Characterization and Biotechnology of Three New Strains of Basidial Fungi as Promising Sources of Biologically Active Substances
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Pure Culture
2.2. Identification of Fungal Strains
2.3. Selection of Nutrient Media and Temperature for Cultivation
2.4. Determination of Radial Growth and Biomass Yield
2.5. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Identification of Fungal Strains of Daedaleopsis tricolor, Pycnoporellus fulgens and Trichaptum abietinum
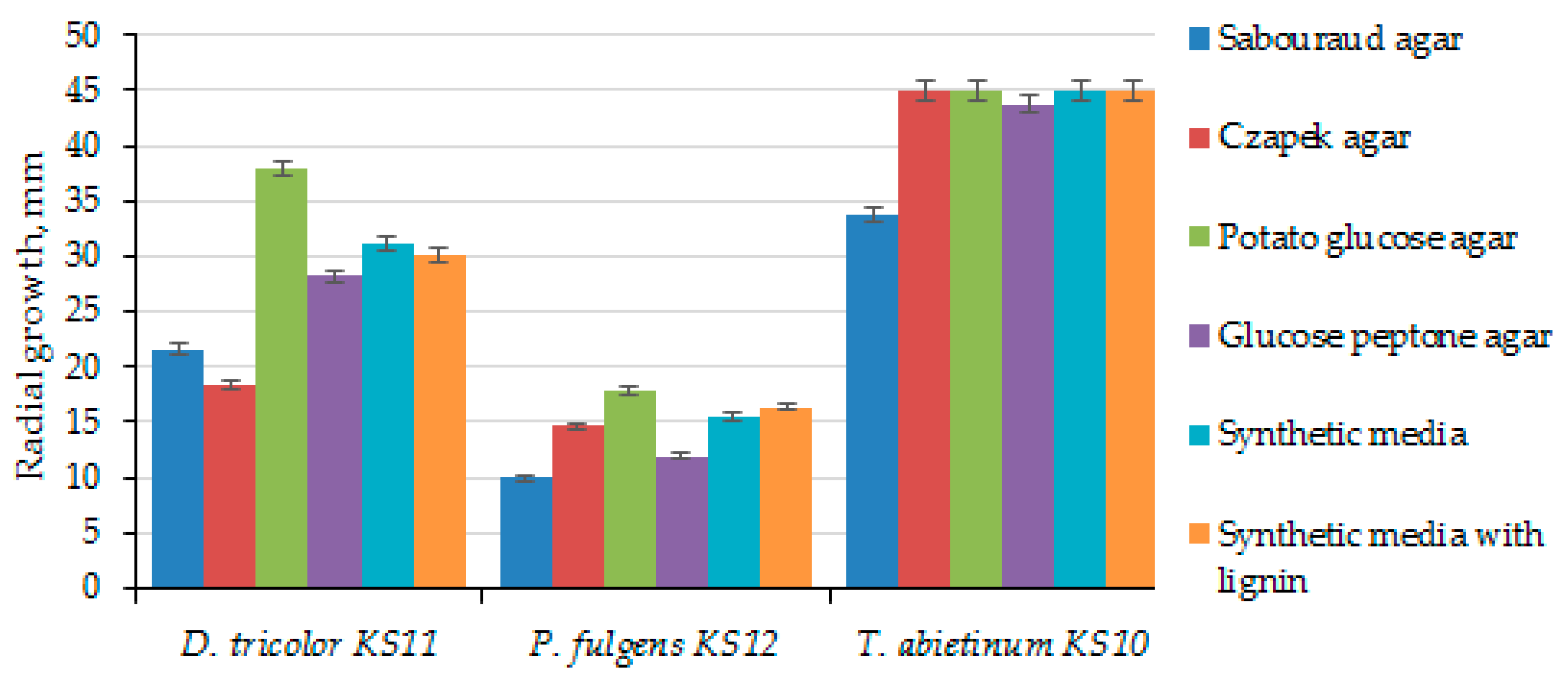
3.2. Selection of Nutritional Media for Culturing Strains of Daedaleopsis tricolor KS11, Pycnoporellus fulgens KS12 and Trichaptum abietinum KS10
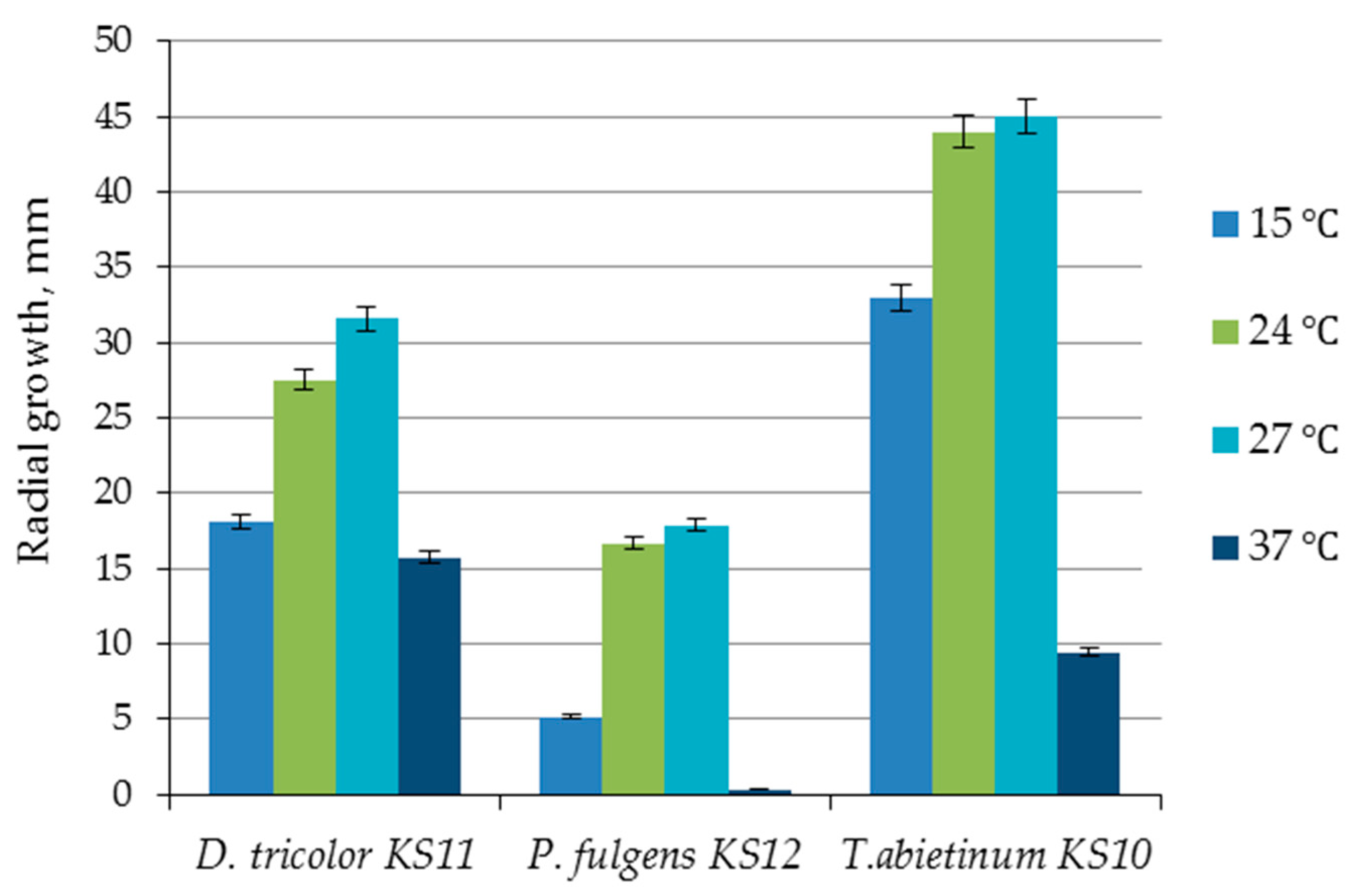
3.3. Effect of Temperature on Growth of Daedaleopsis tricolor KS11, Pycnoporellus fulgens KS12 and Trichaptum abietinum KS10
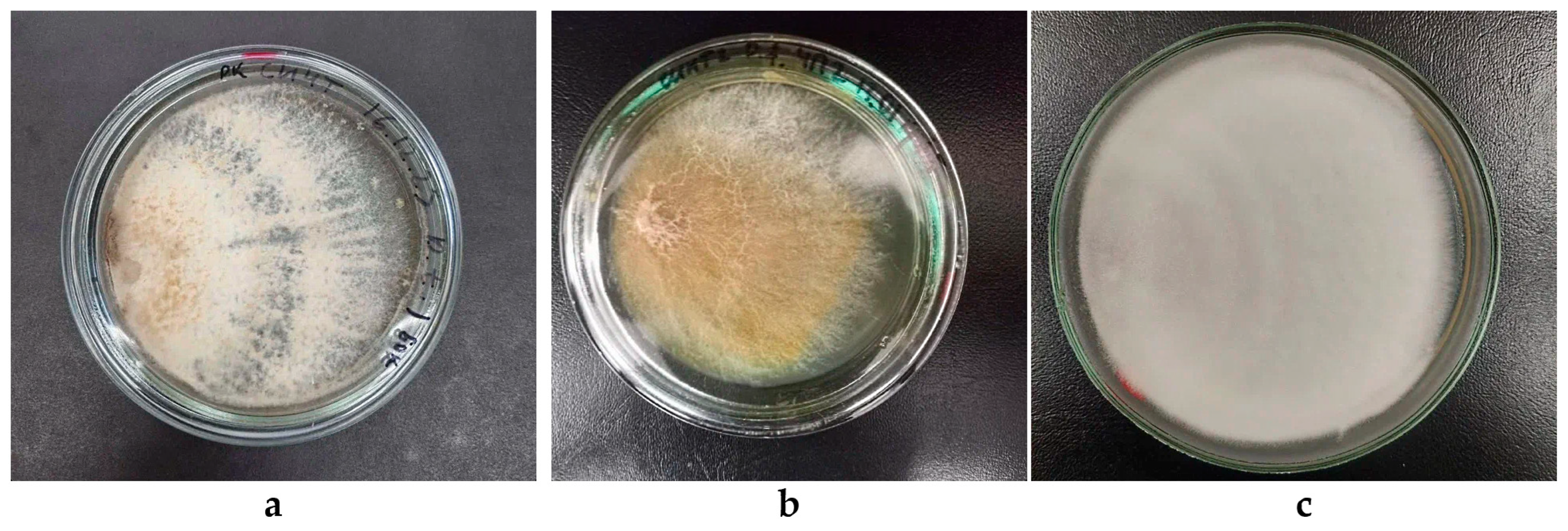
3.4. A Detailed Description of the Morphological Features of the Colonies of Daedaleopsis tricolor KS11, Pycnoporellus fulgens KS12 and Trichaptum abietinum KS10
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belova, N.V. Lanosteroid Terpenoids and Steroids of Higher Fungi. Adv. Biol. Earth Sci. 2016, 1, 107–120. Available online: http://jomardpublishing.com/UploadFiles/Files/journals/ABES/V1No1/Belova.pdf (accessed on 2 November 2024).
- Murphy, E.J.; Masterson, C.; Rezoagli, E.; O’Toole, D.; Major, I.; Stack, G.D.; Lynch, M.; Laffey, J.G.; Rowan, N.J. β-Glucan Extracts from the Same Edible Shiitake Mushroom Lentinus edodes Produce Differential in-Vitro Immunomodulatory and Pulmonary Cytoprotective Effects—Implications for Coronavirus Disease (COVID-19) Immunotherapies. Sci. Total Environ. 2020, 732, 139330. [Google Scholar] [CrossRef]
- Liu, C.; Cheung, P.C.K. Structure and Immunomodulatory Activity of Microparticulate Mushroom Sclerotial β-Glucan Prepared from Polyporus rhinocerus. J. Agric. Food Chem. 2019, 67, 9070–9078. [Google Scholar] [CrossRef]
