Aromatic Amino Acids: Exploring Microalgae as a Potential Biofactory
Abstract
1. Introduction
1.1. Chlamydomonas Reinhardtii
1.2. Phaeodactylum Tricornutum
1.3. Aromatic Amino Acids
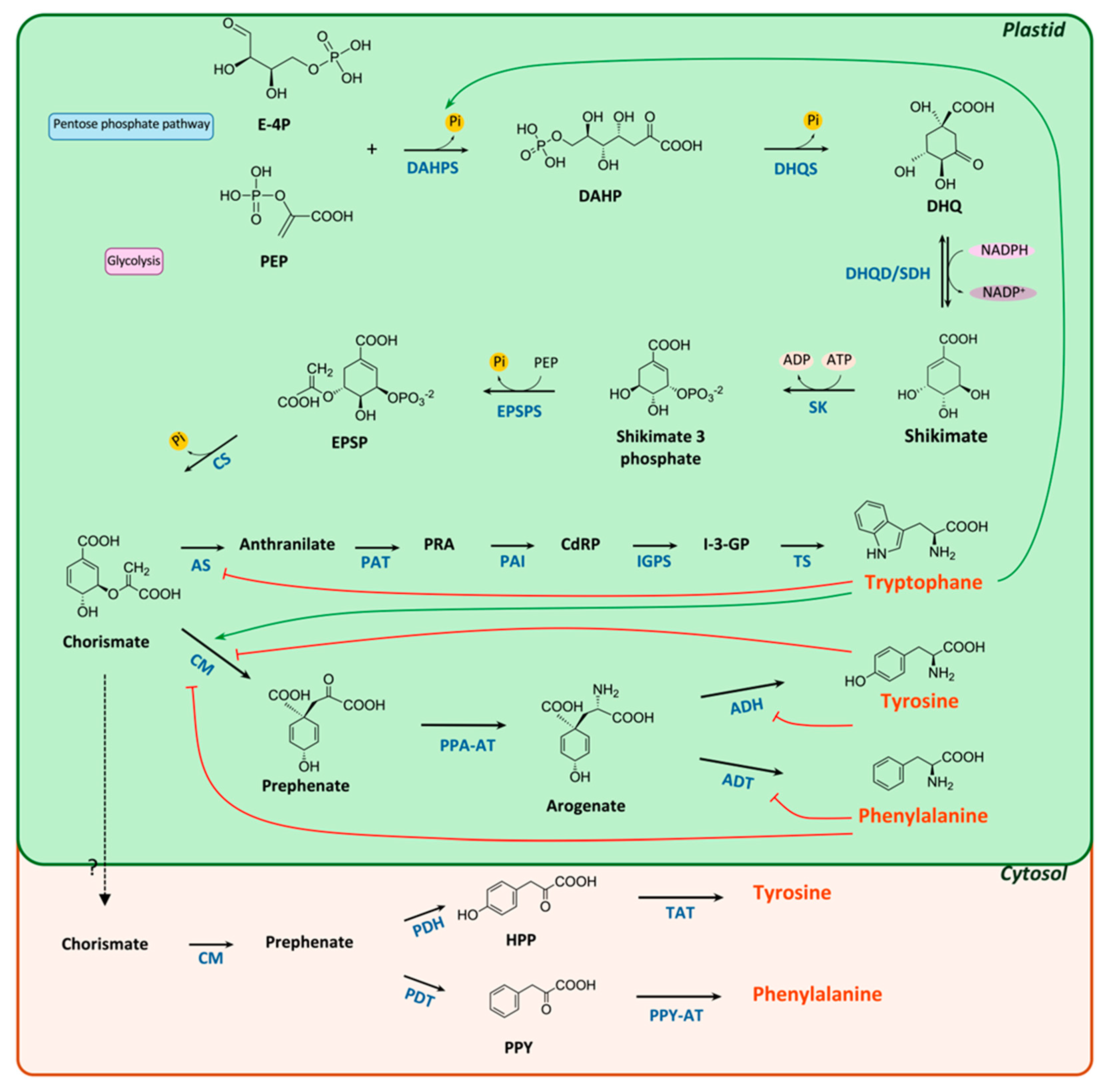
2. Biosynthesis of AAA
2.1. Shikimate/Chorismate Pathway
2.2. Post-Chorismate Pathway
2.3. Phenylalanine and Tyrosine Biosynthesis
2.3.1. Chorismate Mutase (CM)
2.3.2. Prephenate Aminotransferase
2.3.3. Prephenate and Arogenate Dehydratase
2.3.4. Arogenate and Prephenate Dehydrogenase
2.3.5. Phenylalanine Hydroxylase
2.3.6. Phenylpyruvate and Tyrosine Aminotransferases
2.4. The Tryptophan Pathway
3. Metabolic Engineering for Enhancing AAAs in Microalga
3.1. Possible Approaches That Can Be Applied to Enhance AAAs in Microalgae
3.1.1. Increasing Precursors
3.1.2. Modulation of Primary Metabolism
3.1.3. Relieving Allosteric Control
3.1.4. Overexpression of Feedback Insensitive Gene
| Target | Organism | Strategy | Yield | Ref |
|---|---|---|---|---|
| Enhancing PEP level by replacing native PTS systems | E. coli | By combining a non-PTS sugar transport system with the overexpression of several crucial genes responsible for encoding DAHP synthase, transketolase, and chorismate mutase-prephenate dehydratase | Increased DAHP yield by 1.65 times higher than strain having PTS system L-Phe yield of 0.33 g/g glucose | [169] |
| Increasing the E4P pool to increase shikimic acid titer | E. coli | Increasing the E4P level by overexpression of the transketolase gene (tktA) | Increased shikimic acid titer from 38 to 52 g L−1 | [170] |
| Prevent carbon loss and further boost E4P supply | E. coli | Enhance PEP and E4P supply by deletion of the gene zwf1 (encoding glucose 6-phosphate dehydrogenase) and overexpression of gene tkl1 (encoding transketolase) | 14.3 g L−1 L-Trp within 68 h in a fed-batch process from glycerol on a 15 L scale | [171] |
| Enhance the E4P and balance supply between E4P and PEP | S. cerevisiae | Increase the level of E4P by overexpression of transketolase (Tkl1) and ribose-5-phosphate ketol-isomerase (Rki1) | Increased the titer of shikimic acid by 25% | [172] |
| Increase shikimate production | E. coli | Integrate multiple strategies:
| 126.4 g/L of shikimate with a yield of 0.50 g/g glucose and a productivity of 2.63 g/L/h in a 30-L fermenter, highest reported titer | [173] |
| Increase AAA level | E. coli | Overcoming feedback inhibition by construction of a tunable switch by addition/starvation of different inducers and by replacement of feedback sensitive gene with a feedback-resistant (aroGfbr, trpEfbr, and pheAfbr) | 0.32 g/L l-Trp, 0.60 g/L L-Phe, and 0.58 g/L l-L-Tyr | [161] |
| Increase AAA level | S. cerevisiae | Overcoming feedback inhibition by introducing feedback-insensitive DAHP synthase (Aro3fbr/Aro4fbr) and chorismate mutase (Aro7fbr) | 4.5-fold increase of the flux through the AAA biosynthetic pathway | [174] |
| Increase AAA level | Synechocystis | Overcoming feedback inhibition by engineering strain expressing the aroGfbr and tyrAfbr genes from E. coli | 903.8 ± 52.7 mg/gDW of L-Phe and 64.04 ± 3.67 mg/gDW of L-Tyr | [175] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| ID | Transcript ID | Predicted Sub-Cellular Localization | Reference |
|---|---|---|---|
| DAHP synthase | Cre17.g726750_4532.1 Cre17.g726750_4532.2 Cre17.g726750_4532.3 | Chloroplast a,b | [18,176] |
| DHQS | Cre08.g368950_4532.1 Cre08.g368950_4532.2 | Chloroplast a,b Cytosol a,b | |
| DHD/SDH | Cre08.g380201_4532.1 | Chloroplast a,b | |
| SK | Cre10.g436350_4532.1 Cre10.g436350_4532.2 | Chloroplast a Extracellular (Secreted) b | |
| EPSPS | Cre03.g181300_4532.1 | Chloroplast a,b, | |
| CS | Cre03.g145747_4532.1 Cre03.g145747_4532.2 | Mitochondria a Chloroplast a,b | |
| CM | Cre03.g155200_4532.1 Cre03.g155200_4532.2 Cre03.g155200_4532.3 | Chloroplast a,b, Cytosol b | |
| PPA-AT | Cre02.g147302_4532.1 | Chloroplast a,b | |
| ADT/PDT | Cre06.g261800_4532.1 | Chloroplast a,b | |
| ADH/PDH | Cre06.g278350_4532.1 Cre06.g278350_4532.2 | Chloroplast a,b Cytosolic a, Chloroplast b | |
| Phe hydroxylase | Cre01.g029250_4532.1 | Chloroplast a Extracellular (Secreted) b | |
| Asα | Cre06.g306601_4532.1 | Mitochondria a, chloroplast b | |
| Asβ | Cre14.g620300_4532.1 | Chloroplast a,b | |
| PAT | Cre10.g429150_4532.1 | Chloroplast a,b | |
| PAI | Cre12.g519000_4532.1 Cre12.g519000_4532.2 | Chloroplast a,b | |
| IGPS | Cre12.g528700_4532.1 | Mitochondria a Chloroplast b | |
| TSα | Cre12.g528700_4532.1 Cre12.g528700_4532.2 | Cytosol a,b | |
| TSβ | Cre03.g161400_4532.1 | Mitochondria a Chloroplast b | |
| Cationic amino acid transporter | Cre07.g329050_4532.1 Cre07.g329050_4532.2 Cre01.g041050_4532.1 Cre01.g041050_4532.2 Cre01.g041050_4532.3 Cre01.g041050_4532.4 | Plasma membrane a,b Vacuole a, plasma membrane b Mitochondria a, plasma membrane b Chloroplast a, Vacuole b, plasma membrane b Chloroplast a, Vacuole b, plasma membrane b Cytoplasm a, extracellular b | |
| Amino acid transporter transmembrane | Cre16.g801997_4532.1 | Nucleus a, Secreted b |
| ID | Transcript ID | Location | Ref |
|---|---|---|---|
| DAHPS | XP_002177054.1 | Chloroplast ab | [44] |
| DHQS | XP_002180805.1 Phatr3_J20809 | Chloroplast ab | [44] |
| DHQ/SDH | XP_002179655.1 | Chloroplast a, Secreted b | [44] |
| SK | XP_002184173.1 Phatr3_J6807 | Chloroplast a, Secreted b | [44] |
| EPSPS | XP_002178032.1 | Chloroplast ab | [44] |
| CS | XP_002177933.1 Phatr3_J43429 | Chloroplast a, Secreted b | [44] |
| CM | Phatr3_draftJ417 | Chloroplast a, cytosol b | [57] |
| CM | Phatr3_J43277 | Chloroplast a, cytosol b | [57] |
| PPA-AT | XP_002176258.1 | Chloroplast ab | [44] |
| PPY-AT | XP_002186145.1 | Chloroplast a, secreted b | [44] |
| ADT | XP_002181766.1 | Cytosol a, chloroplast b | [44] |
| ADT/PDT | EEC46980.1 Phatr3_J3267 | Cytosol a, chloroplast b | [44] |
| PDH | XP_002177542.1 | Plastid ab | [44] |
| PheH | XP_002181086.1 | Secreted b, nucleus a | [44] |
| AS | XP_002176337.1 Phatr3_Jdraft1682 | Plastid a, extracellular b | [44] |
| PAT | XP_002182064.1 | Chloroplast ab | [44] |
| PAI | XP_002179396.1 | Chloroplast a, extracellular b | [44] |
| IGPS | AAL79536.1 | Chloroplast ab | [177] |
| TS | XP_002176877.1 | Chloroplast ab | [44] |
| TSβ | XP_002182133.1 | Nucleus ab | [44] |
| Cationic amino acid transporter | B7G8I9_PHATC | Membrane b, plastid a | [44] |
| Amino acid transporter transmembrane | B7GA51_PHATC | Secreted b, plastid a | [44] |
References
- Grama, S.B.; Liu, Z.; Li, J. Emerging Trends in Genetic Engineering of Microalgae for Commercial Applications. Mar. Drugs 2022, 20, 285. [Google Scholar] [CrossRef]
- Villanova, V.; Spetea, C. Mixotrophy in diatoms: Molecular mechanism and industrial potential. Physiol. Plant 2021, 173, 603–611. [Google Scholar] [CrossRef]
- Kassaw, T.K.; Paton, A.J.; Peers, G. Episome-Based Gene Expression Modulation Platform in the Model Diatom Phaeodactylum tricornutum. ACS Synth. Biol. 2022, 11, 191–204. [Google Scholar] [CrossRef]
- Gong, Y.; Hu, H.; Gao, Y.; Xu, X.; Gao, H. Microalgae as platforms for production of recombinant proteins and valuable compounds: Progress and prospects. J. Ind. Microbiol. Biotechnol. 2011, 38, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Gimpel, J.A.; Hyun, J.S.; Schoepp, N.G.; Mayfield, S.P. Production of recombinant proteins in microalgae at pilot greenhouse scale. Biotechnol. Bioeng. 2015, 112, 339–345. [Google Scholar] [CrossRef]
