AsNAC Genes: Response to High Mercury Concentrations in Allium sativum Seed Clove
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Stress Treatments
2.2. Germination Percentage
2.3. Analysis of AsNAC Genes
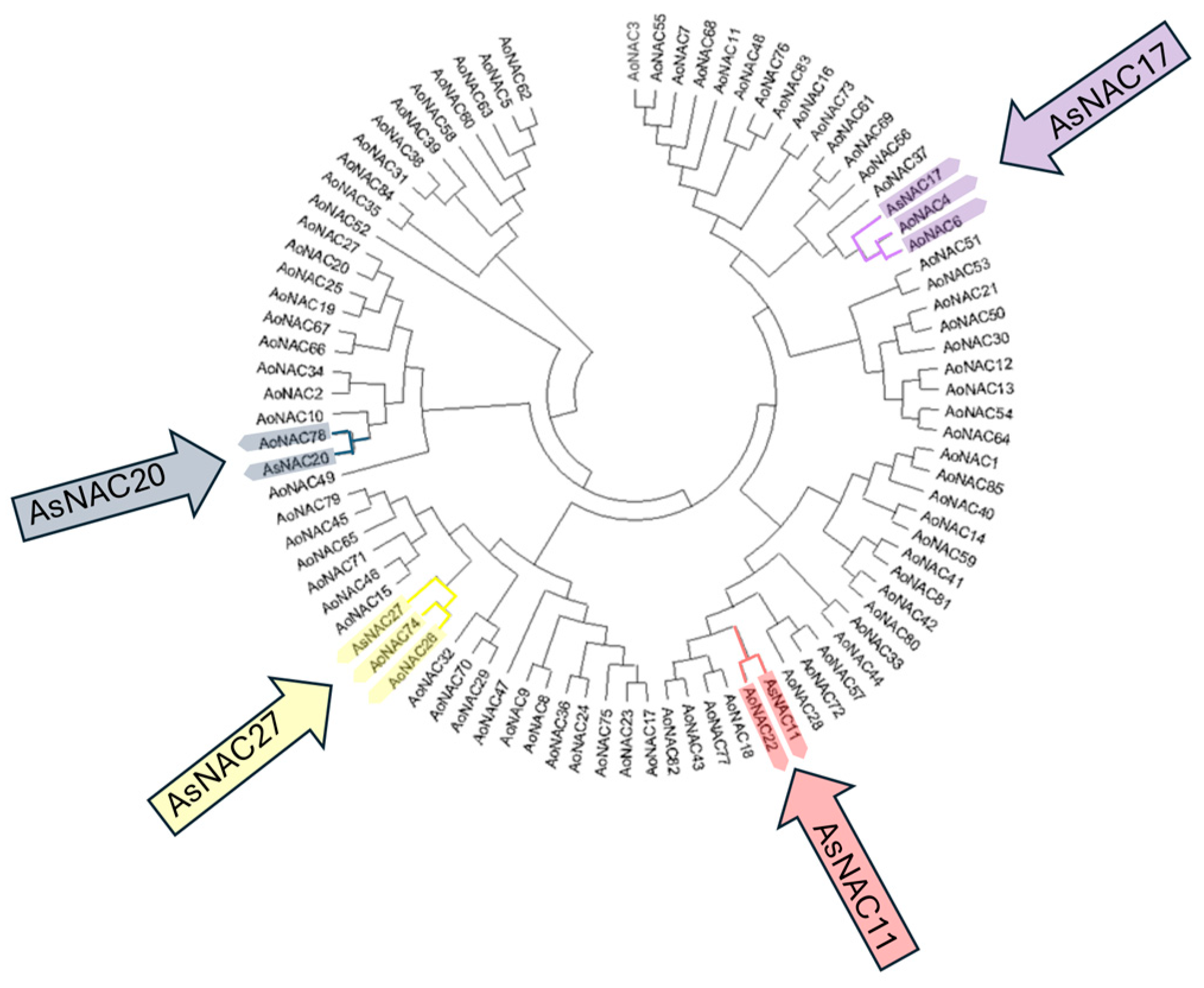
2.4. Phylogenetic Analysis and Classification of AsNAC Genes
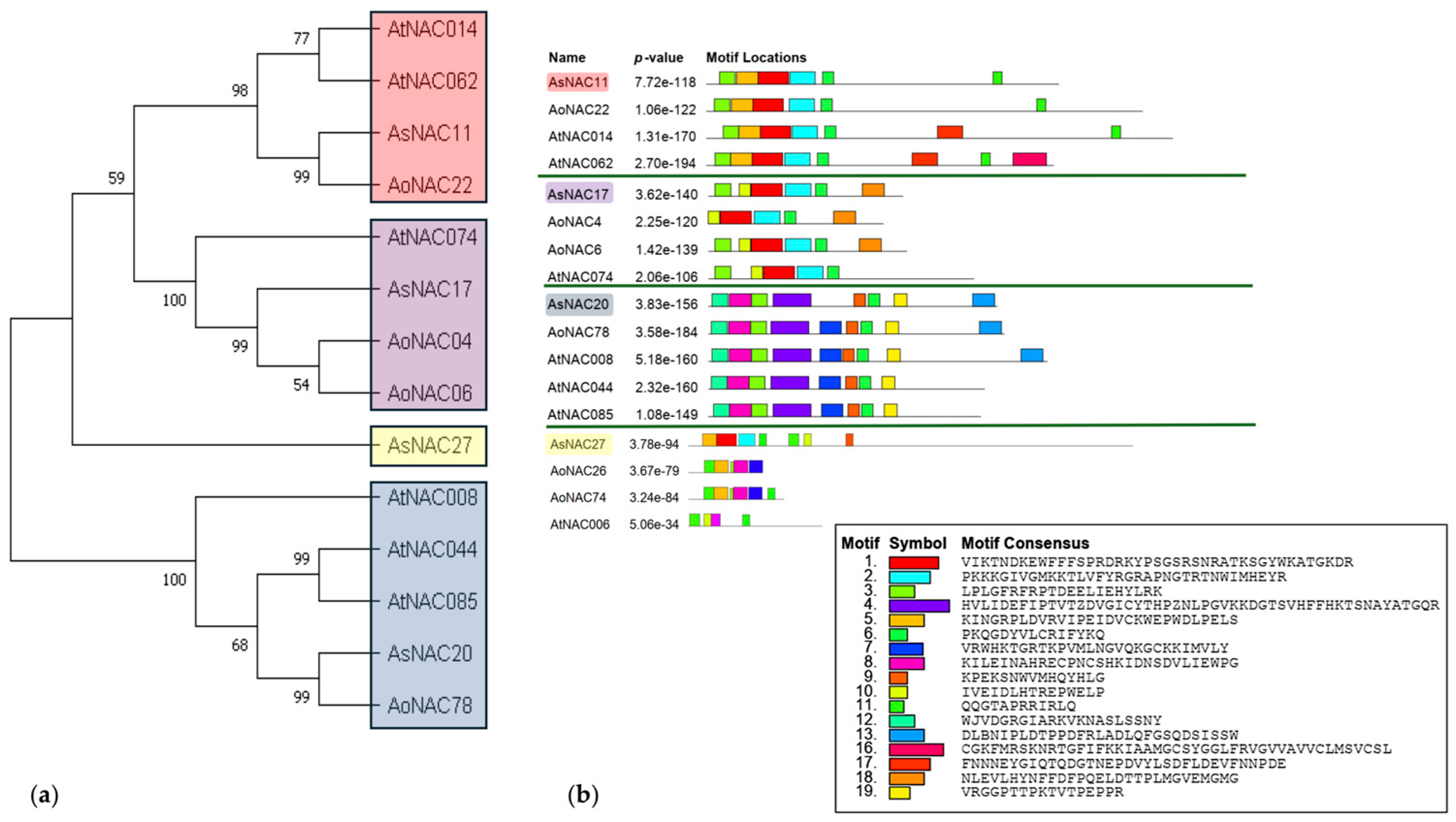
2.5. Gene Structure and Motif Analysis of AsNAC Proteins
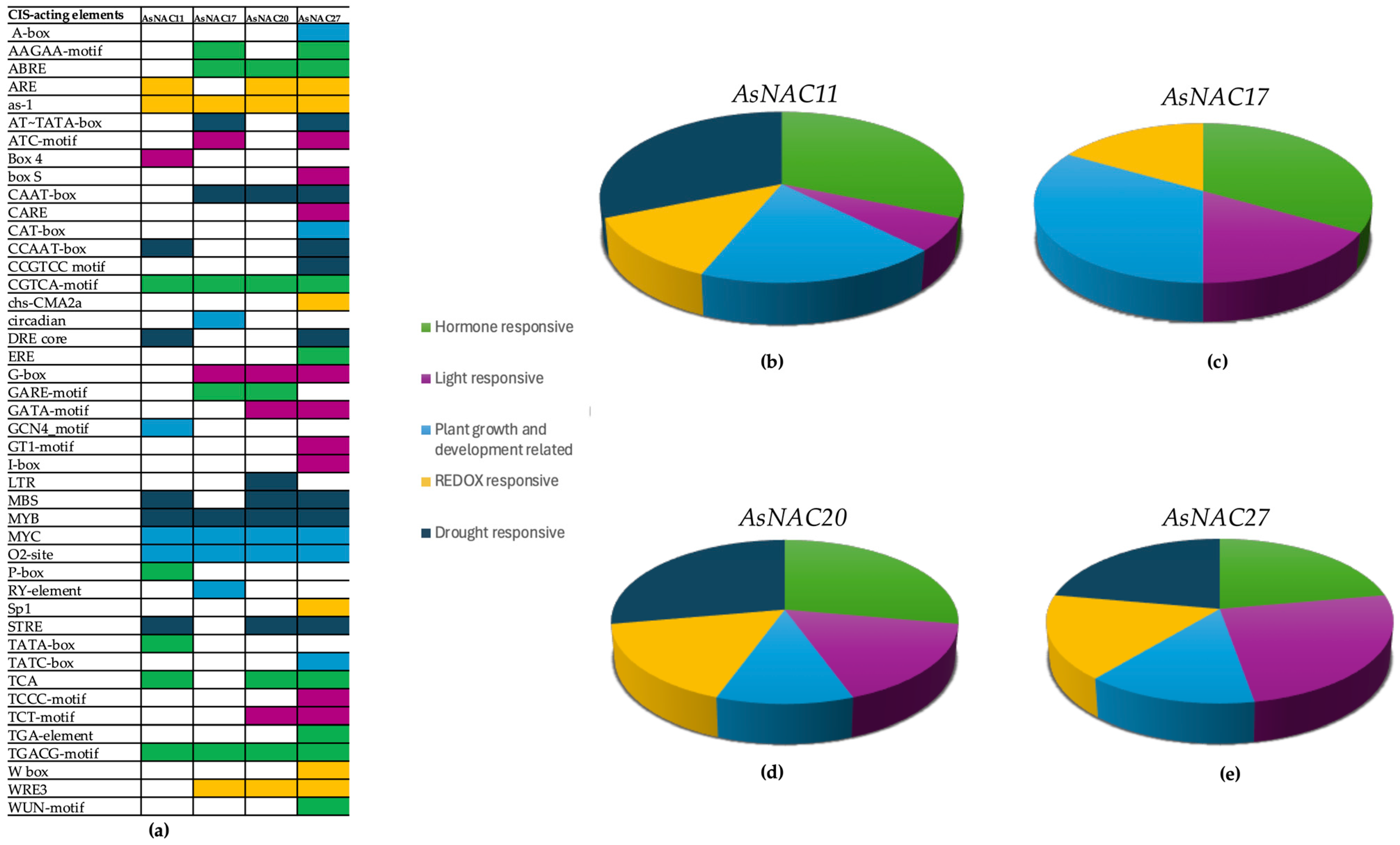
2.6. CIS-Acting Elements on AsNAC Genes
2.7. Protein Interactions