- Ha, D.; Loan, L.; Hung, T.; Han, L.; Khoi, N.; Dung, L.; Min, B.; Nguyen, N. An Improved HPLC-DAD Method for Quantitative Comparisons of Triterpenes in Ganoderma lucidum and Its Five Related Species Originating from Vietnam. Molecules 2015, 20, 1059–1077. [Google Scholar] [CrossRef]
- Risoli, S.; Nali, C.; Sarrocco, S.; Cicero, A.F.G.; Colletti, A.; Bosco, F.; Venturella, G.; Gadaleta, A.; Gargano, M.L.; Marcotuli, I. Mushroom-Based Supplements in Italy: Let’s Open Pandora’s Box. Nutrients 2023, 15, 776. [Google Scholar] [CrossRef]
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Investigation of Antioxidant and Antimicrobial Activities of Different Extracts of Auricularia and Termitomyces Species of Mushrooms. Sci. World J. 2019, 2019, 7357048. [Google Scholar] [CrossRef]
- Bu, H.; Li, X.; Hu, L.; Wang, J.; Li, Y.; Zhao, T.; Wang, H.; Wang, S. The Anti-Inflammatory Mechanism of the Medicinal Fungus Puffball Analysis Based on Network Pharmacology. Inform. Med. Unlocked 2021, 23, 100549. [Google Scholar] [CrossRef]
- Peng, H.; Shahidi, F. Qualitative Analysis of Secondary Metabolites of Chaga Mushroom (Inonotus obliquus): Phenolics, Fatty Acids, and Terpenoids. J. Food Bioact. 2022, 17, 56–72. [Google Scholar] [CrossRef]
- Xu, T.; Li, G.; Wang, X.; Lv, C.; Tian, Y. Inonotus obliquus Polysaccharide Ameliorates Serum Profiling in STZ-Induced Diabetic Mice Model. BMC Chem. 2021, 15, 64. [Google Scholar] [CrossRef]
- Huynh, N.; Beltrame, G.; Tarvainen, M.; Suomela, J.-P.; Yang, B. Supercritical CO2 Extraction of Triterpenoids from Chaga Sterile Conk of Inonotus obliquus. Molecules 2022, 27, 1880. [Google Scholar] [CrossRef]
- Park, J.; Nguyen, T.M.N.; Park, H.; Nguyen, M.T.T.; Lee, N.; Ban, S.; Park, K.; Lee, C.; Kim, J.; Park, J.-T. Protective Effects of Lanostane Triterpenoids from Chaga Mushroom in Human Keratinocytes, HaCaT Cells, against Inflammatory and Oxidative Stresses. Int. J. Mol. Sci. 2023, 24, 12803. [Google Scholar] [CrossRef] [PubMed]
