In Vitro Antioxidant Potential, Antidiabetic Activities, and GC–MS Analysis of Lipid Extracts of Chlorella Microalgae
Abstract
1. Introduction
2. Materials and Methods
2.1. General Chemicals and Materials
2.2. Strains and Culture Conditions
2.3. Preparation of the Crude Extract
2.4. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
2.5. DPPH• Scavenging Activity
2.6. Nitric Oxide Scavenging Activity
2.7. Ferric-Reducing Antioxidant Power (FRAP) Activity
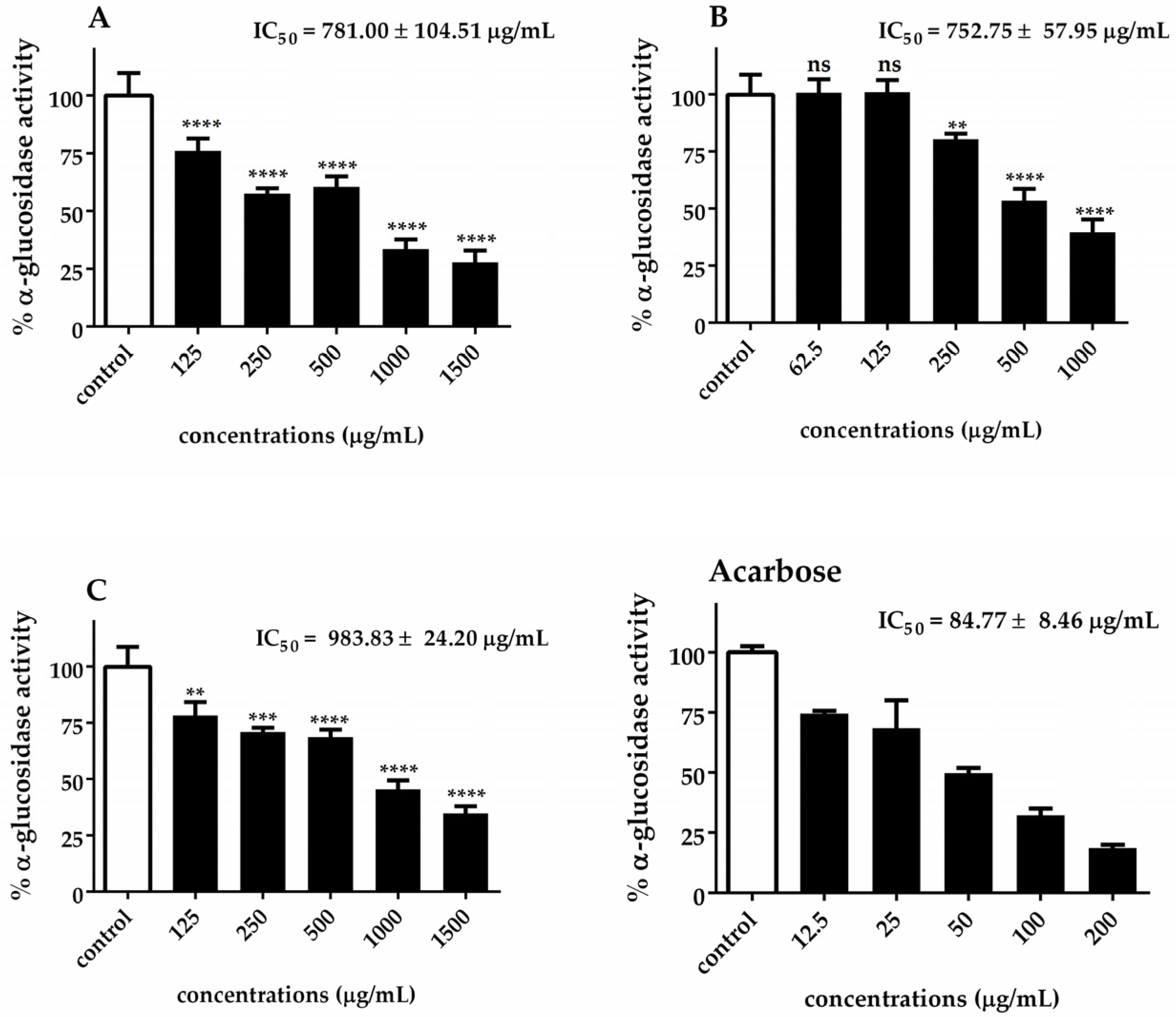
2.8. α-Glucosidase Activity
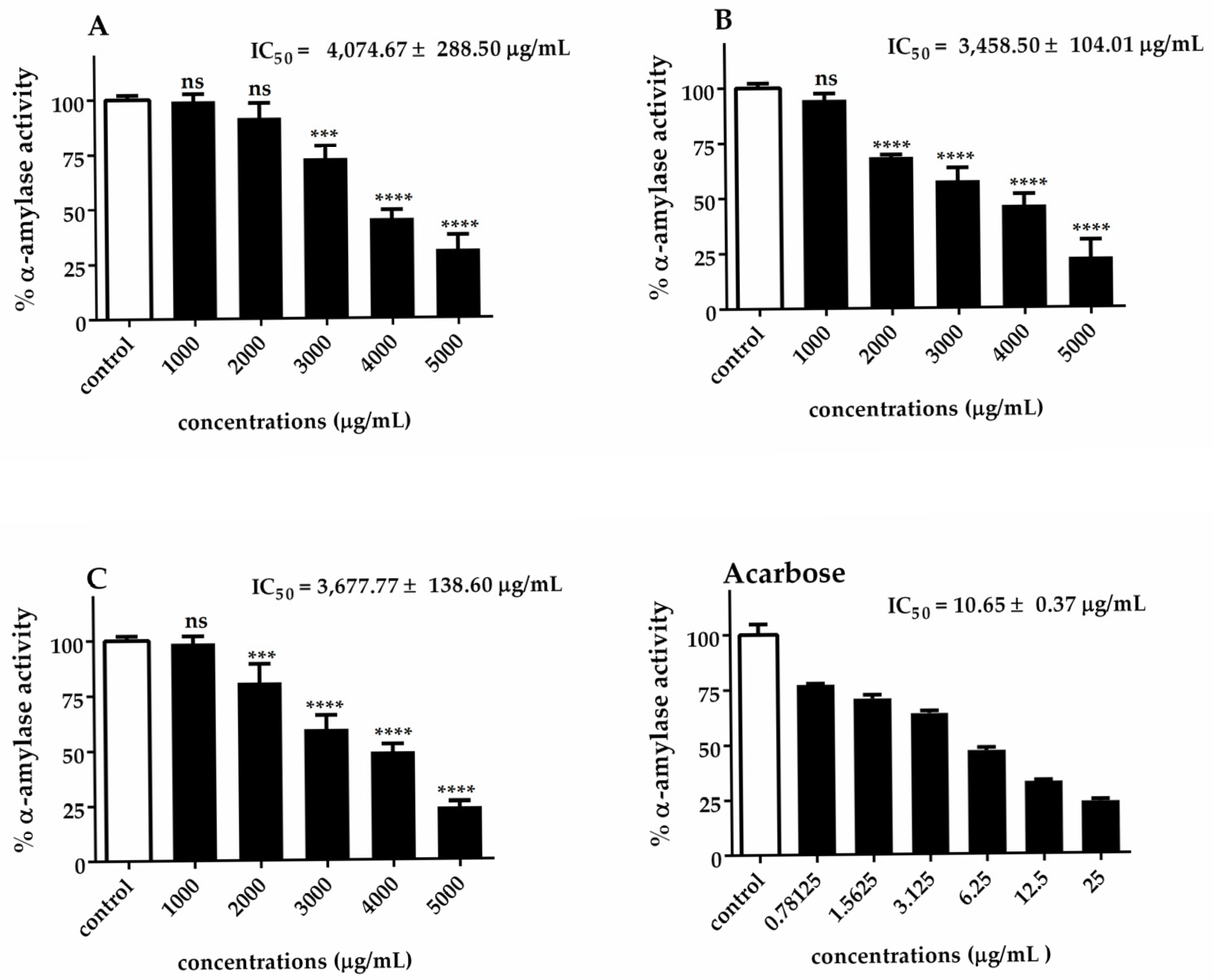
2.9. α-Amylase Activity
2.10. Statistical Analysis
3. Results and Discussion
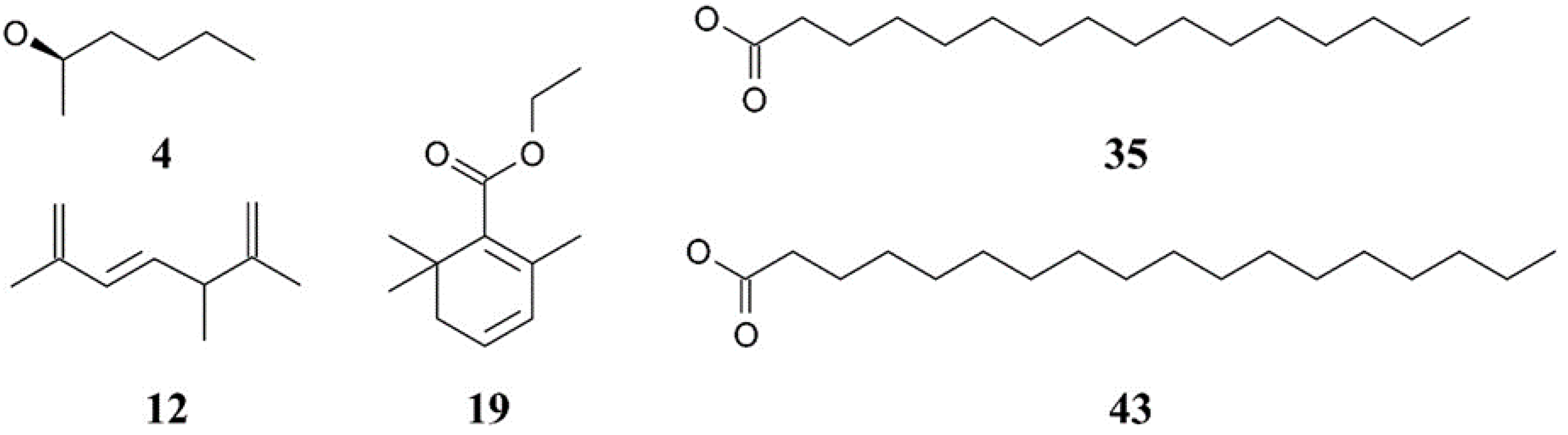
3.1. GC–MS Profiling of Chlorella Lipid Extracts
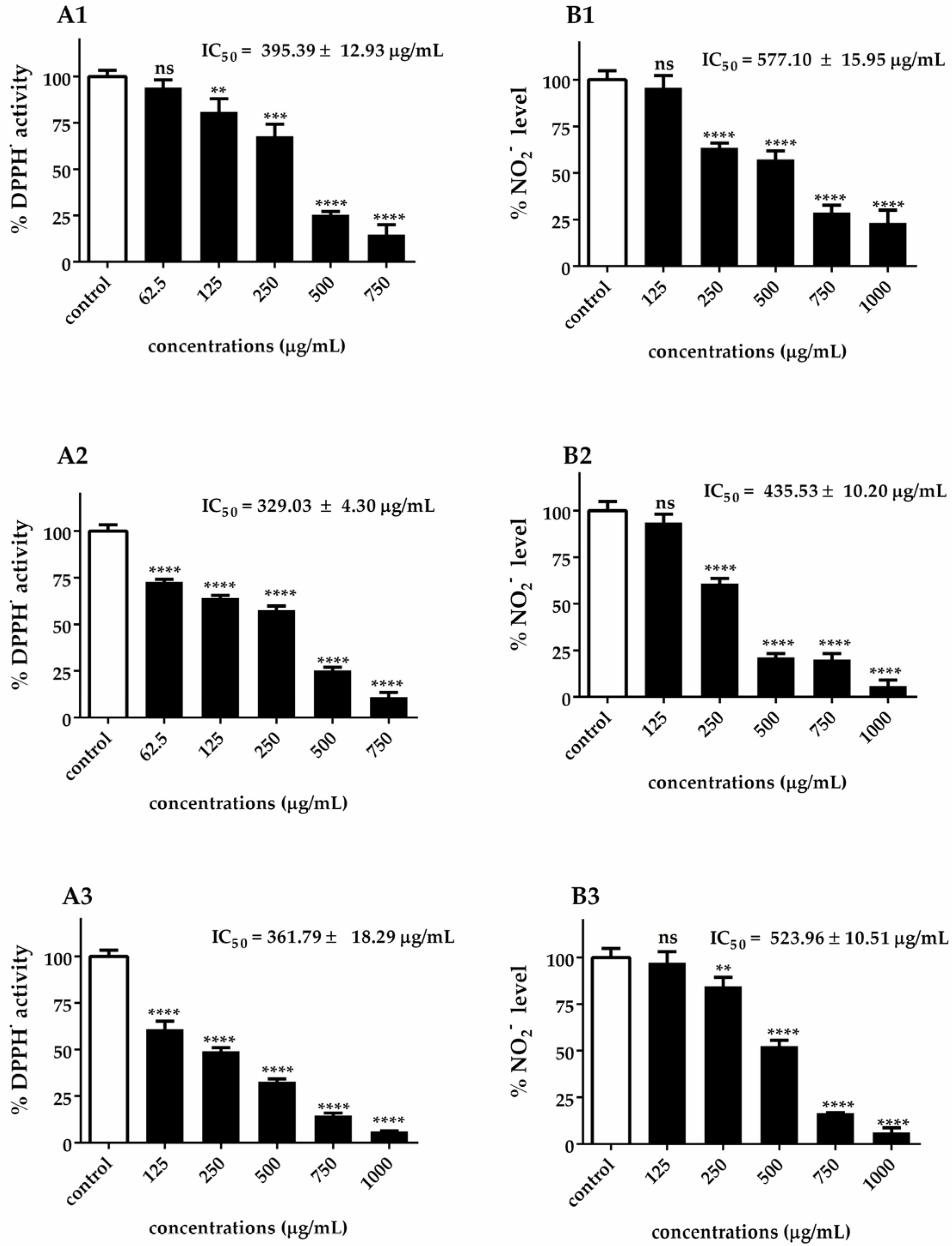
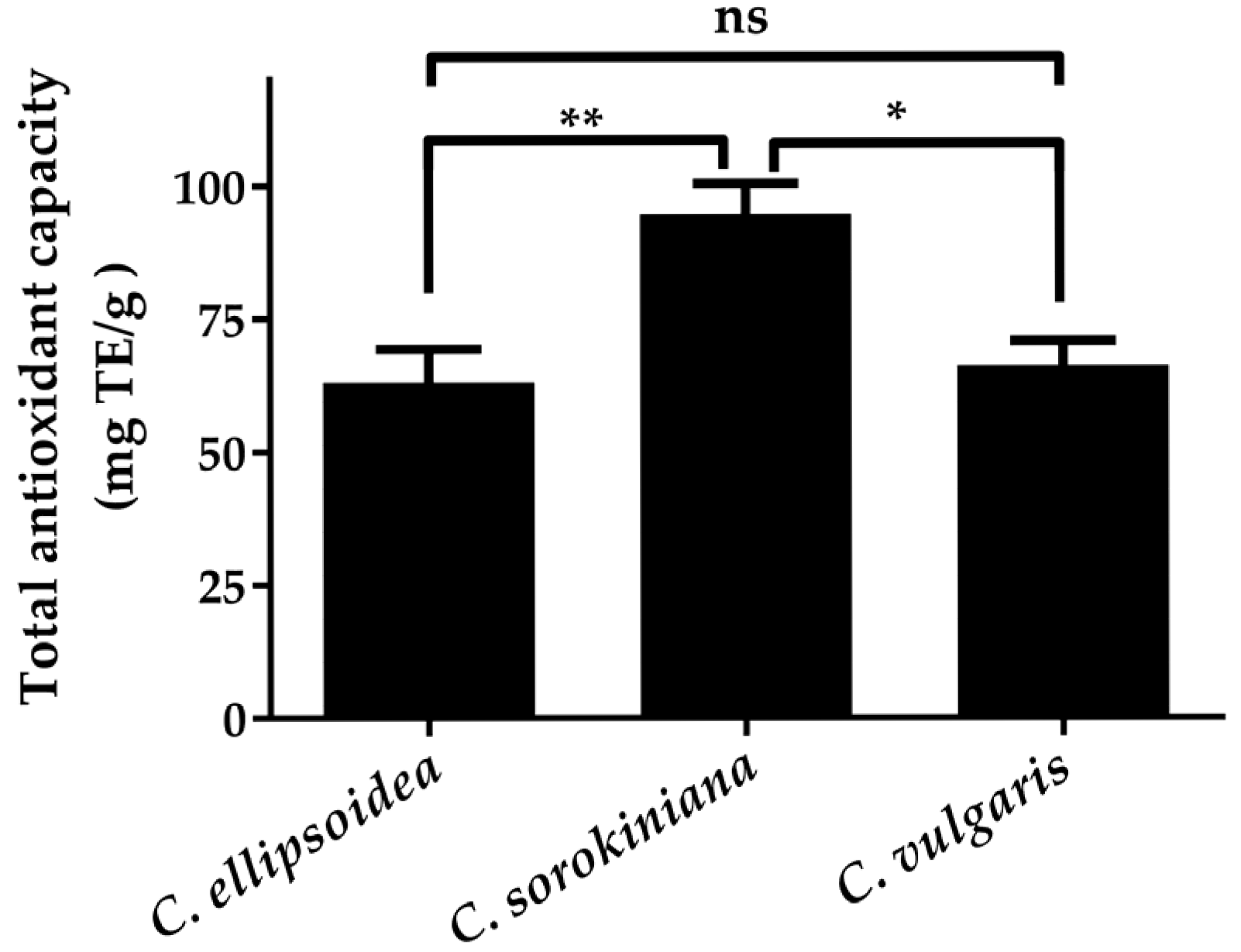
3.2. Antioxidant Activity of Chlorella Extracts
3.3. Antidiabetic Enzyme Inhibition by Chlorella Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jha, N.; Ryu, J.J.; Choi, E.H.; Kaushik, N.K. Generation and Role of Reactive Oxygen and Nitrogen Species Induced by Plasma, Lasers, Chemical Agents, and Other Systems in Dentistry. Oxidative Med. Cell. Longev. 2017, 2017, 7542540. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, M.; Kim, S.J. Reactive oxygen/nitrogen species and their functional correlations in neurodegenerative diseases. J. Neural Transm. 2012, 119, 891–910. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Ni, D.; Rosenkrans, Z.T.; Cai, W. Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res. 2018, 11, 4955–4984. [Google Scholar] [CrossRef] [PubMed]
- Styskal, J.; Van Remmen, H.; Richardson, A.; Salmon, A.B. Oxidative stress and diabetes: What can we learn about insulin resistance from antioxidant mutant mouse models? Free. Radic. Biol. Med. 2012, 52, 46–58. [Google Scholar] [CrossRef]
- Ahmad, W.; Ijaz, B.; Shabbiri, K.; Ahmed, F.; Rehman, S. Oxidative toxicity in diabetes and Alzheimer’s disease: Mechanisms behind ROS/RNS generation. J. Biomed. Sci. 2017, 24, 1–10. [Google Scholar] [CrossRef]
- Magenta, A.; Greco, S.; Capogrossi, M.C.; Gaetano, C.; Martelli, F. Nitric oxide, oxidative stress, and p66Shc interplay in diabetic endothelial dysfunction. BioMed Res. Int. 2014, 2014, 193095. [Google Scholar] [CrossRef]
- Suksungworn, R.; Duangsrisai, S. Phytochemical Contents and Antioxidant Activity of Medicinal Plants from the Rubiaceae Family in Thailand. Plant Sci. Today 2021, 8, 24–31. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Sulaiman Rahman, H. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Artasensi, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Type 2 diabetes mellitus: A review of multi-target drugs. Molecules 2020, 25, 1987. [Google Scholar] [CrossRef]
