Direct Shoot Regeneration from the Finger Millet’s In Vitro-Derived Shoot Apex and Genetic Fidelity Study with ISSR Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Explant Preparation
2.2. Shoot Multiplication and Elongation
2.3. Root Induction
2.4. Acclimatization
2.5. Analysis of ISSR Primers
2.6. Statistical Analysis
3. Results
3.1. Preliminary Study on Shoot Induction by Cytokinin
3.2. Multiple Shoot Induction
3.3. Shoot Elongation and Maturation
3.4. Induction of Roots
3.5. Hardening and Acclimatization
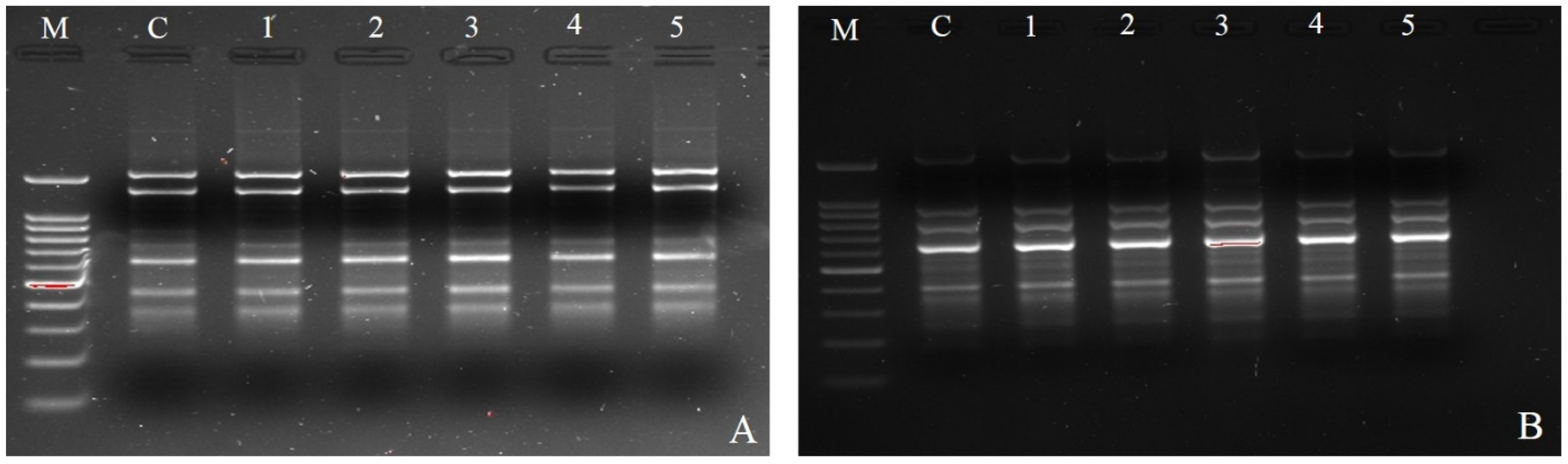
3.6. Examining the Clonal Fidelity of In Vitro Regenerated Plants by ISSR Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BAP | N6-Benzyl amino purine |
| IAA | Indole-3-acetic acid |
| IBA | Indole-3-butyric acid |
| ISSR | Inter-simple sequence repeats |
| Kin | Kinetin |
| MS | Murashige and Skoog’s |
| PGR | Plant growth regulator |
| TDZ | Thidiazuron |
References
- Lydia Pramitha, J.; Ganesan, J.; Francis, N.; Rajasekharan, R.; Thinakaran, J. Revitalization of small millets for nutritional and food security by advanced genetics and genomics approaches. Front. Genet. 2023, 13, 1007552. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.; Vanga, S.K.; Wang, J.; Orsat, V.; Raghavan, V. Millets for food security in the context of climate change: A review. Sustainability 2018, 10, 2228. [Google Scholar] [CrossRef]
- Singh, P.; Raghuvanshi, R.S. Finger millet for food and nutritional security. Afr. J. Food Sci. 2012, 6, 77–84. [Google Scholar]
- Anitha, S.; Givens, D.I.; Botha, R.; Kane-Potaka, J.; Sulaiman, N.L.B.; Tsusaka, T.W.; Bhandari, R.K. Calcium from finger millet—A systematic review and meta-analysis on calcium retention, bone resorption, and in vitro bioavailability. Sustainability 2021, 13, 8677. [Google Scholar] [CrossRef]
- Devi, P.B.; Vijayabharathi, R.; Sathyabama, S.; Malleshi, N.G.; Priyadarisini, V.B. Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber: A review. J. Food Sci. Technol. 2014, 51, 1021–1040. [Google Scholar] [CrossRef]
- Hassan, Z.M.; Sebola, N.A.; Mabelebele, M. The nutritional use of millet grain for food and feed: A review. Agric. Food Secur. 2021, 10, 16. [Google Scholar] [CrossRef]
- Yemets, A.; Klimkina, L.; Tarassenko, L.; Blume, Y. Efficient callus formation and plant regeneration of goosegrass [Eleusine indica (L.) Gaertn.]. Plant Cell Rep. 2003, 21, 503–510. [Google Scholar] [CrossRef]
- Ceasar, S.A.; Maharajan, T.; Krishna, T.P.A.; Ramakrishnan, M.; Victor Roch, G.; Satish, L.; Ignacimuthu, S. Finger millet [Eleusine coracana (L.) Gaertn.] improvement: Current status and future interventions of whole genome sequence. Front. Plant Sci. 2018, 9, 1054. [Google Scholar]
