Abstract
Recurrent vulvovaginal candidiasis (RVVC) is a common condition that affects women of reproductive age. The etiology of RVVC remains largely unknown, but it is believed to be associated with changes in vaginal microbiota composition. This study investigates the vaginal microbiota in 57 women with RVVC and 38 healthy controls. Bacterial DNA was analyzed using high-throughput 16S rRNA gene sequencing, and Candida and Saccharomyces species were determined by PCR. RVVC cases had a higher prevalence of Nakaseomyses glabratus (former Candida glabrata) compared to controls. Alpha diversity metrics were similar between groups, but beta diversity analysis revealed significant differences in vaginal microbiota composition. The Firmicutes abundance was altered in RVVC cases, with genus Bifidobacterium and phylum Actinobacteriota being more abundant than in the controls. At the genus level, Lactobacillus dominated controls using antibiotics, while Bifidobacterium was higher in cases with no antibiotic intake. Our study provides evidence that Nakaseomyses glabratus (former Candida glabrata) is a significant pathogen in RVVC, while Candida albicans was more prevalent in healthy women. The vaginal microbiota composition differs significantly between the two groups, with distinct patterns of bacterial abundance and changes in Firmicutes abundance.
Keywords:
RVVC; Candida albicans; Nakaseomyses glabratus; Candida glabrata; Saccharomyces cerevisiae; vaginal microbiota; 16S rRNA Key Contribution:
The yeast Nakaseomyses glabratus (former Candida glabrata) was more abundant in all cases of recurrent vulvovaginal candidiasis, while C. albicans was more prevalent among healthy women. The presence of N. glabratus was associated with significant changes in the bacterial microbiota diversity with an increased abundance of Bifidobacterium (Actinobacteriota phylum), while the controls exhibited a larger abundance of Lactobacillus (Firmicutes phylum). This adds important information about the perturbation of the vaginal microbial ecology under stress conditions due to yeast pathogens’ presence.
1. Introduction
The human body is inhabited by many microorganisms, bacteria, archaea, and fungi, collectively known as microbiota [1]. Among them, vaginal microbiota constitutes about 9% of total human microbiota and comprises a diverse array of beneficial microbes and opportunistic pathogens that inhabit the vaginal milieu [2,3]. The organisms that inhabit the vagina are dynamic and respond uniquely to various factors such as diet, exercise, medications, and diseases, which cause bacterial dysbiosis. This disruption increases the risk of contracting different urogenital microbial and fungal infections, which can be caused by sexually transmitted infections (STIs) or pelvic inflammatory disease (PID) [2,4].
Some of the characteristic symptoms of vaginal infections occurring in women of reproductive age are foul odor, burning, itching, and presence of discharge [5]. In the case of pregnant women, this microbial ecosystem changes according to the maturation stages of the woman, for example, weeks after childbirth, maternal estrogen metabolism promotes the proliferation of various aerobic and anaerobic bacteria due to mucosa thinning, an increase in vaginal pH, and a reduction in the glycogen substrate [6]. Lactobacillus of genera L. jensenii, L. inerss, L. gasseri, L. crispatus, and Gardnerella vaginalis mainly dominate the vaginal microbial community of healthy women in reproductive age [1,2], and in the last trimester of pregnancy [7]. These microorganisms prevent the invasion of pathogens, keeping the population in control. Previous work focusing on Mexican women using PCR and denaturing gradient gel electrophoresis (PCR-DGGE) reported bacterial communities in the vaginal tract of Mexican women during pregnancy, identifying 21 microorganism species, with 62.5% belonging to the genus Lactobacillus, 8.5% to non-cultivated Ureaplasma, 6.7% to BVAB1, and 3.0% to Gemella bergeriae [8].
In the case of fungi in humans, members of the fungal genera Candida are commonly found on the skin and mucous membranes, producing in some cases an opportunistic vaginal infection called candidiasis. Candida albicans is one of the leading agents associated with vulvovaginal candidiasis (VVC), a multifactorial infectious disease of the female reproductive tract that predominates during fertile years in women [9]. Some studies report that microbial diversity characterized by low Lactobacillus abundance is associated with low levels of inflammation, and high non-Lactobacillus dominated diversity (dysbiosis) is associated with increased risk of infection and obstetric complications [1,10].
Furthermore, fungi, especially yeast Candida, are found in vaginal mucosa as symbionts, forming a complex vaginal ecosystem with other bacteria. Fungi play diverse roles, such as lowering vaginal pH, producing bioactive compounds, competing for nutrients, adhesion sites, and regulating host immune responses [4,11]. It is reported that an overgrowth of Candida, specifically C. albicans, characterizes VVC, one of the most common vaginal infections, and inflammation. This fungus is one of the most abundant species in premenopausal women, pregnant women, and women with acute VVC [4]. The haploid fungi Nakaseomyses glabratus (former Candida glabrata) may be a regular component of the epithelial microbiota of the skin, oral cavity, gastrointestinal, and urogenital tract. Also known as an opportunistic pathogen, it is associated with different vertebrate microbiotas. This fungus is reported in Europe and North America as the second cause of candidiasis and has recently been reclassified as Nakaseomyses glabratus [12,13].
The purpose of this work was to characterize vaginal bacterial diversity in conditions of yeast infection in a sample of Mexican women to identify taxa signatures with a potential association with the presence of Nakaseomyses glabratus (former Candida glabrata) and or Candida albicans.
