Abstract
Microalgae represent promising biotechnological platforms for bioactive compound production with pharmaceutical applications. This study investigated the phytochemical composition and biological activities of lipid extracts from three Chlorella species to evaluate their potential as antioxidant and antidiabetic sources. Lipid extraction using chloroform–methanol (2:1) followed by GC–MS analysis revealed distinct compound distributions: 29 compounds in C. ellipsoidea, 33 in C. sorokiniana, and 19 in C. vulgaris. Major bioactive compounds included 2-hexanol, 1,3,6-heptatriene, 4-(2,3-dimethyl-2-cyclopenten-1-yl)-4-methylpentanal, n-hexadecanoic acid, and octadecanoic acid. Biological activity screening encompassed antioxidant assessment through DPPH• and •NO radical scavenging assays and FRAP analysis, while antidiabetic potential was evaluated using α-glucosidase and α-amylase inhibition assays. C. sorokiniana exhibited superior bioactivity with the highest antioxidant capacity (DPPH• IC50 = 329.03 ± 4.30 µg/mL; •NO IC50 = 435.53 ± 10.20 µg/mL; FRAP = 94.74 ± 5.72 mg TE/g) and strongest enzyme inhibition (α-glucosidase IC50 = 752.75 ± 57.95 µg/mL; α-amylase IC50 = 3458.50 ± 104.01 µg/mL). This is the first report on C. sorokiniana strain KU.B2′s biological properties and phytochemical profile. These findings establish C. sorokiniana as a valuable biotechnological platform for pharmaceutical bioactive compound development.
Key Contribution:
This study reveals that among three Chlorella species, C. sorokiniana demonstrates superior antioxidant and antidiabetic properties, with the highest radical scavenging activity and enzyme inhibition capabilities, establishing it as a promising source of bioactive compounds for pharmaceutical applications.
1. Introduction
Reactive oxygen and nitrogen species (RONS) are free radicals and reactive molecules derived from oxygen and nitrogen, naturally produced within cells and organisms. The generation of RONS can occur both exogenously (e.g., through exposure to ultrasound scanning, drugs, foods, radiation, pollutants, xenobiotics, and toxic chemicals) and endogenously (i.e., produced by plasma, white blood cell components, enzymes, or mitochondria [1,2]. An excessive release of RONS can lead to the impairment of biological structures, disrupting cell membranes, proteins, lipids, and DNA. This oxidative process is associated with the development of numerous chronic degenerative diseases [3,4]. The oxidative damage caused by RONS signaling is implicated in a wide array of conditions, including AIDS, Alzheimer’s disease, atherosclerosis, cancer, diabetes, hypertension, inflammation, neurodegenerative diseases, Parkinson’s disease, stroke, and sepsis [5,6,7,8]. The mitigation of oxidative stress through antioxidants has shown promise in treating RONS-related diseases.
RONS play a role in the pathogenesis of diabetes, particularly type 2 diabetes, which accounts for 90% to 95% of diabetic cases. Type 2 diabetes is characterized by hyperglycemia, insulin deficiency, and insulin resistance [9,10,11]. The effective management of diabetes involves reducing postprandial hyperglycemia, a crucial aspect achieved through inhibiting carbohydrate-hydrolyzing enzymes such as α-amylase and α-glucosidase, which slow down glucose absorption [12,13]. The α-amylase breaks down long-chain carbohydrates, while α-glucosidase breaks down starch and disaccharides into glucose subunits. Therefore, using enzyme inhibitors presents a promising avenue for reducing glucose absorption, representing a critical approach in developing compounds for diabetes treatment [14].
Chlorella, an exceptional microalga, is an established platform for producing bioproducts for producing various bioactive compounds. The species is widely recognized and commercially cultivated for its rich content of bioactive components, including carotenoids, lipids, proteins, polysaccharides, polyphenols, flavonoids, and vitamins [15,16,17,18,19,20,21]. Extracts derived from Chlorella have undergone extensive evaluation for their diverse beneficial properties, such as their anti-aging effects [22] and their potential anticancer [23], antidiabetic [24], antiproliferative [25], antimicrobial [15], anti-inflammatory [26], antioxidant [27], and antitumor [28] activities.
The selection of Chlorella ellipsoidea, C. vulgaris, and C. sorokiniana in this study was based on their taxonomic proximity and distinct ecological origins. C. ellipsoidea and C. vulgaris are commercially available strains that are commonly cultivated for their high nutritional value and bioactive potential, while C. sorokiniana strain KU.B2, isolated from an agricultural drainage system in Thailand, remains underexplored in terms of its biochemical and pharmacological properties. This combination offers a comparative perspective between well-established and potentially novel microalgal sources.
In recent years, advancements in microalgal biotechnology have revealed significant therapeutic potentials of green algae, particularly in the context of chronic metabolic diseases. Cutting-edge studies have focused on identifying bioactive lipids and antioxidants from microalgae species, contributing to a growing interest in their pharmaceutical applications [29]. Moreover, the integration of lipidomic approaches has enhanced the understanding of structure–activity relationships of algal-derived fatty acids and alcohols in oxidative stress regulation and enzyme inhibition [30]. However, despite these advancements, lipid-rich strains such as C. sorokiniana KU.B2 remain underexplored, presenting a unique opportunity for novel compound discovery and functional characterization.
Exploring Chlorella in pharmaceutical research has highlighted its significant potential due to various biological properties. In recent years, interest has been increasing regarding the antioxidant and antidiabetic effects of lipids derived from microalgae [24,31,32,33]. However, few studies have focused on the lipid extracts of specific Chlorella species, particularly regarding their antioxidant and antidiabetic activities. Therefore, this study aims to analyze lipid extracts from the Chlorella species, namely C. ellipsoidea, C. sorokiniana, and C. vulgaris, using GC–MS analysis. The goal is to evaluate the potential antioxidant and antidiabetic properties of these extracts.
2. Materials and Methods
2.1. General Chemicals and Materials
All chemicals used in this study were of analytical grade. Methanol was sourced from Merck (Burlington, MA, USA), chloroform from RCl Labscan Ltd. (Bangkok, Thailand), and hexane from Baker (Center Valley, PA, USA). Additional reagents included sulfanilamide (Carlo Erba Reagents, Milan, France), phosphoric acid (Macron Fine Chemicals, Beijing, China), sodium nitroprusside (Himedia Laboratories, Mumbai, Maharashtra,, India), and naphthylethylenediamine hydrochloride (AppliChem Panreac, Darmstadt, Germany). The FRAP assay utilized 2,4,6-tripyridyl-s-triazine (TPTZ; Fluka, Buchs, Switzerland) and ferric chloride (Chem-supply Pty Ltd., Gillman, SA, Australia). Enzymes and substrates for the antidiabetic assays included α-glucosidase from Saccharomyces cerevisiae, 4-nitrophenyl α-D-glucopyranoside, and α-amylase from the porcine pancreas (all from Sigma-Aldrich, St. Louis, MO, USA), as well as starch (TCI Chemicals, Tokyo, Japan) and dinitrosalicylic acid (DNS). Analytical instruments used were a 96-well microplate reader (Thermo Scientific Multiskan FC, Shanghai, China), Büchi Rotavapor R-210 (Mumbai, India), and Shimadzu GC–MS QP2020 system (Kyoto, Japan).
2.2. Strains and Culture Conditions
The microalgal strains C. ellipsoidea, C. sorokiniana, and C. vulgaris were cultivated in a liquid TAP (Tris–Acetate–Phosphate) medium under previously established protocols [34]. Cultivation was carried out under controlled conditions, including white fluorescent illumination at 330 μmol/m2/s, pH 7.0, and a constant temperature of 30 ± 1 °C, with manual agitation performed five times daily over a 9-day period to ensure uniform growth and adequate nutrient and light distribution [35].
2.3. Preparation of the Crude Extract
After cultivation, three Chlorella species were harvested via centrifugation at 3000 rpm for 5 min. The collected biomass was then dried in a hot-air oven at 60 °C to remove residual moisture and weighed to determine the dry weight. Once dried, the algal material was ground into a coarse powder. For lipid extraction, 20 mg of dried powder from each species was immersed in 50 mL of a chloroform–methanol mixture (2:1 v/v) at room temperature for 7 days. This prolonged extraction period was selected to ensure the complete diffusion and solubilization of lipids from the rigid cell walls of Chlorella, as supported by prior studies employing extended maceration times in microalgal lipid extraction [36].
The lipid content was determined on a dry weight basis and showed significant variation among the three Chlorella species. C. vulgaris exhibited the highest lipid content at 60% dry weight (12 mg from 20 mg dry biomass), followed by C. sorokiniana at 27.25% dry weight (5.15 mg from 20 mg dry biomass), and C. ellipsoidea at 25% dry weight (5 mg from 20 mg dry biomass). These results indicate notable differences in the lipid accumulation capacity among the species, with C. vulgaris demonstrating superior lipid productivity compared to the other two strains. These extracts were stored at −20 °C in the dark until further analysis.