- Ahmad, N.; Mehmood, M.A.; Malik, S. Recombinant protein production in microalgae: Emerging trends. Protein Pept. Lett. 2020, 27, 105–110. [Google Scholar] [CrossRef]
- León, R.; Couso, I.; Fernández, E. Metabolic engineering of ketocarotenoids biosynthesis in the unicelullar microalga Chlamydomonas reinhardtii. J. Biotechnol. 2007, 130, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Rathod, J.P.; Vira, C.; Lali, A.M.; Prakash, G. Metabolic engineering of Chlamydomonas reinhardtii for enhanced β-carotene and lutein production. Appl. Biochem. Biotechnol. 2020, 190, 1457–1469. [Google Scholar] [CrossRef]
- Zhao, M.-L.; Cai, W.-S.; Zheng, S.-Q.; Zhao, J.-L.; Zhang, J.-L.; Huang, Y.; Hu, Z.-L.; Jia, B. Metabolic engineering of the isopentenol utilization pathway enhanced the production of terpenoids in Chlamydomonas reinhardtii. Mar. Drugs 2022, 20, 577. [Google Scholar] [CrossRef]
- Banerjee, C.; Dubey, K.K.; Shukla, P. Metabolic engineering of microalgal based biofuel production: Prospects and challenges. Front. Microbiol. 2016, 7, 432. [Google Scholar] [CrossRef] [PubMed]
- Gangl, D.; Zedler, J.A.; Włodarczyk, A.; Jensen, P.E.; Purton, S.; Robinson, C. Expression and membrane-targeting of an active plant cytochrome P450 in the chloroplast of the green alga Chlamydomonas reinhardtii. Phytochemistry 2015, 110, 22–28. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, S.; Schiano di Visconte, G.; Lowe, G.; Szaub-Newton, J.; Beacham, T.; Landels, A.; Allen, M.J.; Spicer, A.; Matthijs, M. Engineering the unicellular alga Phaeodactylum tricornutum for high-value plant triterpenoid production. Plant Biotechnol. J. 2019, 17, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Gimpel, J.A.; Henriquez, V.; Mayfield, S.P. In Metabolic Engineering of Eukaryotic Microalgae: Potential and Challenges Come with Great Diversity. Front. Microbiol. 2015, 6, 1376. [Google Scholar] [CrossRef] [PubMed]
- Neupert, J.; Karcher, D.; Bock, R. Generation of Chlamydomonas strains that efficiently express nuclear transgenes. Plant J. 2009, 57, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Rochaix, J.-D. Chlamydomonas reinhardtii as the photosynthetic yeast. Annu. Rev. Genet. 1995, 29, 209–230. [Google Scholar] [CrossRef]
- Grossman, A.R.; Harris, E.E.; Hauser, C.; Lefebvre, P.A.; Martinez, D.; Rokhsar, D.; Shrager, J.; Silflow, C.D.; Stern, D.; Vallon, O.; et al. Chlamydomonas reinhardtii at the crossroads of genomics. Eukaryot. Cell 2003, 2, 1137–1150. [Google Scholar] [CrossRef]
- Darwish, R.; Gedi, M.A.; Akepach, P.; Assaye, H.; Zaky, A.S.; Gray, D.A. Chlamydomonas reinhardtii Is a Potential Food Supplement with the Capacity to Outperform Chlorella and Spirulina. Appl. Sci. 2020, 10, 6736. [Google Scholar] [CrossRef]
- Merchant, S.S.; Prochnik, S.E.; Vallon, O.; Harris, E.H.; Karpowicz, S.J.; Witman, G.B.; Terry, A.; Salamov, A.; Fritz-Laylin, L.K.; Marechal-Drouard, L.; et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 2007, 318, 245–250. [Google Scholar] [CrossRef]
- Blaby, I.K.; Blaby-Haas, C.E.; Tourasse, N.; Hom, E.F.; Lopez, D.; Aksoy, M.; Grossman, A.; Umen, J.; Dutcher, S.; Porter, M.; et al. The Chlamydomonas genome project: A decade on. Trends Plant Sci. 2014, 19, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Barahimipour, R.; Neupert, J.; Bock, R. Efficient expression of nuclear transgenes in the green alga Chlamydomonas: Synthesis of an HIV antigen and development of a new selectable marker. Plant Mol. Biol. 2016, 90, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.H. Chlamydomonas as a model organism. Annu. Rev. Plant Biol. 2001, 52, 363–406. [Google Scholar] [CrossRef]
- Jinkerson, R.E.; Jonikas, M.C. Molecular techniques to interrogate and edit the Chlamydomonas nuclear genome. Plant J. 2015, 82, 393–412. [Google Scholar] [CrossRef]
- Cohen, A.; Bruick, R.K.; Mayfield, S.P. Translational regulation of chloroplast gene expression in Chlamydomonas reinhardtii. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1998; Volume 297, pp. 192–208. [Google Scholar]
- Kindle, K.L. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. 1990, 87, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Kindle, K.L.; Schnell, R.A.; Fernández, E.; Lefebvre, P.A. Stable nuclear transformation of Chlamydomonas using the Chlamydomonas gene for nitrate reductase. J. Cell Biol. 1989, 109 Pt 1, 2589–2601. [Google Scholar] [CrossRef]
- Mayfield, S.P.; Kindle, K.L. Stable nuclear transformation of Chlamydomonas reinhardtii by using a C. reinhardtii gene as the selectable marker. Proc. Natl. Acad. Sci. 1990, 87, 2087–2091. [Google Scholar] [CrossRef] [PubMed]
- Mussgnug, J.H. Genetic tools and techniques for Chlamydomonas reinhardtii. Appl. Microbiol. Biotechnol. 2015, 99, 5407–5418. [Google Scholar] [CrossRef] [PubMed]
- Schroda, M. Good News for Nuclear Transgene Expression in Chlamydomonas. Cells 2019, 8, 1534. [Google Scholar] [CrossRef]
- Nguyen, T.H.T.; Park, S.; Jeong, J.; Shin, Y.S.; Sim, S.J.; Jin, E. Enhancing lipid productivity by modulating lipid catabolism using the CRISPR-Cas9 system in Chlamydomonas. J. Appl. Phycol. 2020, 32, 2829–2840. [Google Scholar] [CrossRef]
- Devadasu, E.; Subramanyam, R. Enhanced Lipid Production in Chlamydomonas reinhardtii Caused by Severe Iron Deficiency. Front. Plant Sci. 2021, 12, 615577. [Google Scholar] [CrossRef] [PubMed]
- Karpagam, R.; Preeti, R.; Ashokkumar, B.; Varalakshmi, P. Enhancement of lipid production and fatty acid profiling in Chlamydomonas reinhardtii, CC1010 for biodiesel production. Ecotoxicol. Environ. Saf. 2015, 121, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Rasala, B.A.; Muto, M.; Lee, P.A.; Jager, M.; Cardoso, R.M.; Behnke, C.A.; Kirk, P.; Hokanson, C.A.; Crea, R.; Mendez, M.; et al. Production of therapeutic proteins in algae, analysis of expression of seven human proteins in the chloroplast of Chlamydomonas reinhardtii. Plant Biotechnol. J. 2010, 8, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, S.P.; Franklin, S.E.; Lerner, R.A. Expression and assembly of a fully active antibody in algae. Proc. Natl. Acad. Sci. 2003, 100, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Amendola, S.; Kneip, J.S.; Meyer, F.; Perozeni, F.; Cazzaniga, S.; Lauersen, K.J.; Ballottari, M.; Baier, T. Metabolic Engineering for Efficient Ketocarotenoid Accumulation in the Green Microalga Chlamydomonas reinhardtii. ACS Synth. Biol. 2023, 12, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, J.; Baier, T.; Wentnagel, E.; Lauersen, K.J.; Kruse, O. Tailored carbon partitioning for phototrophic production of (E)-α-bisabolene from the green microalga Chlamydomonas reinhardtii. Metab. Eng. 2018, 45, 211–222. [Google Scholar] [CrossRef]
- Butler, T.; Kapoore, R.V.; Vaidyanathan, S. Phaeodactylum tricornutum: A Diatom Cell Factory. Trends Biotechnol. 2020, 38, 606–622. [Google Scholar] [CrossRef] [PubMed]
- Celi, C.; Fino, D.; Savorani, F. Phaeodactylum tricornutum as a source of value-added products: A review on recent developments in cultivation and extraction technologies. Bioresour. Technol. Rep. 2022, 19, 101–122. [Google Scholar] [CrossRef]