- Olokoba, A.B.; Obateru, O.A.; Olokoba, L.B. Type 2 diabetes mellitus: A review of current trends. Oman Med. J. 2012, 27, 269. [Google Scholar] [CrossRef]
- Tripathi, B.K.; Srivastava, A.K. Diabetes mellitus: Complications and therapeutics. Med. Sci. Monit. 2006, 12, 130–147. [Google Scholar]
- Cheng, A.Y.; Fantus, I.G. Oral antihyperglycemic therapy for type 2 diabetes mellitus. Cmaj 2005, 172, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Hinnen, D.A. Therapeutic options for the management of postprandial glucose in patients with type 2 diabetes on basal insulin. Clin. Diabetes 2015, 33, 175–180. [Google Scholar] [CrossRef]
- Nair, S.S.; Kavrekar, V.; Mishra, A. In vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. Eur. J. Exp. Biol. 2013, 3, 128–132. [Google Scholar]
- Dewi, I.C.; Falaise, C.; Hellio, C.; Bourgougnon, N.; Mouget, J.-L. Anticancer, antiviral, antibacterial, and antifungal properties in microalgae. In Microalgae in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2018; pp. 235–261. [Google Scholar]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef]
- Pantami, H.A.; Ahamad Bustamam, M.S.; Lee, S.Y.; Ismail, I.S.; Mohd Faudzi, S.M.; Nakakuni, M.; Shaari, K. Comprehensive GCMS and LC-MS/MS metabolite profiling of Chlorella vulgaris. Mar. Drugs 2020, 18, 367. [Google Scholar] [CrossRef]
- Sibi, G. Inhibition of lipase and inflammatory mediators by Chlorella lipid extracts for antiacne treatment. J. Adv. Pharm. Technol. Res. 2015, 6, 7–12. [Google Scholar] [CrossRef]
- Wang, H.-M.; Pan, J.-L.; Chen, C.-Y.; Chiu, C.-C.; Yang, M.-H.; Chang, H.-W.; Chang, J.-S. Identification of anti-lung cancer extract from Chlorella vulgaris CC by antioxidant property using supercritical carbon dioxide extraction. Process Biochem. 2010, 45, 1865–1872. [Google Scholar] [CrossRef]
- Yun, H.; Kim, I.; Kwon, S.-H.; Kang, J.-S.; Om, A.-S. Protective effect of Chlorella vulgaris against lead-induced oxidative stress in rat brains. J. Health Sci. 2011, 57, 245–254. [Google Scholar] [CrossRef]
- Chatzikonstantinou, M.; Kalliampakou, A.; Gatzogia, M.; Flemetakis, E.; Katharios, P.; Labrou, N.E. Comparative analyses and evaluation of the cosmeceutical potential of selected Chlorella strains. J. Appl. Phycol. 2017, 29, 179–188. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Tsai, C.-T.; Chuang, W.-L.; Chao, Y.-H.; Pan, I.-H.; Chen, Y.-K.; Lin, C.-C.; Wang, B.-Y. Chlorella sorokiniana induces mitochondrial-mediated apoptosis in human non-small cell lung cancer cells and inhibits xenograft tumor growth in vivo. BMC Complement. Altern. Med. 2017, 17, 88. [Google Scholar] [CrossRef]