2.4. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
Lipid extracts obtained from each Chlorella species were subjected to acid-catalyzed methanolysis by mixing with 1 mL of 1 M HCl in methanol under a gentle nitrogen gas stream for 1 min to facilitate esterification. The reaction mixture was then incubated at 80 °C for 40 min and subsequently allowed to cool to ambient temperature. To enhance the phase separation, 1 mL of 0.9% NaCl solution and 1 mL of hexane were added, followed by centrifugation at 3000 rpm for 3 min. The upper hexane layer was carefully collected and evaporated to dryness in preparation for chromatographic analysis [37].
The derivatized samples were analyzed using a Shimadzu GC–MS QP2020 system equipped with an SH-RXI-5SIL MS column (30 m × 0.25 mm i.d., 0.25 µm film thickness). Helium served as the carrier gas at a constant flow rate of 1.0 mL/min. One microliter of each sample was injected in the split mode at an injector temperature of 250 °C. The oven temperature program began at 40 °C and increased at 5 °C per minute to a final temperature of 300 °C, which was held for 8 min after reaching the target. Compound identification was performed using a post-run analysis software integrated with the NIST14 mass spectral database.
2.5. DPPH• Scavenging Activity
The antioxidant capacity was evaluated using the DPPH• radical scavenging assay following a previously described method [7]. A volume of 150 µL of each algal extract was mixed with 150 µL of 0.2 mM DPPH solution in methanol. The reaction mixture was incubated in the dark at 25 °C for 30 min. After incubation, the absorbance was measured at 520 nm. The DPPH• radical scavenging activity was calculated using the following equation:
where Acontrol is the absorbance of the DPPH solution without extract and Asample is the absorbance in the presence of the algal extract.
%Inhibition = (Acontrol − Asample) × 100/Acontrol
2.6. Nitric Oxide Scavenging Activity
A method described by Suksungworn, et al. [38] was used to determine •NO scavenging activity. A total of 125 µL of 10 mM sodium nitroprusside in PBS was mixed with 25 µL of extract and incubated for 150 min. The reaction was developed with 50 µL of Griess reagent (1% sulfanilamide, 2% phosphoric acid, and 0.1% naphthylethylenediamine), and absorbance was recorded at 546 nm.
2.7. Ferric-Reducing Antioxidant Power (FRAP) Activity
The ferric-reducing antioxidant power (FRAP) activity was determined following the method described by Suksungworn, et al. [7]. A fresh FRAP reagent was prepared by combining 300 mM acetate buffer (pH 3.6), 10 mM TPTZ, and 20 mM ferric chloride in a 10:1:1 ratio. For each reaction, 15 µL of extract was mixed with 285 µL of the FRAP solution, incubated in the dark for 30 min, and measured at 593 nm. Results were based on a calibration curve constructed using Trolox standards (0–250 mg/L), with the regression equation of y = 0.01x + 0.2046 (R2 = 0.9917).
2.8. α-Glucosidase Activity
To assess α-glucosidase activity inhibition, a previously reported procedure was followed, as outlined by Ferreres et al. [39]. Inhibitory activity against α-glucosidase was evaluated by incubating 50 µL of extract with 130 µL of 100 mM phosphate buffer (pH 6.8) and 20 µL of α-glucosidase (0.28 U/mL) at 37 °C for 10 min. Afterward, 100 µL of 0.5 mM 4-nitrophenyl α-D-glucopyranoside was added. The reaction was quantified by measuring the absorbance at 405 nm and this was compared with acarbose as the positive control.
2.9. α-Amylase Activity
The inhibition of α-amylase was evaluated following the method described by Ferreres et al. [39]. To evaluate α-amylase inhibition, 200 µL of 1% starch solution was incubated with 200 µL of extract at 25 °C for 10 min. Then, 200 µL of α-amylase (15 U/mL) was added. After another 10 min, 400 µL of DNS reagent was added, and the mixture was boiled at 100 °C for 5 min. Following cooling, 80 µL of water was added, and the absorbance was measured at 540 nm and compared with acarbose as the positive control.
2.10. Statistical Analysis
Statistical analysis was performed by expressing the data as the mean ± standard deviation of three independent analyses (n = 3). Data were analyzed using a one-way analysis of variance (ANOVA) to compare differences between the treatment groups and concentrations, and this was followed by Tukey’s multiple comparisons test for post hoc analysis. Prior to the ANOVA, the assumptions for parametric testing were verified: the normality of data distribution was assessed using the Shapiro–Wilk test, and homogeneity of variance was confirmed using Levene’s test. The statistical tests were carried out using GraphPad Prism 6.01 (San Diego, CA, USA). A p-value of less than 0.05 (*), p < 0.01 (**), p < 0.001 (***), and p < 0.0001 (****) were considered to be statistically significant, with ns indicating no significant difference.
3. Results and Discussion
3.1. GC–MS Profiling of Chlorella Lipid Extracts
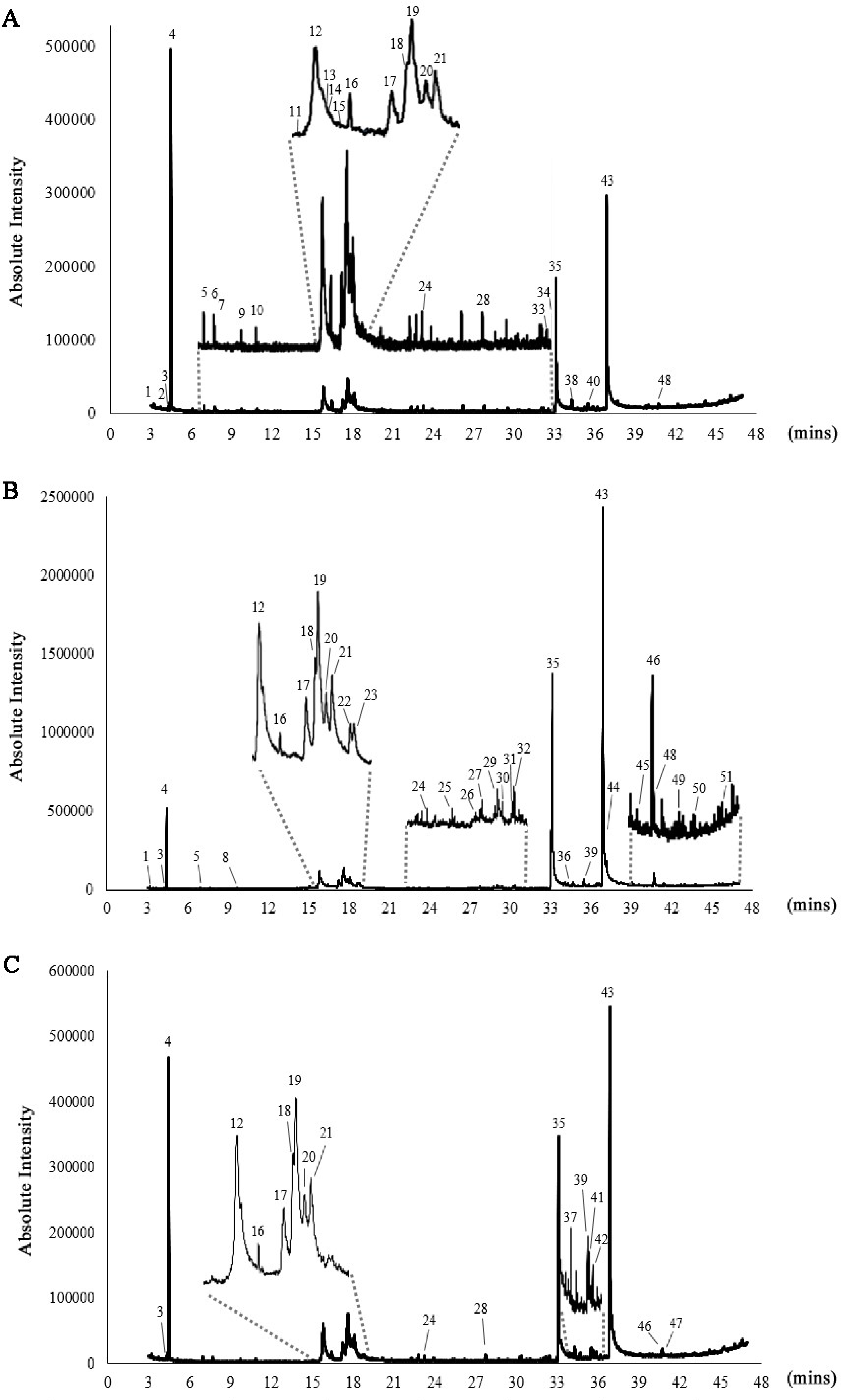
Gas chromatography–mass spectrometry (GC–MS) analysis of the lipid extracts from Chlorella species revealed the presence of phytochemical constituents, tentatively identifying 29 compounds in C. ellipsoidea, 33 compounds in C. sorokiniana, and 19 compounds in C. vulgaris (Figure 1 and Table 1). Among these compounds, the major constituents with peaks greater than 5% were identified as 2-Hexanol, (R)-, 1,3,6-Heptatriene, 2,5,5-trimethyl, 4-(2,3-Dimethyl-2-cyclopenten-1-yl)-4-methylpentanal, n-hexadecanoic acid, and octadecanoic acid, as depicted in Figure 2. The investigation of Chlorella extracts in this study unveiled a diverse array of phytoconstituents, including fatty acids, alcohols, and straight-chain hydrocarbon compounds. Previous studies have reported on the chemical composition of lipid extracts from C. sorokiniana and C. vulgaris [18,21,40]. Our results are consistent with these findings, showing that all three Chlorella species produced n-hexadecanoic and octadecanoic acids as major components, with C. vulgaris extracts containing >92% fatty acids ranging from C16 to C18. The chemical constituents identified in Chlorella extracts may hold significance in the context of pharmacological substances.