2. Materials and Methods
2.1. Experimental Design
This study recruited Mexican women aged 18–45 from gynecological consultations at the ABC Medical Center in Mexico City (19°23′57.4026″ N 99°11′47.57136″ W), using a convenient sampling approach. Participants were divided into two groups. Cases were women with recurrent vulvovaginal candidiasis (RVVC), having at least four episodes in the past 12 months, while controls were healthy women attending routine annual Papanicolaou test (pap smear test) consultations, without a history of recurrent vaginitis. Inclusion criteria for both groups required abstinence from sexual activity for 48 h, no menstruation at the time of sample collection, and no diagnosis of diabetes mellitus, obesity, or autoimmune diseases. Cases were additionally required to have a history of RVVC, while controls were limited to a history of one or fewer vaginitis episodes over their lifetime. Exclusion criteria included menstruation during sample collection or declining to participate. Controls were excluded if they had symptoms of vaginitis within the past six months or a history of more than one vaginitis episode diagnosed as vulvovaginal candidiasis. Cases were excluded if they did not meet the number of RVVC episodes or the specified age range. All participants provided informed consent following the 2013 Declaration of Helsinki after receiving a detailed explanation of the study. The protocol was approved by the Committee of Research and the Ethics in Research Committee of the ABC Medical Center (Project identification code: 2016-06-23). Samples were collected from June 2016 to May 2017.
2.2. Vaginal Swab Sampling
Participants were positioned on a gynecological examination table with stirrups, and a vaginal speculum was inserted for sample collection. Cervicovaginal mucus was collected using Catch-All™ Sample Collection Swabs (Epicentre, Illumina, San Diego, CA, USA, Cat# QEC89100) from the vaginal fornix. The vaginal pH was measured using Hydrion pH test strips (MicroEssential, Brooklyn, NewYork, NY, USA, Cat#165/1-12). The swab trimmed to fit was then placed in a 2 mL tube containing sterile PBS solution pH 7.0 and stored at 4 °C until DNA extraction.
2.3. DNA Extraction from Vaginal Swab Samples
DNA was extracted from vaginal swab samples using the GeneAll Exgene Stool SV Kit (Gene All, Songpa-gu, Seoul, Korea, Cat#115-150). The samples were centrifuged for 5 min at 3800× g, and the supernatant was discarded. The pellet was resuspended in 1.0 mL of PBS buffer pH 7.0, vortexed for 1 min, and incubated at room temperature for 30 s. After centrifugation at maximum speed for 2 min, the supernatant was discarded. The pellet was resuspended in 1.3 mL of Buffer FL, incubated for 5 min at room temperature, and centrifuged at ≥10,000× g for 5 min. The supernatant was transferred to an EzPass filter column, centrifuged, and eluted into a clean 1.5 mL tube with 100 µL of Buffer EB. After further purification steps involving Buffer PB and Buffer NW, the final DNA elution was performed with 50 µL of Buffer EB. DNA integrity and concentration were verified using a Nanodrop 2000 spectrophotometer (Thermo Scientific; Waltham, MA, USA, Cat#ND-2000) and 0.5% agarose gel electrophoresis.
2.4. 16S rRNA Gene Library Preparation
The V3 polymorphic region of bacterial 16S rRNA gene, ~281 bp in length, was amplified using primer V3-341F series (positions 340–356 of Escherichia coli 16S rDNA molecule rrnB GenBank accession number J01859.1), containing different 12 pb Golay barcode, and antisense primer V3-518R (complementary to positions 517–533) (Table S1). The PCR was carried out in the GeneAmp PCR System 2700 Thermal Cycler (Applied Biosystems, ThermoFisher Scientific, Waltham, MA, USA), and the program was 5 min 95 °C; 25 cycles [15 s, 94 °C; 15 s, 62 °C; 15 s, 72 °C] followed by an extension of 10 min at 72 °C [14].
2.5. High-Throughput DNA Semiconductor Sequencing
Equal concentrations of amplicons were pooled and purified, subsequently sequenced as previously reported [14]. DNA library concentration and final fragment size were measured with the 2100 Bioanalyzer Instrument fragment analyzer (Agilent Technologies, Santa Clara, CA, USA), and the average library size obtained was 263 bp. Emulsion PCR was carried out using Ion OneTouchTM 200 Template Kit v2 DL (Life Technologies, Carlsbad, CA, USA). Amplicon enrichment with ionic beads was carried out using Ion OneTouch ES (Life Technologies, Carlsbad, CA, USA). The Ion 318 Kit V2 chip (Cat. 4488146, Life Technologies, Carlsbad, CA, USA) and the Ion Torrent PGM system were used for sequencing. The reads were filtered using PGM software to eliminate polyclonal (homopolymers > 6) and low-quality sequences (quality score ≤ 20).
2.6. Yeast Identification by PCR Targeting ITS1 and ITS2 and MEX67 Gene
To characterize the etiology of RVVC in samples from the participants, PCR reactions were performed using species-specific primers for C. albicans and N. glabratus (former C. glabrata), targeting the ITS1 and ITS2 of C. albicans and C. glabrata, respectively (Table S2) [13,15]. Primers for S. cerevisiae targeting the nuclear export MEX67 gene [16] (Table S2) were included due to the potential involvement of this yeast in vulvovaginitis, which clinically resembles Candida infections. For ITS, the PCR program followed the initial denaturation step at 96 °C for 5 min, followed by 30 cycles [30 s, 94 °C, 30 s, 58 °C, and 30 s, 72 °C], a final extension at 72 °C for 15 min, cooling to 10 °C for 10 min; for MEX67 initial denaturation step at 94 °C for 5 min, followed by 30 cycles [60 s, 94 °C, 60 s, 58 °C, and 60 s, 72 °C], and a final extension at 72 °C for 5 min, cooling to 10 °C for 5 min. Positive controls included C. albicans ATCC, C. glabrata CBS138, and S. cerevisiae S2886, and negative controls used reaction mixtures without DNA templates. Amplicons of ~273 bp, 423 bp, and 150 bp were fractionated in 2.0% agarose gel electrophoresis stained with Midori Green Advanced dye(Nippon Genetics®, Dueren, Germany), and products were visualized using the Molecular Imager Gel Doc XR system Mod. Universal Hood II (Bio-Rad, Hercules, CA, USA, Cat#8195). The three PCR products were cloned using the GeneJET PCR Cloning Kit (ThermoFisher Scientific, Waltham, MA, USA), and plasmid DNA was extracted for Sanger capillary sequencing with pJET1 primers [15]. Resulting sequences were analyzed using VECTOR NTI Advance and BLAST alignments to verify species-specific diagnostic accuracy.
