Phytohormonal Regulation of Abiotic Stress Tolerance, Leaf Senescence and Yield Response in Field Crops: A Comprehensive Review
Abstract
1. Introduction
2. The Molecular Basis of Phytohormone Regulation Activity
2.1. Cytokinins
2.2. Abscisic Acid
2.3. Ethylene
2.4. Gibberellic Acid
2.5. Salicylic Acid
2.6. Jasmonic Acid
3. Phytohormone Regulation of Field Crop Responses to Abiotic Stresses
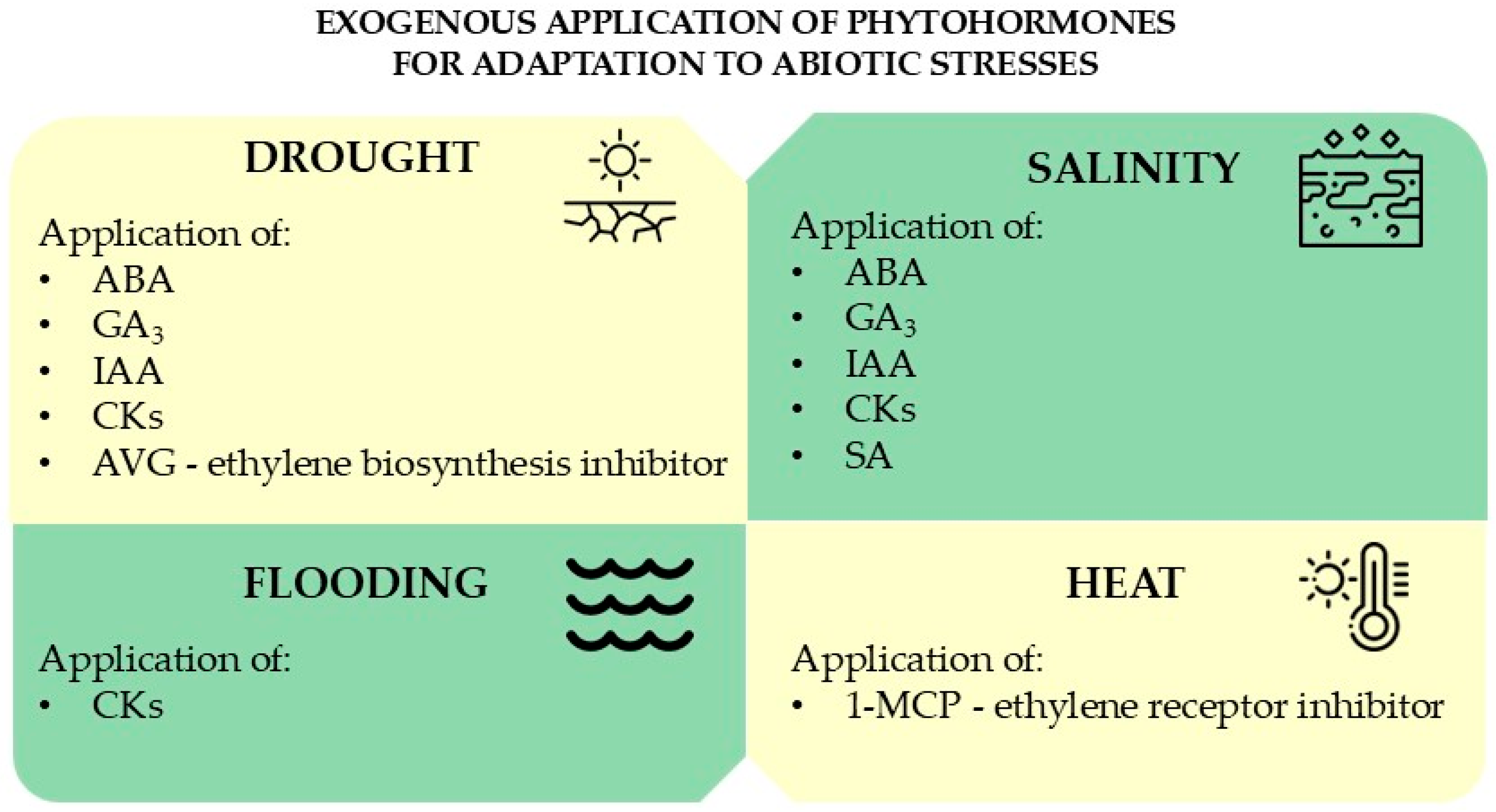
4. Exogeneous Phytohormone Application for Adaptation to Abiotic Stress
4.1. Phytohormone Application and Drought
| Phytohormone | Abiotic Stress Response Regulation of Plants | Effect of Exogenous Phytohormone Application on Crop Response to Abiotic Stress | |
|---|---|---|---|
| DROUGHT | SALINITY | ||
| Cytokinin | Decreased CK levels during abiotic stress occurance. Transient elevation of CK levels with short-term stress [72]. Regulate shoot and root growth ratio. Modulate leaf enzymatic antioxidant activities [74]. Crosstalk with ABA: to regulate stress-response signaling and root growth [11]; reduction in CK content led to ABA hypersensitivity. | Extended maintenance of photosynthesis. Increased leaf relative water content (LRWC), chlorophyll content, membrane stability, root to shoot biomass ratio [15,64]. Decreased lipid peroxidation and induction of antioxidant enzyme activity [126]. | Contrasting results have been reported [62]. |
| Abscisic acid | Increased ABA levels during abiotic stress occurrence. Responsible for stomatal closure, regulation of transpiration and osmotic processes [31]. Maintenance of shoot growth in long-term responses to drought [83]. Induced leaf proline accumulation. Increased xylem water potential and ion accumulation cell vacuoles of roots during salinity events [71]. | Prolonged canopy greenness. Increased chlorophyll and carotene content [6]. Maintenance of photosynthetic membrane integrity [122]. Decreased lipid peroxidation. Higher leaf conductance as long-term effect. | Reduced shoot Na+ and increased K+ concentration [112]. |
