Vaginal Microbiota Patterns Associated with Yeast Infection in Mexican Women, a Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Vaginal Swab Sampling
2.3. DNA Extraction from Vaginal Swab Samples
2.4. 16S rRNA Gene Library Preparation
2.5. High-Throughput DNA Semiconductor Sequencing
2.6. Yeast Identification by PCR Targeting ITS1 and ITS2 and MEX67 Gene
2.7. ASV Determination and Taxonomic Annotation
2.8. Bioinformatic Analyses
3. Results
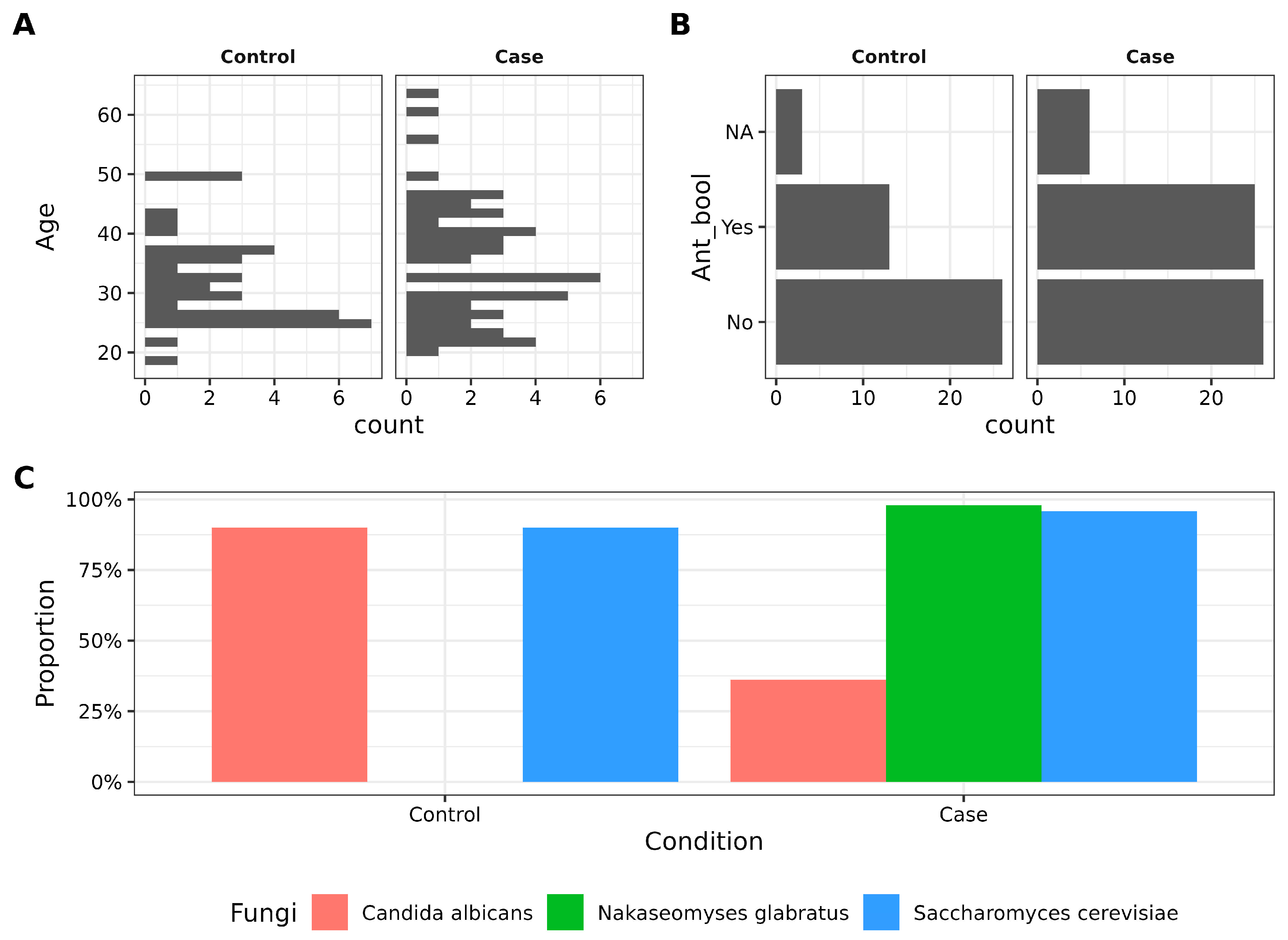
3.1. Characteristics of Participants and Candida Prevalence in Vaginosis Cases vs. Controls
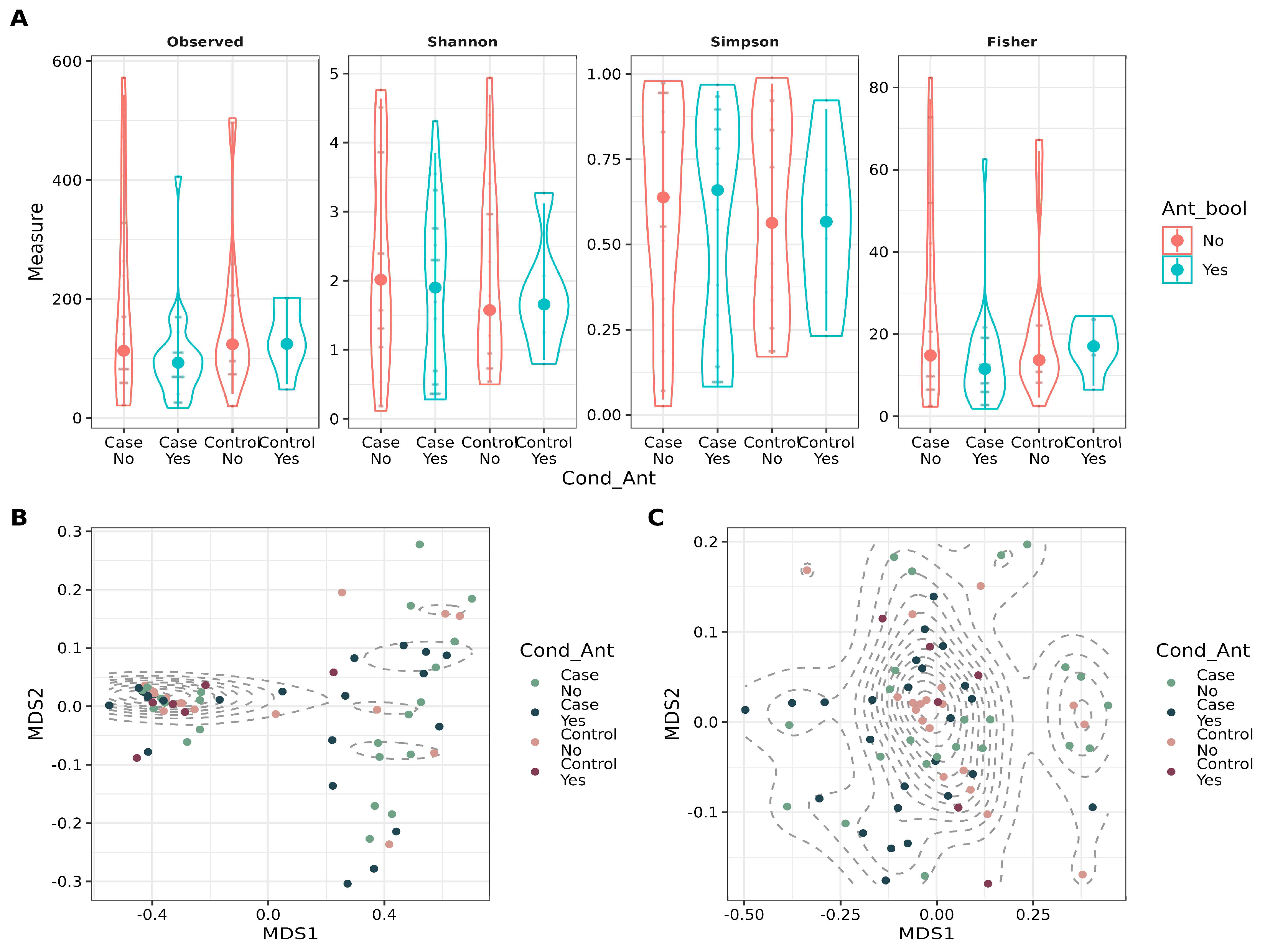
3.2. Antibiotic Intake and Patient Condition Have a Small Effect on Beta Diversity
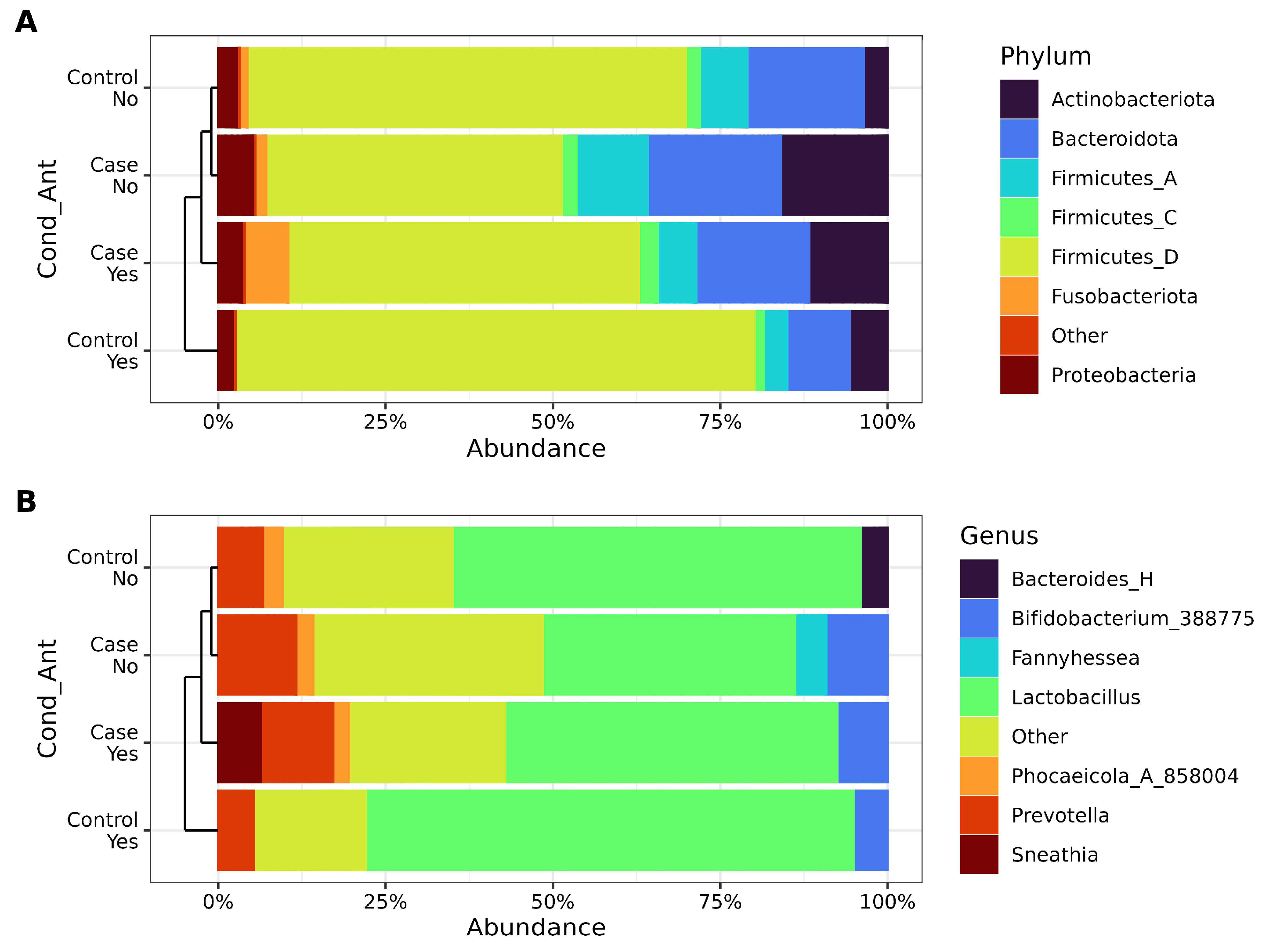
3.3. Vaginal Microbiota Composition Reflects RVVC Condition and Antibiotic Use
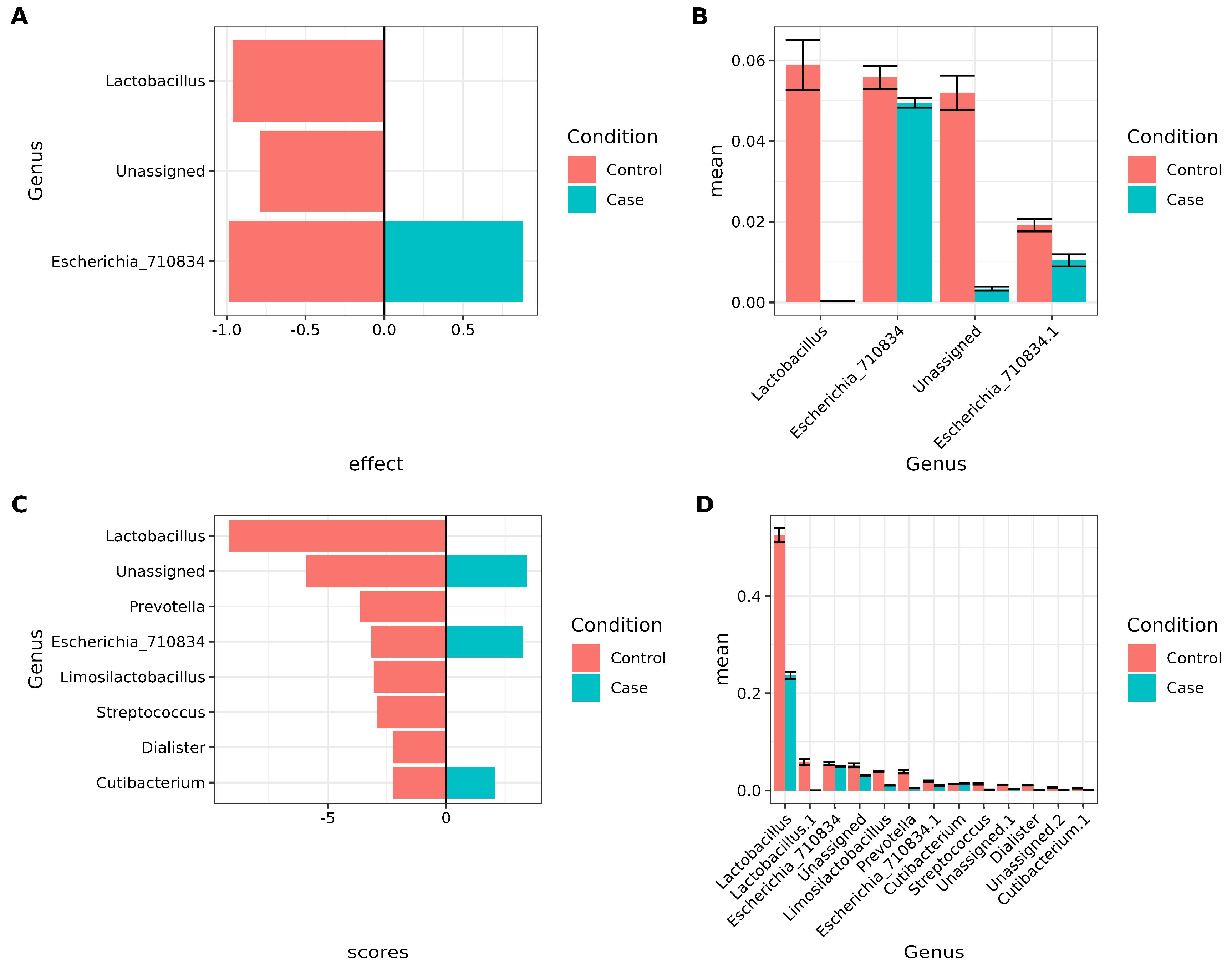
3.4. Lactobacillus and Other Key Bacteria Are Associated with Healthy Vaginal Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moosa, Y.; Kwon, D.; de Oliveira, T.; Wong, E.B. Determinants of Vaginal Microbiota Composition. Front. Cell. Infect. Microbiol. 2020, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Factories 2020, 19, 203. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Chen, T. Vaginal microbiota: Potential targets for vulvovaginal candidiasis infection. Heliyon 2024, 10, e27239. [Google Scholar] [CrossRef]