- Rebolloso-Fuentes, M.M.; Navarro-Pérez, A.; García-Camacho, F.; Ramos-Miras, J.J.; Guil-Guerrero, J.L. Biomass nutrient profiles of the microalga Nannochloropsis. J. Agric. Food Chem. 2001, 49, 2966–2972. [Google Scholar] [CrossRef]
- Slattery, S.S.; Diamond, A.; Wang, H.; Therrien, J.A.; Lant, J.T.; Jazey, T.; Lee, K.; Klassen, Z.; Desgagne-Penix, I.; Karas, B.J.; et al. An Expanded Plasmid-Based Genetic Toolbox Enables Cas9 Genome Editing and Stable Maintenance of Synthetic Pathways in Phaeodactylum tricornutum. ACS Synth. Biol. 2018, 7, 328–338. [Google Scholar] [CrossRef]
- Fantino, E.; Awwad, F.; Merindol, N.; Diaz Garza, A.M.; Gélinas, S.-E.; Gajón Robles, G.C.; Custeau, A.; Meddeb-Mouelhi, F.; Desgagné-Penix, I. Bioengineering Phaeodactylum tricornutum, a marine diatom, for cannabinoid biosynthesis. Algal Res. 2024, 77, 103379. [Google Scholar] [CrossRef]
- Fabris, M.; George, J.; Kuzhiumparambil, U.; Lawson, C.A.; Jaramillo-Madrid, A.C.; Abbriano, R.M.; Vickers, C.E.; Ralph, P. Extrachromosomal Genetic Engineering of the Marine Diatom Phaeodactylum tricornutum Enables the Heterologous Production of Monoterpenoids. ACS Synth. Biol. 2020, 9, 598–612. [Google Scholar] [CrossRef]
- Diamond, A.; Diaz-Garza, A.M.; Li, J.; Slattery, S.S.; Merindol, N.; Fantino, E.; Meddeb-Mouelhi, F.; Karas, B.J.; Barnabé, S.; Desgagné-Penix, I. Instability of extrachromosomal DNA transformed into the diatom Phaeodactylum tricornutum. Algal Res. 2023, 70, 102998. [Google Scholar] [CrossRef]
- Dhaouadi, F.; Awwad, F.; Diamond, A.; Desgagné-Penix, I. Diatoms’ Breakthroughs in Biotechnology: Phaeodactylum tricornutum as a Model for Producing High-Added Value Molecules. Am. J. Plant Sci. 2020, 11, 1632–1670. [Google Scholar] [CrossRef]
- Bowler, C.; Allen, A.E.; Badger, J.H.; Grimwood, J.; Jabbari, K.; Kuo, A.; Maheswari, U.; Martens, C.; Maumus, F.; Otillar, R.P.; et al. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 2008, 456, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lin, X.; Liu, X.; Zhang, J.; Ge, F. Genome Annotation of a Model Diatom Phaeodactylum tricornutum Using an Integrated Proteogenomic Pipeline. Mol. Plant 2018, 11, 1292–1307. [Google Scholar] [CrossRef]
- Filloramo, G.V.; Curtis, B.A.; Blanche, E.; Archibald, J.M. Re-examination of two diatom reference genomes using long-read sequencing. BMC Genom. 2021, 22, 379. [Google Scholar] [CrossRef] [PubMed]
- Giguere, D.J.; Bahcheli, A.T.; Slattery, S.S.; Patel, R.R.; Browne, T.S.; Flatley, M.; Karas, B.J.; Edgell, D.R.; Gloor, G.B. Telomere-to-telomere genome assembly of Phaeodactylum tricornutum. PeerJ 2022, 10, e13607. [Google Scholar] [CrossRef]
- Fabris, M.; Matthijs, M.; Carbonelle, S.; Moses, T.; Pollier, J.; Dasseville, R.; Baart, G.J.E.; Vyverman, W.; Goossens, A. Tracking the sterol biosynthesis pathway of the diatom Phaeodactylum tricornutum. New Phytol. 2014, 204, 521–535. [Google Scholar] [CrossRef]
- Hempel, F.; Bozarth, A.S.; Lindenkamp, N.; Klingl, A.; Zauner, S.; Linne, U.; Steinbüchel, A.; Maier, U.G. Microalgae as bioreactors for bioplastic production. Microb. Cell Fact. 2011, 10, 81. [Google Scholar] [CrossRef]
- Hempel, F.; Lau, J.; Klingl, A.; Maier, U.G. Algae as protein factories: Expression of a human antibody and the respective antigen in the diatom Phaeodactylum tricornutum. PLoS ONE 2011, 6, e28424. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Niu, Y.F.; Huang, T.; Yang, W.D.; Liu, J.S.; Li, H.Y. Genetic improvement of the microalga Phaeodactylum tricornutum for boosting neutral lipid accumulation. Metab. Eng. 2015, 27, 1–9. [Google Scholar] [CrossRef]
- Ma, Y.H.; Wang, X.; Niu, Y.F.; Yang, Z.K.; Zhang, M.H.; Wang, Z.M.; Yang, W.D.; Liu, J.S.; Li, H.Y. Antisense knockdown of pyruvate dehydrogenase kinase promotes the neutral lipid accumulation in the diatom Phaeodactylum tricornutum. Microb. Cell Fact. 2014, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.F.; Zhang, M.H.; Li, D.W.; Yang, W.D.; Liu, J.S.; Bai, W.B.; Li, H.Y. Improvement of neutral lipid and polyunsaturated fatty acid biosynthesis by overexpressing a type 2 diacylglycerol acyltransferase in marine diatom Phaeodactylum tricornutum. Mar. Drugs 2013, 11, 4558–4569. [Google Scholar] [CrossRef] [PubMed]
- Daboussi, F.; Leduc, S.; Marechal, A.; Dubois, G.; Guyot, V.; Perez-Michaut, C.; Amato, A.; Falciatore, A.; Juillerat, A.; Beurdeley, M.; et al. Genome engineering empowers the diatom Phaeodactylum tricornutum for biotechnology. Nat. Commun. 2014, 5, 3831. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Guo, X.; Wan, X.; Liang, Z.; Jiang, M. Characterization of a novel thioesterase (PtTE) from Phaeodactylum tricornutum. J. Basic. Microbiol. 2011, 51, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, S.; Wang, X.; Wang, H.L.; An, C.J.; Li, H.; Li, D.W.; Yang, W.D.; Liu, J.S.; Li, H.Y. Occurrence of plastidial triacylglycerol synthesis and the potential regulatory role of AGPAT in the model diatom Phaeodactylum tricornutum. Biotechnol. Biofuels 2017, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Levitan, O.; Dinamarca, J.; Zelzion, E.; Lun, D.S.; Guerra, L.T.; Kim, M.K.; Kim, J.; Van Mooy, B.A.S.; Bhattacharya, D.; Falkowski, P.G. Remodeling of intermediate metabolism in the diatom Phaeodactylum tricornutum under nitrogen stress. Proc. Natl. Acad. Sci. USA 2015, 112, 412–417. [Google Scholar] [CrossRef]
- Haslam, R.P.; Hamilton, M.L.; Economou, C.K.; Smith, R.; Hassall, K.L.; Napier, J.A.; Sayanova, O. Overexpression of an endogenous type 2 diacylglycerol acyltransferase in the marine diatom Phaeodactylum tricornutum enhances lipid production and omega-3 long-chain polyunsaturated fatty acid content. Biotechnol. Biofuels 2020, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhou, L.; Yang, W.; Wang, L.; Liu, X.; Gong, Y.; Hu, Q.; Wang, G. Overexpression of a novel gene (Pt2015) endows the commercial diatom Phaeodactylum tricornutum high lipid content and grazing resistance. Biotechnol. Biofuels Bioprod. 2022, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.H.; Zhang, R.H.; Lv, N.N.; Yang, G.P.; Wang, Y.S.; Pan, K.H. The Role of Malic Enzyme on Promoting Total Lipid and Fatty Acid Production in Phaeodactylum tricornutum. Front. Plant Sci. 2018, 9, 826. [Google Scholar] [CrossRef]
- Awwad, F.; Fantino, E.I.; Héneault, M.; Diaz-Garza, A.M.; Merindol, N.; Custeau, A.; Gélinas, S.E.; Meddeb-Mouelhi, F.; Li, J.; Lemay, J.F.; et al. Bioengineering of the Marine Diatom Phaeodactylum tricornutum with Cannabis Genes Enables the Production of the Cannabinoid Precursor, Olivetolic Acid. Int. J. Mol. Sci. 2023, 24, 16624. [Google Scholar] [CrossRef]
- Baker, C.M.; Grant, G.H. Role of aromatic amino acids in protein-nucleic acid recognition. Biopolymers 2007, 85, 456–470. [Google Scholar] [CrossRef]
- Han, Q.; Phillips, R.S.; Li, J. Editorial: Aromatic Amino Acid Metabolism. Front. Mol. Biosci. 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Stepanov, S.S.; Zolotareva, E.K. Methanol-induced stimulation of growth, intracellular amino acids, and protein content in Chlamydomonas reinhardtii. J. Appl. Phycol. 2015, 27, 1509–1516. [Google Scholar] [CrossRef]
- Tietel, Z.; Wikoff, W.R.; Kind, T.; Ma, Y.; Fiehn, O. Hyperosmotic stress in Chlamydomonas induces metabolomic changes in biosynthesis of complex lipids. Eur. J. Phycol. 2020, 55, 11–29. [Google Scholar] [CrossRef]