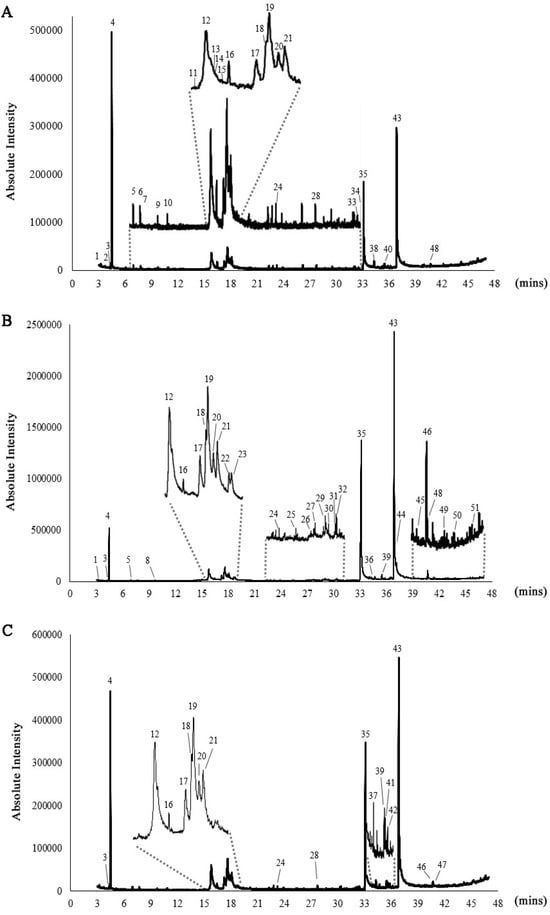
Figure 1.
GC–MS chromatograms of C. ellipsoidea (A), C. sorokiniana (B), and C. vulgaris (C). The numbered peaks represent identified compounds, with their corresponding retention times (Rt) and relative abundances (%) provided in Table 1.

Table 1.
Chemical composition of C. ellipsoidea, C. sorokiniana, and C. vulgaris identified by GC–MS analysis. Compounds are listed with their retention time (Rt) and relative abundance (ratio) in each Chlorella extract. “—” indicates not detected.
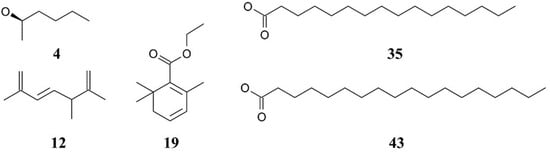
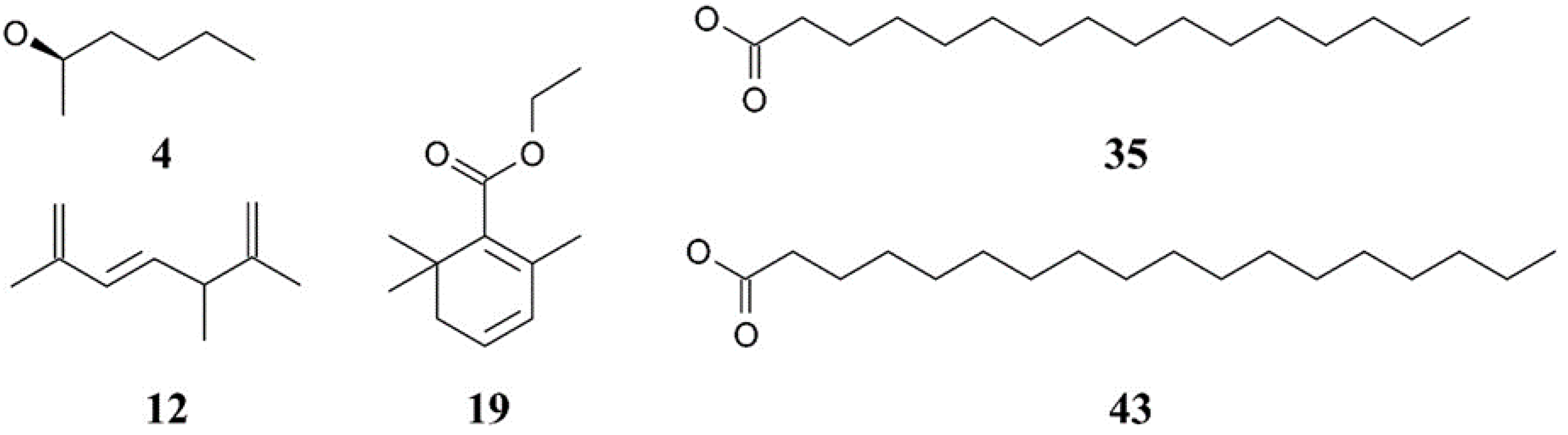
Figure 2.
Chemical structures of the major compounds identified in Chlorella extracts. The compounds include 4—Butanal, 3-methyl- (isovaleraldehyde), 12—2,6-Octadiene, 2,6-dimethyl- (myrcene), 19—2,4-Hexadienoic acid, 3-(2,6,6-trimethyl-1-cyclohexen-1-yl)-, ethyl ester, 35—Hexadecanoic acid, ethyl ester (ethyl palmitate), and 43—9,12-Octadecadienoic acid (Z,Z)-, ethyl ester (ethyl linoleate). These compounds were identified using GC–MS analysis.
The chemical constituents identified in Chlorella extracts play significant roles in their observed antioxidant and antidiabetic activities. n-Hexadecanoic acid (palmitic acid) and octadecanoic acid (stearic acid), the dominant saturated fatty acids in our extracts, are well-documented for their bioactive properties. These fatty acids contribute to antioxidant activity through multiple mechanisms: they can donate hydrogen atoms to neutralize free radicals, chelate metal ions that catalyze oxidative reactions, and stabilize cell membrane structures against lipid peroxidation [41]. In terms of antidiabetic effects, these fatty acids have been shown to inhibit α-glucosidase and α-amylase enzymes by binding to their active sites, thereby reducing carbohydrate digestion and postprandial glucose spikes [42].
2-Hexanol, though not typically reported as a major microalgae component, demonstrated notable contributions to the bioactivity profiles. This volatile alcohol exhibits antioxidant potential through its ability to scavenge hydroxyl radicals and inhibit lipid peroxidation processes [43]. Its presence in microalgae extracts may result from metabolic processes involving fatty acid degradation or as part of the volatile organic compound (VOC) profile that microalgae naturally produce [29,44]. The antidiabetic properties of hexanol compounds have been attributed to their capacity to modulate glucose metabolism and enhance insulin sensitivity through interaction with cellular signaling pathways.
The synergistic effects of these constituents likely enhance the overall bioactivity of Chlorella extracts. The combination of saturated fatty acids and alcohols creates a multi-target approach for combating oxidative stress and managing diabetes-related enzyme activities. n-Hexadecanoic and octadecanoic acids provide the primary antioxidant and enzyme inhibitory framework, while compounds like 2-hexanol may act as supporting agents that enhance the radical scavenging capacity and membrane protection. This synergistic interaction explains why C. sorokiniana, despite having a diverse phytochemical profile with 33 identified compounds, demonstrated superior bioactivity compared to the other species. The therapeutic prospects of these Chlorella extracts are therefore supported by the complementary mechanisms of action exhibited by their constituent compounds, making them promising candidates for natural antioxidant and antidiabetic applications.