2.8. RNA Isolation from Allium sativum Seed Tissue
2.9. cDNA Synthesis
2.10. qPCR Conditions
2.11. Mercury Concentrations in Allium sativum Seed Tissue
2.12. Statistics
3. Results
3.1. Germination Percentage
3.2. Phylogenetic Relationships and Classification of NAC Family TFs in Garlic
3.3. Conserved Motifs in NAC Genes
3.4. CIS-Acting Elements on AsNAC Genes
3.5. Analysis of the Interaction of AsNAC Proteins
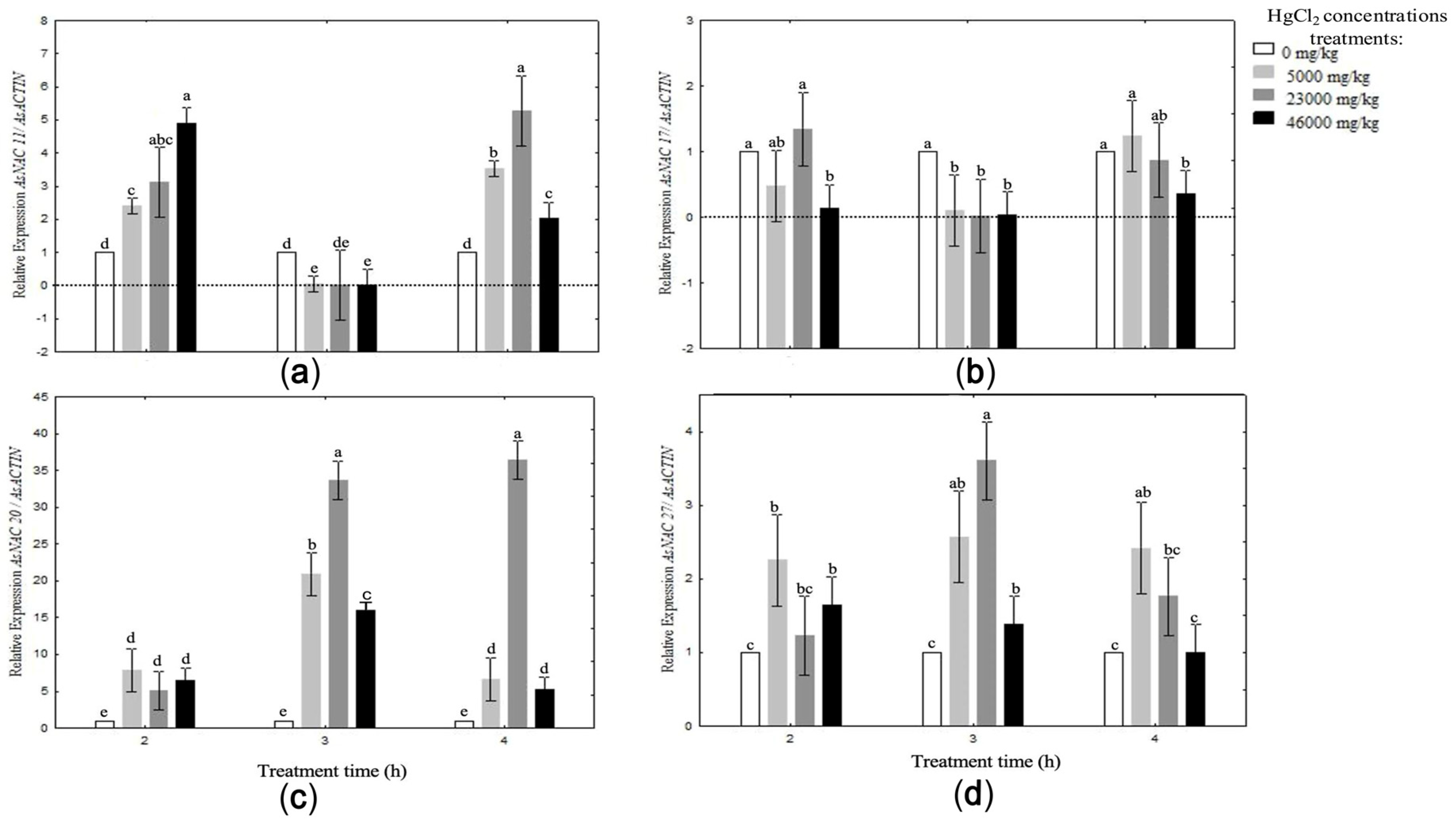
3.6. Expression Patterns of AsNAC Genes in Garlic Seed Cloves Exposed to High Mercury Concentrations
3.7. Mercury Concentrations in Garlic Seed Clove Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy metal contamination in agricultural soil: Environmental pollutants affecting crop health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Hu, B.; Jia, X.; Hu, J.; Xu, D.; Xia, F.; Li, Y. Assessment of heavy metal pollution and health risks in the Soil-Plant-Human system in the Yangtze River Delta, China. Int. J. Environ. Res. Public Health 2017, 14, 1042. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Arya, S.; Kumar, S. Industrial wastewater treatment: Current trends, bottlenecks, and best practices. Chemosphere 2021, 285, 131245. [Google Scholar] [CrossRef]
- López-Tejedor, I.; Sierra, M.J.; Rodríguez, J.; Millán, R. Estudio de la Absorción y Distribución de Mercurio en Nerium Oleander L. en la Ribera del río Valdeazogues (Estación de Chillón-Almadén). 2014. Available online: http://documenta.ciemat.es/handle/123456789/124 (accessed on 14 May 2024).