2.7. ASV Determination and Taxonomic Annotation
The FASTQ files were further processed and analyzed using QIIME 2022.2 [17]. Amplicon Sequence Variants (ASVs) were determined with the QIIME dada2 denoise-single plugin, with sequence truncation at 238 nt. Taxonomic assignment was performed using the feature-classifier classify-consensus-blast plugin with a 99% percentage identity. The Greengenes 2 database was used for BLAST analysis (QIIME 2022.2) [18].
2.8. Bioinformatic Analyses
The analyses were conducted with RStudio in R 4.2.0 [19,20]. The following packages were employed for relative abundance, diversity and differential analyses: phyloseq 1.4.0 [21], DESeq2 1.3.6 [22], and ALDEx2 1.36.0; qiime2R [23] to import the qiime artifacts [24]; for alpha and beta diversity analyses vegan 2.6-2 [25]; lefser 1.15.9 [26] for discriminatory analyses; ComplexHeatmap 2.12.0 [27] for heatmap elaboration; and tidyverse 1.3.1 [28], dplyr 1.09, ggplot2 3.3.6, scales 1.2.0, ggpubr 0.4.4, and gridExtra 2.3 [29] for graphical images.
3. Results
3.1. Characteristics of Participants and Candida Prevalence in Vaginosis Cases vs. Controls
In this study, 38 controls and 57 cases met the inclusion criteria, of which 27 controls and 54 cases were successfully sequenced (Table S3). No statistically significant differences were observed between groups regarding age, age of menarche, sexual life start, or vaginal pH, and other variables showed similar distribution between groups (Figure S1). A subset of participants, 13 cases (30.95%), and 25 controls (43.86%) reported the use of antibiotics in the last three months, and this factor was considered in subsequent analyses. Additionally, PCR detection revealed significant differences in the prevalence of C. albicans (90% in controls vs. 36.17% in cases) and N. glabratus (0.0% controls vs. 97.87% cases) based on two-proportion z-tests (Figure 1C, Table 1). On the other hand, S. cerevisiae showed a comparable abundance in both groups (90.0% controls vs. 95.74% cases).
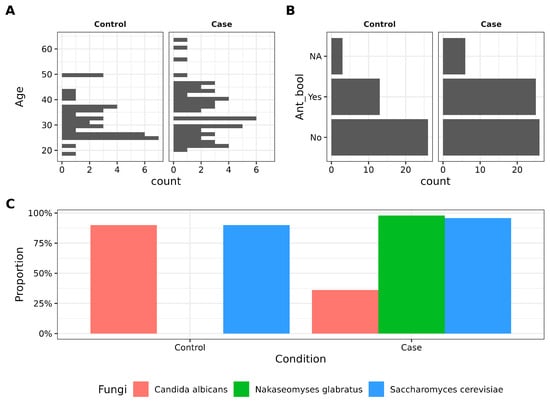
Figure 1.
Main variables driving the study. (A). Histogram depicting the age (in years) distribution between control and case groups. No statistical difference was found according to the Student’s t-test. (B). Barplot showing antibiotic intake in women. (C). Barplot showing the proportion of control and case groups with three different fungi detected by PCR.

Table 1.
General data for participants of the study.
3.2. Antibiotic Intake and Patient Condition Have a Small Effect on Beta Diversity
The sequencing data were processed as described in the Section 2. For samples with low sequencing depth, re-sequencing was performed, and the resulting reads were combined by summation (Figure S2). Both alpha and beta diversity metrics were analyzed to investigate the impact of antibiotic use and patient condition on global bacterial diversity. While alpha diversity appeared unaffected by these factors, beta diversity showed significant associations based on ADONIS tests. Weighted beta diversity was influenced primarily by patient condition (p = 0.042), whereas unweighted beta diversity was significantly associated with both patient condition (p = 0.012) and antibiotic use (p = 0.018). Despite these findings, the low values indicate a small effect (Figure 2). Additionally, sequencing depth was examined with antibiotic consumption to determine whether it contributed to reduced read counts, yet no evidence of such an effect was found (Figure S3).
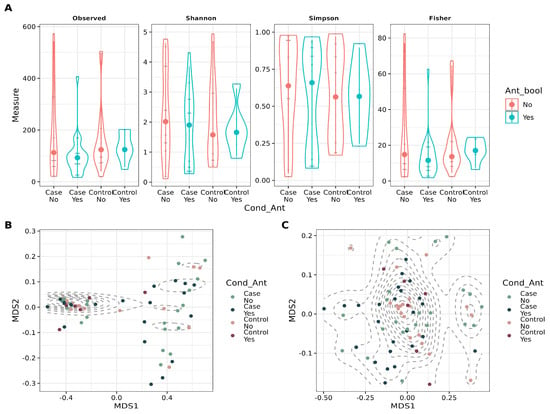
Figure 2.