- Wan, X.-Z.; Li, T.-T.; Zhong, R.-T.; Chen, H.-B.; Xia, X.; Gao, L.-Y.; Gao, X.-X.; Liu, B.; Zhang, H.-Y.; Zhao, C. Anti-diabetic activity of PUFAs-rich extracts of Chlorella pyrenoidosa and Spirulina platensis in rats. Food Chem. Toxicol. 2019, 128, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Cha, K.H.; Koo, S.Y.; Lee, D.-U. Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J. Agric. Food Chem. 2008, 56, 10521–10526. [Google Scholar] [CrossRef] [PubMed]
- Soontornchaiboon, W.; Joo, S.S.; Kim, S.M. Anti-inflammatory effects of violaxanthin isolated from microalga Chlorella ellipsoidea in RAW 264.7 macrophages. Biol. Pharm. Bull. 2012, 35, 1137–1144. [Google Scholar] [CrossRef]
- Takyar, M.B.T.; Khajavi, S.H.; Safari, R. Evaluation of antioxidant properties of Chlorella vulgaris and Spirulina platensis and their application in order to extend the shelf life of rainbow trout (Oncorhynchus mykiss) fillets during refrigerated storage. LWT 2019, 100, 244–249. [Google Scholar] [CrossRef]
- Reyna-Martinez, R.; Gomez-Flores, R.; López-Chuken, U.; Quintanilla-Licea, R.; Caballero-Hernandez, D.; Rodríguez-Padilla, C.; Beltrán-Rocha, J.C.; Tamez-Guerra, P. Antitumor activity of Chlorella sorokiniana and Scenedesmus sp. microalgae native of Nuevo León State, México. PeerJ 2018, 6, e4358. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, Y.; Tham, Y.J.; Zou, S. Emission of marine volatile organic compounds (VOCs) by phytoplankton—A review. Mar. Environ. Res. 2023, 191, 106177. [Google Scholar] [CrossRef]
- Huang, J.J.; Xu, W.; Lin, S.; Cheung, P.C.K. The bioactivities and biotechnological production approaches of carotenoids derived from microalgae and cyanobacteria. Crit. Rev. Biotechnol. 2025, 45, 276–304. [Google Scholar] [CrossRef]
- Hamed, I. The evolution and versatility of microalgal biotechnology: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1104–1123. [Google Scholar] [CrossRef]
- Hartweg, J.; Perera, R.; Montori, V.M.; Dinneen, S.F.; Neil, A.H.; Farmer, A.J. Omega-3 polyunsaturated fatty acids (PUFA) for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2008, 2008, CD003205. [Google Scholar] [CrossRef] [PubMed]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef] [PubMed]