- Slaveykova, V.I.; Majumdar, S.; Regier, N.; Li, W.; Keller, A.A. Metabolomic responses of green alga Chlamydomonas reinhardtii exposed to sublethal concentrations of inorganic and methylmercury. Environ. Sci. Technol. 2021, 55, 3876–3887. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, C.; Fu, L.; Xu, L.; Cui, X.; Li, Q.; Crittenden, J.C. Responses of the Microalga Chlorophyta sp. to Bacterial Quorum Sensing Molecules (N-Acylhomoserine Lactones): Aromatic Protein-Induced Self-Aggregation. Env. Environ. Sci. Technol. 2017, 51, 3490–3498. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino Acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Schenck, C.A.; Holland, C.K.; Schneider, M.R.; Men, Y.; Lee, S.G.; Jez, J.M.; Maeda, H.A. Molecular basis of the evolution of alternative tyrosine biosynthetic routes in plants. Nat. Chem. Biol. 2017, 13, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Galili, G.; Aharoni, A. Shikimate Pathway and Aromatic Amino Acid Biosynthesis. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2012. [Google Scholar]
- Lynch, J.H.; Dudareva, N. Aromatic Amino Acids: A Complex Network Ripe for Future Exploration. Trends Plant Sci. 2020, 25, 670–681. [Google Scholar] [CrossRef]
- Kroth, P.G.; Chiovitti, A.; Gruber, A.; Martin-Jezequel, V.; Mock, T.; Parker, M.S.; Stanley, M.S.; Kaplan, A.; Caron, L.; Weber, T.; et al. A model for carbohydrate metabolism in the diatom Phaeodactylum tricornutum deduced from comparative whole genome analysis. PLoS ONE 2008, 3, e1426. [Google Scholar] [CrossRef]
- Sprenger, G.A. Aromatic Amino Acids. In Amino Acid Biosynthesis—Pathways, Regulation and Metabolic Engineering; Wendisch, V.F., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 93–127. [Google Scholar]
- Herrmann, K. The Shikimate Pathway: Early Steps in the Biosynthesis of Aromatic Compounds. Plant Cell 1995, 7, 907–919. [Google Scholar] [CrossRef]
- Konig, V.; Pfeil, A.; Braus, G.H.; Schneider, T.R. Substrate and metal complexes of 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase from Saccharomyces cerevisiae provide new insights into the catalytic mechanism. J. Mol. Biol. 2004, 337, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Gruenberg, M.; Irla, M.; Myllek, S.; Draths, K. Characterization of two 3-deoxy-d-Arabino-Heptulosonate 7-phosphate synthases from Bacillus methanolicus. Protein Expr. Purif. 2021, 188, 105972. [Google Scholar] [CrossRef]
- Ogino, T.; Garner, C.; Markley, J.L.; Herrmann, K.M. Biosynthesis of aromatic compounds: 13C NMR spectroscopy of whole Escherichia coli cells. Proc. Natl. Acad. Sci. 1982, 79, 5828–5832. [Google Scholar] [CrossRef]
- Yokoyama, R.; de Oliveira, M.V.V.; Kleven, B.; Maeda, H.A. The entry reaction of the plant shikimate pathway is subjected to highly complex metabolite-mediated regulation. Plant Cell 2021, 33, 671–696. [Google Scholar] [CrossRef] [PubMed]
- Mir, R.; Jallu, S.; Singh, T.P. The shikimate pathway: Review of amino acid sequence, function and three-dimensional structures of the enzymes. Crit. Rev. Microbiol. 2015, 41, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Deng, A.; Bai, H.; Yang, Z.; Liang, Y.; Liu, Z.; Qiu, Q.; Wang, L.; Liu, S.; Zhang, Y.; et al. Molecular basis for feedback inhibition of tyrosine-regulated 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase from Escherichia coli. J. Struct. Biol. 2019, 206, 322–334. [Google Scholar] [CrossRef]
- Jayaraman, K.; Trachtmann, N.; Sprenger, G.A.; Gohlke, H. Protein engineering for feedback resistance in 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase. Appl. Microbiol. Biotechnol. 2022, 106, 6505–6517. [Google Scholar] [CrossRef]
- Simpson, R.J.; Davidson, B.E. Studies on 3-deoxy-D-arabinoheptulosonate-7-phosphate synthetase(phe)from Escherichia coli K12. 2. Kinetic properties. Eur. J. Biochem. 1976, 70, 501–507. [Google Scholar] [CrossRef]
- Ramilo, C.A.; Evans, J.N. Overexpression, purification, and characterization of tyrosine-sensitive 3-deoxy-D-arabino-heptulosonic acid 7-phosphate synthase from Escherichia coli. Protein Expr. Purif. 1997, 9, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Paravicini, G.; Schmidheini, T.; Braus, G. Purification and properties of the 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase (phenylalanine-inhibitable) of Saccharomyces cerevisiae. Eur. J. Biochem. 1989, 186, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Schnappauf, G.; Hartmann, M.; Künzler, M.; Braus, G.H. The two 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase isoenzymes from Saccharomyces cerevisiae show different kinetic modes of inhibition. Arch. Microbiol. 1998, 169, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Webby, C.J.; Baker, H.M.; Lott, J.S.; Baker, E.N.; Parker, E.J. The Structure of 3-Deoxy-d-arabino-heptulosonate 7-phosphate Synthase from Mycobacterium tuberculosis Reveals a Common Catalytic Scaffold and Ancestry for Type I and Type II Enzymes. J. Mol. Biol. 2005, 354, 927–939. [Google Scholar] [CrossRef]
- Light, S.H.; Anderson, W.F. The diversity of allosteric controls at the gateway to aromatic amino acid biosynthesis. Protein Sci. 2013, 22, 395–404. [Google Scholar] [CrossRef]
- Kanaris, M.; Poulin, J.; Shahinas, D.; Johnson, D.; Crowley, V.M.; Fucile, G.; Provart, N.; Christendat, D. Elevated tyrosine results in the cytosolic retention of 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase in Arabidopsis thaliana. Plant J. 2022, 109, 789–803. [Google Scholar] [CrossRef]
- Neetu, N.; Katiki, M.; Dev, A.; Gaur, S.; Tomar, S.; Kumar, P. Structural and Biochemical Analyses Reveal that Chlorogenic Acid Inhibits the Shikimate Pathway. J. Bacteriol. 2020, 202, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, A.R.; Lamb, H.K.; Moore, J.D.; Charles, I.G.; Roberts, C.F. The pre-chorismate (shikimate) and quinate pathways in filamentous fungi: Theoretical and practical aspects. Microbiology 1993, 139, 2891–2899. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.A.; Christendat, D. Structure of Arabidopsis Dehydroquinate Dehydratase-Shikimate Dehydrogenase and Implications for Metabolic Channeling in the Shikimate Pathway. Biochemistry 2006, 45, 7787–7796. [Google Scholar] [CrossRef] [PubMed]
- de Avila, M.B.; de Azevedo, W.F., Jr. Development of machine learning models to predict inhibition of 3-dehydroquinate dehydratase. Chem. Biol. Drug Des. 2018, 92, 1468–1474. [Google Scholar] [CrossRef]
- Han, J.W.; Lee, K.P.; Yoon, M.; Kang, S.H.; Kim, G.H. Cold stress regulation of a bi-functional 3-dehydroquinate dehydratase/shikimate dehydrogenase (DHQ/SDH)-like gene in the freshwater green alga Spirogyra varians. Bot. Mar. 2009, 52, 178–185. [Google Scholar] [CrossRef]
- Díaz, J.; Merino, F. Wound-induced shikimate dehydrogenase and peroxidase related to lignification in pepper (Capsicum annuum L.) leaves. J. Plant Physiol. 1998, 152, 51–57. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, H. Engineering the shikimate pathway for biosynthesis of molecules with pharmaceutical activities in E. coli. Curr. Opin. Biotechnol. 2016, 42, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Widhalm, J.R.; Dudareva, N. A familiar ring to it: Biosynthesis of plant benzoic acids. Mol. Plant 2015, 8, 83–97. [Google Scholar] [CrossRef]