- Environmental Protection Agency (EPA). Mercury; initial inventory report of supply, use, and trade. Fed. Regist. 2017, 82, 15522–15523. [Google Scholar]
- Hussain, S.; Yang, J.; Hussain, J.; Sattar, A.; Ullah, S.; Hussain, I.; Zhang, L. Mercury fractionation, bioavailability, and the major factors predicting its transfer and accumulation in soil–wheat systems. Sci. Total Environ. 2022, 847, 157432. [Google Scholar]
- Kamal, M.; Ghaly, A.E.; Mahmoud, N.; Côté, R. Phytoaccumulation of heavy metals by aquatic plants. Environ. Int. 2004, 29, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Piyatida, P.; Da Silva, J.a.T.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 2012, 872875. [Google Scholar] [CrossRef]
- Ajsuvakova, O.; Tinkov, A.; Aschner, M.; Rocha, J.; Michalke, B.; Skalnaya, M.; Skalny, A.; Butnariu, M.; Dadar, M.; Sarac, I.; et al. Sulfhydryl groups as targets of mercury toxicity. Coord. Chem. Rev. 2020, 417, 213343. [Google Scholar] [CrossRef]
- Bechaieb, R.; Akacha, A.; Gérard, H. Quantum chemistry insight into Mg-substitution in chlorophyll by toxic heavy metals: Cd, Hg and Pb. Chem. Phys. Lett. 2016, 663, 27–32. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali, A.; Kour, N.; Bornhorst, J.; Alharbi, K.; Rinklebe, J.; Moneim, D.; Ahmad, P.; Chung, Y. Heavy Metal Induced Oxidative Stress Mitigation and ROS Scavenging in Plants. Plants 2023, 12, 3003. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Liang, Y.; Guo, J.; Gong, H.; Xu, Z. Silicon alleviates mercury toxicity in garlic plants. J. Plant Nutr. 2020, 43, 2508–2517. [Google Scholar] [CrossRef]
- Munzuroğlu, O.; Geçkil, H. Effects of metals on seed germination, root elongation, and coleoptile and hypocotyl growth in Triticum aestivum and Cucumis sativus. Arch. Environ. Contam. Toxicol. 2002, 43, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Buscaroli, A. An overview of indexes to evaluate terrestrial plants for phytoremediation purposes (Review). Ecol. Indic. 2017, 82, 367–380. [Google Scholar] [CrossRef]
- Boim, A.; Melo, L.; Moreno, F.; Alleoni, L. Bioconcentration factors and the risk concentrations of potentially toxic elements in garden soils. J. Environ. Manag. 2016, 170, 21–27. [Google Scholar] [CrossRef]
- Yu, J.; Mao, C.; Zhong, Q.; Yao, X.; Li, P.; Liu, C.; Ming, F. OsNAC2 Is Involved in Multiple Hormonal Pathways to Mediate Germination of Rice Seeds and Establishment of Seedling. Front. Plant Sci. 2021, 12, 699303. [Google Scholar] [CrossRef]
- Huang, C.; Zhao, J.; Huang, Q.; Peng, L.; Huang, Z.; Li, W.; Sun, S.; He, Y.; Wang, Z. OsNAC3 regulates seed germination involving abscisic acid pathway and cell elongation in rice. New Phytol. 2024, 241, 650. [Google Scholar] [CrossRef]
- Cobbett, C.S. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000, 123, 825–832. [Google Scholar]
- Singh, S.; Koyama, H.; Bhati, K.K.; Alok, A. The biotechnological importance of the plant-specific NAC transcription factor family in crop improvement. J. Plant Res. 2021, 134, 475–495. [Google Scholar]
- Kianoush, S.; Balali-Mood, M.; Mousavi, S.R.; Moradi, V.; Sadeghi, M.; Dadpour, B.; Rajabi, O.; Shakeri, M.T. Comparison of therapeutic effects of garlic and d-Penicillamine in patients with chronic occupational lead poisoning. Basic. Clin. Pharmacol. Toxicol. 2012, 110, 476–481. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Qiu, M.; Wang, A.; Ye, J.; Fu, S. Garlic (Allium sativum) and Fu-ling (Poria cocos) mitigate lead toxicity by improving antioxidant defense mechanisms and chelating ability in the liver of grass carp (Ctenopharyngodon idella). Ecotoxicology 2021, 30, 885–898. [Google Scholar] [CrossRef]
- Liu, D.; Zou, J.; Meng, Q.; Zou, J.; Jiang, W. Uptake and accumulation and oxidative stress in garlic (Allium sativum L.) under lead phytotoxicity. Ecotoxicology 2009, 18, 134–143. [Google Scholar] [PubMed]
- Zhang, H.; Jiang, Y.; He, Z.; Ma, M. Cadmium accumulation and oxidative burst in garlic (Allium sativum). J. Plant Physiol. 2005, 162, 977–984. [Google Scholar] [CrossRef]
- Wang, G.L.; An, Y.H.; Zhou, C.L.; Hu, Z.Z.; Ren, X.Q.; Xiong, A.S. Transcriptome-wide identification of NAC (no apical meristem/Arabidopsis transcription activation factor/cup-shaped cotyledon) transcription factors potentially involved in salt stress response in garlic. PeerJ 2022, 10, e14602. [Google Scholar]
- Li, C.; Zhang, J.; Zhang, Q.; Dong, A.; Wu, Q.; Zhu, X.; Zhu, X. Genome-Wide Identification and Analysis of the NAC Transcription Factor Gene Family in Garden Asparagus (Asparagus officinalis). Genes 2022, 13, 976. [Google Scholar] [CrossRef] [PubMed]
- TAIR. Available online: https://www.arabidopsis.org (accessed on 14 May 2024).