- Kaeoboon, S.; Suksungworn, R.; Sanevas, N. Toxicity response of Chlorella microalgae to glyphosate herbicide exposure based on biomass, pigment contents and photosynthetic efficiency. Plant Sci. Today 2021, 8, 293–300. [Google Scholar] [CrossRef]
- Andersen, R.A. Algal Culturing Techniques; Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Songserm, R.; Kaeoboon, S.; Suksungworn, R.; Duangsrisai, S.; Sanevas, N. GC-MS profiling, anti-oxidant and anti-diabetic assessments of extracts from microalgae Scenedesmus falcatus (KU.B1) and Chlorella sorokiniana (KU.B2). Plant Sci. Today 2022, 9, 632–641. [Google Scholar] [CrossRef]
- Benning, C.; Somerville, C. Isolation and genetic complementation of a sulfolipid-deficient mutant of Rhodobacter sphaeroides. J. Bacteriol. 1992, 174, 2352–2360. [Google Scholar] [CrossRef]
- Suksungworn, R.; Andrade, P.B.; Oliveira, A.P.; Valentão, P.; Duangsrisai, S.; Gomes, N.G. Inhibition of proinflammatory enzymes and attenuation of IL-6 in LPS-challenged RAW 264.7 macrophages substantiates the ethnomedicinal use of the herbal drug Homalium bhamoense Cubitt & WW Sm. Int. J. Mol. Sci. 2020, 21, 2421. [Google Scholar]
- Ferreres, F.; Andrade, C.; Gomes, N.G.; Andrade, P.B.; Gil-Izquierdo, A.; Pereira, D.M.; Suksungworn, R.; Duangsrisai, S.; Videira, R.A.; Valentão, P. Valorisation of kitul, an overlooked food plant: Phenolic profiling of fruits and inflorescences and assessment of their effects on diabetes-related targets. Food Chem. 2021, 342, 128323. [Google Scholar] [CrossRef]
- Kurnia, D.; Yuliantini, A.; Cendana, I.; Nurachman, Z. Fatty acid analysis of marine microalgae Chlorella vulgaris in modified medium used GC-FID. In Journal of Physics: Conference Series, Proceedings of the 2nd International Conference on Applied Sciences Mathematics and Informatics, Bandar Lampung, Indonesia, 9–11 August 2018; IOP Publishing: Bristol, UK, 2019; p. 012007. [Google Scholar]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Siaut, M.; Cuiné, S.; Cagnon, C.; Fessler, B.; Nguyen, M.; Carrier, P.; Beyly, A.; Beisson, F.; Triantaphylidès, C.; Li-Beisson, Y.; et al. Oil accumulation in the model green alga Chlamydomonas reinhardtii: Characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol. 2011, 11, 7. [Google Scholar] [CrossRef]
- Budavari, S.; O’Neil, M.; Smith, A.; Heckelman, P.; Kinneary, J. The Merck Index; Merck Research Laboratories Division of Merck & Co.: Whitehouse Station, NJ, USA, 2001. [Google Scholar]
- Moran, L.; Bou, G.; Aldai, N.; Ciardi, M.; Morillas-España, A.; Sánchez-Zurano, A.; Barron, L.J.R.; Lafarga, T. Characterisation of the volatile profile of microalgae and cyanobacteria using solid-phase microextraction followed by gas chromatography coupled to mass spectrometry. Sci. Rep. 2022, 12, 3661. [Google Scholar] [CrossRef]
