Identifying Antimicrobial Agents from Chlorella sorokiniana: A Biotechnological Approach Utilizing Eco-Friendly Extraction and Characterization Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgae Strain Identification and Growth Conditions
2.2. Preparation of Extracts
2.3. Antimicrobial Tests of the Extracts
2.3.1. Agar Well Diffusion Method
2.3.2. Broth Dilution Method
2.4. Extract Characterization—GC-MS Analysis
Mean minus U-CLF = Total chromatogram mean − Normalized U-CLF
2.5. Statistical Analysis and PCA
3. Results and Discussion
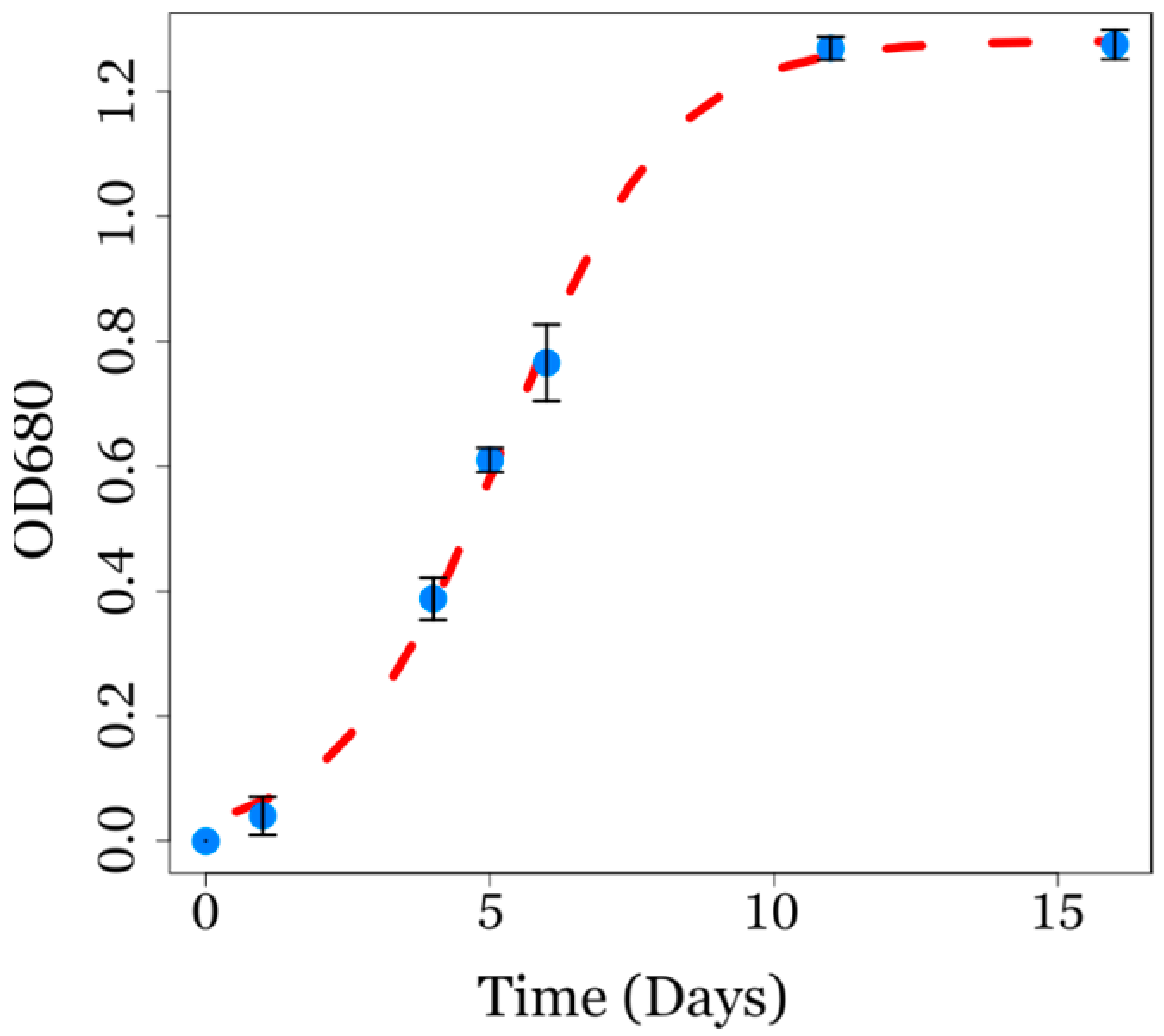
3.1. Microalgae Biomass Production
3.2. Extract Preparations
3.2.1. Extract Preparation from Pre-Treated Microalgae Biomass and Influence of the Solvents on Chlorophyll Removal
3.2.2. Extracts from Untreated Microalgae Biomass
3.2.3. Assessment of Extract Sustainability
3.3. Antimicrobial Tests of the Extracts
3.3.1. Agar Well Diffusion Method
3.3.2. Broth Dilution Method
3.4. Extract Characterization
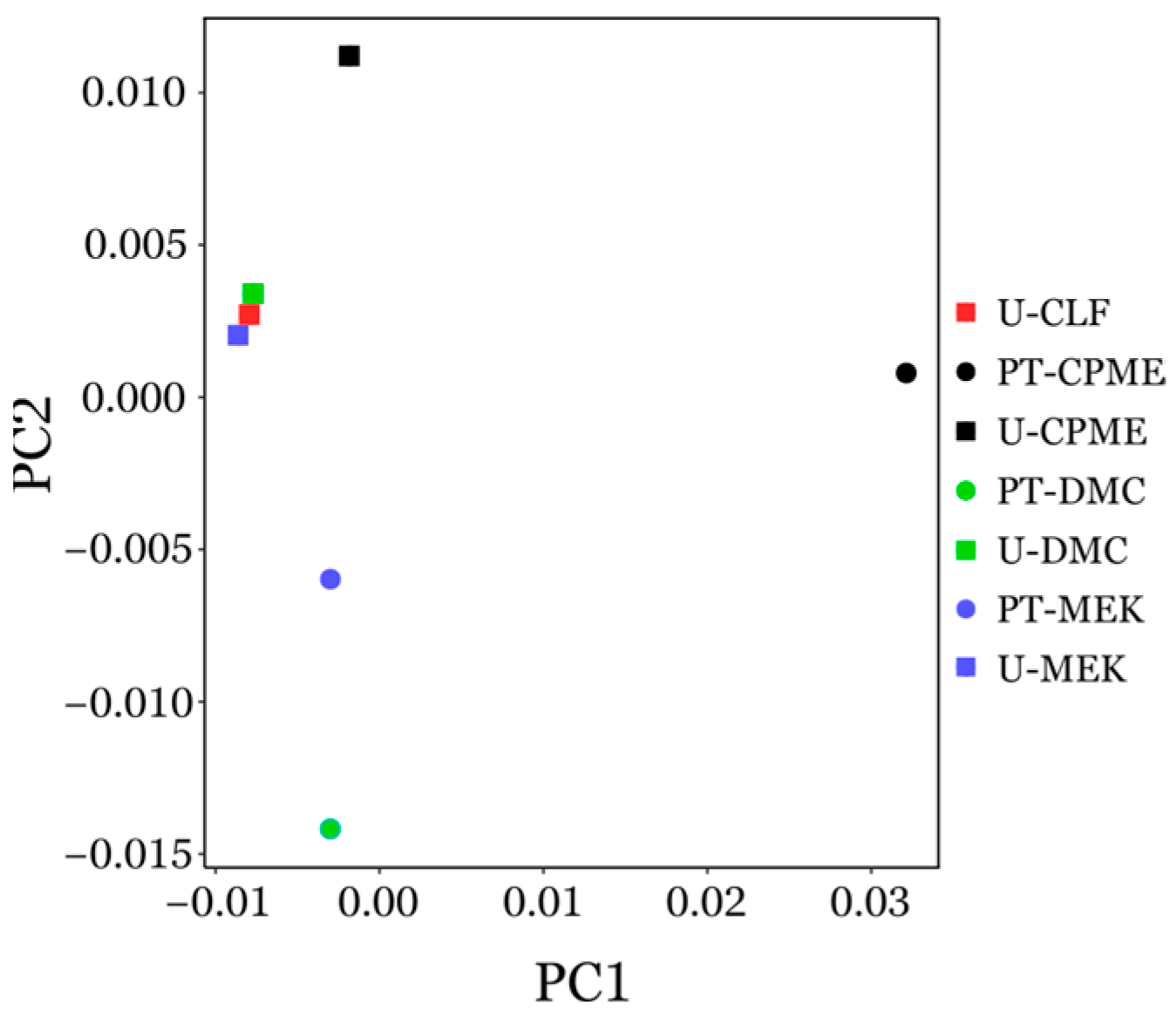
3.4.1. Principal Component Analysis (PCA) of GC-MS Chromatogram
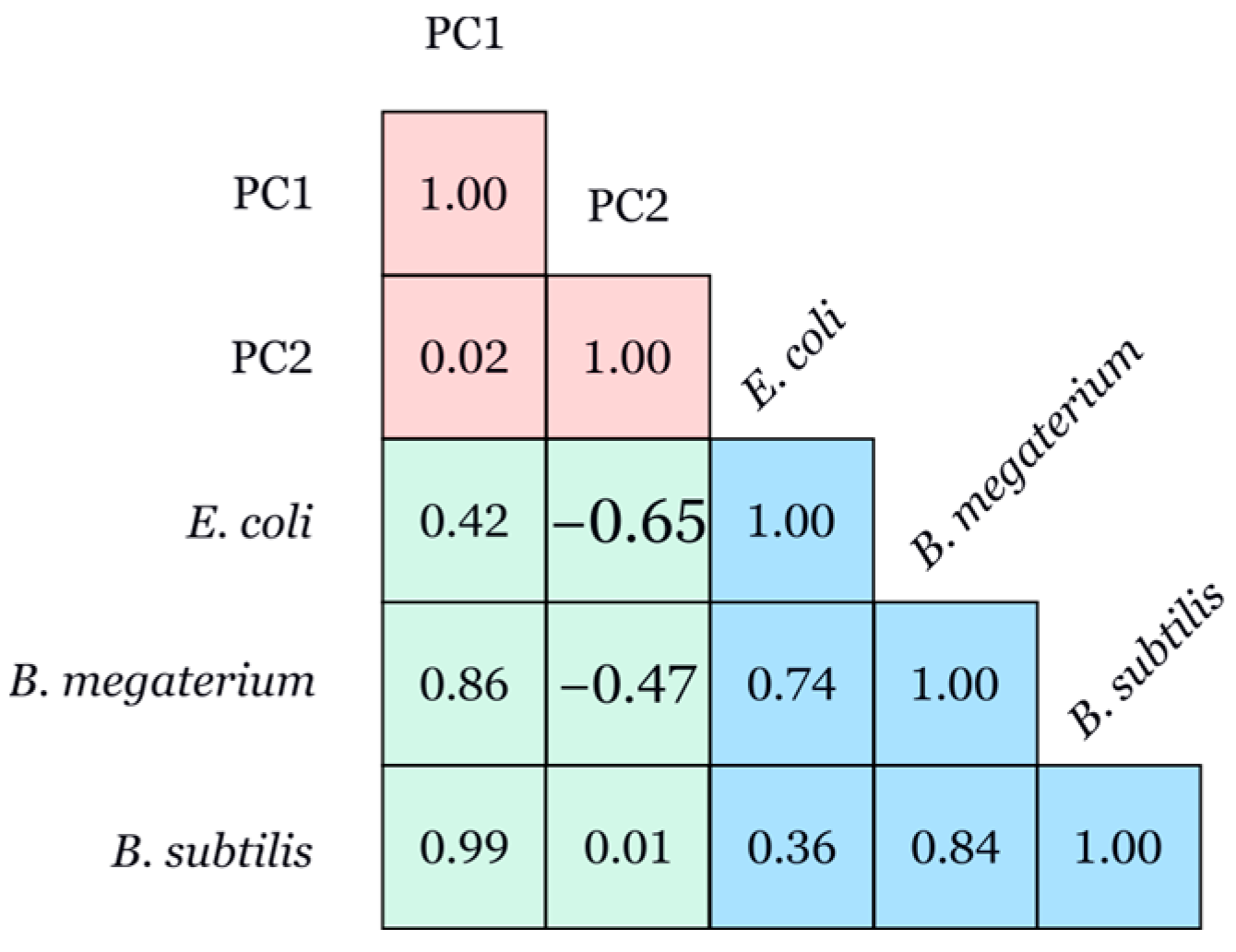
3.4.2. Pearson Correlation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, F.; Akhlaq, A.; Rasool, M.H.; Srinuanpan, S. Cyanobacterial Bioactive Compounds: Synthesis, Extraction, and Applications. In Pharmaceutical and Nutraceutical Potential of Cyanobacteria; Springer International Publishing: Cham, Switzerland, 2024; pp. 215–243. [Google Scholar] [CrossRef]
- Ljubic, A.; Holdt, S.L.; Jakobsen, J.; Bysted, A.; Jacobsen, C. Fatty acids, carotenoids, and tocopherols from microalgae: Targeting the accumulation by manipulating the light during growth. J. Appl. Phycol. 2021, 33, 2783–2793. [Google Scholar] [CrossRef]
- Barone, G.D.; Cernava, T.; Ullmann, J.; Liu, J.; Lio, E.; Germann, A.T.; Nakielski, A.; Russo, D.A.; Chavkin, T.; Knufmann, K.; et al. Recent developments in the production and utilization of photosynthetic microorganisms for food applications. Heliyon 2023, 9, e14708. [Google Scholar] [CrossRef]