- Sun, Z.; Ge, X.; Qiu, B.; Xiang, Z.; Jiang, C.; Wu, J.; Li, Y. Vulvovaginal candidiasis and vaginal microflora interaction: Microflora changes and probiotic therapy. Front. Cell. Infect. Microbiol. 2023, 13, 1123026. [Google Scholar] [CrossRef]
- Kalia, N.; Singh, J.; Kaur, M. Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: A critical review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 5. [Google Scholar] [CrossRef]
- Yamamoto, T.; Zhou, X.; Williams, C.J.; Hochwalt, A.; Forney, L.J. Bacterial Populations in the Vaginas of Healthy Adolescent Women. J. Pediatr. Adolesc. Gynecol. 2009, 22, 11–18. [Google Scholar] [CrossRef]
- González-Sánchez, A.; Reyes-Lagos, J.J.; Peña-Castillo, M.A.; Nirmalkar, K.; García-Mena, J.; Pacheco-López, G. Vaginal Microbiota Is Stable and Mainly Dominated by Lactobacillus at Third Trimester of Pregnancy and Active Childbirth: A Longitudinal Study of Ten Mexican Women. Curr. Microbiol. 2022, 79, 230. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, C.; Romero-González, R.; Albani-Campanario, M.; Figueroa-Damián, R.; Meraz-Cruz, N.; Hernández-Guerrero, C. Vaginal Microbiota of Healthy Pregnant Mexican Women is Constituted by Four Lactobacillus Species and Several Vaginosis-Associated Bacteria. Infect. Dis. Obstet. Gynecol. 2011, 2011, 851485. [Google Scholar] [CrossRef]
- Willems, H.M.E.; Ahmed, S.S.; Liu, J.; Xu, Z.; Peters, B.M. Vulvovaginal Candidiasis: A Current Understanding and Burning Questions. J. Fungi 2020, 6, 27. [Google Scholar] [CrossRef]
- Anahtar, M.N.; Gootenberg, D.B.; Mitchell, C.M.; Kwon, D.S. Cervicovaginal Microbiota and Reproductive Health: The Virtue of Simplicity. Cell Host Microbe 2018, 23, 159–168. [Google Scholar] [CrossRef]
- Younes, J.A.; Lievens, E.; Hummelen, R.; van der Westen, R.; Reid, G.; Petrova, M.I. Women and Their Microbes: The Unexpected Friendship. Trends Microbiol. 2018, 26, 16–32. [Google Scholar] [CrossRef]
- Granada, M.; Cook, E.; Sherlock, G.; Rosenzweig, F. Microbe Profile: Candida glabrata—A master of deception: This article is part of the Microbe Profiles collection. Microbiology 2024, 170, 001518. [Google Scholar] [CrossRef]