3.2. Antioxidant Activity of Chlorella Extracts
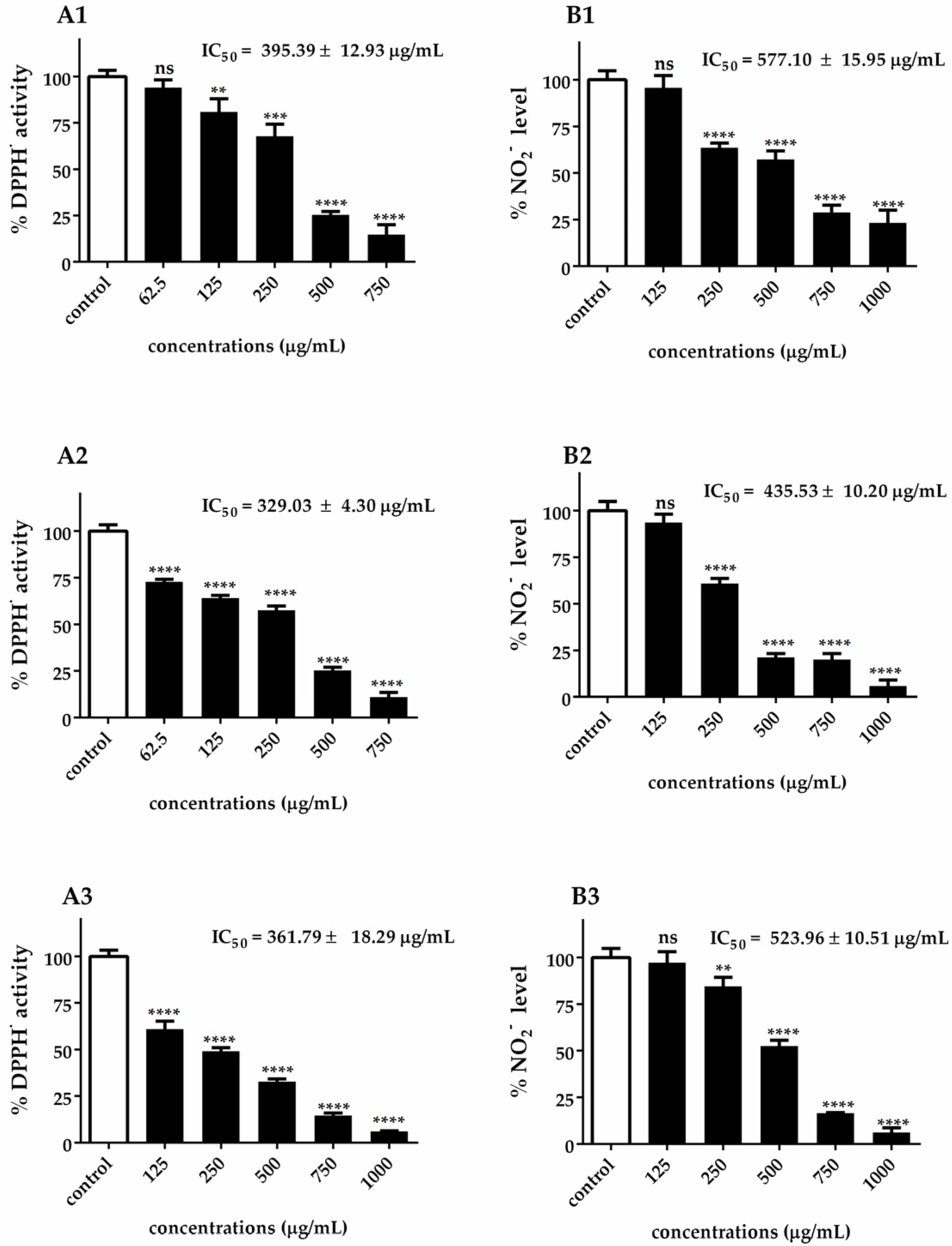
The assessment of antioxidant activity requires a multifaceted approach due to the intricate nature of chemical constituents and their diverse mechanisms of action. In this study, the similar patterns observed between DPPH• radical scavenging activity (A1–A3) and •NO scavenging activity (B1–B3) in Figure 3 can be attributed to several interconnected antioxidant mechanisms inherent in Chlorella extracts. Both DPPH• and •NO radical scavenging assays rely on the hydrogen-donating capacity of antioxidant compounds, explaining why extracts with a high DPPH• scavenging activity also demonstrate strong •NO scavenging properties. The parallel dose–response relationships observed across both assays suggest that the same bioactive compounds are responsible for both radical scavenging activities.
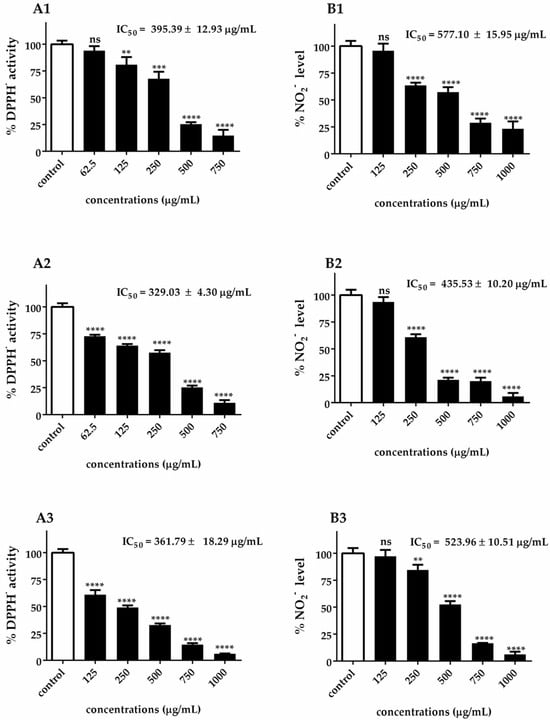
Figure 3.
DPPH• radical scavenging activity (A1–A3) and •NO scavenging activity (B1–B3) of C. ellipsoidea (1), C. sorokiniana (2), and C. vulgaris (3) at various concentrations. Each value represents the mean ± SD (n = 3). IC₅₀ values are shown in each panel. Statistical significance was determined by two-way ANOVA followed by Tukey’s post hoc test for concentration-dependent effects within each species: p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****), ns = not significant.
This correlation is particularly evident in the ranking of species efficacy, where C. ellipsoidea consistently demonstrated the lowest antioxidant capacity (DPPH• IC₅₀ = 395.39 ± 12.93 μg/mL; •NO IC50 = 577.10 ± 15.95 μg/mL), C. vulgaris showed intermediate performance (DPPH• IC50 = 361.79 ± 18.29 μg/mL; •NO IC50 = 523.96 ± 10.51 μg/mL), and C. sorokiniana exhibited the highest efficacy (DPPH• IC50 = 329.03 ± 4.30 μg/mL; •NO IC50 = 435.53 ± 10.20 μg/mL). This ranking pattern is consistently maintained across both assay systems, reinforcing the concept that similar antioxidant mechanisms are operating.
It is important to note that while the DPPH• assay measures the general free radical scavenging capacity against stable synthetic radicals, the •NO scavenging activity specifically targets nitric oxide radicals, which are predominantly generated during inflammatory processes. The similar efficacy patterns observed between these two distinct assays suggest that Chlorella extracts possess broad-spectrum antioxidant properties capable of neutralizing both general oxidative stress and inflammation-associated reactive nitrogen species. This dual functionality is particularly significant from a therapeutic perspective, as it indicates that these extracts may provide comprehensive protection against both general oxidative damage and inflammation-mediated nitrosative stress.
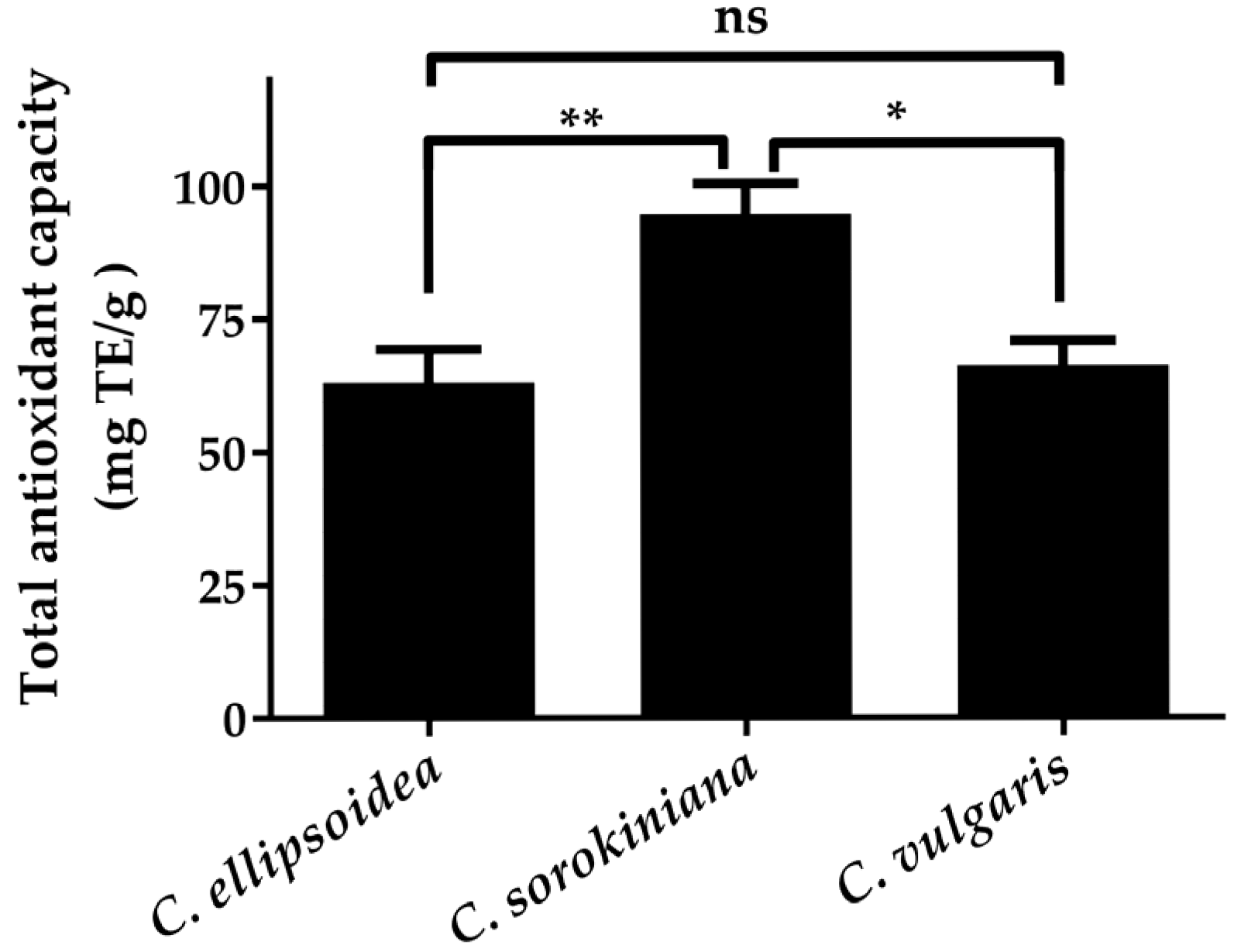
The consistent ranking across different radical scavenging assays is further validated by the FRAP results (Figure 4), where C. sorokiniana demonstrated the highest total antioxidant capacity (94.74 ± 5.72 mg TE/g), followed by C. vulgaris (66.21 ± 4.60 mg TE/g) and C. ellipsoidea (63.11 ± 6.23 mg TE/g). Although the FRAP ranking differs slightly from the DPPH• and •NO scavenging patterns, all three assays consistently identify C. ellipsoidea as having the lowest overall antioxidant performance, supporting the reliability of the observed trends.
