxel-based chemotherapy. Med. Oncol. 2012, 29, 3207–3215. [Google Scholar] [CrossRef]
- Gan, R.; Yang, Y.; Yang, X.; Zhao, L.; Lu, J.; Meng, Q. Downregulation of miR-221/222 enhances sensitivity of breast cancer cells to tamoxifen through upregulation of TIMP3. Cancer Gene Ther. 2014, 21, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Zhang, K.; Savadelis, A.; Zmina, P.; Aguila, B.; Welford, S.M.; Abdul-Karim, F.; Bonk, K.W.; Keri, R.A.; Bedogni, B. The membrane tethered matrix metalloproteinase MT1-MMP triggers an outside-in DNA damage response that impacts chemo-and radiotherapy responses of breast cancer. Cancer Lett. 2019, 443, 115–124. [Google Scholar] [CrossRef]
- Olivares-Urbano, M.A.; Griñán-Lisón, C.; Zurita, M.; Del Moral, R.; Ríos-Arrabal, S.; Artacho-Cordón, F.; Arrebola, J.P.; González, A.R.; León, J.; Antonio Marchal, J.; et al. Matrix metalloproteases and TIMPs as prognostic biomarkers in breast cancer patients treated with radiotherapy: A pilot study. J. Cell. Mol. Med. 2020, 24, 139–148. [Google Scholar] [CrossRef]
- Yuan, J.; Xiao, C.; Lu, H.; Yu, H.; Hong, H.; Guo, C.; Wu, Z. Effects of various treatment approaches for treatment efficacy for late stage breast cancer and expression level of TIMP-1 and MMP-9. Cancer Biomark. 2018, 23, 1–7. [Google Scholar] [CrossRef]
- Saxena, M.; Stephens, M.A.; Pathak, H.; Rangarajan, A. Transcription factors that mediate epithelial–mesenchymal transition lead to multidrug resistance by upregulating ABC transporters. Cell Death Dis. 2011, 2, e179. [Google Scholar] [CrossRef]
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015, 527, 472–476. [Google Scholar] [CrossRef]
- Debien, V.; De Caluwe, A.; Wang, X.; Piccart-Gebhart, M.; Tuohy, V.K.; Romano, E.; Buisseret, L. Immunotherapy in breast cancer: An overview of current strategies and perspectives. NPJ Breast Cancer 2023, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Vathiotis, I.A.; Trontzas, I.; Gavrielatou, N.; Gomatou, G.; Syrigos, N.K.; Kotteas, E.A. Immune Checkpoint Blockade in Hormone Receptor-Positive Breast Cancer: Resistance Mechanisms and Future Perspectives. Clin. Breast Cancer 2022, 22, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Hai, L.; Jiang, Z.; Zhang, H.; Sun, Y. From multi-omics to predictive biomarker: AI in tumor microenvironment. Front. Immunol. 2024, 15, 1514977. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalili-Tanha, G.; Shoari, A. Innovative Approaches to EMT-Related Biomarker Identification in Breast Cancer: Multi-Omics and Machine Learning Methods. BioTech 2025, 14, 75. https://doi.org/10.3390/biotech14030075
Khalili-Tanha G, Shoari A. Innovative Approaches to EMT-Related Biomarker Identification in Breast Cancer: Multi-Omics and Machine Learning Methods. BioTech. 2025; 14(3):75. https://doi.org/10.3390/biotech14030075
Chicago/Turabian StyleKhalili-Tanha, Ghazaleh, and Alireza Shoari. 2025. "Innovative Approaches to EMT-Related Biomarker Identification in Breast Cancer: Multi-Omics and Machine Learning Methods" BioTech 14, no. 3: 75. https://doi.org/10.3390/biotech14030075
APA StyleKhalili-Tanha, G., & Shoari, A. (2025). Innovative Approaches to EMT-Related Biomarker Identification in Breast Cancer: Multi-Omics and Machine Learning Methods. BioTech, 14(3), 75. https://doi.org/10.3390/biotech14030075