- Fu, W.; Nelson, D.; Yi, Z.; Xu, M.; Khraiwesh, B.; Jijakli, K.; Chaiboonchoe, A.; Alzahmi, A.; Al-Khairy, D.; Brynjolfsson, S.; et al. Bioactive Compounds from Microalgae: Current Development and Prospects. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 54, pp. 199–225. [Google Scholar]
- Zuorro, A.; Lavecchia, R.; Contreras-Ropero, J.E.; Martínez, J.B.G.; Barajas-Ferreira, C.; Barajas-Solano, A.F. Natural Antimicrobial Agents from Algae: Current Advances and Future Directions. Int. J. Mol. Sci. 2024, 25, 11826. [Google Scholar] [CrossRef]
- Reynolds, D.; Huesemann, M.; Edmundson, S.; Sims, A.; Hurst, B.; Cady, S.; Beirne, N.; Freeman, J.; Berger, A.; Gao, S. Viral inhibitors derived from macroalgae, microalgae, and cyanobacteria: A review of antiviral potential throughout pathogenesis. Algal Res. 2021, 57, 102331. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.R.; Spínola, M.P.; Lordelo, M.; Prates, J.A.M. Chemical Compounds, Bioactivities, and Applications of Chlorella vulgaris in Food, Feed and Medicine. Appl. Sci. 2024, 14, 10810. [Google Scholar] [CrossRef]
- Temnov, M.S.; Ustinskaya, Y.V.; Meronyuk, K.I.; Dvoretsky, D.S. Biological Activity of Endometabolites of Microalgae Chlorella sorokiniana. Ind. Biotechnol. 2024, 20, 67–76. [Google Scholar] [CrossRef]
- Usman, M.; Nakagawa, M.; Cheng, S. Emerging Trends in Green Extraction Techniques for Bioactive Natural Products. Processes 2023, 11, 3444. [Google Scholar] [CrossRef]
- Martins, R.; Barbosa, A.; Advinha, B.; Sales, H.; Pontes, R.; Nunes, J. Green Extraction Techniques of Bioactive Compounds: A State-of-the-Art Review. Processes 2023, 11, 2255. [Google Scholar] [CrossRef]