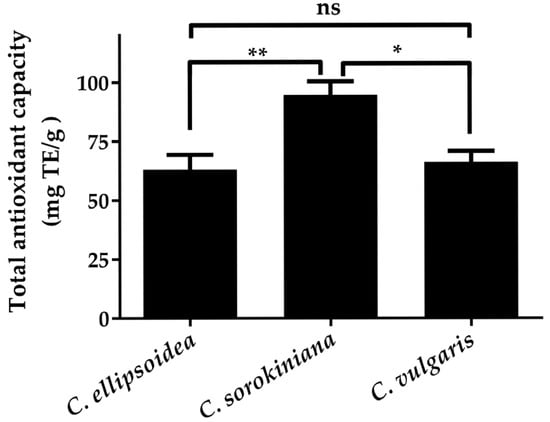
Figure 4.
Total antioxidant capacity of C. ellipsoidea, C. sorokiniana, and C. vulgaris measured by FRAP assay. Data are presented as mean ± standard deviation (n = 3). Statistical differences were evaluated using two-way ANOVA followed by Tukey’s post hoc test. Significant differences: p < 0.05 (*), p < 0.01 (**), ns = not significant.
Our findings align with previous studies on Chlorella species, which have extensively explored the in vitro and in vivo antioxidant activities of these microalgae. For instance, a previous report highlighted the peptide from C. ellipsoidea as an inhibitor of free-radical-induced oxidative stress [45]. In the case of C. sorokiniana, antioxidant enzymes including ascorbate peroxidase (APX), glutathione reductase (GR), glutathione S transferase (GST), peroxidase (POX), and superoxide dismutase (SOD) were presented [46]. Moreover, C. sorokiniana also demonstrates antioxidant properties, including studies involving a cell-based assay assessing the survival of Caenorhabditis elegans under oxidative stress [47], the inhibition of radical scavenging [48], the reduction in ROS products in the mitochondria [49], and reversible physiological oxidative perturbation [50]. Evaluation of the physiological response of C. vulgaris has revealed the presence of oxidative enzymes such as SOD and catalase (CAT) [51]. Furthermore, C. vulgaris has been investigated in vivo studies, including naphthalene-induced lipid peroxidation in the serum, liver, and kidneys of rats [52], and malondialdehyde (MDA), SOD, and glutathione peroxidase (GPx) in the livers of rats [53].
Despite the known presence of n-hexadecanoic and octadecanoic acid in Chlorella species, their antioxidant effects have been reported [54,55]. Previous studies have highlighted the antioxidant properties of hexanol, and its derivatives found in extracts from various sources such as pomegranate, mung bean, ripe coffee bean, and soybean [56,57,58]. These studies demonstrated that extracts with high concentrations of hexanol and its derivatives contained potent antioxidant compounds. The antioxidant activity observed in Chlorella extracts may be attributed to major constituents such as n-hexadecanoic and octadecanoic acid. Similarly, other algae species known to contain significant amounts of these fatty acids, such as Spirulina platensis and Dunaliella salina, have also demonstrated potential antioxidant activity [59,60]. Previous studies have indicated that algae’s fatty acid composition, including n-hexadecanoic and octadecanoic acid, contributes to their antioxidant properties [61]. This suggests that the antioxidant potential of algae like S. platensis and D. salina could be linked to their content of n-hexadecanoic acid and octadecanoic acid, supporting their role as natural sources of antioxidants. Therefore, our results support the potential antioxidant effects of Chlorella species. Notably, our findings indicated that the highest level of inhibition was observed with C. sorokiniana.
3.3. Antidiabetic Enzyme Inhibition by Chlorella Extracts
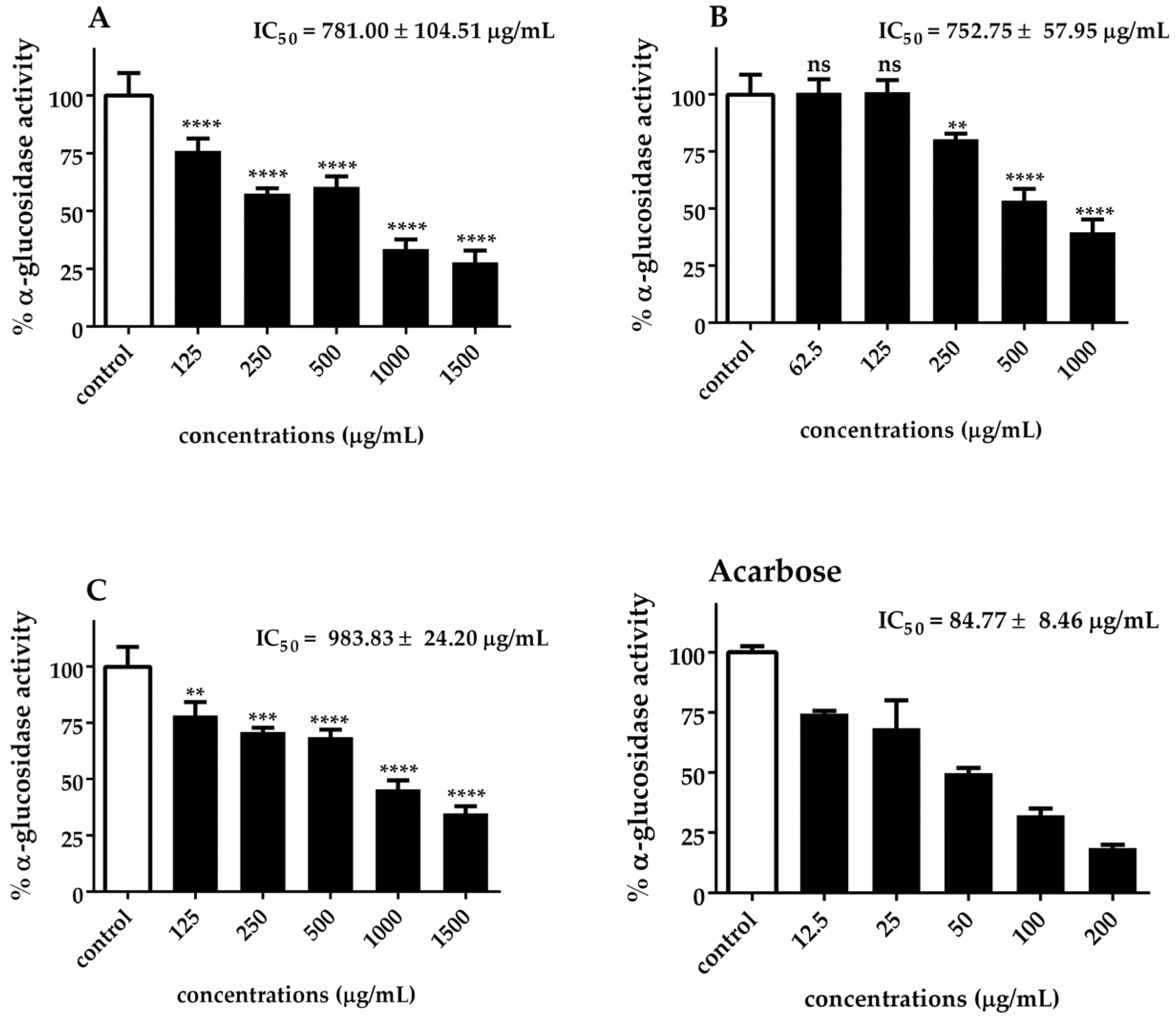
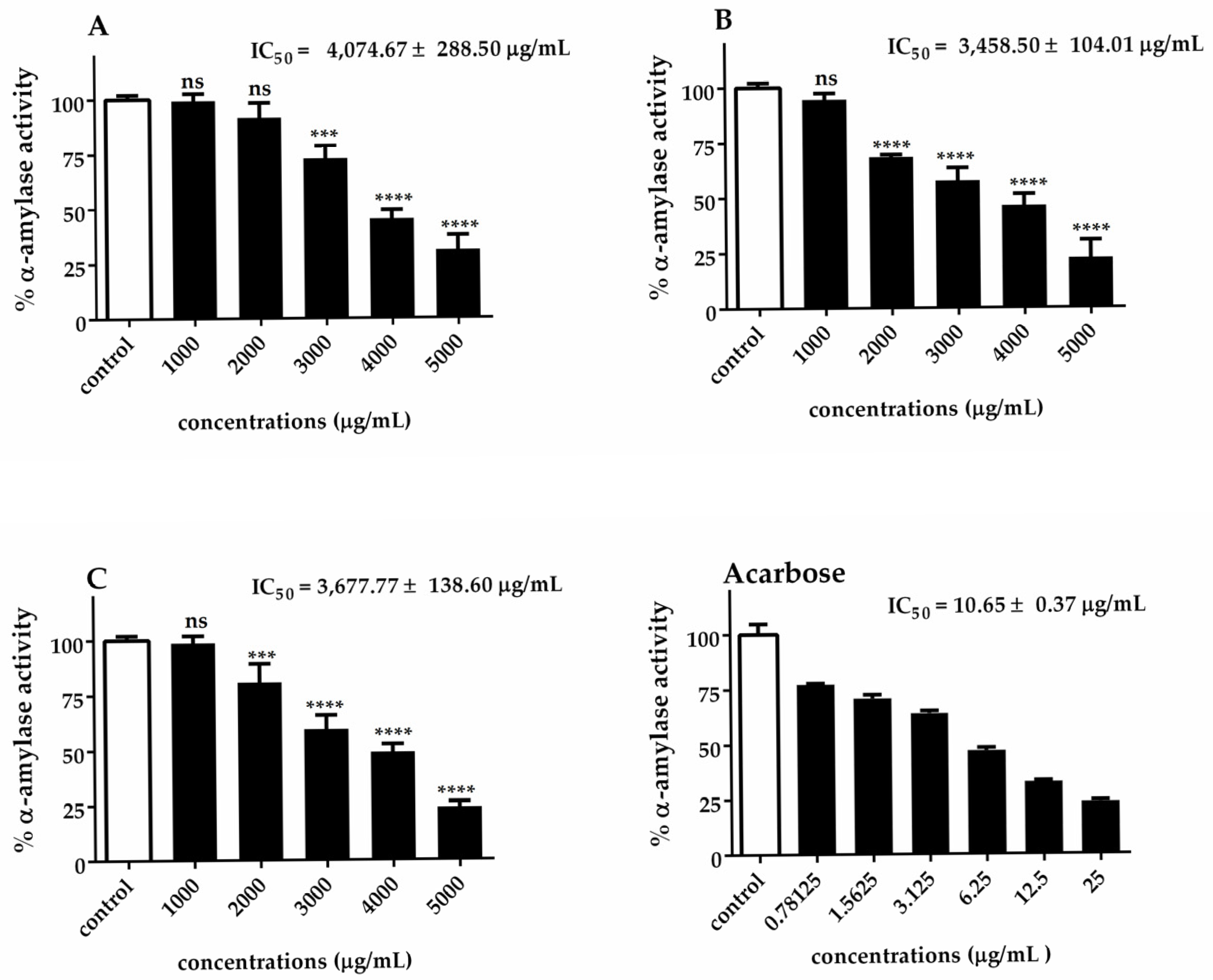