- Ooka, H.; Satoh, K.; Doi, K.; Nagata, T.; Otomo, Y.; Murakami, K.; Matsubara, K.; Osato, N.; Kawai, J.; Carninci, P. Comprehensive analysis of NAC family genes in Oryza sativa and Arabidopsis thaliana. DNA Res. 2003, 10, 239–247. [Google Scholar]
- MEME. Available online: https://meme-suite.org/meme/tools/meme (accessed on 30 May 2024).
- Plant Care. Available online: https://bioinformatics.psb.ugent.be/webtools/plantcare/html/ (accessed on 5 June 2024).
- Zklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Annika, G.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2∆∆C(T) Method. Methods 2001, 25, 402–408. [Google Scholar] [PubMed]
- The Fitness for Purpose of Analytical Methods: A Laboratory Guide to Method Validation and Related Topics. Eurachem, 2nd ed. Available online: https://www.eurachem.org/images/stories/Guides/pdf/MV_guide_2nd_ed_ES.pdf (accessed on 14 May 2024).
- Hanasoge, S.; Ljungman, M. H2AX phosphorylation after UV irradiation is triggered by DNA repair intermediates and is mediated by the ATR kinase. Carcinogenesis 2007, 28, 2298–2304. [Google Scholar] [CrossRef]
- Szurman-Zubrzycka, M.; Jędrzejek, P.; Szarejko, I. How Do Plants Cope with DNA Damage? A Concise Review on the DDR Pathway in Plants. Int. J. Mol. Sci. 2023, 24, 2404. [Google Scholar] [CrossRef]
- Yan, S.; Wang, W.; Marqués, J.; Mohan, R.; Saleh, A.; Durrant, W.E.; Song, J.; Dong, X. Salicylic acid activates DNA damage responses to potentiate plant immunity. Mol. Cell 2013, 52, 602–610. [Google Scholar] [CrossRef]
- Iftode, C.; Daniely, Y.; Borowiec, J. Replication protein A (RPA): The eukaryotic SSB. Crit. Rev. Biochem. Mol. Biol. 1999, 34, 141–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hossain, G.S.; Islas-Osuna, M.A.; Mitchell, D.L.; Mount, D.W. Repair of UV damage in plants by nucleotide excision repair: Arabidopsis UVH1 DNA repair gene is a homolog of Saccharomyces cerevisiae Rad1. Plant J. 2000, 21, 519–528. [Google Scholar] [CrossRef]
- Shang, X.; Wang, Z.; Liu, L. Small heat shock proteins (sHSPs) in Arabidopsis: Characterization, function, and response to stress. J. Integr. Plant Biol. 2020, 62, 744–759. [Google Scholar] [CrossRef]
- Manfield, I.W.; Devlin, P.F.; Jen, C.H.; Westhead, D.R.; Gilmartin, P.M. Conservation, convergence, and divergence of light-responsive, circadian-regulated, and tissue-specific expression patterns during evolution of the Arabidopsis GATA gene family. Plant Physiol. 2007, 143, 941–958. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional control of flavonoid biosynthesis by MYB–bHLH–WDR complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 65, 667–676. [Google Scholar] [CrossRef]
- Liao, Y.; Zou, H.F.; Wang, H.W. GmMYB76, GmMYB92, and GmMYB177 genes confer stress tolerance in transgenic Arabidopsis plants. Cell Res. 2008, 18, 1047–1060. [Google Scholar] [CrossRef]
- Colinas, M.; Shaw, H.V.; Loubéry, S.; Kaufmann, M.; Moulin, M.; Fitzpatrick, T.B. A pathway for repair of NAD(P)H in plants. J. Biol. Chem. 2014, 289, 14692–14706. [Google Scholar] [CrossRef]
- Yi, D.; Alvim Kamei, C.L.; Cools, T.; Vanderauwera, S.; Takahashi, N.; Okushima, Y.; Eekhout, T.; Yoshiyama, K.O.; Larkin, J.; Van den Daele, H. The Arabidopsis SIAMESE-RELATED cyclin-dependent kinase inhibitors SMR5 and SMR7 regulate the DNA damage checkpoint in response to reactive oxygen species. Plant Cell 2014, 26, 296–309. [Google Scholar] [CrossRef]