- Alfieri, M.; Mascheretti, I.; Kentsop, R.A.D.; Mattana, M.; Laura, M.; Ottolina, G. Optimized Ultrasound-Assisted Extraction of Lignans from Linum Species with Green Solvents. Molecules 2022, 27, 2732. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Rutkowska, M.; Owczarek, K.; Tobiszewski, M.; Namieśnik, J. Extraction with environmentally friendly solvents. TrAC Trends Anal. Chem. 2017, 91, 12–25. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, L.; Mei, L.; Kanda, H. Extraction and Separation of Natural Products from Microalgae and Other Natural Sources Using Liquefied Dimethyl Ether, a Green Solvent: A Review. Foods 2024, 13, 352. [Google Scholar] [CrossRef] [PubMed]
- Pyo, S.-H.; Park, J.H.; Chang, T.-S.; Hatti-Kaul, R. Dimethyl carbonate as a green chemical. Curr. Opin. Green Sustain. Chem. 2017, 5, 61–66. [Google Scholar] [CrossRef]
- Shah, P.; Parikh, S.; Shah, M.; Dharaskar, S. A holistic review on application of green solvents and replacement study for conventional solvents. Biomass-Convers. Biorefinery 2022, 12, 1985–1999. [Google Scholar] [CrossRef]
- Errichiello, F.; Cucciniello, R.; Tomasini, M.; Falivene, L.; Gambuti, A.; Cassiano, C.; Forino, M. Efficient and selective extraction of oleanolic acid from grape pomace with dimethyl carbonate. Green Chem. 2024, 26, 10177–10188. [Google Scholar] [CrossRef]
- Ambriz-Pérez, D.L.; Luna-Avelar, K.D.; de Jesús Gárate-Osuna, A.; Betancourt-Lozano, M.; Santos-Ballardo, D.U. Bioactive Compounds Extraction from Microalgae by Biotechnological Processes. In Bioactive Compounds Extraction from Marine Resources and Wastes; Springer: Singapore, 2025; pp. 91–123. [Google Scholar] [CrossRef]
- Songserm, R.; Kaeoboon, S.; Suksungworn, R.; Duangsrisai, S.; Sanevas, N. GC-MS profiling, anti-oxidant and anti-diabetic assessments of extracts from microalgae Scenedesmus falcatus (KU.B1) and Chlorella sorokiniana (KU.B2). Plant Sci. Today 2022, 9, 632–641. [Google Scholar] [CrossRef]
- Karamizadeh, S.; Abdullah, S.M.; Manaf, A.A.; Zamani, M.; Hooman, A. An Overview of Principal Component Analysis. J. Signal Inf. Process. 2013, 4, 173–175. [Google Scholar] [CrossRef]