- Janjušević, L.; Karaman, M.; Šibul, F.; Tommonaro, G.; Iodice, C.; Jakovljević, D.; Pejin, B. The Lignicolous Fungus Trametes versicolor (L.) Lloyd (1920): A Promising Natural Source of Antiradical and AChE Inhibitory Agents. J. Enzym. Inhib. Med. Chem. 2017, 32, 355–362. [Google Scholar] [CrossRef]
- Lee, I.; Ahn, B.; Choi, J.; Hattori, M.; Min, B.; Bae, K. Selective Cholinesterase Inhibition by Lanostane Triterpenes from Fruiting Bodies of Ganoderma lucidum. Bioorganic Med. Chem. Lett. 2011, 21, 6603–6607. [Google Scholar] [CrossRef]
- Papinutti, L. Effects of Nutrients, pH and Water Potential on Exopolysaccharides Production by a Fungal Strain Belonging to Ganoderma lucidum Complex. Bioresour. Technol. 2010, 101, 1941–1946. [Google Scholar] [CrossRef]
- Abdullah, N.R.; Mohd Nasir, M.H.; Azizan, N.H.; Wan-Mohtar, W.A.A.Q.I.; Sharif, F. Bioreactor-Grown Exo- and Endo-β-Glucan from Malaysian Ganoderma lucidum: An in Vitro and in Vivo Study for Potential Antidiabetic Treatment. Front. Bioeng. Biotechnol. 2022, 10, 960320. [Google Scholar] [CrossRef]
- Supramani, S.; Rejab, N.A.; Ilham, Z.; Ahmad, R.; Show, P.-L.; Ibrahim, M.F.; Wan-Mohtar, W.A.A.Q.I. Performance of Biomass and Exopolysaccharide Production from the Medicinal Mushroom Ganoderma lucidum in a New Fabricated Air-L-Shaped Bioreactor (ALSB). Processes 2023, 11, 670. [Google Scholar] [CrossRef]
- Angelova, G.; Brazkova, M.; Mihaylova, D.; Slavov, A.; Petkova, N.; Blazheva, D.; Deseva, I.; Gotova, I.; Dimitrov, Z.; Krastanov, A. Bioactivity of Biomass and Crude Exopolysaccharides Obtained by Controlled Submerged Cultivation of Medicinal Mushroom Trametes versicolor. J. Fungi 2022, 8, 738. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Grgić, J.; Perković, G.; Koceva Komlenić, D.; Bucić-Kojić, A. A Comparative Study of the Influence of Various Fungal-Based Pretreatments of Grape Pomace on Phenolic Compounds Recovery. Foods 2022, 11, 1665. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.Y.; Jung, J.Y.; Park, H.M.; Yang, J.-K. Comparison of the Metabolic Profile of the Mycelia and Fruiting Bodies of Artificially Cultured Cordyceps Militaris. J. Mushroom 2022, 20, 13–21. [Google Scholar] [CrossRef]