- Satish, L.; Ceasar, S.A.; Shilpha, J.; Rency, A.S.; Rathinapriya, P.; Ramesh, M. Direct plant regeneration from in vitro-derived shoot apical meristems of finger millet (Eleusine coracana (L.) Gaertn.). In Vitro Cell Dev. Biol. Plant 2015, 51, 192–200. [Google Scholar] [CrossRef]
- Arockiasamy, S.; Ignacimuthu, S. Regeneration of transgenic plants from two indica rice (Oryza sativa L.) cultivars using shoot apex explants. Plant Cell Rep. 2007, 26, 1745–1753. [Google Scholar] [CrossRef]
- Thapa, C.B.; Pant, K.K.; Bhattarai, H.D.; Ghimire, M.; Sah, A.K.; Pant, B. In vitro propagation and evaluation of genetic homogeneity using RAPD, ISSR, and SCoT markers in Piper longum L. S. Afr. J. Bot. 2024, 172, 609–618. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, N.S.; Dixon, B.; Sierra, I.; Kan, S.; Layton, A.; Qin, H. Reliable callus-induced plantlet regeneration from leaf explants of Lagerstroemia speciosa and genetic fidelity assessment through ISSR markers. Plant Cell Tissue Organ Cult. 2024, 157, 76. [Google Scholar] [CrossRef]
- Bisht, V.; Rawat, J.M.; Gaira, K.S.; Purohit, S.; Anand, J.; Sinha, S.; Rawat, B. Assessment of genetic homogeneity of in-vitro propagated apple root stock MM 104 using ISSR and SCoT primers. BMC Plant Biol. 2024, 24, 240. [Google Scholar] [CrossRef] [PubMed]
- Krishna, T.A.; Maharajan, T.; David, R.H.A.; Ramakrishnan, M.; Ceasar, S.A.; Duraipandiyan, V.; Ignacimuthu, S. Microsatellite markers of finger millet (Eleusine coracana (L.) Gaertn) and foxtail millet (Setaria italica (L.) Beauv) provide resources for cross-genome transferability and genetic diversity analyses in other millets. Biocatal. Agric. Biotechnol. 2018, 16, 493–501. [Google Scholar] [CrossRef]
- Ceasar, S.A.; Ignacimuthu, S. Efficient somatic embryogenesis and plant regeneration from shoot apex explants of different Indian genotypes of finger millet (Eleusine coracana (L.) Gaertn.). In Vitro Cell Dev. Biol. Plant 2008, 44, 427–435. [Google Scholar] [CrossRef]
- Dey, M.; Bakshi, S.; Galiba, G.; Sahoo, L.; Panda, S.K. Development of a genotype independent and transformation amenable regeneration system from shoot apex in rice (Oryza sativa spp. indica) using TDZ. 3 Biotech 2012, 2, 233–240. [Google Scholar] [CrossRef]
- Devi, P.; Zhong, H.; Sticklen, M. In vitro morphogenesis of pearl millet [Pennisetum glaucum (L.) R. Br.]: Efficient production of multiple shoots and inflorescences from shoot apices. Plant Cell Rep. 2000, 19, 546–550. [Google Scholar] [CrossRef]
- Zhong, H.; Srinivasan, C.; Sticklen, M.B. In-vitro morphogenesis of corn (Zea mays L.): II. Differentiation of ear and tassel clusters from cultured shoot apices and immature inflorescences. Planta 1992, 187, 490–497. [Google Scholar] [CrossRef]