- Détain, A.; Redecker, D.; Leborgne-Castel, N. Structural conservation of WEE1 and its role in cell cycle regulation in plants. Sci. Rep. 2021, 11, 23862. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, J.; Wang, L.; Wang, H.; Long, H.; Yang, L.; Shao, R. Water deficit aggravated the inhibition of photosynthetic performance of maize under mercury stress but is alleviated by brassinosteroids. J. Hazard. Mater. 2023, 443, 130365. [Google Scholar] [PubMed]
- Sircar, S.; Parekh, N. Functional characterization of drought-responsive modules and genes in Oryza sativa: A network-based approach. Front. Genet. 2015, 6, 256. [Google Scholar] [CrossRef]
- Han, K.; Zhao, Y.; Sun, Y.; Li, Y. NACs, generalist in plant life. Plant Biotechnol. J. 2023, 21, 2433–2457. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lindsey-Boltz, L.; Kemp, M.; Mason, A.; Wold, M.; Sancar, A. Reconstitution of RPA-covered single-stranded DNA-activated ATR-Chk1 signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 13660–13665. [Google Scholar] [CrossRef]
- Heckathorn, S.A.; Mueller, J.K.; LaGuidice, S.; Zhu, B.; Barrett, T.; Blair, B.; Dong, Y. Chloroplast small heat-shock proteins protect photosynthesis during heavy metal stress. Am. J. Bot. 2004, 91, 1312–1318. [Google Scholar]
- Ezeh, O.S.; Yamamoto, Y.Y. Combinatorial Effects of Cis-Regulatory Elements and Functions in Plants. Rev. Agric. Sci. 2024, 12, 79–92. [Google Scholar]
- Ng, S.; Ivanova, A.; Duncan, O.; Law, S.; Van Aken, O.; De Clercq, I.; Wang, Y.; Carrie, C.; Xu, L.; Kmiec, B. A Membrane-Bound NAC Transcription Factor, ANAC017, Mediates Mitochondrial Retrograde Signaling in Arabidopsis. Plant Cell 2013, 25, 3450–3471. [Google Scholar] [CrossRef]
- Ho, L.; Giraud, E.; Uggalla, V.; Lister, R.; Clifton, R.; Glen, A.; Thirkettle-Watts, D.; Van Aken, O.; Whelan, J. Identification of Regulatory Pathways Controlling Gene Expression of Stress-Responsive Mitochondrial Proteins in Arabidopsis. Plant Physiol. 2008, 147, 1858–1873. [Google Scholar] [CrossRef]
- Fujita, M.; Fujita, Y.; Maruyama, K.; Seki, M.; Hiratsu, K.; Ohme-Takagi, M.; Tran, L.S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway. Plant J. 2004, 39, 863–876. [Google Scholar]
- Carabulea, V.; Motelica, D.; Vrînceanu, N.O.; Plopeanu, G.; Costea, M.; Oprea, B.S.; Tanase, V. Bioaccumulation of heavy metals in garlic bulbs (Allium sativum L.) In correlation with soil from private gardens in the Copșa Mică area, Romania. J. Appl. Life Sci. Environ. 2023, 55, 245–255. [Google Scholar] [CrossRef]
- Tegegne, W.A.; Mengiste, A.A. Determination of essential and non-essential metals concentration in garlic (Allium sativum L.) bulb and leaf cultivated in Ambo Woreda, Ethiopia. Sci. J. Anal. Chem. 2016, 4, 84–94. [Google Scholar]
- Jiang, W.; Liu, D. Pb-induced cellular defense system in the root meristematic cells of Allium sativum L. BMC Plant Biol. 2010, 10, 40. [Google Scholar]



| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Alignment Temp (°C) |
|---|---|---|---|
| AsNAC11 | CTACACCATTGAACCAAGCATCTCC | GAGCACTTCATCATTAGCCACATTACA | 59 |
| AsNAC17 | CTCATACACCACCTAAGGAGGACTG | CCGAAGCATCCACCTAACATTGATTG | 59 |
| AsNAC20 | CAAGAGAAGAAGAGATGGAGCAAGTCA | CAACTAGATATGCTGTCCTGAGAACCA | 61 |
| AsNAC27 | GCTTGGTACACTGCAACGGTAGTAA | TTGACTTCTCGGACTGGAGGATGG | 61 |
| AsACTIN | TGCTCTGGATTATGAACAGGAACTTGA | CAATCATTGAAGGCTGGAACAACACT | 58 |
| AsNAC | Protein | Function | References |
|---|---|---|---|
| 11 | DAU1 | Key role in regulating sperm cell development. | [30] |
| 11 | DAW1 | Preserved in plants and involved in the regulation of cell polarity and growth. | [34] |
| 11, 17 | JHS1 | This gene plays a crucial role in the response to DNA damage, specifically in the repair of double-strand breaks, and helps maintain the integrity of the root and shoot apical meristem (RAM and SAM). | [35] |