- Abell, C. 1.22—Enzymology and Molecular Biology of the Shikimate Pathway. In Comprehensive Natural Products Chemistry; Barton, S.D., Nakanishi, K., Meth-Cohn, O., Eds.; Pergamon: Oxford, UK, 1999; pp. 573–607. [Google Scholar]
- Herrmann, K.M.; Weaver, L.M. The shikimate pathway. Annu. Rev. Plant Biol. 1999, 50, 473–503. [Google Scholar] [CrossRef]
- Tohge, T.; Watanabe, M.; Hoefgen, R.; Fernie, A.R. Shikimate and phenylalanine biosynthesis in the green lineage. Front. Plant Sci. 2013, 4, 62. [Google Scholar] [CrossRef]
- Steinrücken, H.; Amrhein, N. The herbicide glyphosate is a potent inhibitor of 5-enolpyruvylshikimic acid-3-phosphate synthase. Biochem. Biophys. Res. Commun. 1980, 94, 1207–1212. [Google Scholar] [CrossRef]
- Anderson, K.S.; Johnson, K.A. Kinetic and structural analysis of enzyme intermediates: Lessons from EPSP synthase. Chem. Rev. 1990, 90, 1131–1149. [Google Scholar] [CrossRef]
- Knaggs, A.R. The biosynthesis of shikimate metabolites. Nat. Prod. Rep. 2000, 17, 269–292. [Google Scholar] [CrossRef] [PubMed]
- Macheroux, P.; Schmid, J.; Amrhein, N.; Schaller, A. A unique reaction in a common pathway: Mechanism and function of chorismate synthase in the shikimate pathway. Planta 1999, 207, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Kalra, I.; Wang, X.; Cvetkovska, M.; Jeong, J.; McHargue, W.; Zhang, R.; Hüner, N.; Yuan, J.S.; Morgan-Kiss, R. Chlamydomonas sp. UWO 241 Exhibits High Cyclic Electron Flow and Rewired Metabolism under High Salinity1 [OPEN]. Plant Physiol. 2020, 183, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Desgagné-Penix, I. Biosynthesis of alkaloids in Amaryllidaceae plants: A review. Phytochem. Rev. 2020, 20, 409–431. [Google Scholar] [CrossRef]
- Lee, A.Y. Structural Studies of Ligand-Protein Interactions in Trypsin and Chorismate Mutase. Ph.D. Thesis, Cornell University, New York, NY, USA, 1995. [Google Scholar]
- Khanapur, M.; Alvala, M.; Prabhakar, M.; Shiva Kumar, K.; Edwin, R.K.; Sri Saranya, P.S.; Patel, R.K.; Bulusu, G.; Misra, P.; Pal, M. Mycobacterium tuberculosis chorismate mutase: A potential target for TB. Bioorg Med. Chem. 2017, 25, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Westfall, C.S.; Xu, A.; Jez, J.M. Structural evolution of differential amino acid effector regulation in plant chorismate mutases. J. Biol. Chem. 2014, 289, 28619–28628. [Google Scholar] [CrossRef] [PubMed]
- Winston, D.S.; Gorman, S.D.; Boehr, D.D. Conformational Transitions in Yeast Chorismate Mutase Important for Allosteric Regulation as Identified by Nuclear Magnetic Resonance Spectroscopy. J. Mol. Biol. 2022, 434, 167531. [Google Scholar] [CrossRef] [PubMed]
- Pittard, J.; Yang, J. Biosynthesis of the Aromatic Amino Acids. EcoSal Plus 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Kroll, K.; Holland, C.K.; Starks, C.M.; Jez, J.M. Evolution of allosteric regulation in chorismate mutases from early plants. Biochem. J. 2017, 474, 3705–3717. [Google Scholar] [CrossRef] [PubMed]
- Sträter, N.; Schnappauf, G.; Braus, G.; Lipscomb, W.N. Mechanisms of catalysis and allosteric regulation of yeast chorismate mutase from crystal structures. Structure 1997, 5, 1437–1452. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Lonergan, S.G.; Conn, E.E. Chorismate Mutase Isoenzymes from Selected Plants and Their Immunological Comparison with the Isoenzymes from Sorghum bicolor. Plant Physiol. 1986, 81, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Woodin, T.S.; Nishioka, L.; Hsu, A. Comparison of chorismate mutase isozyme patterns in selected plants. Plant Physiol. 1978, 61, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.H. Revisiting the dual pathway hypothesis of Chorismate production in plants. Hortic. Res. 2022, 9, uhac052. [Google Scholar] [CrossRef] [PubMed]
- Abolhassani Rad, S.; Clayton, E.J.; Cornelius, E.J.; Howes, T.R.; Kohalmi, S.E. Moonlighting proteins: Putting the spotlight on enzymes. Plant Signal Behav. 2018, 13, e1517075. [Google Scholar] [CrossRef]
- Wang, M.; Toda, K.; Maeda, H.A. Biochemical properties and subcellular localization of tyrosine aminotransferases in Arabidopsis thaliana. Phytochemistry 2016, 132, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Thorbjørnsrud, H.V.; Bressan, L.; Khatanbaatar, T.; Carrer, M.; Würth-Roderer, K.; Cordara, G.; Kast, P.; Cascella, M.; Krengel, U. What Drives Chorismate Mutase to Top Performance? Insights from a Combined In Silico and In Vitro Study. Biochemistry 2023, 62, 782–796. [Google Scholar] [CrossRef]
- Yoo, H.; Widhalm, J.R.; Qian, Y.; Maeda, H.; Cooper, B.R.; Jannasch, A.S.; Gonda, I.; Lewinsohn, E.; Rhodes, D.; Dudareva, N. An alternative pathway contributes to phenylalanine biosynthesis in plants via a cytosolic tyrosine:phenylpyruvate aminotransferase. Nat. Commun. 2013, 4, 2833. [Google Scholar] [CrossRef] [PubMed]
- El-Azaz, J.; de la Torre, F.; Avila, C.; Canovas, F.M. Identification of a small protein domain present in all plant lineages that confers high prephenate dehydratase activity. Plant J. 2016, 87, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, T.A.; Schimmel, B.C.; Kim, J.Y.; Reinhardt, D.; Cline, K.; Clark, D.G. A petunia chorismate mutase specialized for the production of floral volatiles. Plant J. 2010, 61, 145–155. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qian, Y.; Lynch, J.H.; Guo, L.; Rhodes, D.; Morgan, J.A.; Dudareva, N. Completion of the cytosolic post-chorismate phenylalanine biosynthetic pathway in plants. Nat. Commun. 2019, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Schnappauf, G.; Krappmann, S.; Braus, G.H. Tyrosine and tryptophan act through the same binding site at the dimer interface of yeast chorismate mutase. J. Biol. Chem. 1998, 273, 17012–17017. [Google Scholar] [CrossRef]
- Krappmann, S.; Pries, R.; Gellissen, G.; Hiller, M.; Braus, G.H. HARO7 encodes chorismate mutase of the methylotrophic yeast Hansenula polymorpha and is derepressed upon methanol utilization. J. Bacteriol. 2000, 182, 4188–4197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krappmann, S.; Helmstaedt, K.; Gerstberger, T.; Eckert, S.; Hoffmann, B.; Hoppert, M.; Schnappauf, G.; Braus, G.H. The aroC gene of Aspergillus nidulans codes for a monofunctional, allosterically regulated chorismate mutase. J. Biol. Chem. 1999, 274, 22275–22282. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, F.; Medina-Morales, B.; Blanca-Reyes, I.; Pascual, M.B.; Ávila, C.; Cánovas, F.M.; Castro-Rodríguez, V. Properties and Functional Analysis of Two Chorismate Mutases from Maritime Pine. Cells 2024, 13, 929. [Google Scholar] [CrossRef] [PubMed]
- Dornfeld, C.; Weisberg, A.J.; K, C.R.; Dudareva, N.; Jelesko, J.G.; Maeda, H.A. Phylobiochemical characterization of class-Ib aspartate/prephenate aminotransferases reveals evolution of the plant arogenate phenylalanine pathway. Plant Cell 2014, 26, 3101–3114. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Yoo, H.; Dudareva, N. Prephenate aminotransferase directs plant phenylalanine biosynthesis via arogenate. Nat. Chem. Biol. 2011, 7, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Corea, O.R.; Bedgar, D.L.; Davin, L.B.; Lewis, N.G. The arogenate dehydratase gene family: Towards understanding differential regulation of carbon flux through phenylalanine into primary versus secondary metabolic pathways. Phytochemistry 2012, 82, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Shasany, A.K.; Schnepp, J.; Orlova, I.; Taguchi, G.; Cooper, B.R.; Rhodes, D.; Pichersky, E.; Dudareva, N. RNAi suppression of Arogenate Dehydratase1 reveals that phenylalanine is synthesized predominantly via the arogenate pathway in petunia petals. Plant Cell 2010, 22, 832–849. [Google Scholar] [CrossRef] [PubMed]