- Zhang, S.; Cho, M.J.; Koprek, T.; Yun, R.; Bregitzer, P.; Lemaux, P. Genetic transformation of commercial cultivars of oat (Avena sativa L.) and barley (Hordeum vulgare L.) using in vitro shoot meristematic cultures derived from germinated seedlings. Plant Cell Rep. 1999, 18, 959–966. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Zhang, M. Production of Multiple Shoots from Shoot Apical Meristems of Oat (Avena sativa L.). J. Plant Physiol. 1996, 148, 667–671. [Google Scholar] [CrossRef]
- Malik, K.A.; Saxena, P. Thidiazuron induces high-frequency shoot regeneration in intact seedlings of pea (Pisum sativum), chickpea (Cicer arietinum) and lentil (Lens culinaris). Funct. Plant Biol. 1992, 19, 731–740. [Google Scholar] [CrossRef]
- Sujatha, G.; Kumari, B.R. High-frequency shoot multiplication in Artemisia vulgaris L. using thidiazuron. Plant Biotechnol. Rep. 2007, 1, 149–154. [Google Scholar] [CrossRef]
- Khawar, K.M.; Sancak, C.; Uranbey, S.; Özcan, S. Effect of thidiazuron on shoot regeneration from different explants of lentil (Lens culinaris Medik.) via organogenesis. Turk. J. Bot. 2004, 28, 421–426. [Google Scholar]
- Li, H.; Murch, S.; Saxena, P. Thidiazuron-induced de novo shoot organogenesis on seedlings, etiolated hypocotyls and stem segments of Huang-qin. Plant Cell Tissue Organ Cult. 2000, 62, 169–173. [Google Scholar] [CrossRef]
- Faisal, M.; Ahmad, N.; Anis, M. Shoot multiplication in Rauvolfia tetraphylla L. using thidiazuron. Plant Cell Tissue Organ Cult. 2005, 80, 187–190. [Google Scholar] [CrossRef]
- Basalma, D.; Uranbey, S.; Mirici, S.; Kolsarici, Ö. TDZ x IBA induced shoot regeneration from cotyledonary leaves and in vitro multiplication in safflower (Carthamus tinctorius L.). Afr. J. Biotechnol. 2008, 7, 1–12. [Google Scholar]
- Eapen, S.; Tivarekar, S.; George, L. Thidiazuron-induced shoot regeneration in pigeonpea (Cajanus cajan L.). Plant Cell Tissue Organ Cult. 1998, 53, 217–220. [Google Scholar] [CrossRef]
- Gallo-Meagher, M.; English, R.; Abouzid, A. Thidiazuron stimulates shoot regeneration of sugarcane embryogenic callus. In Vitro Cell Dev. Biol. Plant 2000, 36, 37–40. [Google Scholar] [CrossRef]
- Ganeshan, S.V.; Chodaparambil, S.; Båga, M.; Fowler, D.B.; Hucl, P.; Rossnagel, B.G.; Chibbar, R.N. In vitro regeneration of cereals based on multiple shoot induction from mature embryos in response to thidiazuron. Plant Cell Tissue Organ Cult. 2006, 85, 63–73. [Google Scholar] [CrossRef]
- Ganeshan, S.; Båga, M.; Harvey, B.L.; Rossnagel, B.G.; Scoles, G.J.; Chibbar, R.N. Production of multiple shoots from thidiazuron-treated mature embryos and leaf-base/apical meristems of barley (Hordeum vulgare). Plant Cell Tissue Organ Cult. 2003, 73, 57–64. [Google Scholar] [CrossRef]