- He, H.; Li, Y.; Fang, M.; Li, T.; Liang, Y.; Mei, Y. Carbon Source Affects Synthesis, Structures, and Activities of Mycelial Polysaccharides from Medicinal Fungus Inonotus obliquus. J. Microbiol. Biotechnol. 2021, 31, 855–866. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, P.; Zhu, Z.; Chen, C.; Xu, X. Production of Phenolic Compounds and Antioxidant Activity via Bioconversion of Wheat Straw by Inonotus obliquus under Submerged Fermentation with the Aid of a Surfactant. J. Sci. Food Agric. 2021, 101, 1021–1029. [Google Scholar] [CrossRef]
- Poyedinok, N.; Mykhaylova, O.; Sergiichuk, N.; Tugay, T.; Tugay, A.; Lopatko, S.; Matvieieva, N. Effect of Colloidal Metal Nanoparticles on Biomass, Polysaccharides, Flavonoids, and Melanin Accumulation in Medicinal Mushroom Inonotus obliquus (Ach.:Pers.) Pilát. Appl. Biochem. Biotechnol. 2020, 191, 1315–1325. [Google Scholar] [CrossRef]
- Srivastava, M.; Kumari, M.; Karn, S.K.; Bhambri, A.; Mahale, V.G.; Mahale, S. Submerged Cultivation and Phytochemical Analysis of Medicinal Mushrooms (Trametes Sp.). Front. Fungal Biol. 2024, 5, 1414349. [Google Scholar] [CrossRef]
- Abd Razak, D.L.; Jamaluddin, A.; Abd Rashid, N.Y.; Sani, N.A.; Abdul Manan, M. Assessment of Cosmeceutical Potentials of Selected Mushroom Fruitbody Extracts Through Evaluation of Antioxidant, Anti-Hyaluronidase and Anti-Tyrosinase Activity. J 2020, 3, 329–342. [Google Scholar] [CrossRef]
- Roca-Lema, D.; Martinez-Iglesias, O.; Portela, C.F.D.A.; Rodríguez-Blanco, A.; Valladares-Ayerbes, M.; Díaz-Díaz, A.; Casas-Pais, A.; Prego, C.; Figueroa, A. In Vitro Anti-Proliferative and Anti-Invasive Effect of Polysaccharide-Rich Extracts from Trametes Versicolor and Grifola Frondosa in Colon Cancer Cells. Int. J. Med. Sci. 2019, 16, 231–240. [Google Scholar] [CrossRef]
- Tima, S.; Tapingkae, T.; To-anun, C.; Noireung, P.; Intaparn, P.; Chaiyana, W.; Sirithunyalug, J.; Panyajai, P.; Viriyaadhammaa, N.; Nirachonkul, W.; et al. Antileukaemic Cell Proliferation and Cytotoxic Activity of Edible Golden Cordyceps (Cordyceps militaris) Extracts. Evid.-Based Complement. Altern. Med. 2022, 2022, 5347718. [Google Scholar] [CrossRef]