- Ko, S.-C.; Kang, N.; Kim, E.-A.; Kang, M.C.; Lee, S.-H.; Kang, S.-M.; Lee, J.-B.; Jeon, B.-T.; Kim, S.-K.; Park, S.-J. A novel angiotensin I-converting enzyme (ACE) inhibitory peptide from a marine Chlorella ellipsoidea and its antihypertensive effect in spontaneously hypertensive rats. Process Biochem. 2012, 47, 2005–2011. [Google Scholar] [CrossRef]
- Hamed, S.M.; Selim, S.; Klöck, G.; AbdElgawad, H. Sensitivity of two green microalgae to copper stress: Growth, oxidative and antioxidants analyses. Ecotoxicol. Environ. Saf. 2017, 144, 19–25. [Google Scholar] [CrossRef]
- Petruk, G.; Gifuni, I.; Illiano, A.; Roxo, M.; Pinto, G.; Amoresano, A.; Marzocchella, A.; Piccoli, R.; Wink, M.; Olivieri, G. Simultaneous production of antioxidants and starch from the microalga Chlorella sorokiniana. Algal Res. 2018, 34, 164–174. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Mabinya, L.V.; Olaniran, A.O.; Okoh, A.I. Chemical characterization of sulfated polysaccharides from Gracilaria gracilis and Ulva lactuca and their radical scavenging, metal chelating, and cholinesterase inhibitory activities. Int. J. Food Prop. 2019, 22, 100–110. [Google Scholar] [CrossRef]
- Napolitano, G.; Fasciolo, G.; Salbitani, G.; Venditti, P. Chlorella sorokiniana dietary supplementation increases antioxidant capacities and reduces ros release in mitochondria of hyperthyroid rat liver. Antioxidants 2020, 9, 883. [Google Scholar] [CrossRef]
- Salbitani, G.; Vona, V.; Bottone, C.; Petriccione, M.; Carfagna, S. Sulfur deprivation results in oxidative perturbation in Chlorella sorokiniana (211/8k). Plant Cell Physiol. 2015, 56, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Delgado, I.; Mora-Solarte, D.A.; Velasco-Santamaría, Y. Respuestas fisiológicas y capacidad antioxidante de Chlorella vulgaris (Chlorellaceae) expuesta a fenantreno. Acta Biológica Colomb. 2020, 25, 225–234. [Google Scholar] [CrossRef]
- Vijayavel, K.; Anbuselvam, C.; Balasubramanian, M.P. Antioxidant effect of the marine algae Chlorella vulgaris against naphthalene-induced oxidative stress in the albino rats. Mol. Cell. Biochem. 2007, 303, 39–44. [Google Scholar] [CrossRef]
- Hernayanti, H.; Simanjuntak, S.B.I. Antioxidant effect of Chlorella vulgaris on physiological response of rat induced by carbon tetrachloride. Biosaintifika J. Biol. Biol. Educ. 2019, 11, 84–90. [Google Scholar] [CrossRef]