- Guo, B.; Abbasi, B.H.; Zeb, A.; Xu, L.L.; Wei, Y.H. Thidiazuron: A multi-dimensional plant growth regulator. Afr. J. Biotechnol. 2011, 10, 8984–9000. [Google Scholar]
- Lata, H.; Chandra, S.; Wang, Y.H.; Raman, V.; Khan, I.A. TDZ-induced high frequency plant regeneration through direct shoot organogenesis in Stevia rebaudiana Bertoni: An important medicinal plant and a natural sweetener. Am. J. Plant Sci. 2013, 4, 117–128. [Google Scholar] [CrossRef]
- Ebrahimie, E.; Habashy, A.; Mohammadie-Dehcheshmeh, M.; Ghannadha, M.; Ghareyazie, B.; Yazdi-Amadi, B. Direct shoot regeneration from mature embryo as a rapid and genotype-independent pathway in tissue culture of heterogeneous diverse sets of cumin (Cuminum cyminum L.) genotypes. In Vitro Cell Dev. Biol. Plant 2006, 42, 455–460. [Google Scholar] [CrossRef]
- Lin, H.; De Jeu, M.; Jacobsen, E. Direct shoot regeneration from excised leaf explants of in vitro grown seedlings of Alstroemeria L. Plant Cell Rep. 1997, 16, 770–774. [Google Scholar] [CrossRef]
- Stamp, J.A.; Colby, S.M.; Meredith, C.P. Direct shoot organogenesis and plant regeneration from leaves of grape (Vitis spp.). Plant Cell Tissue Organ Cult. 1990, 22, 127–133. [Google Scholar] [CrossRef]
- Chitra, D.V.; Padmaja, G. Shoot regeneration via direct organogenesis from in vitro derived leaves of mulberry using thidiazuron and 6-benzylaminopurine. Sci. Hortic. 2005, 106, 593–602. [Google Scholar] [CrossRef]
- Raghu, A.; Geetha, S.; Martin, G.; Balachandran, I.; Ravindran, P. Direct shoot organogenesis from leaf explants of Embelia ribes Burm. f.: A vulnerable medicinal plant. J. For. Res. 2006, 11, 57–60. [Google Scholar] [CrossRef]
- Tilkat, E.; Onay, A.; Yıldırım, H.; Ayaz, E. Direct plant regeneration from mature leaf explants of pistachio, Pistacia vera L. Sci. Hortic. 2009, 121, 361–365. [Google Scholar] [CrossRef]
- Rout, G. Direct plant regeneration from leaf explants of Plumbago species and its genetic fidelity through RAPD markers. Ann. Appl. Biol. 2002, 140, 305–313. [Google Scholar] [CrossRef]
- Ahmad, N.; Anis, M. Direct plant regeneration from encapsulated nodal segments of Vitex negundo. Biol. Plant. 2010, 54, 748–752. [Google Scholar] [CrossRef]
- Agrawal, D.; Banerjee, A.; Kolala, R.; Dhage, A.; Kulkarni, A.; Nalawade, S.; Hazra, S.; Krishnamurthy, K. In vitro induction of multiple shoots and plant regeneration in cotton (Gossypium hirsutum L.). Plant Cell Rep. 1997, 16, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Geetha, N.; Venkatachalam, P.; Prakash, V.; Sita, G.L. High frequency induction of multiple shoots and plant regeneration from seedling explants of pigeonpea (Cajanus cajan L.). Curr. Sci. 1998, 25, 1036–1041. [Google Scholar]