- Kirrolia, A.; Bishnoi, N.; Singh, R. Effect of shaking, incubation temperature, salinity and media composition on growth traits of green microalgae Chlorococcum sp. J. Algal Biomass Util. 2012, 3, 46–53. [Google Scholar]
- Tundo, P.; Selva, M. The Chemistry of Dimethyl Carbonate. Accounts Chem. Res. 2002, 35, 706–716. [Google Scholar] [CrossRef]
- Santoro, I.; Nardi, M.; Benincasa, C.; Costanzo, P.; Giordano, G.; Procopio, A.; Sindona, G. Sustainable and Selective Extraction of Lipids and Bioactive Compounds from Microalgae. Molecules 2019, 24, 4347. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; McElroy, C.R.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 7. [Google Scholar] [CrossRef]
- Yew, G.Y.; Chew, K.W.; Malek, M.A.; Ho, Y.-C.; Chen, W.-H.; Ling, T.C.; Show, P.L. Hybrid liquid biphasic system for cell disruption and simultaneous lipid extraction from microalgae Chlorella sorokiniana CY-1 for biofuel production. Biotechnol. Biofuels 2019, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xu, J.; Wu, H.; Wang, G.; Dai, S.; Fan, J.; He, H.; Xiang, W. A Saponification Method for Chlorophyll Removal from Microalgae Biomass as Oil Feedstock. Mar. Drugs 2016, 14, 162. [Google Scholar] [CrossRef] [PubMed]
- Phaisan, S.; Yusakul, G.; Sakdamas, A.; Taluengjit, N.; Sakamoto, S.; Putalun, W. A green and effective method using oils to remove chlorophyll from Chromolaena odorata (L.) R.M. King & H. Rob. Songklanakarin J. Sci. Technol. 2020, 42, 1084–1090. [Google Scholar]
- Sheldon, R.A. The E Factor: Fifteen years on. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Valgas, C.; De Souza, S.M.; Smânia, E.F.A.; Smânia, A., Jr. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef]
- Rubin, J.E. Antimicrobial Susceptibility Testing Methods and Interpretation of Results. In Antimicrobial Therapy in Veterinary Medicine; Wiley: Hoboken, NJ, USA, 2013; pp. 11–20. [Google Scholar] [CrossRef]