Diversity metrics for stratified groups, considering antibiotic intake and condition (case vs. control). (A). Violin plot depicting alpha diversity metrics (Observed number of ASVs, Shannon, Simpson, and Fisher) for the groups. (B). Scatter plot showing Non-Metric Multidimensional Scaling (NMDS) for weighted Unifrac distance for beta diversity assessment in the groups. ADONIS was applied to account for the variance explained by Condition (p = 0.036, = 0.05) and Antibiotic intake (p = 0.39, = 0.01). (C). Scatter plot showing Non-Metric Multidimensional Scaling (NMDS) for unweighted Unifrac distance for beta diversity assessment in the groups. ADONIS was applied to account for the variance explained by Condition (p = 0.012, = 0.03) and Antibiotic intake (p = 0.018, = 0.03).
3.3. Vaginal Microbiota Composition Reflects RVVC Condition and Antibiotic Use
The composition of the vaginal microbiota was analyzed at both the phylum (Figure 3A) and genus levels (Figure 3B) to identify changes in key bacterial taxa across samples. Hierarchical clustering using weighted beta diversity was employed to compare taxa and assess similarities among bacterial communities in the groups. Samples were first grouped by antibiotic intake and subsequently by RVVC condition. Significant alterations were observed in the Firmicutes abundance, particularly between cases and controls. Cases without antibiotic use exhibited the highest abundance of Bacteroidota and Actinobacteriota phyla, whereas controls who had taken antibiotics were dominated by the Firmicutes_D phylum.
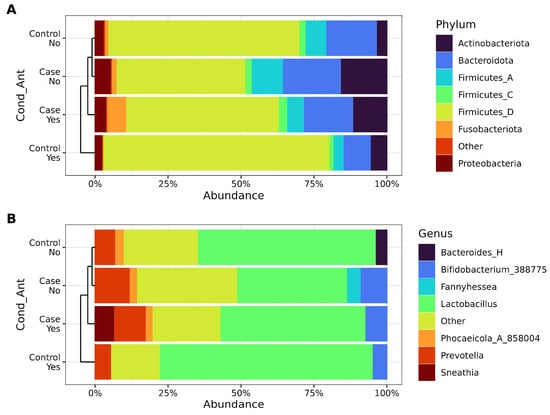
Figure 3.
Relative abundance for stratified groups, considering antibiotic intake and condition (case vs. control). (A). Phylum relative abundance as percentages. (B). Genus relative abundance as percentages. Taxa are identified on the right side of each figure.
At the genus level, the members of the phylum Firmicutes_D were predominantly represented by Lactobacillus, which also dominated controls with antibiotic use. In contrast, cases without antibiotic intake showed a higher prevalence of Bifidobacterium, while Bacteroides_H was found only in controls who had not taken antibiotics. Notably, Fannyhessea was characteristic of cases without antibiotic use, whereas Sneathia was specific to cases with antibiotic use. Additionally, a marked increase in genus Prevotella was observed in cases where Lactobacillus was replaced by a diverse array of low-abundance taxa (<2%, grouped as “Other”). These findings highlight the dynamic shifts in microbial composition associated with antibiotic use and patient condition (Figure 3B).
3.4. Lactobacillus and Other Key Bacteria Are Associated with Healthy Vaginal Microbiota
Differential abundance analyses were performed to identify bacteria associated with vaginosis while accounting for antibiotic intake among participants. Two approaches were used: ALDEx2, with the formula “ antibiotic intake + RVVC condition”, and linear discriminant analysis (LEfSe), using RVVC as the class variable and antibiotic intake as the subclass. The results from ALDEx2 are presented in Figure 4A, with corresponding square root-transformed relative abundance values shown in Figure 4B. In the control group, Lactobacillus and an unassigned taxon were prominent. Interestingly, two distinct ASVs classified as Escherichia_710834 were identified in both the control and case groups.
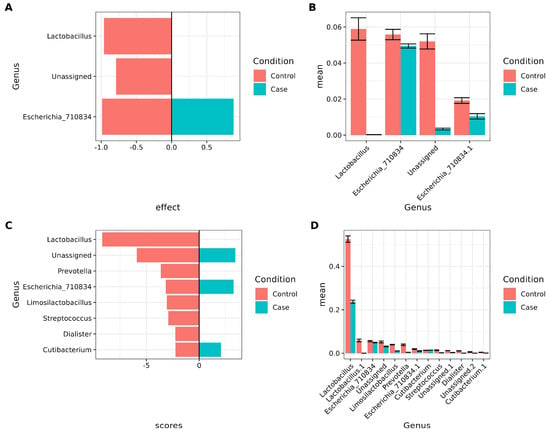
Figure 4.
Important biomarker bacteria considering antibiotic intake and condition. (A,B), Using ALDEx2 (“ antibiotic intake + RVVC condition”). (C,D), using LEFSE (Class = RVVC, Subclass = antibiotic intake). Only features with p-values that passed FDR correction were included in the graphs. (A). Barplot showing ALDEx2 results for condition (case vs. control). The X-axis shows the effect, and the Y-axis shows the genus. Negative values correspond to control, positive values correspond to cases. (B). Barplot of mean sqrt of relative abundance of taxa found by ALDEx2. The X-axis shows the mean sqrt of relative abundance, and the Y-axis shows the genera. (C). Barplot showing LEFSE results for condition (case vs. control). The X-axis shows the LDA scores, and the Y-axis shows the genus. Negative values correspond to control; positive values correspond to cases. (D). Barplot of mean sqrt of relative abundance of taxa found by LEFSE. The X-axis shows the mean sqrt of relative abundance, and the Y-axis shows genus.