- Rai, M.K.; Jaiswal, V.S.; Jaiswal, U. Shoot multiplication and plant regeneration of guava (Psidium guajava L.) from nodal explants of in vitro raised plantlets. J. Fruit Ornam. Plant Res. 2009, 17, 29–38. [Google Scholar]
- Sharma, V.K.; Hänsch, R.; Mendel, R.R.; Schulze, J. A highly efficient plant regeneration system through multiple shoot differentiation from commercial cultivars of barley (Hordeum vulgare L.) using meristematic shoot segments excised from germinated mature embryos. Plant Cell Rep. 2004, 23, 9–16. [Google Scholar] [CrossRef]
- Thomas, T.D. Thidiazuron induced multiple shoot induction and plant regeneration from cotyledonary explants of mulberry. Biol. Plant. 2003, 46, 529–533. [Google Scholar] [CrossRef]
- Martins, M.; Sarmento, D.; Oliveira, M. Genetic stability of micropropagated almond plantlets, as assessed by RAPD and ISSR markers. Plant Cell Rep. 2004, 23, 492–496. [Google Scholar] [CrossRef]
- Joshi, P.; Dhawan, V. Assessment of genetic fidelity of micropropagated Swertia chirayita plantlets by ISSR marker assay. Biol. Plant. 2007, 51, 22–26. [Google Scholar] [CrossRef]
- Rai, M.K.; Phulwaria, M.; Gupta, A.K.; Shekhawat, N.; Jaiswal, U. Genetic homogeneity of guava plants derived from somatic embryogenesis using SSR and ISSR markers. Plant Cell Tissue Organ Cult. 2012, 111, 259–264. [Google Scholar] [CrossRef]
- Tawar, P.; Sawant, R.; Dalvi, S.; Nikam, A.; Kawar, P.; Devarumath, R. An assessment of somaclonal variation in micropropagated plants of sugarcane by RAPD markers. Sugar Tech 2008, 10, 124–127. [Google Scholar] [CrossRef]
- Bhatia, R.; Singh, K.; Jhang, T.; Sharma, T. Assessment of clonal fidelity of micropropagated gerbera plants by ISSR markers. Sci. Hortic. 2009, 119, 208–211. [Google Scholar] [CrossRef]
- Kumar, S.; Mangal, M.; Dhawan, A.; Singh, N. Assessment of genetic fidelity of micropropagated plants of Simmondsia chinensis (Link) Schneider using RAPD and ISSR markers. Acta Physiol. Plant 2011, 33, 2541–2545. [Google Scholar] [CrossRef]

| PGR Used in Shoot Induction Medium (µM) | Name of the Genotype | ||||
|---|---|---|---|---|---|
| BAP | TDZ | Kin | IE-2606 | ||
| Explants Formed Shoots % | Mean Number of Shoots/Shoot Apex Explants | Shoot Length (cm) | |||
| 2.2 | - | - | 20.0 ± 4.7 d | 3.6 ± 0.2 f | 1.6 ± 0.2 e |
| 4.4 | - | - | 56.6 ± 7.2 c | 6.3 ± 0.5 e,f | 4.1 ± 0.3 c,d |
| 6.6 | - | - | 63.3 ± 7.2 b,c | 8.0 ± 0.4 d,e,f | 5.2 ± 0.2 b,c |
| 8.8 | - | - | 73.3 ± 2.7 a,b,c | 9.3 ± 0.5 c,d,e | 5.9 ± 0.3 a,b,c |
| 8.8 | - | 1.1 | 26.6 ± 2.7 d | 8.3 ± 1.1 d,e,f | 1.5 ± 0.1 e |