The α-glucosidase and α-amylase IC50 values of Chlorella extracts are presented in Figure 5 and Figure 6. Our results indicated that C. sorokiniana exhibited the highest potential α-glucosidase inhibitory activity (IC50 = 752.75 ± 57.95 μg/mL), followed by C. ellipsoidea (IC50 = 781.00 ± 104.51 μg/mL) and C. vulgaris (IC50 = 983.83 ± 24.20 μg/mL). The observed inhibitory effects were statistically significant for each treatment, except for 62.5 and 125 μg/mL of C. sorokiniana. Regarding the inhibition of α-amylase activity, it was noted that C. sorokiniana displayed the greatest inhibitory activity (IC50 = 3458.50 ± 104.01 μg/mL), followed by C. vulgaris (IC50 = 3677.77 ± 138.60 μg/mL) and C. ellipsoidea (IC50 = 4074.67 ± 288.50 μg/mL). Comparatively, the α-amylase and α-glucosidase inhibitory activities of Chlorella extracts were lower than those of acarbose (standard reference). The inhibitory effects exhibited concentration-dependent trends for different concentrations of the extracts and the positive control, acarbose. These results highlight the potential of Chlorella extracts, particularly C. sorokiniana, as inhibitors of α-glucosidase and α-amylase activities, which are crucial targets in managing diabetes.
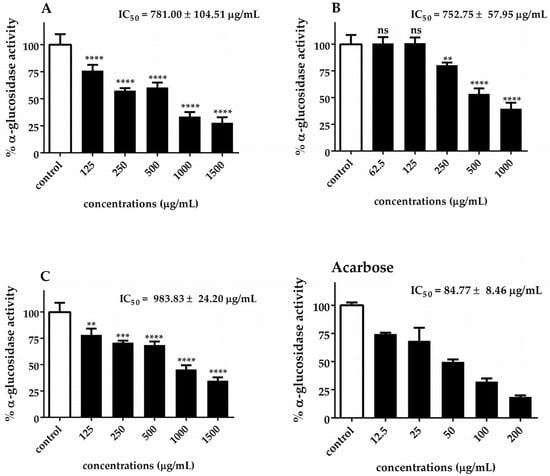
Figure 5.
Inhibitory effect of C. ellipsoidea (A), C. sorokiniana (B), and C. vulgaris (C) extracts on α-glucosidase activity at various concentrations. Acarbose was used as a positive control. Data are expressed as mean ± SD (n = 3). IC₅₀ values are shown in each panel. Statistical significance was determined by two-way ANOVA followed by Tukey’s post hoc test: p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****), ns = not significant.
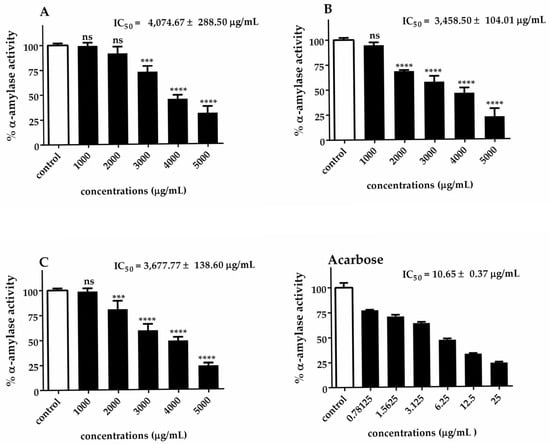
Figure 6.
Inhibitory effect of C. ellipsoidea (A), C. sorokiniana (B), and C. vulgaris (C) extracts on α-amylase activity at various concentrations. Acarbose was used as a positive control. Data are expressed as mean ± SD (n = 3). IC₅₀ values are shown in each panel. Statistical significance was determined by two-way ANOVA followed by Tukey’s post hoc test: p < 0.001 (***), p < 0.0001 (****), ns = not significant.
Several research studies have explored the in vivo effects of antidiabetic agents [62,63]. However, the available literature is limited in terms of the specific effects of Chlorella extracts on the inhibition of α-glucosidase and α-amylase activities [36]. Some bioactive compounds have been identified for their ability to inhibit these enzymes. For example, hexanol from Vitis vinifera and Agaricus campestris has demonstrated hypoglycemic potential and insulin activity [64,65]. Notably, hexanol has not previously been associated with α-glucosidase and α-amylase inhibitors. On the other hand, previous studies on fatty acids and their effects on antidiabetic enzymes have shown that n-hexadecanoic and octadecanoic acids, identified as chemical constituents, exhibit the potent inhibition of α-glucosidase and α-amylase [41,42].
4. Conclusions
This study comparatively evaluated the phytochemical composition and bioactivities of lipid extracts from three Chlorella species (C. ellipsoidea, C. sorokiniana, and C. vulgaris), with GC–MS profiling revealing dominant fatty acids including n-hexadecanoic and octadecanoic acids across all species. Among the three species, C. sorokiniana KU.B2 demonstrated superior antioxidant activity (DPPH• IC50 = 329.03 ± 3.30 μg/mL; NO• IC50 = 455.53 ± 10.20 μg/mL) and the strongest antidiabetic potential (α-glucosidase IC50 = 752.75 ± 57.95 μg/mL; α-amylase IC50 = 3.458 ± 0.104 μg/mL), while C. ellipsoidea and C. vulgaris showed moderate activities.
Importantly, our study provides the first report of antioxidant and antidiabetic activities from the C. sorokiniana KU.B2 strain, highlighting its novelty and value as a local microalgal resource. The identification of bioactive constituents in this novel isolate opens new opportunities for the bioprospecting of microalgae from natural and agricultural environments. These findings establish a foundation for pharmaceutical development applications and demonstrate the significant potential of underexplored local Chlorella strains as sources of natural antioxidants and antidiabetic compounds for therapeutic applications.