- Pribat, A.; Noiriel, A.; Morse, A.M.; Davis, J.M.; Fouquet, R.; Loizeau, K.; Ravanel, S.; Frank, W.; Haas, R.; Reski, R.; et al. Nonflowering plants possess a unique folate-dependent phenylalanine hydroxylase that is localized in chloroplasts. Plant Cell 2010, 22, 3410–3422. [Google Scholar] [CrossRef] [PubMed]
- Rippert, P.; Matringe, M. Purification and kinetic analysis of the two recombinant arogenate dehydrogenase isoforms of Arabidopsis thaliana. Eur. J. Biochem. 2002, 269, 4753–4761. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Toda, K.; Block, A.; Maeda, H.A. TAT1 and TAT2 tyrosine aminotransferases have both distinct and shared functions in tyrosine metabolism and degradation in Arabidopsis thaliana. J. Biol. Chem. 2019, 294, 3563–3576. [Google Scholar] [CrossRef]
- Tzin, V.; Galili, G. New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef]
- Huccetogullari, D.; Luo, Z.W.; Lee, S.Y. Metabolic engineering of microorganisms for production of aromatic compounds. Microb. Cell Fact. 2019, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.P.; Niu, F.X.; Yan, Z.B.; Fong, L.S.; Huang, Y.B.; Liu, J.Z. Recent Advances in Metabolically Engineered Microorganisms for the Production of Aromatic Chemicals Derived from Aromatic Amino Acids. Front. Bioeng. Biotechnol. 2020, 8, 407. [Google Scholar] [CrossRef]
- Braga, A.; Faria, N. Biotechnological production of specialty aromatic and aromatic-derivative compounds. World J. Microbiol. Biotechnol. 2022, 38, 80. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, C.; Yang, J.; Nian, R.; Xian, M.; Li, F.; Zhang, H. Common problems associated with the microbial productions of aromatic compounds and corresponding metabolic engineering strategies. Biotechnol. Adv. 2020, 41, 107548. [Google Scholar] [CrossRef]
- Guo, L.; Ding, S.; Liu, Y.; Gao, C.; Hu, G.; Song, W.; Liu, J.; Chen, X.; Liu, L. Enhancing tryptophan production by balancing precursors in Escherichia coli. Biotechnol. Bioeng. 2022, 119, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Gosset, G. Improvement of Escherichia coli production strains by modification of the phosphoenolpyruvate: Sugar phosphotransferase system. Microb. Cell Factories 2005, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M. Sugar transport systems in Corynebacterium glutamicum: Features and applications to strain development. Appl. Microbiol. Biotechnol. 2012, 96, 1191–1200. [Google Scholar] [CrossRef]
- Yi, J.; Draths, K.; Li, K.; Frost, J. Altered glucose transport and shikimate pathway product yields in E. coli. Biotechnol. Prog. 2003, 19, 1450–1459. [Google Scholar] [CrossRef]
- Elston, R.; Mulligan, C.; Thomas, G.H. Flipping the switch: Dynamic modulation of membrane transporter activity in bacteria. Microbiology 2023, 169, 001412. [Google Scholar] [CrossRef]
- Wu, S.; Chen, W.; Lu, S.; Zhang, H.; Yin, L. Metabolic engineering of shikimic acid biosynthesis pathway for the production of shikimic acid and its branched products in microorganisms: Advances and prospects. Molecules 2022, 27, 4779. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xiao, Q.; Wu, X.; Ma, H.; Li, J.; Guo, X.; Liu, Z.; Zhang, Y.; Luo, Y. Mechanistic investigation of a D to N mutation in DAHP synthase that dictates carbon flux into the shikimate pathway in yeast. Commun. Chem. 2023, 6, 152. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Matsuoka, Y. Feedback regulation and coordination of the main metabolism for bacterial growth and metabolic engineering for amino acid fermentation. Biotechnol. Adv. 2022, 55, 107887. [Google Scholar] [CrossRef]
- Rodriguez, A.; Martnez, J.A.; Flores, N.; Escalante, A.; Gosset, G.; Bolivar, F. Engineering Escherichia coli to overproduce aromatic amino acids and derived compounds. Microb. Cell Factories 2014, 13, 126. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Y.; Ding, D.; Wen, J.; Zhu, B.; Zhang, D. Genetic engineering of Escherichia coli to improve L-phenylalanine production. BMC Biotechnol. 2018, 18, 5. [Google Scholar] [CrossRef]
- Chen, M.; Liang, H.; Han, C.; Zhou, P.; Xing, Z.; Chen, Q.; Liu, Y.; Xie, G.A.; Xie, R. Engineering of global transcription factor FruR to redirect the carbon flow in Escherichia coli for enhancing L-phenylalanine biosynthesis. Microb. Cell Fact. 2022, 21, 222. [Google Scholar] [CrossRef] [PubMed]
- Kohlstedt, M.; Starck, S.; Barton, N.; Stolzenberger, J.; Selzer, M.; Mehlmann, K.; Schneider, R.; Pleissner, D.; Rinkel, J.; Dickschat, J.S.; et al. From lignin to nylon: Cascaded chemical and biochemical conversion using metabolically engineered Pseudomonas putida. Metab. Eng. 2018, 47, 279–293. [Google Scholar] [CrossRef]
- Deng, X.; Cai, J.; Li, Y.; Fei, X. Expression and knockdown of the PEPC1 gene affect carbon flux in the biosynthesis of triacylglycerols by the green alga Chlamydomonas reinhardtii. Biotechnol. Lett. 2014, 36, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Cravens, A.; Payne, J.; Smolke, C.D. Synthetic biology strategies for microbial biosynthesis of plant natural products. Nat. Commun. 2019, 10, 2142. [Google Scholar] [CrossRef]
- Moroney, J.V.; Ynalvez, R.A. Proposed carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii. Eukaryot. Cell 2007, 6, 1251–1259. [Google Scholar] [CrossRef]
- Johnson, X.; Alric, J. Central carbon metabolism and electron transport in Chlamydomonas reinhardtii: Metabolic constraints for carbon partitioning between oil and starch. Eukaryot. Cell 2013, 12, 776–793. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ding, D.; Wang, H.; Liu, L.; Fang, H.; Chen, T.; Zhang, D. Engineering Escherichia coli to improve tryptophan production via genetic manipulation of precursor and cofactor pathways. Synth. Syst. Biotechnol. 2020, 5, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Wulf, D.; Krüger, F.J.; Klages, L.J.; Viehöver, P.; Jin, E.; Wobbe, L.; Eisenhut, M.; Kruse, O.; Blifernez-Klassen, O.; Bräutigam, A. Multiple transcription factors mediate acclimation of Chlamydomonas to light stress. bioRxiv 2023. [Google Scholar] [CrossRef]
- Liu, L.; Duan, X.; Wu, J. Modulating the direction of carbon flow in Escherichia coli to improve l-tryptophan production by inactivating the global regulator FruR. J. Biotechnol. 2016, 231, 141–148. [Google Scholar] [CrossRef]
- Dhakarey, R.; Yaritz, U.; Tian, L.; Amir, R. A Myb transcription factor, PgMyb308-like, enhances the level of shikimate, aromatic amino acids, and lignins, but represses the synthesis of flavonoids and hydrolyzable tannins, in pomegranate (Punica granatum L.). Hortic. Res. 2022, 9, uhac008. [Google Scholar] [CrossRef]
- Hu, C.; Jiang, P.; Xu, J.; Wu, Y.; Huang, W. Mutation analysis of the feedback inhibition site of phenylalanine-sensitive 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase of Escherichia coli. J. Basic. Microbiol. 2003, 43, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Jossek, R.; Bongaerts, J.; Sprenger, G.A. Characterization of a new feedback-resistant 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase AroF of Escherichia coli. FEMS Microbiol. Lett. 2001, 202, 145–148. [Google Scholar] [CrossRef]