- Takashima, M.; Sugita, T. Taxonomy of Pathogenic Yeasts Candida, Cryptococcus, Malassezia, and Trichosporon: Current Status, Future Perspectives, and Proposal for Transfer of Six Candida Species to the Genus Nakaseomyces. Med. Mycol. J. 2022, 63, 119–132. [Google Scholar] [CrossRef]
- Hernández-Quiroz, F.; Murugesan, S.; Flores-Rivas, C.; Piña-Escobedo, A.; Juárez-Hernández, J.I.; García-Espitia, M.; Chávez-Carbajal, A.; Nirmalkar, K.; García-Mena, J. A high-throughput DNA sequencing study of fecal bacteria of seven Mexican horse breeds. Arch. Microbiol. 2022, 204, 382. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Mitchell, T.G. Rapid identification of pathogenic fungi directly from cultures by using multiplex PCR. J. Clin. Microbiol. 2002, 40, 2860–2865. [Google Scholar] [CrossRef]
- Muir, A.; Harrison, E.; Wheals, A. A multiplex set of species-specific primers for rapid identification of members of the genus Saccharomyces. FEMS Yeast Res. 2011, 11, 552–563. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- McDonald, D.; Jiang, Y.; Balaban, M.; Cantrell, K.; Zhu, Q.; Gonzalez, A.; Morton, J.T.; Nicolaou, G.; Parks, D.H.; Karst, S.M.; et al. Greengenes2 unifies microbial data in a single reference tree. Nat. Biotechnol. 2023, 42, 715–718. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 17 November 2024).