- Wei, C.; Khan, M.A.; Du, J.; Cheng, J.; Tania, M.; Leung, E.L.-H.; Fu, J. Cordycepin Inhibits Triple-Negative Breast Cancer Cell Migration and Invasion by Regulating EMT-TFs SLUG, TWIST1, SNAIL1, and ZEB1. Front. Oncol. 2022, 12, 898583. [Google Scholar] [CrossRef]
- Song, H.-N. Functional Cordyceps Coffee Containing Cordycepin and β-Glucan. Prev. Nutr. Food Sci. 2020, 25, 184–193. [Google Scholar] [CrossRef]
- Sharpe, E.; Farragher-Gnadt, A.P.; Igbanugo, M.; Huber, T.; Michelotti, J.C.; Milenkowic, A.; Ludlam, S.; Walker, M.; Hanes, D.; Bradley, R.; et al. Comparison of Antioxidant Activity and Extraction Techniques for Commercially and Laboratory Prepared Extracts from Six Mushroom Species. J. Agric. Food Res. 2021, 4, 100130. [Google Scholar] [CrossRef]
- Vladykina, V.D.; Mukhin, V.A.; Badalyan, S.M. Daedaleopsis Genus in Siberia and the Far East of Russia. In Proceedings of the III Russian National Conference “Information Technology in Biodiversity Research”, Yekaterinburg, Russia, 5–10 October 2020; pp. 17–26. [Google Scholar] [CrossRef]
- Kotkova, V.M.; Popov, E.S. Aphyllophoraceous fungi of Pskov Region. Nov. Sist. Nizshikh Rastenii 2013, 47, 87–121. [Google Scholar] [CrossRef]
- Seierstad, K.S.; Fossdal, R.; Miettinen, O.; Carlsen, T.; Skrede, I.; Kauserud, H. Contrasting Genetic Structuring in the Closely Related Basidiomycetes Trichaptum Abietinum and Trichaptum Fuscoviolaceum (Hymenochaetales). Fungal Biol. 2021, 125, 269–275. [Google Scholar] [CrossRef]
- Buratti, S.; Girometta, C.E.; Savino, E.; Gorjón, S.P. An Example of the Conservation of Wood Decay Fungi: The New Research Culture Collection of Corticioid and Polyporoid Strains of the University of Salamanca (Spain). Forests 2023, 14, 2029. [Google Scholar] [CrossRef]
- Karasiński, D.; Wołkowycki, M. An Annotated And Illustrated Catalogue Of Polypores (Agaricomycetes) Of The Białowieża Forest (NE Poland). Pol. Bot. J. 2015, 60, 217–292. [Google Scholar] [CrossRef][Green Version]