| 11, 17 | ATR | Plays a central role in cell cycle regulation by transmitting DNA damage signals to downstream effectors of cell cycle progression. | [33] |
| 11, 17 | RPA2A, RPA2B | Involved in the repair of DNA lesions, particularly those resulting from oxidative stress. | [36] |
| 11, 17 | UVH1 | Involved in nucleotide excision repair (NER) of damaged DNA (dark repair mechanism). Involved in the repair of UV light and probably oxidative damage. | [37] |
| 17 | F4J030_ARATH | Member of the small heat shock proteins (sHSPs). In addition to heat stress, sHSPs can also be induced by other types of abiotic stress, such as dehydration, salinity, and oxidative stress. | [38] |
| 17 | GATA11 | The main function of GATA TFs is to regulate gene expression in response to environmental and hormonal stimuli, as well as in developmental processes. | [39] |
| 17 | HH06 | It associates with basic helix–loop–helix (bHLH) transcription factors, allowing the formation of dimers that regulate genes involved in hormonal signaling and in response to abiotic stresses through the synthesis of anthocyanins. | [40] |
| 17 | PUX1, PUX6 | These play crucial roles in regulating the structure and function of essential proteins such as CDC48 and in modulating GA hormone signaling. | [41] |
| 20 | MYB3R3, MYB3R4, MYB3R5 | The MYB family is involved in diverse processes such as developmental control, the determination of cell fate, plant responses to environmental factors and hormones, signal transduction in plant growth processes, pathogen defense, and xylogenesis and lignin biosynthesis. | [42] |
| 20 | Q8L637_ARATH | A pyridoxamine 5′-phosphate oxidase family protein involved in the regulation of cellular metabolism. They can oxidize 6-NADH and 6-NADPH, suggesting a role in the elimination of damaged forms of NAD(P)H. | [43] |
| 20 | SMR5, SMR7 | These SIAMESE-RELATION (SMR)-type regulators modulate cell cycle arrest in response to DNA damage or oxidative stress. SMR5 has been shown to play a crucial role in cell cycle arrest in situations of water or genotoxic stress. | [44] |
| 20 | WEE1 | Encodes a protein kinase that plays a crucial role in regulating the cell cycle. | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Almanza, B.; Guerrero-González, M.d.l.L.; Loredo-Tovias, M.; García-Arreola, M.E.; Loredo-Osti, C.; Padilla-Ortega, E.; Delgado-Sánchez, P. AsNAC Genes: Response to High Mercury Concentrations in Allium sativum Seed Clove. BioTech 2025, 14, 27. https://doi.org/10.3390/biotech14020027
Mendoza-Almanza B, Guerrero-González MdlL, Loredo-Tovias M, García-Arreola ME, Loredo-Osti C, Padilla-Ortega E, Delgado-Sánchez P. AsNAC Genes: Response to High Mercury Concentrations in Allium sativum Seed Clove. BioTech. 2025; 14(2):27. https://doi.org/10.3390/biotech14020027
Chicago/Turabian StyleMendoza-Almanza, Brenda, María de la Luz Guerrero-González, Marcos Loredo-Tovias, María Elena García-Arreola, Catarina Loredo-Osti, Erika Padilla-Ortega, and Pablo Delgado-Sánchez. 2025. "AsNAC Genes: Response to High Mercury Concentrations in Allium sativum Seed Clove" BioTech 14, no. 2: 27. https://doi.org/10.3390/biotech14020027
APA StyleMendoza-Almanza, B., Guerrero-González, M. d. l. L., Loredo-Tovias, M., García-Arreola, M. E., Loredo-Osti, C., Padilla-Ortega, E., & Delgado-Sánchez, P. (2025). AsNAC Genes: Response to High Mercury Concentrations in Allium sativum Seed Clove. BioTech, 14(2), 27. https://doi.org/10.3390/biotech14020027