- Posit Team. RStudio: Integrated Development Environment for R; Posit Software, PBC: Boston, MA, USA, 2023; Available online: http://www.posit.co/ (accessed on 17 October 2024).
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Bisanz, J.E. qiime2R: Importing QIIME2 Artifacts and Associated Data into R Sessions. 2018. Available online: https://github.com/jbisanz/qiime2R (accessed on 17 October 2024).
- Nixon, M.P.; McGovern, K.C.; Letourneau, J.; David, L.A.; Lazar, N.A.; Mukherjee, S.; Silverman, J.D. Scale Reliant Inference. arXiv 2024, arXiv:2201.03616. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package. 28 de Agosto de 2024. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 17 December 2024).
- Khleborodova, A.; Gamboa-Tuz, S.D.; Ramos, M.; Segata, N.; Waldron, L.; Oh, S. Lefser: Implementation of metagenomic biomarker discovery tool, LEfSe, in R. Bioinformatics 2024, 40, btae707. [Google Scholar] [CrossRef]
- Gu, Z. Complex heatmap visualization. iMeta 2022, 1, e43. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Auguie, B.; Antonov, A. gridExtra: Miscellaneous Functions for «Grid» Graphics. 9 de Septiembre de 2017. Available online: https://cran.r-project.org/web/packages/gridExtra/ (accessed on 17 December 2024).