- Karadzic, D.; Radulovic, Z.; Jovanovic, D.; Milenkovic, I. A Contribution to the Knowledge of Fungi Daedaleopsis Confragosa (Bolton) Schröt the Cause of White Rot of Hardwood. Glas. Sumar. Fak. 2023, 128, 31–46. [Google Scholar] [CrossRef]
- Koukol, O.; Kotlaba, F.; Pouzar, Z. Taxonomic Evaluation of the Polypore Daedaleopsis tricolor Based on Morphology and Molecular Data. Czech Mycol. 2014, 66, 107–119. [Google Scholar] [CrossRef]
- Mukhin, V.A.; Knudsen, H.; Corfixen, P.; Zhuykova, E.V.; Nepryakhin, I.O.; Diyarova, D.K. The Genus Trichaptum in North Asia. Mikol. Fitopatol. 2023, 57, 255–266. [Google Scholar] [CrossRef]
- Cho, D.H.; Chung, J.Y. Study of Fungal Diversity and Fungal Resources in Mt. Gwangdeok. Korean J. Nat. Conserv. 2023, 22, 1–26. [Google Scholar] [CrossRef]
- Cartabia, M.; Girometta, C.E.; Milanese, C.; Baiguera, R.M.; Buratti, S.; Branciforti, D.S.; Vadivel, D.; Girella, A.; Babbini, S.; Savino, E.; et al. Collection and Characterization of Wood Decay Fungal Strains for Developing Pure Mycelium Mats. J. Fungi 2021, 7, 1008. [Google Scholar] [CrossRef]
- Shakhova, N. Revealing New Active and Biotechnologically Perspective Producers of Oxidative and Cellulolytic Enzymes among Pure Cultures of Xylotrophic Agaricomycetes from the Southern Non-Chernozem Zone of the European Part of Russia. Curr. Res. Environ. Appl. Mycol. 2020, 10, 113–119. [Google Scholar] [CrossRef]
- Mali, T.; Kuuskeri, J.; Shah, F.; Lundell, T.K. Interactions Affect Hyphal Growth and Enzyme Profiles in Combinations of Coniferous Wood-Decaying Fungi of Agaricomycetes. PLoS ONE 2017, 12, e0185171. [Google Scholar] [CrossRef]
- Protsenko, M.A.; Troshkova, G.P.; Kosogova, T.A.; Teplyakova, T.V. Biologically Active Compounds Fruiting Bodies and Mycelium of the Basidiomycete Daedaleopsis tricolor. Fundam. Res. 2014, 12, 136–140. Available online: https://fundamental-research.ru/ru/article/view?id=36083 (accessed on 2 November 2024).