- Hashem, E.Z.; Khodadadi, M.; Asadi, F.; Koohi, M.K.; Eslami, M.; Hasani-Dizaj, S.; Zadeh, R.T. The antioxidant activity of palmitoleic acid on the oxidative stress parameters of palmitic acid in adult rat cardiomyocytes. Ann. Mil. Health Sci. Res. 2016, 14, e11467. [Google Scholar]
- Wang, Z.-J.; Liang, C.-L.; Li, G.-M.; Yu, C.-Y.; Yin, M. Stearic acid protects primary cultured cortical neurons against oxidative stress. Acta Pharmacol. Sin. 2007, 28, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Hernández Escarcega, G.; Sánchez-Chávez, E.; Pérez Álvarez, S.; Soto Caballero, M.; Soto Parra, J.M.; Flores-Córdova, M.A.; Salas Salazar, N.A.; Ojeda Barrios, D.L. Determination of antioxidant phenolic, nutritional quality and volatiles in pomegranates (Punica granatum L.) cultivated in Mexico. Int. J. Food Prop. 2020, 23, 979–991. [Google Scholar] [CrossRef]
- Kulapichitr, F.; Borompichaichartkul, C.; Pratontep, S.; Lopetcharat, K.; Boonbumrung, S.; Suppavorasatit, I. Differences in volatile compounds and antioxidant activity of ripe and unripe green coffee beans (Coffea arabica L. ‘Catimor’). In Proceedings of the III Southeast Asia Symposium on Quality Management in Postharvest Systems 1179, Siem Reap, Cambodia, 13–15 August 2015; pp. 261–268. [Google Scholar]
- Lee, K.-G.; Shibamoto, T. Antioxidant properties of aroma compounds isolated from soybeans and mung beans. J. Agric. Food Chem. 2000, 48, 4290–4293. [Google Scholar] [CrossRef]
- Gabr, G.A.; El-Sayed, S.M.; Hikal, M.S. Antioxidant Activities of Phycocyanin: A Bioactive Compound from Spirulina platensis. J. Pharm. Res. Int. 2020, 32, 73–85. [Google Scholar] [CrossRef]
- Roy, U.K.; Nielsen, B.V.; Milledge, J.J. Antioxidant Production in Dunaliella. Appl. Sci. 2021, 11, 3959. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Goswami, S. Microalgae—A green multi-product biorefinery for future industrial prospects. Biocatal. Agric. Biotechnol. 2020, 25, 101580. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, J.-S.; Hyun, T.K.; Yang, J.; Kang, H.-H.; Cho, J.-C.; Yeom, H.-M.; Kim, M. In vitro antioxidant and antidiabetic activities of Rehmannia glutinosa tuberous root extracts. ScienceAsia 2013, 39, 605. [Google Scholar] [CrossRef][Green Version]
- Park, H.; Hwang, K.Y.; Kim, Y.H.; Oh, K.H.; Lee, J.Y.; Kim, K. Discovery and biological evaluation of novel alpha-glucosidase inhibitors with in vivo antidiabetic effect. Bioorganic Med. Chem. Lett. 2008, 18, 3711–3715. [Google Scholar] [CrossRef]