- Liu, X.; Niu, H.; Huang, Z.; Li, Q.; Gu, P. Construction of a switchable synthetic Escherichia coli for aromatic amino acids by a tunable switch. J. Ind. Microbiol. Biotechnol. 2020, 47, 233–242. [Google Scholar] [CrossRef]
- Niu, H.; Li, R.; Liang, Q.; Qi, Q.; Li, Q.; Gu, P. Metabolic engineering for improving L-tryptophan production in Escherichia coli. J. Ind. Microbiol. Biotechnol. 2019, 46, 55–65. [Google Scholar] [CrossRef]
- Guo, X.; Wu, X.; Ma, H.; Liu, H.; Luo, Y. Yeast: A platform for the production of (L)-tyrosine derivatives. Yeast 2023, 40, 214–230. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Malitsky, S.; Aharoni, A.; Galili, G. Expression of a bacterial bi-functional chorismate mutase/prephenate dehydratase modulates primary and secondary metabolism associated with aromatic amino acids in Arabidopsis. Plant J. 2009, 60, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.; Guy, A.; Galili, G.; Dor, E.; Schweitzer, R.; Amir, R.; Hacham, Y. Enhanced Production of Aromatic Amino Acids in Tobacco Plants Leads to Increased Phenylpropanoid Metabolites and Tolerance to Stresses. Front. Plant Sci. 2020, 11, 604349. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Rogachev, I.; Meir, S.; Moyal Ben Zvi, M.; Masci, T.; Vainstein, A.; Aharoni, A.; Galili, G. Tomato fruits expressing a bacterial feedback-insensitive 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase of the shikimate pathway possess enhanced levels of multiple specialized metabolites and upgraded aroma. J. Exp. Bot. 2013, 64, 4441–4452. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.; Bar, E.; Ovadia, R.; Perl, A.; Galili, G.; Lewinsohn, E.; Oren-Shamir, M. Phenylpyruvate Contributes to the Synthesis of Fragrant Benzenoid-Phenylpropanoids in Petunia x hybrida Flowers. Front. Plant Sci. 2017, 8, 769. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.H.; Qian, Y.; Guo, L.; Maoz, I.; Huang, X.Q.; Garcia, A.S.; Louie, G.; Bowman, M.E.; Noel, J.P.; Morgan, J.A.; et al. Modulation of auxin formation by the cytosolic phenylalanine biosynthetic pathway. Nat. Chem. Biol. 2020, 16, 850–856. [Google Scholar] [CrossRef]
- Báez-Viveros, J.L.; Flores, N.; Juárez, K.; Castillo-España, P.; Bolivar, F.; Gosset, G. Metabolic transcription analysis of engineered Escherichia coli strains that overproduce L-phenylalanine. Microb. Cell Fact. 2007, 6, 30. [Google Scholar] [CrossRef]
- Knop, D.R.; Draths, K.M.; Chandran, S.S.; Barker, J.L.; von Daeniken, R.; Weber, W.; Frost, J.W. Hydroaromatic equilibration during biosynthesis of shikimic acid. J. Am. Chem. Soc. 2001, 123, 10173–10182. [Google Scholar] [CrossRef] [PubMed]
- Tröndle, J.; Schoppel, K.; Bleidt, A.; Trachtmann, N.; Sprenger, G.A.; Weuster-Botz, D. Metabolic control analysis of L-tryptophan production with Escherichia coli based on data from short-term perturbation experiments. J. Biotechnol. 2020, 307, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Suástegui, M.; Guo, W.; Feng, X.; Shao, Z. Investigating strain dependency in the production of aromatic compounds in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2016, 113, 2676–2685. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, C.; Ye, C.; Guo, L.; Liu, J.; Chen, X.; Song, W.; Wu, J.; Liu, L. Systems engineering of Escherichia coli for high-level shikimate production. Metab. Eng. 2023, 75, 1–11. [Google Scholar] [CrossRef]
- Luttik, M.A.; Vuralhan, Z.; Suir, E.; Braus, G.H.; Pronk, J.T.; Daran, J.M. Alleviation of feedback inhibition in Saccharomyces cerevisiae aromatic amino acid biosynthesis: Quantification of metabolic impact. Metab. Eng. 2008, 10, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Brey, L.F.; Wlodarczyk, A.J.; Bang Thofner, J.F.; Burow, M.; Crocoll, C.; Nielsen, I.; Zygadlo Nielsen, A.J.; Jensen, P.E. Metabolic engineering of Synechocystis sp. PCC 6803 for the production of aromatic amino acids and derived phenylpropanoids. Metab. Eng. 2020, 57, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Craig, R.J.; Gallaher, S.D.; Shu, S.; Salomé, P.A.; Jenkins, J.W.; Blaby-Haas, C.E.; Purvine, S.O.; O’Donnell, S.; Barry, K.; Grimwood, J.; et al. The Chlamydomonas Genome Project, version 6: Reference assemblies for mating-type plus and minus strains reveal extensive structural mutation in the laboratory. Plant Cell 2022, 35, 644–672. [Google Scholar] [CrossRef] [PubMed]
- Lippmeier, J.C.; Brown, A.M.; Apt, K.E. Isolation of algal genes by functional complementation of yeast. J. Phycol. 2002, 38, 529–533. [Google Scholar] [CrossRef]
| Product | Application | Yield | Strategy/Method | Ref |
|---|---|---|---|---|
| Lipids | Biofuel production | Oleic acid (C18:1) increased by 27.2%; total lipid accumulated up to 28% of dried biomass | CRISPR-Cas9 technology generated esterase/lipase/thioesterase ELT1 knockout (Cre01.g00030) mutants (nuclear transformation via electroporation in strain CC-4349). | [29] |
| 4-fold increase in lipid content as compared to control | Severe iron deficiency induced triacylglycerols accumulation (modified media in strain CC-125). | [30] | ||
| 2.34-fold increase in lipid content; more than 80% of total SFA (saturated fatty acid) and MUFA (mono-unsaturated fatty acid) content | Nutrient starved (nitrogen, phosphorous), glucose supplementation (0.1%) increased lipid content and appropriate profile for biodiesel production (media modification in CC1010 strain). | [31] | ||
| Therapeutic protein: fourteenth human fibronectin type III domain, human vascular endothelial growth factor isoform121, and high mobility group protein B1 | Pharmaceutical | 2–3% of total soluble protein | Potential human therapeutic protein production (tenth human fibronectin type III domain, fourteenth human fibronectin type III domain, human vascular endothelial growth factor isoform121, and high mobility group protein B1) by recombinant DNA technology (chloroplast transformation by particle bombardment in strain 137c). | [32] |
| Antibodies: monoclonal antibody directed against a glycoprotein of the herpes simplex virus D (HSV8). | Biotechnological | High levels of protein accumulation | Expression of a large single chain coding sequence, IgA heavy chain protein fused to the light chain by a flexible linker peptide (chloroplast transformation by particle bombardment in strain 137c). | [33] |
| Antigens: HIV antigen P24 | Biotechnological | 0.25% of total soluble proteins (TSP) | Expression of codon-optimized the HIV antigen P24 gene variant (nuclear transformation via glass bead method in strain Elow47 and UVM11). | [20] |
| Carotenoids: CrtYB (phytoene–β-carotene synthase—PBS) gene Production of ‘Asthaxanthin’ | Pharmaceutical | B-carotene:22.8 mg g−1 and Lutein: 8.9 mg g−1 up to 4.3 mg/L/day | Heterologous expression of phytoene–β-carotene synthase gene from red yeast Xanthophyllomyces dendrorhous. (nuclear transformation using chloroplast transit peptide via particle bombardment in strain CC-124). Synthetic redesign of ß-carotene ketolase gene, avoiding bottlenecking phytoene synthase and increasing activity of ß-carotene hydroxylase (Electroporation transformation in strain CC-125) | [8] [34] |