LEfSe results are shown in Figure 4C, with the corresponding square root-transformed relative abundance values in Figure 4D. These findings corroborate the ALDEx2 results for Lactobacillus. In addition, Prevotella, Limosilactobacillus, Streptococcus, and Dialister were predominant in controls, whereas two distinct Escherichia_710834 ASVs, unassigned taxa, and Cutibacterium were characteristic of cases.
4. Discussion
This work aims to characterize the vaginal microbiota of women suffering from RVVC. We found no differences between cases and control in women associated with metadata. For instance, no differences in vaginal pH were consistent with the disease diagnosis [30]. The age prevalence in women is also consistent with the literature, which indicates that most episodes occur in the range from 19 to 35 years with lower prevalence rates after 50 years [31]. In our sample, we observed a broader range between 20 to 50 years, with most cases around 35. Additionally, an association between RVVC incidence and new sexual partners is reported [32]. In our work, there is a tendency among cases to have more sexual partners, but our results are not conclusive in this matter.
In this work, we detected by PCR N. glabratus (former C. glabrata) exclusive to RVVC cases, while C. albicans were found in higher proportion in the control group. Interestingly, some reports mention C. albicans as the major cause of infection, with 80–95% of isolates identified as this species [30]. Some other species, such as N. glabratus (formerly C. glabrata), Candida tropicalis, and Candida parapsilosis, are now frequently identified as human pathogens [33]. Moreover, Candida spp. has been previously isolated from healthy women [34,35], and it has been reported that Candida infections are common and, in most cases, occur in non-symptomatic healthy individuals [36]. Due to the contrasting results in this study, there might be an effect of the vaginal bacteria in our participants, which will be Fexplored further. It was also interesting, for the scope of this study, that a significant portion of participants in both groups reported the use of antibiotics. It has been reported that the intake of broad-spectrum antibiotics might reduce gut microbiota diversity [37]. For this reason, we had to take antibiotic intake into account when evaluating the bacterial profile.
We found no major differences in alpha diversity between cases and controls in our work. However, a small effect was observed due to both antibiotic intake and RVVC. As mentioned before, it is expected that antibiotics have an impact on microbiota diversity, also in the context of vaginal microbiota, and in the long term might develop resistance and cause recurrent infections [38]. Overall, the diversity in all groups was low, which is expected in the case of vaginal microbiota [4], for instance, Clostridioides difficile infection in the gut could occur due to dysbiosis caused by antibiotics [39]. Previous reports evaluating microbial diversity during RVVC conditions found major differences in alpha and beta diversity, with lower diversity in the cases [40,41].
Our relative abundance results indicate an increase in Bifidobacterium in association with RVVC, while antibiotic intake exhibited a mixed effect, increasing Bifidobacterium in controls and decreasing in the cases. In a previous study, a reduction in gut Bifidobacterium was observed in response to amoxicillin and azithromycin [42]. Gardnerella vaginalis has been widely associated with vaginal infections [43,44], and recently, ATCC Genome Portal reclassified Gardnerella vaginalis as part of Bifidobacterium [45]. In two previous works, studying women with bacterial vaginosis exposed to metronidazole or clindamycin, mixed effects in Gardnerella abundance were observed after the treatment, increasing in some women and decreasing in others [46,47]. Furthermore, we found Sneathia in cases where the participant consumed oral antibiotics. Previous work showed Sneathia was unchanged by antibiotic intake [47]. There is sufficient evidence pointing to Sneathia as an emerging pathogen in female reproductive disease with adverse perinatal outcomes [48].
Additionally, we identified Lactobacillus with ALDEx2 and LEFSE as characteristic of healthy women when comparing to RVVC. This is consistent with other works [2,49], which have linked Lactobacillus to a healthy vagina when studying bacterial vaginosis. Remarkably, we found different ASVs classified as Escherichia in our study, characterizing both cases and controls. Another report found Escherichia to be predominant in patients with aerobic vaginitis [49,50]. Particularly, other bacteria identified by LEFSE analysis and associated with healthy women were Prevotella, Limosilactobacillus, Streptococcus, and Dialister. The association between Bacterial Vaginosis and increased abundance of anaerobes like Prevotella, which is an important source of lipopolysaccharide and ammonia in vaginal mucus, enhances the growth of other bacterial vaginosis-associated organisms like Gardnerella vaginalis [50,51]. In the case of probiotics, Limosilactobacillus, one ex-member of the Lactobacillus species, is a probiotic bacterium that can be found in various parts of the human body, like the gastrointestinal tract (GI), urinary tract, skin, and breast milk, and suggests its metabolites promote human health [51,52]. In the case of Streptococcus, it has been associated with Bacterial Vaginosis in pregnant women, which also presents a dysbiosis of Lactobacillus [53]. The genus Dialister also has been associated with higher prevalence and abundance in Bacterial vaginosis [41].