- Mansjö, M.; Espinosa-Gongora, C.; Samanci, I.; Groenheit, R.; Werngren, J. Performance of a broth microdilution assay for routine minimum inhibitory concentration determination of 14 anti-tuberculous drugs against the Mycobacterium tuberculosis complex based on the EUCAST reference protocol. Antimicrob. Agents Chemother. 2025, 69, e0094624. [Google Scholar] [CrossRef]
- Lee, M. Data analysis with RStudio: An easygoing introduction by Franz Kronthaler and Silke Zöllner Berlin, Germany: Springer-Verlag GmbH, DE, 2021. pp. 131. Biometrics 2021, 77, 1502–1503. [Google Scholar] [CrossRef]
- Tallarida, R.J.; Murray, R.B. Duncan Multiple Range Test. In Manual of Pharmacologic Calculations; Springer: New York, NY, USA, 1987; pp. 125–127. [Google Scholar] [CrossRef]
- Cuevas, A.; Febrero, M.; Fraiman, R. An anova test for functional data. Comput. Stat. Data Anal. 2004, 47, 111–122. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; Downey, G.; Rawson, A.; Warriner, K.; Gernigon, G. Application of principal component and hierarchical cluster analysis to classify fruits and vegetables commonly consumed in Ireland based on in vitro antioxidant activity. J. Food Compos. Anal. 2011, 24, 250–256. [Google Scholar] [CrossRef]
- Kumar, V. Evaluation of Various Growth Conditions for the Cultivation of Microalgae Species in the Arid Regions. J. Mech. Cont. Math. Sci. 2019, 4, 255–265. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Aguilar-Hernandez, I.; Cardenas-Chavez, D.L.; Ornelas-Soto, N.; Romero-Ogawa, M.A.; Parra-Saldivar, R. Extraction and purification of high-value metabolites from microalgae: Essential lipids, astaxanthin and phycobiliproteins. Microb. Biotechnol. 2015, 8, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Jalalizadeh, M.; Zhang, Q. Growth kinetic models for microalgae cultivation: A review. Algal Res. 2015, 12, 497–512. [Google Scholar] [CrossRef]