| 8.8 | - | 2.3 | 56.6 ± 2.7 c | 12.0 ± 0.9 a,b,c,d | 4.3 ± 0.5 b,c,d |
| 8.8 | - | 3.4 | 70.0 ± 4.7 a,b,c | 14.6 ± 1.1 a,b,c | 5.7 ± 0.3 a,b,c |
| 8.8 | - | 4.6 | 66.6 ± 2.7 b,c | 12.3 ± 1.5 a,b,c,d | 4.9 ± 0.3 b,c,d |
| - | 2.2 | - | 60.0 ± 4.7 b,c | 10.0 ± 0. 4 b,c,e | 3.1 ± 0.4 d,e |
| - | 4.5 | - | 96.6 ± 2.7 a | 17.3 ± 0.7 a | 7.4 ± 0.2 a |
| - | 6.8 | - | 86.6 ± 2.7 a,b | 15.0 ± 0.4 a,b,d | 6.3 ± 0.3 a,b |
| - | 9.0 | - | 76.6 ± 2.7 a,b,c | 13.3 ± 1.0 a,b,c,d | 6.0 ± 0.1 a,b,c |
| PGRs Used in Root Induction Medium | Concentrations of Auxin (µM) | Rooted Cultures (%) | Mean Number of Roots | Root Length (in cm) |
|---|---|---|---|---|
| 0.0 | 5.5 ± 4.5 d | 0.3 ± 0.27 d | 0.2 ± 0.1 d | |
| IAA | 0.25 µM | 33.2 ± 7.8 c,d | 2.0 ± 0.47 c,d | 3.9 ± 0.4 c |
| 2.85 µM | 77.4 ± 4.5 a,b | 4.6 ± 0.47 a | 6.9 ± 0.4 a,b | |
| 5.70 µM | 66.4 ± 7.8 a,b,c | 4.0 ± 0.27 a,b | 5.6 ± 0.3 b,c | |
| 0.0 | 5.5 ± 4.5 d | 0.3 ± 0.2 d | 0.3 ± 0.2 d | |
| 0.25 µM | 44.2 ± 4.5 b,c | 2.6 ± 0.2 b,c | 4.6 ± 0.4 c | |
| IBA | 2.46 µM | 94.3 ± 4.6 a | 5.6 ± 0.2 a | 8.2 ± 0.3 a |
| 4.9 µM | 71.9 ± 4.5 a,b | 4.3 ± 0.2 a,b | 5.7 ± 0.3 b,c |
| S. No | Primers Name | Primers Sequences (5′-3′) | Annealing Tm (°C) | Number of Scoreable Bands Per Primer | Monomorphism (%) |
|---|---|---|---|---|---|
| 1 | UBC 834 | AGAGAGAGAGAGAGAGYT | 50.5 | 5 | 100 |
| 2 | UBC 841 | GAGAGAGAGAGAGAGAGC | 51.5 | 7 | 100 |
| 3 | UBC 848 | CACACACACACACACARG | 55.0 | 5 | 100 |
| 4 | UBC 856 | ACACACACACACACACYA | 55.0 | 4 | 100 |
| 5 | UBC 857 | ACACACACACACACACYG | 54.0 | 6 | 100 |
| Total number of bands produced | 27 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maharajan, T.; Duraipandiyan, V.; Krishna, T.P.A. Direct Shoot Regeneration from the Finger Millet’s In Vitro-Derived Shoot Apex and Genetic Fidelity Study with ISSR Markers. BioTech 2025, 14, 29. https://doi.org/10.3390/biotech14020029
Maharajan T, Duraipandiyan V, Krishna TPA. Direct Shoot Regeneration from the Finger Millet’s In Vitro-Derived Shoot Apex and Genetic Fidelity Study with ISSR Markers. BioTech. 2025; 14(2):29. https://doi.org/10.3390/biotech14020029
Chicago/Turabian StyleMaharajan, Theivanayagam, Veeramuthu Duraipandiyan, and Thumadath Palayullaparambil Ajeesh Krishna. 2025. "Direct Shoot Regeneration from the Finger Millet’s In Vitro-Derived Shoot Apex and Genetic Fidelity Study with ISSR Markers" BioTech 14, no. 2: 29. https://doi.org/10.3390/biotech14020029
APA StyleMaharajan, T., Duraipandiyan, V., & Krishna, T. P. A. (2025). Direct Shoot Regeneration from the Finger Millet’s In Vitro-Derived Shoot Apex and Genetic Fidelity Study with ISSR Markers. BioTech, 14(2), 29. https://doi.org/10.3390/biotech14020029