Author Contributions
S.K. cultivated the microalgae, conducted the experiments, analyzed the data and wrote the first draft of the manuscript. S.K., R.S. (Rattanaporn Songserm) and R.S. (Rungcharn Suksungworn) conducted the experiments and analyzed the data. S.D. provided resources and guidelines for the experiments and reviewed the manuscript. N.S. provided conceptualizations and resources, and reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by The Mahidol Wittayanusorn School Scholarship from the Mahidol Wittayanusorn School, Kasetsart University Research and Development Institute, KURDI Program Number FF(KU-SRIU)1.67, and International SciKU Branding (ISB), Faculty of Science, Kasetsart University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jha, N.; Ryu, J.J.; Choi, E.H.; Kaushik, N.K. Generation and Role of Reactive Oxygen and Nitrogen Species Induced by Plasma, Lasers, Chemical Agents, and Other Systems in Dentistry. Oxidative Med. Cell. Longev. 2017, 2017, 7542540. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, M.; Kim, S.J. Reactive oxygen/nitrogen species and their functional correlations in neurodegenerative diseases. J. Neural Transm. 2012, 119, 891–910. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Ni, D.; Rosenkrans, Z.T.; Cai, W. Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res. 2018, 11, 4955–4984. [Google Scholar] [CrossRef] [PubMed]
- Styskal, J.; Van Remmen, H.; Richardson, A.; Salmon, A.B. Oxidative stress and diabetes: What can we learn about insulin resistance from antioxidant mutant mouse models? Free. Radic. Biol. Med. 2012, 52, 46–58. [Google Scholar] [CrossRef]
- Ahmad, W.; Ijaz, B.; Shabbiri, K.; Ahmed, F.; Rehman, S. Oxidative toxicity in diabetes and Alzheimer’s disease: Mechanisms behind ROS/RNS generation. J. Biomed. Sci. 2017, 24, 1–10. [Google Scholar] [CrossRef]
- Magenta, A.; Greco, S.; Capogrossi, M.C.; Gaetano, C.; Martelli, F. Nitric oxide, oxidative stress, and p66Shc interplay in diabetic endothelial dysfunction. BioMed Res. Int. 2014, 2014, 193095. [Google Scholar] [CrossRef]
- Suksungworn, R.; Duangsrisai, S. Phytochemical Contents and Antioxidant Activity of Medicinal Plants from the Rubiaceae Family in Thailand. Plant Sci. Today 2021, 8, 24–31. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Sulaiman Rahman, H. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Artasensi, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Type 2 diabetes mellitus: A review of multi-target drugs. Molecules 2020, 25, 1987. [Google Scholar] [CrossRef]
- Olokoba, A.B.; Obateru, O.A.; Olokoba, L.B. Type 2 diabetes mellitus: A review of current trends. Oman Med. J. 2012, 27, 269. [Google Scholar] [CrossRef]
- Tripathi, B.K.; Srivastava, A.K. Diabetes mellitus: Complications and therapeutics. Med. Sci. Monit. 2006, 12, 130–147. [Google Scholar]
- Cheng, A.Y.; Fantus, I.G. Oral antihyperglycemic therapy for type 2 diabetes mellitus. Cmaj 2005, 172, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Hinnen, D.A. Therapeutic options for the management of postprandial glucose in patients with type 2 diabetes on basal insulin. Clin. Diabetes 2015, 33, 175–180. [Google Scholar] [CrossRef]
- Nair, S.S.; Kavrekar, V.; Mishra, A. In vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. Eur. J. Exp. Biol. 2013, 3, 128–132. [Google Scholar]
- Dewi, I.C.; Falaise, C.; Hellio, C.; Bourgougnon, N.; Mouget, J.-L. Anticancer, antiviral, antibacterial, and antifungal properties in microalgae. In Microalgae in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2018; pp. 235–261. [Google Scholar]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef]
- Pantami, H.A.; Ahamad Bustamam, M.S.; Lee, S.Y.; Ismail, I.S.; Mohd Faudzi, S.M.; Nakakuni, M.; Shaari, K. Comprehensive GCMS and LC-MS/MS metabolite profiling of Chlorella vulgaris. Mar. Drugs 2020, 18, 367. [Google Scholar] [CrossRef]
- Sibi, G. Inhibition of lipase and inflammatory mediators by Chlorella lipid extracts for antiacne treatment. J. Adv. Pharm. Technol. Res. 2015, 6, 7–12. [Google Scholar] [CrossRef]
- Wang, H.-M.; Pan, J.-L.; Chen, C.-Y.; Chiu, C.-C.; Yang, M.-H.; Chang, H.-W.; Chang, J.-S. Identification of anti-lung cancer extract from Chlorella vulgaris CC by antioxidant property using supercritical carbon dioxide extraction. Process Biochem. 2010, 45, 1865–1872. [Google Scholar] [CrossRef]
- Yun, H.; Kim, I.; Kwon, S.-H.; Kang, J.-S.; Om, A.-S. Protective effect of Chlorella vulgaris against lead-induced oxidative stress in rat brains. J. Health Sci. 2011, 57, 245–254. [Google Scholar] [CrossRef]
- Chatzikonstantinou, M.; Kalliampakou, A.; Gatzogia, M.; Flemetakis, E.; Katharios, P.; Labrou, N.E. Comparative analyses and evaluation of the cosmeceutical potential of selected Chlorella strains. J. Appl. Phycol. 2017, 29, 179–188. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Tsai, C.-T.; Chuang, W.-L.; Chao, Y.-H.; Pan, I.-H.; Chen, Y.-K.; Lin, C.-C.; Wang, B.-Y. Chlorella sorokiniana induces mitochondrial-mediated apoptosis in human non-small cell lung cancer cells and inhibits xenograft tumor growth in vivo. BMC Complement. Altern. Med. 2017, 17, 88. [Google Scholar] [CrossRef]
- Wan, X.-Z.; Li, T.-T.; Zhong, R.-T.; Chen, H.-B.; Xia, X.; Gao, L.-Y.; Gao, X.-X.; Liu, B.; Zhang, H.-Y.; Zhao, C. Anti-diabetic activity of PUFAs-rich extracts of Chlorella pyrenoidosa and Spirulina platensis in rats. Food Chem. Toxicol. 2019, 128, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Cha, K.H.; Koo, S.Y.; Lee, D.-U. Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J. Agric. Food Chem. 2008, 56, 10521–10526. [Google Scholar] [CrossRef] [PubMed]
- Soontornchaiboon, W.; Joo, S.S.; Kim, S.M. Anti-inflammatory effects of violaxanthin isolated from microalga Chlorella ellipsoidea in RAW 264.7 macrophages. Biol. Pharm. Bull. 2012, 35, 1137–1144. [Google Scholar] [CrossRef]
- Takyar, M.B.T.; Khajavi, S.H.; Safari, R. Evaluation of antioxidant properties of Chlorella vulgaris and Spirulina platensis and their application in order to extend the shelf life of rainbow trout (Oncorhynchus mykiss) fillets during refrigerated storage. LWT 2019, 100, 244–249. [Google Scholar] [CrossRef]
- Reyna-Martinez, R.; Gomez-Flores, R.; López-Chuken, U.; Quintanilla-Licea, R.; Caballero-Hernandez, D.; Rodríguez-Padilla, C.; Beltrán-Rocha, J.C.; Tamez-Guerra, P. Antitumor activity of Chlorella sorokiniana and Scenedesmus sp. microalgae native of Nuevo León State, México. PeerJ 2018, 6, e4358. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, Y.; Tham, Y.J.; Zou, S. Emission of marine volatile organic compounds (VOCs) by phytoplankton—A review. Mar. Environ. Res. 2023, 191, 106177. [Google Scholar] [CrossRef]
- Huang, J.J.; Xu, W.; Lin, S.; Cheung, P.C.K. The bioactivities and biotechnological production approaches of carotenoids derived from microalgae and cyanobacteria. Crit. Rev. Biotechnol. 2025, 45, 276–304. [Google Scholar] [CrossRef]
- Hamed, I. The evolution and versatility of microalgal biotechnology: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1104–1123. [Google Scholar] [CrossRef]
- Hartweg, J.; Perera, R.; Montori, V.M.; Dinneen, S.F.; Neil, A.H.; Farmer, A.J. Omega-3 polyunsaturated fatty acids (PUFA) for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2008, 2008, CD003205. [Google Scholar] [CrossRef] [PubMed]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef] [PubMed]
- Kaeoboon, S.; Suksungworn, R.; Sanevas, N. Toxicity response of Chlorella microalgae to glyphosate herbicide exposure based on biomass, pigment contents and photosynthetic efficiency. Plant Sci. Today 2021, 8, 293–300. [Google Scholar] [CrossRef]
- Andersen, R.A. Algal Culturing Techniques; Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Songserm, R.; Kaeoboon, S.; Suksungworn, R.; Duangsrisai, S.; Sanevas, N. GC-MS profiling, anti-oxidant and anti-diabetic assessments of extracts from microalgae Scenedesmus falcatus (KU.B1) and Chlorella sorokiniana (KU.B2). Plant Sci. Today 2022, 9, 632–641. [Google Scholar] [CrossRef]