| Terpenoids (E)-α-bisabolene, the sesquiterpene biodiesel precursor | Sustainable energy production | 10.3 ± 0.7 mg g−1 DCW of (E)-α-bisabolene 11.0 mg L−1) titer of (E)-α-bisabolene ((under light/dark cycle) | Overexpression of Abies grandis bisabolene synthase gene; downregulation of competing pathways via amiRNA knockdown and modified culture conditions (Glass bead transformation in strain UVM4) | [35] |
| Product | Application of Products | Yield | Methods | Ref |
|---|---|---|---|---|
| Lipid | Biofuel | 2.5-fold more lipid production, 57.8% DW | Overexpression of the endogenous P. tricornutum malic enzyme, transformed via electroporation. | [51] |
| 82% increase in the lipid production | Knockdown of pyruvate dehydrogenase kinase, transformed via electroporation. | [52] | ||
| 35% increase in neutral lipid accumulation, 76% increase in the valuable omega-3, eicosapentaenoic acid (EPA) | Overexpression of the endogenous diacylglycerol acyltransferase 2, transformed via electroporation. | [53] | ||
| 45-fold increase in triacylglycerol accumulation | Modification of the genome of the P. tricornutum, disruption of the UDP-glucose pyrophosphorylase gene using meganucleases and transcription activator-like effector nucleases. | [54] | ||
| Enhanced total fatty acid (C18:0 and C18:1) content by 72% | Overexpression of P. tricornutum thioesterase, transformed using microparticle bombardment. | [55] | ||
| Increased TAG content by 1.81-fold with a significant increase in polyunsaturated fatty acids | Overexpression of 1-acyl-sn-glycerol-3-phosphate acyltransferase designated AGPAT1, transformed via electroporation. | [56] | ||
| 43% increase in cellular lipid content | Knocked down the gene encoding for nitrate reductase, transformed via biolistic transformation. | [57] | ||
| 2–3-fold increase in TAG production | Overexpression of an endogenous type 2 diacylglycerol acyltransferase, transformed via biolistic transformation. | [58] | ||
| Lipids increased by 30%, and 95% of the population changed the morphotype from fusiform to triradiate | Overexpression of a novel gene (Pt2015), transformed via biolistic transformation. | [59] | ||
| Increased 23.19 and 25.32% in SFAs and between 49.02 and 54.04% in PUFAs | Overexpression of the endogenous P. tricornutum malic enzyme, transformed via biolistic transformation. | [60] | ||
| Geraniol | Pharmaceutical application (key intermediate in the biosynthesis of monoterpenoid indole alkaloids (MIAs)) | Geraniol titer of 0.309 mg/L | Engineering P. tricornutum through extrachromosomal, episome-based expression for the heterologous biosynthesis of geraniol, transformed by bacterial conjugation. | [41] |
| Plant triterpenoids (Betulin, Lupeol) | Pharmaceutical application (antiprotozoal, antimicrobial, antitumor, precursor for the treatment of certain cancers and HIV) | Successful production of betulin and its precursor lupeol (0.1 mg/L over 2 days of culturing). | Introducing three plant enzymes in P. tricornutum: a Lotus japonicus oxidosqualene cyclase (lupeol synthase) and a Medicago truncatula cytochrome P450 along with its native reductase, transformed via biolistic transformation. | [12] |
| CBGA | Pharmaceutical application (precursor to several cannabinoids (CB) such as well-known cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC)) | Production of cannabigerolic acid (CBGA) up to 4.1 (±0.2) mg/kg of microalgae fresh biomass weight. | Engineering P. tricornutum to express a mutant version of the Streptomyces sp. NphB, a non-cannabis aromatic prenyltransferase enzyme, either by random integrated chromosomal expression (RICE) or extrachromosomal expression (EE). | [40] |
| Olivetolic acid (OA) | Pharmaceutical application (cannabinoid precursor) | Successful integration and functionality of the heterologous cannabis genes TKS and OAC, and significant olivetolic acid accumulation (0.6–2.6 mg/L). | Engineering P. tricornutum through the introduction of C. sativa tetraketide synthase and olivetolic acid cyclase, olivetolic acid. Transformed via bacterial conjugation. | [61] |
| Antibodies | Pharmaceutical application (Hepatitis B vaccine) | Antibody concentration about 8.7% of total soluble protein, which complies with 21 mg antibody per gram algal DW or 400 mg antibody in a 250 mL culture. | Heterologous expression of a fully assembled human IgG antibody against Hepatitis B surface antigen in P. tricornutum, transformed with biolistic transformation. | [50] |
| Polyhydroxybutyrate (PHB) | Biodegradable plastics | Sufficient production in PHB levels of up to 10.6% of algal DW. | Introducing the bacterial PHB pathway of R. eutropha H16. The enzymes PhaA (ketothiolase), PhaB (acetoacetyl-CoA reductase), and PhaC (PHB synthase) were expressed with stable nuclear transformation. | [49] |
| Species | Isozymes | Inhibitors | Substrate | Km [mM] | Ref |
|---|---|---|---|---|---|
| E. coli | AroG | Phe | PEP E4P | 0.08 0.9 | [82] |
| AroF AroH | Tyr Trp | PEP E4P | 0.013 0.0814 | [83] | |
| S. cerevisiae | Aro3 | Phe | PEP E4P | 0.018 0.13 | [84] |
| Aro4 | Tyr | PEP E4P | 0.125 0.5 | [85] | |
| Mycobacterium tuberculosis | mtDAHPS | Phe | PEP E4P | 0.025 0.037 | [86] |
| Arabidopsis thaliana | DHS1 | Chorismate; Caffeate | PEP E4P | 0.25 2.842 | [78] |
| DHS2 | Tyr, Trp, Chorismate; Caffeate | PEP E4P | 0.36 1.755 | ||
| DHS3 | Chorismate; Caffeate | PEP E4P | 0.706 1.55 |
| Enzyme | Species | kcat (s−1) | Km (mM) | kcat/Km (M/s) | Ref |
|---|---|---|---|---|---|
| AtCM1 | Arabidopsis thaliana | 16 | 0.55 | 29,090 | [108] |
| AtCM2 | Arabidopsis thaliana | 39 | 0.15 | 260,000 | [108] |
| AtCM3 | Arabidopsis thaliana | 13 | 1.10 | 11,818 | [108] |
| PhCM1 | Petunia hybrida | 25 | 0.174 | 143,678 | [122] |
| PhCM2 | Petunia hybrida | 64 | 0.009 | 7,136,000 | [122] |
| PpCM1 | Physcomitrella patens | 21 | 2.39 | 8660 | [111] |
| PpCM2 | Physcomitrella patens | 19.5 | 2.33 | 8370 | [111] |
| SmCM | Selaginella moellendorffii | 18.8 | 5.19 | 3620 | [111] |
| ScCM | Saccharomyces cerevisiae | 360 | 3.8 | 94,736 | [123] |
| HpCM | Hansenula polymorpha | 319.1 | 16.7 | 19,107 | [124] |
| AnCM | Aspergillus nidulans | 82 | 2.3 | 35,652 | [125] |
| PpCM1 | Pinus pinaster | 29.4 | 1.6 | 18.4 | [126] |
| PpCM2 | Pinus pinaster | 35 | 1.7 | 20.6 | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niraula, A.; Danesh, A.; Merindol, N.; Meddeb-Mouelhi, F.; Desgagné-Penix, I. Aromatic Amino Acids: Exploring Microalgae as a Potential Biofactory. BioTech 2025, 14, 6. https://doi.org/10.3390/biotech14010006
Niraula A, Danesh A, Merindol N, Meddeb-Mouelhi F, Desgagné-Penix I. Aromatic Amino Acids: Exploring Microalgae as a Potential Biofactory. BioTech. 2025; 14(1):6. https://doi.org/10.3390/biotech14010006
Chicago/Turabian StyleNiraula, Archana, Amir Danesh, Natacha Merindol, Fatma Meddeb-Mouelhi, and Isabel Desgagné-Penix. 2025. "Aromatic Amino Acids: Exploring Microalgae as a Potential Biofactory" BioTech 14, no. 1: 6. https://doi.org/10.3390/biotech14010006
APA StyleNiraula, A., Danesh, A., Merindol, N., Meddeb-Mouelhi, F., & Desgagné-Penix, I. (2025). Aromatic Amino Acids: Exploring Microalgae as a Potential Biofactory. BioTech, 14(1), 6. https://doi.org/10.3390/biotech14010006