- Zhao, J.-Y.; Feng, T.; Li, Z.-H.; Dong, Z.-J.; Zhang, H.-B.; Liu, J.-K. Sesquiterpenoids and an Ergosterol from Cultures of the Fungus Daedaleopsis Tricolor. Nat. Prod. Bioprospect. 2013, 3, 271–276. [Google Scholar] [CrossRef]
- Weather and Climate. Available online: https://weatherandclimate.com/russia/mariy-el/volzhsk (accessed on 13 April 2025).
- Reference and Information Portal “Weather and Climate”. Available online: http://www.pogodaiklimat.ru/weather.php?id=27595 (accessed on 13 April 2025).
- World Weather. Available online: https://world-weather.ru/pogoda/russia/kazan/2021/ (accessed on 13 April 2025).
- Sysoeva, M.A.; Urazlina, L.N.; Khabibrakhmanova, V.R.; Grigoryeva, T.V.; Sysoeva, E.V. Isolation of the Inonotus Obliquus Chaga Mushroom Strain and Intensification of a Culture Growth during Solid-Phase Cultivation. Proc. Univ. Appl. Chem. Biotechnol. 2020, 10, 95–106. [Google Scholar] [CrossRef]
- Kizitska, T.; Barshteyn, V.; Sevindik, M.; Krupodorova, T. Evaluation of Fomitopsis Betulina Strains for Growth on Different Media and Exopolysaccharide Production. Arch. Biol. Sci. 2024, 76, 257–265. [Google Scholar] [CrossRef]
- Cartabia, M.; Girometta, C.E.; Baiguera, R.M.; Buratti, S.; Babbini, S.; Bernicchia, A.; Savino, E. Lignicolous Fungi Collected in Northern Italy: Identification and Morphological Description of Isolates. Diversity 2022, 14, 413. [Google Scholar] [CrossRef]
- Maramokhin, E.; Sirotina, M.; Zontikov, D. Cultivation of Mycelium and the Study of the Phytopathogenicity of Certain Xylotrophic Basidiomycetes under in vitro Conditios. Bull. Nizhnevartovsk State Univ. 2020, 2, 12–18. [Google Scholar] [CrossRef]
- Mustafin, K.G.; Ahmetsadykov, N.N.; Bisko, N.A.; Suleimenova, Z.B.; Narmuratova, Z.B.; Saduyeva, Z.K. Selection of the optimal culture conditions for high biomass synthesis by Trametes versicolor. KazNU Bull. Biol. Ser. 2016, 2, 151–158. Available online: https://bb.kaznu.kz/index.php/biology/article/view/1190/1131 (accessed on 14 April 2025).
- Shirokikh, A.A.; Zaripova, G.F.; Ustyuzhanin, I.A.; Zlobin, A.A.; Shirokikh, I.G. Influence of nutrient medium components and cultivation conditions on the growth of Trametes versicolor in mycelial culture. Theor. Appl. Ecol. 2014, 3, 86–93. Available online: http://envjournal.ru/ari/v2014/v3/files/14313.pdf?ysclid=m9bwshlvyu434085304 (accessed on 14 April 2025).