- Sobel, J.D. Vulvovaginal candidosis. Lancet 2007, 369, 1961–1971. [Google Scholar] [CrossRef]
- Blostein, F.; Levin-Sparenberg, E.; Wagner, J.; Foxman, B. Recurrent vulvovaginal candidiasis. Ann. Epidemiol. 2017, 27, 575–582.e3. [Google Scholar] [CrossRef]
- Yano, J.; Sobel, J.D.; Nyirjesy, P.; Sobel, R.; Williams, V.L.; Yu, Q.; Noverr, M.C.; Fidel, P.L., Jr. Current patient perspectives of vulvovaginal candidiasis: Incidence, symptoms, management and post-treatment outcomes. BMC Women’s Health 2019, 19, 48. [Google Scholar] [CrossRef]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2012, 36, 288–305. [Google Scholar] [CrossRef]
- Chow, A.W.; Percival-Smith, R.; Bartlett, K.H.; Goldring, A.M.; Morrison, B.J. Vaginal colonization with Escherichia coli in healthy women: Determination of relative risks by quantitative culture and multivariate statistical analysis. Am. J. Obstet. Gynecol. 1986, 154, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Goldacre, M.J.; Watt, B.; Loudon, N.; Milne, L.J.; Loudon, J.D.; Vessey, M.P. Vaginal microbial flora in normal young women. BMJ 1979, 1, 1450–1455. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gow, N.A.; Hube, B. Importance of the Candida albicans cell wall during commensalism and infection. Curr. Opin. Microbiol. 2012, 15, 406–412. [Google Scholar] [CrossRef]
- Dubourg, G.; Lagier, J.C.; Robert, C.; Armougom, F.; Hugon, P.; Metidji, S.; Dione, N.; Dangui, N.P.M.; Pfleiderer, A.; Abrahao, J.; et al. Culturomics and pyrosequencing evidence of the reduction in gut microbiota diversity in patients with broad-spectrum antibiotics. Int. J. Antimicrob. Agents 2014, 44, 117–124. [Google Scholar] [CrossRef]
- Lev-Sagie, A.; Goldman-Wohl, D.; Cohen, Y.; Dori-Bachash, M.; Leshem, A.; Mor, U.; Strahilevitz, J.; Moses, A.E.; Shapiro, H.; Yagel, S.; et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 2019, 25, 1500–1504. [Google Scholar] [CrossRef]
- Theriot, C.M.; Koenigsknecht, M.J.; Carlson, P.E., Jr.; Hatton, G.E.; Nelson, A.M.; Li, B.; Huffnagle, G.B.; Li, J.Z.; Young, V.B. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat. Commun. 2014, 5, 3114. [Google Scholar] [CrossRef]
- Ceccarani, C.; Foschi, C.; Parolin, C.; D’antuono, A.; Gaspari, V.; Consolandi, C.; Laghi, L.; Camboni, T.; Vitali, B.; Severgnini, M.; et al. Diversity of vaginal microbiome and metabolome during genital infections. Sci. Rep. 2019, 9, 14095. [Google Scholar] [CrossRef]
- Liu, C.M.; Hungate, B.A.; Tobian, A.A.R.; Ravel, J.; Prodger, J.L.; Serwadda, D.; Kigozi, G.; Galiwango, R.M.; Nalugoda, F.; Keim, P.; et al. Penile Microbiota and Female Partner Bacterial Vaginosis in Rakai, Uganda. mBio 2015, 6, e00589-15. [Google Scholar] [CrossRef]
- Chopyk, J.; Güemes, A.G.C.; Ramirez-Sanchez, C.; Attai, H.; Ly, M.; Jones, M.B.; Liu, R.; Liu, C.; Yang, K.; Tu, X.M.; et al. Common antibiotics, azithromycin and amoxicillin, affect gut metagenomics within a household. BMC Microbiol. 2023, 23, 206. [Google Scholar] [CrossRef]
- Bagnall, P.; Rizzolo, D. Bacterial vaginosis: A practical review. J. Am. Acad. Physician Assist. 2017, 30, 15–21. [Google Scholar] [CrossRef]
- Morrill, S.; Gilbert, N.M.; Lewis, A.L. Gardnerella vaginalis as a Cause of Bacterial Vaginosis: Appraisal of the Evidence From in vivo Models. Front. Cell. Infect. Microbiol. 2020, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Reclassification of the Bifidobacterium and Gardnerella Genera. 2024. Available online: https://www.atcc.org/resources/posters/2019-posters/reclassification-of-the-bifidobacterium-and-gardnerella-genera (accessed on 17 December 2024).