- Benning, C.; Somerville, C. Isolation and genetic complementation of a sulfolipid-deficient mutant of Rhodobacter sphaeroides. J. Bacteriol. 1992, 174, 2352–2360. [Google Scholar] [CrossRef]
- Suksungworn, R.; Andrade, P.B.; Oliveira, A.P.; Valentão, P.; Duangsrisai, S.; Gomes, N.G. Inhibition of proinflammatory enzymes and attenuation of IL-6 in LPS-challenged RAW 264.7 macrophages substantiates the ethnomedicinal use of the herbal drug Homalium bhamoense Cubitt & WW Sm. Int. J. Mol. Sci. 2020, 21, 2421. [Google Scholar]
- Ferreres, F.; Andrade, C.; Gomes, N.G.; Andrade, P.B.; Gil-Izquierdo, A.; Pereira, D.M.; Suksungworn, R.; Duangsrisai, S.; Videira, R.A.; Valentão, P. Valorisation of kitul, an overlooked food plant: Phenolic profiling of fruits and inflorescences and assessment of their effects on diabetes-related targets. Food Chem. 2021, 342, 128323. [Google Scholar] [CrossRef]
- Kurnia, D.; Yuliantini, A.; Cendana, I.; Nurachman, Z. Fatty acid analysis of marine microalgae Chlorella vulgaris in modified medium used GC-FID. In Journal of Physics: Conference Series, Proceedings of the 2nd International Conference on Applied Sciences Mathematics and Informatics, Bandar Lampung, Indonesia, 9–11 August 2018; IOP Publishing: Bristol, UK, 2019; p. 012007. [Google Scholar]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Siaut, M.; Cuiné, S.; Cagnon, C.; Fessler, B.; Nguyen, M.; Carrier, P.; Beyly, A.; Beisson, F.; Triantaphylidès, C.; Li-Beisson, Y.; et al. Oil accumulation in the model green alga Chlamydomonas reinhardtii: Characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol. 2011, 11, 7. [Google Scholar] [CrossRef]
- Budavari, S.; O’Neil, M.; Smith, A.; Heckelman, P.; Kinneary, J. The Merck Index; Merck Research Laboratories Division of Merck & Co.: Whitehouse Station, NJ, USA, 2001. [Google Scholar]
- Moran, L.; Bou, G.; Aldai, N.; Ciardi, M.; Morillas-España, A.; Sánchez-Zurano, A.; Barron, L.J.R.; Lafarga, T. Characterisation of the volatile profile of microalgae and cyanobacteria using solid-phase microextraction followed by gas chromatography coupled to mass spectrometry. Sci. Rep. 2022, 12, 3661. [Google Scholar] [CrossRef]
- Ko, S.-C.; Kang, N.; Kim, E.-A.; Kang, M.C.; Lee, S.-H.; Kang, S.-M.; Lee, J.-B.; Jeon, B.-T.; Kim, S.-K.; Park, S.-J. A novel angiotensin I-converting enzyme (ACE) inhibitory peptide from a marine Chlorella ellipsoidea and its antihypertensive effect in spontaneously hypertensive rats. Process Biochem. 2012, 47, 2005–2011. [Google Scholar] [CrossRef]
- Hamed, S.M.; Selim, S.; Klöck, G.; AbdElgawad, H. Sensitivity of two green microalgae to copper stress: Growth, oxidative and antioxidants analyses. Ecotoxicol. Environ. Saf. 2017, 144, 19–25. [Google Scholar] [CrossRef]
- Petruk, G.; Gifuni, I.; Illiano, A.; Roxo, M.; Pinto, G.; Amoresano, A.; Marzocchella, A.; Piccoli, R.; Wink, M.; Olivieri, G. Simultaneous production of antioxidants and starch from the microalga Chlorella sorokiniana. Algal Res. 2018, 34, 164–174. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Mabinya, L.V.; Olaniran, A.O.; Okoh, A.I. Chemical characterization of sulfated polysaccharides from Gracilaria gracilis and Ulva lactuca and their radical scavenging, metal chelating, and cholinesterase inhibitory activities. Int. J. Food Prop. 2019, 22, 100–110. [Google Scholar] [CrossRef]
- Napolitano, G.; Fasciolo, G.; Salbitani, G.; Venditti, P. Chlorella sorokiniana dietary supplementation increases antioxidant capacities and reduces ros release in mitochondria of hyperthyroid rat liver. Antioxidants 2020, 9, 883. [Google Scholar] [CrossRef]
- Salbitani, G.; Vona, V.; Bottone, C.; Petriccione, M.; Carfagna, S. Sulfur deprivation results in oxidative perturbation in Chlorella sorokiniana (211/8k). Plant Cell Physiol. 2015, 56, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Delgado, I.; Mora-Solarte, D.A.; Velasco-Santamaría, Y. Respuestas fisiológicas y capacidad antioxidante de Chlorella vulgaris (Chlorellaceae) expuesta a fenantreno. Acta Biológica Colomb. 2020, 25, 225–234. [Google Scholar] [CrossRef]
- Vijayavel, K.; Anbuselvam, C.; Balasubramanian, M.P. Antioxidant effect of the marine algae Chlorella vulgaris against naphthalene-induced oxidative stress in the albino rats. Mol. Cell. Biochem. 2007, 303, 39–44. [Google Scholar] [CrossRef]
- Hernayanti, H.; Simanjuntak, S.B.I. Antioxidant effect of Chlorella vulgaris on physiological response of rat induced by carbon tetrachloride. Biosaintifika J. Biol. Biol. Educ. 2019, 11, 84–90. [Google Scholar] [CrossRef]
- Hashem, E.Z.; Khodadadi, M.; Asadi, F.; Koohi, M.K.; Eslami, M.; Hasani-Dizaj, S.; Zadeh, R.T. The antioxidant activity of palmitoleic acid on the oxidative stress parameters of palmitic acid in adult rat cardiomyocytes. Ann. Mil. Health Sci. Res. 2016, 14, e11467. [Google Scholar]
- Wang, Z.-J.; Liang, C.-L.; Li, G.-M.; Yu, C.-Y.; Yin, M. Stearic acid protects primary cultured cortical neurons against oxidative stress. Acta Pharmacol. Sin. 2007, 28, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Hernández Escarcega, G.; Sánchez-Chávez, E.; Pérez Álvarez, S.; Soto Caballero, M.; Soto Parra, J.M.; Flores-Córdova, M.A.; Salas Salazar, N.A.; Ojeda Barrios, D.L. Determination of antioxidant phenolic, nutritional quality and volatiles in pomegranates (Punica granatum L.) cultivated in Mexico. Int. J. Food Prop. 2020, 23, 979–991. [Google Scholar] [CrossRef]
- Kulapichitr, F.; Borompichaichartkul, C.; Pratontep, S.; Lopetcharat, K.; Boonbumrung, S.; Suppavorasatit, I. Differences in volatile compounds and antioxidant activity of ripe and unripe green coffee beans (Coffea arabica L. ‘Catimor’). In Proceedings of the III Southeast Asia Symposium on Quality Management in Postharvest Systems 1179, Siem Reap, Cambodia, 13–15 August 2015; pp. 261–268. [Google Scholar]
- Lee, K.-G.; Shibamoto, T. Antioxidant properties of aroma compounds isolated from soybeans and mung beans. J. Agric. Food Chem. 2000, 48, 4290–4293. [Google Scholar] [CrossRef]
- Gabr, G.A.; El-Sayed, S.M.; Hikal, M.S. Antioxidant Activities of Phycocyanin: A Bioactive Compound from Spirulina platensis. J. Pharm. Res. Int. 2020, 32, 73–85. [Google Scholar] [CrossRef]
- Roy, U.K.; Nielsen, B.V.; Milledge, J.J. Antioxidant Production in Dunaliella. Appl. Sci. 2021, 11, 3959. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Goswami, S. Microalgae—A green multi-product biorefinery for future industrial prospects. Biocatal. Agric. Biotechnol. 2020, 25, 101580. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, J.-S.; Hyun, T.K.; Yang, J.; Kang, H.-H.; Cho, J.-C.; Yeom, H.-M.; Kim, M. In vitro antioxidant and antidiabetic activities of Rehmannia glutinosa tuberous root extracts. ScienceAsia 2013, 39, 605. [Google Scholar] [CrossRef][Green Version]
- Park, H.; Hwang, K.Y.; Kim, Y.H.; Oh, K.H.; Lee, J.Y.; Kim, K. Discovery and biological evaluation of novel alpha-glucosidase inhibitors with in vivo antidiabetic effect. Bioorganic Med. Chem. Lett. 2008, 18, 3711–3715. [Google Scholar] [CrossRef]
- Collado-González, J.; Grosso, C.; Valentão, P.; Andrade, P.B.; Ferreres, F.; Durand, T.; Guy, A.; Galano, J.M.; Torrecillas, A.; Gil-Izquierdo, Á. Inhibition of α-glucosidase and α-amylase by Spanish extra virgin olive oils: The involvement of bioactive compounds other than oleuropein and hydroxytyrosol. Food Chem. 2017, 235, 298–307. [Google Scholar] [CrossRef]
- Liu, B.; Kongstad, K.T.; Wiese, S.; Jäger, A.K.; Staerk, D. Edible seaweed as future functional food: Identification of α-glucosidase inhibitors by combined use of high-resolution α-glucosidase inhibition profiling and HPLC–HRMS–SPE–NMR. Food Chem. 2016, 203, 16–22. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).