- Sriragavi, G.; Sangeetha, M.; Santhakumar, M.; Lokesh, E.; Nithyalakshmi, M.; Saleel, C.A.; Balagurunathan, R. Exploring Antibacterial Properties of Bioactive Compounds Isolated from Streptomyces sp. in Bamboo Rhizosphere Soil. ACS Omega 2023, 8, 36333–36343. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Nobre, B.P.; Ertekin, F.; Hayes, M.; Garciá-Vaquero, M.; Vieira, F.; Koc, M.; Gouveia, L.; Aires-Barros, M.R.; Palavra, A.M.F. Extraction of value-added compounds from microalgae. In Microalgae-Based Biofuels and Bioproducts; Elsevier: Amsterdam, The Netherlands, 2017; pp. 461–483. [Google Scholar] [CrossRef]
- Gallego, R.; Montero, L.; Cifuentes, A.; Ibáñez, E.; Herrero, M. Green Extraction of Bioactive Compounds from Microalgae. J. Anal. Test. 2018, 2, 109–123. [Google Scholar] [CrossRef]
- Sarkar, S.; Manna, M.S.; Bhowmick, T.K.; Gayen, K. Priority-based multiple products from microalgae: Review on techniques and strategies. Crit. Rev. Biotechnol. 2020, 40, 590–607. [Google Scholar] [CrossRef]
- Svenning, J.B.; Dalheim, L.; Vasskog, T.; Matricon, L.; Vang, B.; Olsen, R.L. Lipid yield from the diatom Porosira glacialis is determined by solvent choice and number of extractions, independent of cell disruption. Sci. Rep. 2020, 10, 22229. [Google Scholar] [CrossRef]
- Ahmed, E.A.; Elenin, S.A.; Hamouda, A.; Mahamoud, H. Solvent Extraction Techniques of Lipid from Algal species in Wastewater Treatment Station. Egypt. J. Chem. 2019, 62, 1951–1962. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor at 30: A passion for pollution prevention. Green Chem. 2023, 25, 1704–1728. [Google Scholar] [CrossRef]
- Shaima, A.F.; Yasin, N.H.M.; Ibrahim, N.; Takriff, M.S.; Gunasekaran, D.; Ismaeel, M.Y. Unveiling antimicrobial activity of microalgae Chlorella sorokiniana (UKM2), Chlorella sp. (UKM8) and Scenedesmus sp. (UKM9). Saudi J. Biol. Sci. 2022, 29, 1043–1052. [Google Scholar] [CrossRef]
- Pasalari, H.; Gharibi, H.; Darvishali, S.; Farzadkia, M. The effects of different pretreatment technologies on microbial community in anaerobic digestion process: A systematic review. J. Environ. Health Sci. Eng. 2024, 22, 439–453. [Google Scholar] [CrossRef]