5. Conclusions
We observed some significant differences in vaginal microbiota composition between women with recurrent vulvovaginal candidiasis (RVVC) and healthy controls. N. glabratus (formerly C. glabrata) was exclusively detected in RVVC cases, while C. albicans was more prevalent in the control group. The vaginal microbiota diversity was relatively low, but we found distinct patterns of bacterial abundance between the two groups. Bifidobacterium exhibited an increase in response to RVVC, while antibiotic intake had a mixed effect on bacterial abundance, decreasing it in cases and increasing it in controls. The pathogen G. vaginalis (classified as part of Bifidobacterium) showed variable responses after antibiotic treatment in both groups of this study. Lactobacillus was more prevalent in healthy women when contrasted to RVVC. Other bacteria associated with healthy women included Prevotella, Limosilactobacillus, Streptococcus, and Dialister. These observations underscore the importance of further research into the mechanisms behind these associations, with a particular focus on the interactions between N. glabratus and vaginal bacteria.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biotech14020031/s1, Figure S1: Typical Q-Q plot showing normal distribution of age in the studied women; Figure S2: Bar plot showing sequencing depth for each sample, and Figure S3: Bar plot showing sample counts and antibiotic intake in both case and control groups. Table S1: 5′ to 3′ sequence of barcoded primers for bacteria used in this study; Table S2: Diagnostic primers for yeast detection, and Table S3: Sequencing summary.
Author Contributions
Conceptualization, C.M.-B. and J.G.-M.; methodology, J.P.-D., C.M.-B., C.J.J.-C., A.P.-E., N.d.S.L.-P., A.d.l.C.-M., D.R.-S., Y.G.-M., J.M.V.-I. and J.G.-M.; software, J.M.V.-I.; validation, C.J.J.-C.; formal analysis, J.M.V.-I.; investigation, J.P.-D., C.M.-B., C.J.J.-C., A.P.-E., N.d.S.L.-P., A.d.l.C.-M., D.R.-S., Y.G.-M., J.M.V.-I. and J.G.-M.; resources, Y.G.-M. and J.G.-M.; data curation, C.M.-B., C.J.J.-C. and J.G.-M.; writing—original draft preparation, C.M.-B., C.J.J.-C., D.R.-S., J.M.V.-I. and J.G.-M.; writing—review and editing, J.P-D., C.M.-B., C.J.J.-C., A.P.-E., N.d.S.L.-P., A.d.l.C.-M., D.R.-S., Y.G.-M., J.M.V.-I. and J.G.-M.; visualization, J.G.-M. and J.M.V.-I.; supervision, J.G.-M., A.P.-E. and C.J.J.-C.; project administration, C.M.-B. and J.G.-M.; funding acquisition, J.G.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Cinvestav, Secretaría de Ciencia, Humanidades, Tecnología e Innovación (SECIHTI): CONACYT-163235, Laboratorio de Referencia y Apoyo para la Caracterización de Genomas, Transcriptomas y Microbiomas en la Unidad Zacatenco del Cinvestav; by CONACYT FORDECYT-PRONACES/6669/2020_Programa Presupuestario F003-Ciencia de Frontera 2019 “Estudio de la vía Tracto-Digestivo-Materno Leche-Materna Tracto-digestivo-neonato, una aproximación ómica”, and by SECTEI/249/2019—CM-SECTEI/109/2020—CM-SECTEI/124/2021, Convocatoria 2019, Proyectos Científicos, Tecnológicos y/o de Innovación para la atención a problemas específicos de la Ciudad de México relacionados con la investigación y atención de enfermedades crónicas no transmisibles (ECNT). The funding body was not involved in study design; collection, management, analysis, and interpretation of data; or the decision to submit for publication.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Committee of Research and the Ethics in Research Committee of the ABC Medical Center (Project identification code: 2016-06-23).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated for this study can be found in the NCBI BioProject ID PRJNA1199545 Link https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1199545 (accessed on 10 April 2025).
Acknowledgments
We thank SECIHTI for Postdoctoral Fellowship Estancias-postdoctorales-por Mexico (321600) granted to CJJ-C, Fellowships granted to JMV-I (959984). Fellowship granted to NdSL-P (1227574), AdlC-M (1311414), and SNI-Ayudante Fellowships granted to DR-S (629184). We are grateful to Rodrigo García-Gutiérrez for technical support and Viridiana Rosas-Ocegueda for administrative assistance.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Moosa, Y.; Kwon, D.; de Oliveira, T.; Wong, E.B. Determinants of Vaginal Microbiota Composition. Front. Cell. Infect. Microbiol. 2020, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Factories 2020, 19, 203. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Chen, T. Vaginal microbiota: Potential targets for vulvovaginal candidiasis infection. Heliyon 2024, 10, e27239. [Google Scholar] [CrossRef]