- Collado-González, J.; Grosso, C.; Valentão, P.; Andrade, P.B.; Ferreres, F.; Durand, T.; Guy, A.; Galano, J.M.; Torrecillas, A.; Gil-Izquierdo, Á. Inhibition of α-glucosidase and α-amylase by Spanish extra virgin olive oils: The involvement of bioactive compounds other than oleuropein and hydroxytyrosol. Food Chem. 2017, 235, 298–307. [Google Scholar] [CrossRef]
- Liu, B.; Kongstad, K.T.; Wiese, S.; Jäger, A.K.; Staerk, D. Edible seaweed as future functional food: Identification of α-glucosidase inhibitors by combined use of high-resolution α-glucosidase inhibition profiling and HPLC–HRMS–SPE–NMR. Food Chem. 2016, 203, 16–22. [Google Scholar] [CrossRef]
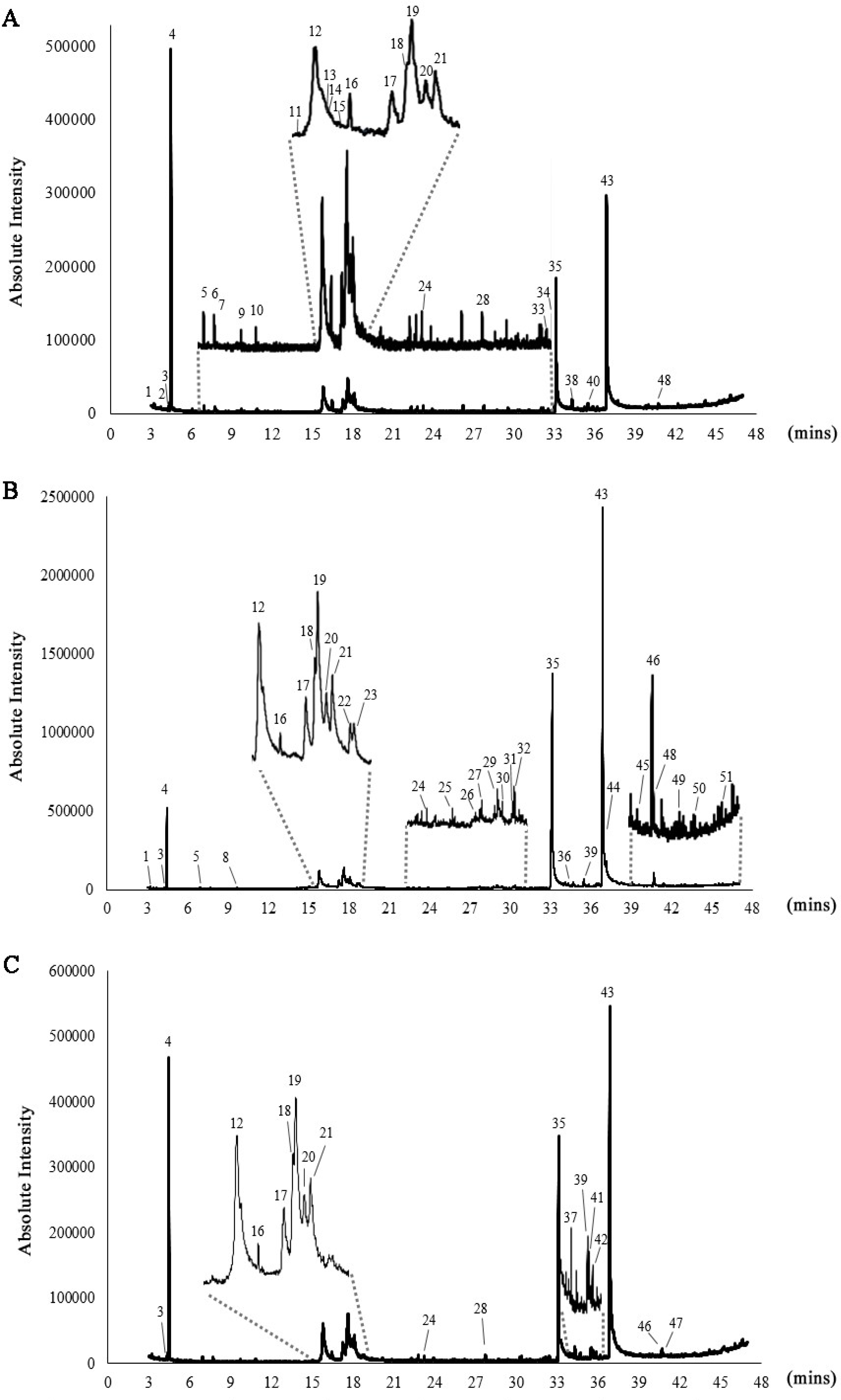
| No. | Rt | Compound | Ratio | ||
|---|---|---|---|---|---|
| C. ellipsoidea | C. sorokiniana | C. vulgaris | |||
| 1 | 3.237 | 3-Hydroxy-3-methyl-2-butanone | 0.24 | 0.06 | — |
| 2 | 4.305 | 2,3-Dimethyl-1-hexene | 0.24 | — | — |
| 3 | 4.422 | Propanoic acid, 2-hydroxy-2-methyl- | 0.43 | 0.08 | 0.31 |
| 4 * | 4.475 | 2-Hexanol, (R)- | 22.19 | 4.11 | 15.35 |
| 5 | 6.952 | 2-Butenal, 3-methyl- | 0.38 | 0.07 | — |
| 6 | 7.724 | Diisoamyl ether | 0.35 | — | — |
| 7 | 7.773 | 4-Octen-3-one | 0.22 | — | — |
| 8 | 9.702 | Hexanal, 4-methyl- | — | 0.06 | — |
| 9 | 9.74 | Methacrolein | 0.20 | — | — |
| 10 | 10.856 | 4-Heptanone, 3-methyl- | 0.21 | — | — |
| 11 | 15.62 | Carbamic acid, 3-methylphenyl-, butyl ester | 0.37 | — | — |
| 12 * | 15.815 | 1,3,6-Heptatriene, 2,5,5-trimethyl | 6.23 | 2.64 | 5.96 |
| 13 | 16.035 | Propanoic acid, 2,2-dimethyl-, 2-(1,1-dimethylethyl)phenyl ester | 0.23 | — | — |
| 14 | 16.06 | Methanol, (cyclohexyl)(2,3-dimethylphenyl)- | 0.33 | — | — |
| 15 | 16.21 | Carbamic acid, N,N-dimethyl-, 4-isopropylphenyl ester | 0.19 | — | — |
| 16 | 16.469 | p-Pentylacetophenone | 0.79 | 0.14 | 0.37 |
| 17 | 17.268 | 5-Isopropyl-2,8-dimethyl-9-oxatricyclo[4.4.0.0(2,8)]decan-7-one | 1.86 | 1.16 | 1.35 |
| 18 | 17.565 | Cyclohexane, 1,1,4,4-tetramethyl-2,5-dimethylene- | 2.20 | 1.17 | 2.45 |
| 19 * | 17.644 | 4-(2,3-Dimethyl-2-cyclopenten-1-yl)-4-methylpentanal | 6.16 | 3.53 | 6.26 |
| 20 | 17.907 | 1,3-Cyclohexadiene-1-carboxylic acid, 2,6,6-trimethyl-, ethyl ester | 2.32 | 0.87 | 0.75 |
| 21 | 18.093 | 4-(1,2-Dimethyl-cyclopent-2-enyl)-butan-2-one | 3.02 | 1.11 | 1.29 |
| 22 | 18.659 | 1-Cyclohexyl-2,2-dimethyl-1-propanol acetate | — | 0.39 | — |
| 23 | 18.761 | 1H-Imidazolecarboxylic acid-, (1-methylethyl) ester | — | 0.49 | — |
| 24 | 23.238 | Pentanoic acid, 5-hydroxy-, 2,4-di-t-butylphenyl esters | 0.43 | 0.11 | 0.34 |
| 25 | 25.319 | 3,4,5-Trimethyl-4-heptanol | — | 0.10 | — |
| 26 | 27.579 | 1-Tridecyn-4-ol | — | 0.06 | — |
| 27 | 27.738 | Dodecane, 2,6,11-trimethyl- | — | 0.11 | — |