- Krupodorova, T.; Barshteyn, V.; Sekan, A. Review of the Basic Cultivation Conditions Influence on the Growth of Basidiomycetes. Curr. Res. Environ. Appl. Mycol. 2021, 11, 494–531. [Google Scholar] [CrossRef]
- Gwon, J.H.; Park, H.; Eom, A.H. Effect of Temperature, pH, and Media on the Mycelial Growth of Tuber koreanum. Mycobiology 2022, 50, 238–243. [Google Scholar] [CrossRef] [PubMed]



| Fungus | Strain | Year of Collection | Place of Collection | Year of Deposition | GenBank Number |
|---|---|---|---|---|---|
| Daedaleopsis tricolor | KS11 | 2021 | Republic of Tatarstan | 2023 | OR804093 |
| Pycnoporellus fulgens | KS12 | 2021 | Republic of Tatarstan | 2023 | OR805526 |
| Trichaptum abietinum | KS10 | 2019 | Republic of Mari El | 2023 | OR610852 |
| Nutrient Media | D. tricolor KS11 | P. fulgens KS12 | T. abietinum KS10 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Days | Amount of Biomass | Days | Amount of Biomass | Days | Amount of Biomass | ||||
| mg | mg/cm2 | mg | mg/cm2 | mg | mg/cm2 | ||||
| Sabouraud agar | 13 | 168.67 ± 9.87 | 2.76 ± 0.18 | - | - | - | 9 | 262.33 ± 52.54 | 4.13 ± 0.83 |
| Czapek agar | 16 | 33.00 ± 18.03 | 0.54 ± 0.28 | 17 | 2.00 ± 1.00 | 0.04 ± 0.02 | 6 | 1.00 ± 0.05 | 0.02 ± 0.01 |
| Potato glucose agar | 9 | 85.00 ± 9.17 | 1.34 ± 0.14 | 12 | 30.00 ± 0.75 | 0.47 ± 0.02 | 6 | 127.67 ± 23.35 | 2.01 ± 0.37 |
| Glucose peptone agar | 9 | 57.00 ± 3.61 | 0.96 ± 0.10 | 17 | 79.67 ± 22.85 | 1.33 ± 0.33 | 7 | 108.00 ± 44.51 | 1.70 ± 0.70 |
| Synthetic medium | 9 | 125.00 ± 24.58 | 1.97 ± 0.39 | 16 | 70.00 ± 10.00 | 1.01 ± 0.01 | 6 | 171.50 ± 60.10 | 2.70 ± 0.95 |
| Synthetic medium with lignin | 9 | 125.33 ± 24.95 | 2.04 ± 0.28 | 16 | 81.33 ± 16.01 | 1.37 ± 0.25 | 6 | 157.00 ± 26.21 | 2.47 ± 0.41 |
| Temperature, °C | D. tricolor KS11 | P. fulgens KS12 | T. abietinum KS10 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Days | Amount of Biomass | Days | Amount of Biomass | Days | Amount of Biomass | ||||
| mg | mg/cm2 | mg | mg/cm2 | mg | mg/cm2 | ||||
| 15 | 10 | 84.75 ± 21.90 | 1.33 ± 0.34 | - | - | - | 9 | 195.33 ± 2.08 | 3.07 ± 0.03 |
| 24 | 10 | 130.25 ± 25.12 | 2.05 ± 0.40 | 13 | 70.00 ± 17.32 | 1.23 ± 0.31 | 7 | 189.67 ± 20.21 | 3.13 ± 0.32 |
| 27 | 9 | 133.25 ± 8.66 | 2.10 ± 0.14 | 16 | 86.73 ± 11.01 | 1.42 ± 0.24 | 6 | 227.33 ± 36.53 | 3.58 ± 0.57 |
| 37 | Inhibits growth | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sysoeva, M.A.; Prozorova, I.S.; Sysoeva, E.V.; Grigoryeva, T.V.; Ismagilova, R.K. Characterization and Biotechnology of Three New Strains of Basidial Fungi as Promising Sources of Biologically Active Substances. BioTech 2025, 14, 30. https://doi.org/10.3390/biotech14020030
Sysoeva MA, Prozorova IS, Sysoeva EV, Grigoryeva TV, Ismagilova RK. Characterization and Biotechnology of Three New Strains of Basidial Fungi as Promising Sources of Biologically Active Substances. BioTech. 2025; 14(2):30. https://doi.org/10.3390/biotech14020030
Chicago/Turabian StyleSysoeva, Maria Alexandrovna, Ilyuza Shamilevna Prozorova, Elena Vladislavovna Sysoeva, Tatyana Vladimirovna Grigoryeva, and Ruzilya Kamilevna Ismagilova. 2025. "Characterization and Biotechnology of Three New Strains of Basidial Fungi as Promising Sources of Biologically Active Substances" BioTech 14, no. 2: 30. https://doi.org/10.3390/biotech14020030
APA StyleSysoeva, M. A., Prozorova, I. S., Sysoeva, E. V., Grigoryeva, T. V., & Ismagilova, R. K. (2025). Characterization and Biotechnology of Three New Strains of Basidial Fungi as Promising Sources of Biologically Active Substances. BioTech, 14(2), 30. https://doi.org/10.3390/biotech14020030