- Sun, Z.; Ge, X.; Qiu, B.; Xiang, Z.; Jiang, C.; Wu, J.; Li, Y. Vulvovaginal candidiasis and vaginal microflora interaction: Microflora changes and probiotic therapy. Front. Cell. Infect. Microbiol. 2023, 13, 1123026. [Google Scholar] [CrossRef]
- Kalia, N.; Singh, J.; Kaur, M. Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: A critical review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 5. [Google Scholar] [CrossRef]
- Yamamoto, T.; Zhou, X.; Williams, C.J.; Hochwalt, A.; Forney, L.J. Bacterial Populations in the Vaginas of Healthy Adolescent Women. J. Pediatr. Adolesc. Gynecol. 2009, 22, 11–18. [Google Scholar] [CrossRef]
- González-Sánchez, A.; Reyes-Lagos, J.J.; Peña-Castillo, M.A.; Nirmalkar, K.; García-Mena, J.; Pacheco-López, G. Vaginal Microbiota Is Stable and Mainly Dominated by Lactobacillus at Third Trimester of Pregnancy and Active Childbirth: A Longitudinal Study of Ten Mexican Women. Curr. Microbiol. 2022, 79, 230. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, C.; Romero-González, R.; Albani-Campanario, M.; Figueroa-Damián, R.; Meraz-Cruz, N.; Hernández-Guerrero, C. Vaginal Microbiota of Healthy Pregnant Mexican Women is Constituted by Four Lactobacillus Species and Several Vaginosis-Associated Bacteria. Infect. Dis. Obstet. Gynecol. 2011, 2011, 851485. [Google Scholar] [CrossRef]
- Willems, H.M.E.; Ahmed, S.S.; Liu, J.; Xu, Z.; Peters, B.M. Vulvovaginal Candidiasis: A Current Understanding and Burning Questions. J. Fungi 2020, 6, 27. [Google Scholar] [CrossRef]
- Anahtar, M.N.; Gootenberg, D.B.; Mitchell, C.M.; Kwon, D.S. Cervicovaginal Microbiota and Reproductive Health: The Virtue of Simplicity. Cell Host Microbe 2018, 23, 159–168. [Google Scholar] [CrossRef]
- Younes, J.A.; Lievens, E.; Hummelen, R.; van der Westen, R.; Reid, G.; Petrova, M.I. Women and Their Microbes: The Unexpected Friendship. Trends Microbiol. 2018, 26, 16–32. [Google Scholar] [CrossRef]
- Granada, M.; Cook, E.; Sherlock, G.; Rosenzweig, F. Microbe Profile: Candida glabrata—A master of deception: This article is part of the Microbe Profiles collection. Microbiology 2024, 170, 001518. [Google Scholar] [CrossRef]
- Takashima, M.; Sugita, T. Taxonomy of Pathogenic Yeasts Candida, Cryptococcus, Malassezia, and Trichosporon: Current Status, Future Perspectives, and Proposal for Transfer of Six Candida Species to the Genus Nakaseomyces. Med. Mycol. J. 2022, 63, 119–132. [Google Scholar] [CrossRef]
- Hernández-Quiroz, F.; Murugesan, S.; Flores-Rivas, C.; Piña-Escobedo, A.; Juárez-Hernández, J.I.; García-Espitia, M.; Chávez-Carbajal, A.; Nirmalkar, K.; García-Mena, J. A high-throughput DNA sequencing study of fecal bacteria of seven Mexican horse breeds. Arch. Microbiol. 2022, 204, 382. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Mitchell, T.G. Rapid identification of pathogenic fungi directly from cultures by using multiplex PCR. J. Clin. Microbiol. 2002, 40, 2860–2865. [Google Scholar] [CrossRef]
- Muir, A.; Harrison, E.; Wheals, A. A multiplex set of species-specific primers for rapid identification of members of the genus Saccharomyces. FEMS Yeast Res. 2011, 11, 552–563. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- McDonald, D.; Jiang, Y.; Balaban, M.; Cantrell, K.; Zhu, Q.; Gonzalez, A.; Morton, J.T.; Nicolaou, G.; Parks, D.H.; Karst, S.M.; et al. Greengenes2 unifies microbial data in a single reference tree. Nat. Biotechnol. 2023, 42, 715–718. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 17 November 2024).
- Posit Team. RStudio: Integrated Development Environment for R; Posit Software, PBC: Boston, MA, USA, 2023; Available online: http://www.posit.co/ (accessed on 17 October 2024).
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Bisanz, J.E. qiime2R: Importing QIIME2 Artifacts and Associated Data into R Sessions. 2018. Available online: https://github.com/jbisanz/qiime2R (accessed on 17 October 2024).
- Nixon, M.P.; McGovern, K.C.; Letourneau, J.; David, L.A.; Lazar, N.A.; Mukherjee, S.; Silverman, J.D. Scale Reliant Inference. arXiv 2024, arXiv:2201.03616. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package. 28 de Agosto de 2024. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 17 December 2024).
- Khleborodova, A.; Gamboa-Tuz, S.D.; Ramos, M.; Segata, N.; Waldron, L.; Oh, S. Lefser: Implementation of metagenomic biomarker discovery tool, LEfSe, in R. Bioinformatics 2024, 40, btae707. [Google Scholar] [CrossRef]
- Gu, Z. Complex heatmap visualization. iMeta 2022, 1, e43. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Auguie, B.; Antonov, A. gridExtra: Miscellaneous Functions for «Grid» Graphics. 9 de Septiembre de 2017. Available online: https://cran.r-project.org/web/packages/gridExtra/ (accessed on 17 December 2024).