- Ebbensgaard, A.; Mordhorst, H.; Aarestrup, F.M.; Hansen, E.B. The Role of Outer Membrane Proteins and Lipopolysaccharides for the Sensitivity of Escherichia coli to Antimicrobial Peptides. Front. Microbiol. 2018, 9, 2153. [Google Scholar] [CrossRef] [PubMed]
- Choi, U.; Lee, C.-R. Distinct Roles of Outer Membrane Porins in Antibiotic Resistance and Membrane Integrity in Escherichia coli. Front. Microbiol. 2019, 10, 953. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; van Staden, J. Bioprospecting for bioactive compounds in microalgae: Antimicrobial compounds. Biotechnol. Adv. 2022, 59, 107977. [Google Scholar] [CrossRef]
- Fornazier, M.; de Oliveira Rodrigues, P.; Pasquini, D.; Baffi, M.A. Effects of Alkaline Pretreatment with Sodium Hydroxide with and Without Anthraquinone on the Enzymatic Hydrolysis of Corncob and Corn Stover and Ethanol Production. Waste Biomass Valoriz. 2024, in press. [Google Scholar] [CrossRef]
- Effects of Sodium Hydroxide Pretreatment on Structural Components of Biomass. Trans. ASABE 2014, 57, 1187–1198. [CrossRef]
- Santhakumari, S.; Jayakumar, R.; Logalakshmi, R.; Prabhu, N.M.; Nazar, A.K.A.; Pandian, S.K.; Ravi, A.V. In vitro and in vivo effect of 2,6-Di-tert-butyl-4-methylphenol as an antibiofilm agent against quorum sensing mediated biofilm formation of Vibrio spp. Int. J. Food Microbiol. 2018, 281, 60–71. [Google Scholar] [CrossRef]



| Extraction Methods | E-Factor (mg Waste/mg Extract) 1 |
|---|---|
| PT-DMC | 21.9 |
| U-DMC | 17.4 |
| PT-CPME | 28.7 |
| U-CPME | 7.7 |
| PT-CLF | 49.9 |
| U-CLF | 12.4 |
| PT-MEK | 12.8 |
| U-MEK | 12.2 |
| Extracted Sample | MIC (µg/mL) | ||
|---|---|---|---|
| E. coli | B. megaterium | B. subtilis | |
| PT-DMC | 118.02 ± 6.17 | 356.52 ± 109.46 | 63.91 ± 32.81 |
| U-DMC | 29.74 ± 12.57 | 22.02 ± 1.45 | 17.18 ± 2.67 |
| PT-CPME | 139.28 ± 34.35 | 638.87 ± 8.69 | 716.12 ± 1.62 |