- Ahrens, P.; Andersen, L.O.; Lilje, B.; Johannesen, T.B.; Dahl, E.G.; Baig, S.; Jensen, J.S.; Falk, L. Changes in the vaginal microbiota following antibiotic treatment for Mycoplasma genitalium, Chlamydia trachomatis and bacterial vaginosis. PLoS ONE 2020, 15, e0236036. [Google Scholar] [CrossRef]
- Zwittink, R.D.; Munckhof, E.H.A.v.D.; Hall, M.A.L.-V.; Boers, K.; Molijn, A.; Knetsch, C.W.; Kuijper, E.J. The vaginal microbiota in the course of bacterial vaginosis treatment. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 651–656. [Google Scholar] [CrossRef]
- Theis, K.R.; Florova, V.; Romero, R.; Borisov, A.B.; Winters, A.D.; Galaz, J.; Gomez-Lopez, N. Sneathia: An emerging pathogen in female reproductive disease and adverse perinatal outcomes. Crit. Rev. Microbiol. 2021, 47, 517–542. [Google Scholar] [CrossRef]
- Pramanick, R.; Nathani, N.; Warke, H.; Mayadeo, N.; Aranha, C. Vaginal Dysbiotic Microbiome in Women with No Symptoms of Genital Infections. Front. Cell. Infect. Microbiol. 2021, 11, 760459. [Google Scholar] [CrossRef]
- Prasad, D.; Parween, S.; Kumari, K.; Singh, N. Prevalence, Etiology, and Associated Symptoms of Vaginal Discharge During Pregnancy in Women Seen in a Tertiary Care Hospital in Bihar. Cureus 2021, 13, e12700. [Google Scholar] [CrossRef]
- Randis, T.M.; Ratner, A.J. Gardnerella and Prevotella: Co-conspirators in the Pathogenesis of Bacterial Vaginosis. J. Infect. Dis. 2019, 220, 1085–1088. [Google Scholar] [CrossRef]
- Abuqwider, J.; Altamimi, M.; Mauriello, G. Limosilactobacillus reuteri in Health and Disease. Microorganisms 2022, 10, 522. [Google Scholar] [CrossRef]
- Mohamed, I.; Zakeer, S.; Azab, M.; Hanora, A. Changes in Vaginal Microbiome in Pregnant and Nonpregnant Women with Bacterial Vaginosis: Toward Microbiome Diagnostics? OMICS J. Integr. Biol. 2020, 24, 602–614. [Google Scholar] [CrossRef]
| Variable | Control | Cases | p-Value |
|---|---|---|---|
| Number | 38 | 57 | - |
| Age | 31.92 (±7.67) (38) | 35.31 (±10.22) (51) | 0.08 |
| Menarche Age | 12.53 (±1.59) (38) | 12.64 (±1.44) (51) | 0.71 |
| Age Start Sexual Life | 19.67 (±3.00) (36) | 18.62 (±2.89) (48) | 0.11 |
| Contraceptive Method | |||
| None | 14/32 (43.75%) | 29/57 (50.88%) | - |
| Tubal ligation | 1/32 (3.12%) | 8/57 (14.03%) | - |
| Condom | 6/32 (18.75%) | 10/57 (17.54%) | - |
| Intrauterine device | 2/32 (6.25%) | 6/57 (10.53%) | - |
| Oral | 7/32 (21.88%) | 4/57 (7.02%) | - |
| Implant | 2/32 (6.25%) | 0/57 (0.00%) | - |
| Menstrual Cycle Phase | |||
| Luteal | 19/32 (59.38%) | 32/55 (58.18%) | - |
| Follicular | 11/32 (34.38%) | 17/55 (30.91%) | - |
| Ovulation | 2/32 (6.25%) | 6/55 (10.91%) | - |
| Sex Partners | |||
| 1 | 15/32 (46.88%) | 21/54 (38.89%) | - |
| 2 | 17/32 (53.13%) | 33/54 (61.11%) | - |
| Gestations | |||
| 0 | 17/31 (54.84%) | 17/52 (32.69%) | - |