- Sobel, J.D. Vulvovaginal candidosis. Lancet 2007, 369, 1961–1971. [Google Scholar] [CrossRef]
- Blostein, F.; Levin-Sparenberg, E.; Wagner, J.; Foxman, B. Recurrent vulvovaginal candidiasis. Ann. Epidemiol. 2017, 27, 575–582.e3. [Google Scholar] [CrossRef]
- Yano, J.; Sobel, J.D.; Nyirjesy, P.; Sobel, R.; Williams, V.L.; Yu, Q.; Noverr, M.C.; Fidel, P.L., Jr. Current patient perspectives of vulvovaginal candidiasis: Incidence, symptoms, management and post-treatment outcomes. BMC Women’s Health 2019, 19, 48. [Google Scholar] [CrossRef]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2012, 36, 288–305. [Google Scholar] [CrossRef]
- Chow, A.W.; Percival-Smith, R.; Bartlett, K.H.; Goldring, A.M.; Morrison, B.J. Vaginal colonization with Escherichia coli in healthy women: Determination of relative risks by quantitative culture and multivariate statistical analysis. Am. J. Obstet. Gynecol. 1986, 154, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Goldacre, M.J.; Watt, B.; Loudon, N.; Milne, L.J.; Loudon, J.D.; Vessey, M.P. Vaginal microbial flora in normal young women. BMJ 1979, 1, 1450–1455. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gow, N.A.; Hube, B. Importance of the Candida albicans cell wall during commensalism and infection. Curr. Opin. Microbiol. 2012, 15, 406–412. [Google Scholar] [CrossRef]
- Dubourg, G.; Lagier, J.C.; Robert, C.; Armougom, F.; Hugon, P.; Metidji, S.; Dione, N.; Dangui, N.P.M.; Pfleiderer, A.; Abrahao, J.; et al. Culturomics and pyrosequencing evidence of the reduction in gut microbiota diversity in patients with broad-spectrum antibiotics. Int. J. Antimicrob. Agents 2014, 44, 117–124. [Google Scholar] [CrossRef]
- Lev-Sagie, A.; Goldman-Wohl, D.; Cohen, Y.; Dori-Bachash, M.; Leshem, A.; Mor, U.; Strahilevitz, J.; Moses, A.E.; Shapiro, H.; Yagel, S.; et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 2019, 25, 1500–1504. [Google Scholar] [CrossRef]
- Theriot, C.M.; Koenigsknecht, M.J.; Carlson, P.E., Jr.; Hatton, G.E.; Nelson, A.M.; Li, B.; Huffnagle, G.B.; Li, J.Z.; Young, V.B. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat. Commun. 2014, 5, 3114. [Google Scholar] [CrossRef]
- Ceccarani, C.; Foschi, C.; Parolin, C.; D’antuono, A.; Gaspari, V.; Consolandi, C.; Laghi, L.; Camboni, T.; Vitali, B.; Severgnini, M.; et al. Diversity of vaginal microbiome and metabolome during genital infections. Sci. Rep. 2019, 9, 14095. [Google Scholar] [CrossRef]
- Liu, C.M.; Hungate, B.A.; Tobian, A.A.R.; Ravel, J.; Prodger, J.L.; Serwadda, D.; Kigozi, G.; Galiwango, R.M.; Nalugoda, F.; Keim, P.; et al. Penile Microbiota and Female Partner Bacterial Vaginosis in Rakai, Uganda. mBio 2015, 6, e00589-15. [Google Scholar] [CrossRef]
- Chopyk, J.; Güemes, A.G.C.; Ramirez-Sanchez, C.; Attai, H.; Ly, M.; Jones, M.B.; Liu, R.; Liu, C.; Yang, K.; Tu, X.M.; et al. Common antibiotics, azithromycin and amoxicillin, affect gut metagenomics within a household. BMC Microbiol. 2023, 23, 206. [Google Scholar] [CrossRef]
- Bagnall, P.; Rizzolo, D. Bacterial vaginosis: A practical review. J. Am. Acad. Physician Assist. 2017, 30, 15–21. [Google Scholar] [CrossRef]
- Morrill, S.; Gilbert, N.M.; Lewis, A.L. Gardnerella vaginalis as a Cause of Bacterial Vaginosis: Appraisal of the Evidence From in vivo Models. Front. Cell. Infect. Microbiol. 2020, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Reclassification of the Bifidobacterium and Gardnerella Genera. 2024. Available online: https://www.atcc.org/resources/posters/2019-posters/reclassification-of-the-bifidobacterium-and-gardnerella-genera (accessed on 17 December 2024).
- Ahrens, P.; Andersen, L.O.; Lilje, B.; Johannesen, T.B.; Dahl, E.G.; Baig, S.; Jensen, J.S.; Falk, L. Changes in the vaginal microbiota following antibiotic treatment for Mycoplasma genitalium, Chlamydia trachomatis and bacterial vaginosis. PLoS ONE 2020, 15, e0236036. [Google Scholar] [CrossRef]
- Zwittink, R.D.; Munckhof, E.H.A.v.D.; Hall, M.A.L.-V.; Boers, K.; Molijn, A.; Knetsch, C.W.; Kuijper, E.J. The vaginal microbiota in the course of bacterial vaginosis treatment. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 651–656. [Google Scholar] [CrossRef]
- Theis, K.R.; Florova, V.; Romero, R.; Borisov, A.B.; Winters, A.D.; Galaz, J.; Gomez-Lopez, N. Sneathia: An emerging pathogen in female reproductive disease and adverse perinatal outcomes. Crit. Rev. Microbiol. 2021, 47, 517–542. [Google Scholar] [CrossRef]
- Pramanick, R.; Nathani, N.; Warke, H.; Mayadeo, N.; Aranha, C. Vaginal Dysbiotic Microbiome in Women with No Symptoms of Genital Infections. Front. Cell. Infect. Microbiol. 2021, 11, 760459. [Google Scholar] [CrossRef]
- Prasad, D.; Parween, S.; Kumari, K.; Singh, N. Prevalence, Etiology, and Associated Symptoms of Vaginal Discharge During Pregnancy in Women Seen in a Tertiary Care Hospital in Bihar. Cureus 2021, 13, e12700. [Google Scholar] [CrossRef]
- Randis, T.M.; Ratner, A.J. Gardnerella and Prevotella: Co-conspirators in the Pathogenesis of Bacterial Vaginosis. J. Infect. Dis. 2019, 220, 1085–1088. [Google Scholar] [CrossRef]
- Abuqwider, J.; Altamimi, M.; Mauriello, G. Limosilactobacillus reuteri in Health and Disease. Microorganisms 2022, 10, 522. [Google Scholar] [CrossRef]
- Mohamed, I.; Zakeer, S.; Azab, M.; Hanora, A. Changes in Vaginal Microbiome in Pregnant and Nonpregnant Women with Bacterial Vaginosis: Toward Microbiome Diagnostics? OMICS J. Integr. Biol. 2020, 24, 602–614. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).