| U-CPME | 4.89 ± 0.05 | 38.05 ± 9.17 | 43.11 ± 17.18 |
| PT-MEK | 207.37 ± 6.67 | 280.92 ± 27.10 | 29.05 ± 1.19 |
| U-MEK | 22.68 ± 2.24 | 4.18 ± 0.42 | 2.39 ± 1.05 |
| PT-CLF | 860.63 ± 1.90 | 750.60 ± 3.61 | 7490 ± 3186 a |
| U-CLF | 32.66 ± 8.51 | 37.58 ± 0.32 | 25.79 ± 0.28 |
| VAN b | 1.55 ± 0.03 | 36.25 ± 1.09 | 2.63 ± 0.23 |
| RT | Compound 1 |
|---|---|
| 6.00 | Unknown |
| 6.71 | Unknown |
| 9.71 | Unknown |
| 10.45 | Unknown |
| 12.29 | 2,6-ditert-butylcyclohexa-2,5-diene-1,4-dione |
| 12.42 | 2,6-ditert-butyl-4-hydroxy-4-methylcyclohexa-2,5-dien-1-one |
| 13.00 | 2,6-ditert-butyl-4-methylphenol |
| 13.15 | Unknown |
| 13.80 | Unknown |
| 16.16 | Unknown |
| 16.78 | Unknown |
| 18.99 | Unknown |
| 19.48 | Unknown |
| 21.94 | Unknown |
| 27.48 | bis(2-ethylhexyl) benzene-1,4-dicarboxylate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lio, E.; Dramis, M.; Ottolina, G.; Secundo, F. Identifying Antimicrobial Agents from Chlorella sorokiniana: A Biotechnological Approach Utilizing Eco-Friendly Extraction and Characterization Methods. BioTech 2025, 14, 22. https://doi.org/10.3390/biotech14010022
Lio E, Dramis M, Ottolina G, Secundo F. Identifying Antimicrobial Agents from Chlorella sorokiniana: A Biotechnological Approach Utilizing Eco-Friendly Extraction and Characterization Methods. BioTech. 2025; 14(1):22. https://doi.org/10.3390/biotech14010022
Chicago/Turabian StyleLio, Elia, Martina Dramis, Gianluca Ottolina, and Francesco Secundo. 2025. "Identifying Antimicrobial Agents from Chlorella sorokiniana: A Biotechnological Approach Utilizing Eco-Friendly Extraction and Characterization Methods" BioTech 14, no. 1: 22. https://doi.org/10.3390/biotech14010022
APA StyleLio, E., Dramis, M., Ottolina, G., & Secundo, F. (2025). Identifying Antimicrobial Agents from Chlorella sorokiniana: A Biotechnological Approach Utilizing Eco-Friendly Extraction and Characterization Methods. BioTech, 14(1), 22. https://doi.org/10.3390/biotech14010022