| 1–2 | 7/31 (22.58%) | 18/52 (34.62%) | - |
| 3 | 7/31 (22.58%) | 17/52 (32.69%) | - |
| Delivers | |||
| 0 | 22/31 (70.97%) | 32/52 (61.54%) | - |
| 1–2 | 4/31 (12.90%) | 13/52 (25.00%) | - |
| 3 | 5/31 (16.13%) | 7/52 (13.46%) | - |
| Cesarean sections | |||
| 0 | 25/31 (80.65%) | 30/52 (57.69%) | - |
| 1–2 | 5/31 (16.13%) | 21/52 (40.38%) | - |
| 3 | 1/31 (3.23%) | 1/52 (1.92%) | - |
| Abortions | |||
| 0 | 29/31 (93.54%) | 39/52 (75.00%) | - |
| 1–2 | 1/31 (3.22%) | 12/52 (23.07%) | - |
| 3 | 1/31 (3.22%) | 1/52 (1.92%) | - |
| Vaginal pH | |||
| mean pH | 4.48 ± 0.57 (29) | 4.78 ± 1.25 (43) | 0.18 |
| 4 | 14/25 (56.00%) | 17/40 (42.50%) | - |
| 5 | 11/25 (44.00%) | 23/40 (57.50%) | - |
| Risk factors | |||
| Smoking | 8/42 (19.05%) | 19/58 (33.33%) | - |
| Medication | |||
| Antibiotics | 13/42 (30.95%) | 25/57 (43.86%) | - |
| Antifungal | 0/33 (0.00%) | 5/57 (8.77%) | - |
| Vulvovaginal Candidiasis episodes | |||
| 0 | 31/31 (100.00%) | 0/52 (0.00%) | - |
| 1–2 | 0/31 (0.00%) | 38/52 (73.08%) | - |
| 3 | 0/31 (0.00%) | 14/52 (26.92%) | - |
| PCR Candida detection | |||
| Candida albicans | 27/30 (90.00%) | 17/47 (36.17%) | 9.93 × 10−6 |
| Nakaseomyses glabratus * | 0/30 (0.00%) | 46/47 (97.87%) | <2.2 × 10−16 |
| Saccharomyces cerevisiae | 27/30 (90.00%) | 45/47 (95.74%) | 0.6000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pineda-Díaz, J.; Miranda-Brito, C.; Juárez-Castelán, C.J.; Piña-Escobedo, A.; Lázaro-Pérez, N.d.S.; de la Cruz-Munguía, A.; Ramírez-Sánchez, D.; Gómez-Meraz, Y.; Vélez-Ixta, J.M.; García-Mena, J. Vaginal Microbiota Patterns Associated with Yeast Infection in Mexican Women, a Pilot Study. BioTech 2025, 14, 31. https://doi.org/10.3390/biotech14020031
Pineda-Díaz J, Miranda-Brito C, Juárez-Castelán CJ, Piña-Escobedo A, Lázaro-Pérez NdS, de la Cruz-Munguía A, Ramírez-Sánchez D, Gómez-Meraz Y, Vélez-Ixta JM, García-Mena J. Vaginal Microbiota Patterns Associated with Yeast Infection in Mexican Women, a Pilot Study. BioTech. 2025; 14(2):31. https://doi.org/10.3390/biotech14020031
Chicago/Turabian StylePineda-Díaz, Janet, Carolina Miranda-Brito, Carmen Josefina Juárez-Castelán, Alberto Piña-Escobedo, Noemí del Socorro Lázaro-Pérez, Alejandra de la Cruz-Munguía, Daniela Ramírez-Sánchez, Yuliana Gómez-Meraz, Juan Manuel Vélez-Ixta, and Jaime García-Mena. 2025. "Vaginal Microbiota Patterns Associated with Yeast Infection in Mexican Women, a Pilot Study" BioTech 14, no. 2: 31. https://doi.org/10.3390/biotech14020031
APA StylePineda-Díaz, J., Miranda-Brito, C., Juárez-Castelán, C. J., Piña-Escobedo, A., Lázaro-Pérez, N. d. S., de la Cruz-Munguía, A., Ramírez-Sánchez, D., Gómez-Meraz, Y., Vélez-Ixta, J. M., & García-Mena, J. (2025). Vaginal Microbiota Patterns Associated with Yeast Infection in Mexican Women, a Pilot Study. BioTech, 14(2), 31. https://doi.org/10.3390/biotech14020031