| 28 | 27.743 | 3,5-Dimethyl-4-octanone | 0.48 | — | 0.30 |
| 29 | 29.001 | Tetradecanoic acid | — | 0.17 | — |
| 30 | 29.381 | 1,4-Benzenedimethanol, α,α′-dimethyl- | — | 0.08 | — |
| 31 | 30.246 | Cyclohexanepropanol- | — | 0.05 | — |
| 32 | 30.38 | Isopropyl myristate | — | 0.18 | — |
| 33 | 32.151 | 3-Hexanone, 2,5-dimethyl- | 0.22 | — | — |
| 34 | 32.995 | Phthalic acid, 4-cyanophenyl nonyl ester | 0.46 | — | — |
| 35 * | 33.112 | n-Hexadecanoic acid | 17.81 | 33.30 | 23.55 |
| 36 | 34.025 | Glutaric acid, tridec-2-yn-1-yl 4-methylcyclohexyl ester | — | 0.11 | — |
| 37 | 34.307 | Hexadecanal | — | — | 0.84 |
| 38 | 34.314 | 14-Heptadecenal | 0.69 | — | — |
| 39 | 35.476 | n-Pentadecanol | — | 0.85 | 0.86 |
| 40 | 35.492 | 1-Undecene, 9-methyl- | 0.62 | — | — |
| 41 | 35.618 | 7-Tetradecyne | — | — | 0.63 |
| 42 | 35.847 | 2-Heptadecenal | — | — | 0.45 |
| 43 * | 36.868 | Octadecanoic acid | 30.70 | 46.39 | 38.14 |
| 44 | 37.127 | Hexadecanamide | — | 0.59 | — |
| 45 | 39.563 | 2-Propenoic acid, 3-(4-methoxyphenyl)-, 2-ethylhexyl ester | — | 0.08 | — |
| 46 | 40.668 | Octadecanamide | — | 1.08 | 0.50 |
| 47 | 40.675 | 9-Octadecenamide, (Z)- | — | — | 0.28 |
| 48 | 40.725 | Hexanedioic acid, bis(2-ethylhexyl) ester | 0.42 | 0.50 | — |
| 49 | 42.713 | 1-Dimethylaminohexane | — | 0.13 | — |
| 50 | 43.756 | 1-Pentadecyne | — | 0.10 | — |
| 51 | 45.772 | Phthalic acid, di(3-methylphenyl) ester | — | 0.11 | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaeoboon, S.; Songserm, R.; Suksungworn, R.; Duangsrisai, S.; Sanevas, N. In Vitro Antioxidant Potential, Antidiabetic Activities, and GC–MS Analysis of Lipid Extracts of Chlorella Microalgae. BioTech 2025, 14, 46. https://doi.org/10.3390/biotech14020046
Kaeoboon S, Songserm R, Suksungworn R, Duangsrisai S, Sanevas N. In Vitro Antioxidant Potential, Antidiabetic Activities, and GC–MS Analysis of Lipid Extracts of Chlorella Microalgae. BioTech. 2025; 14(2):46. https://doi.org/10.3390/biotech14020046
Chicago/Turabian StyleKaeoboon, Somruthai, Rattanaporn Songserm, Rungcharn Suksungworn, Sutsawat Duangsrisai, and Nuttha Sanevas. 2025. "In Vitro Antioxidant Potential, Antidiabetic Activities, and GC–MS Analysis of Lipid Extracts of Chlorella Microalgae" BioTech 14, no. 2: 46. https://doi.org/10.3390/biotech14020046
APA StyleKaeoboon, S., Songserm, R., Suksungworn, R., Duangsrisai, S., & Sanevas, N. (2025). In Vitro Antioxidant Potential, Antidiabetic Activities, and GC–MS Analysis of Lipid Extracts of Chlorella Microalgae. BioTech, 14(2), 46. https://doi.org/10.3390/biotech14020046