| Ethylene | Increased ethylene release associated with stress symptoms. Crosstalk with ABA: shoot and root growth regulation; ethylene production regulated by ABA levels [97]. | Application of ethylene inhibitors. Contrasting results: seldom completely reverses the effects of drought [86,127]. | |
| Gibberellins | Increased levels during abiotic stress occurrence. Maintainance of plant growth [62,100]. | Increased chlorophyll content and LRWC [102,127]. | Positive effects on both cell division and elongation [117]. |
| Salicylic acid | Increased SA levels during abiotic stress occurrence. Enhanced antioxidative capacity, accumulation of proline [103]. | Increased cell division in apical meristem of seedling roots and decreased root Na+ [119,134]. | |
4.2. Phytohormone Application and Salinity
4.3. Phytohormone Application and Flooding
4.4. Phytohormone Applications and Heat Stress
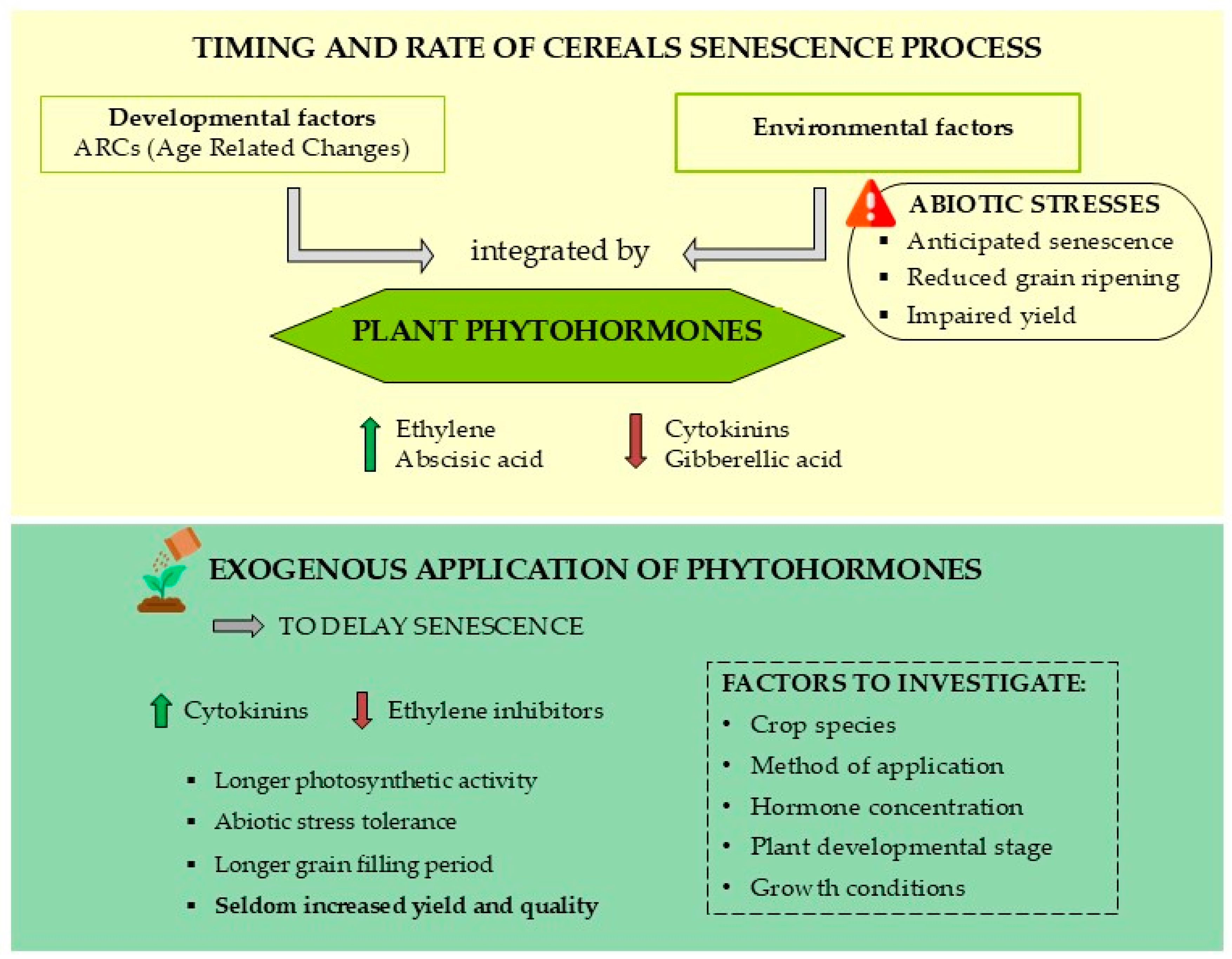
4.5. Phytohormonal Regulation of Leaf Senescence
5. Phytohormone Applications for Senescence Regulation
| Phytohormone | Senescence Process Regulation | Agronomic Effects of Exogenous Phytohormone Application | |
|---|---|---|---|
| Cytokinin | Decreased CK contents in senescing leaves. Regulates senescence onset and process by mediating the movement of tZ acropetally through the xylem and of iP basipetally through the phloem [11]. Regulates senescence by increasing the root sink strength [146]. | Delay of leaf senescence Prolonged photosynthetic activity, increased endosperm cell division rate and delayed nutrient remobilization to grains. Inhibition of amino acid and sugar export to the phloem. It maintains the sink activity of older leaves: protein, chlorophyll and Rubisco increased in older leaves and decreased in young leaves [15]. | |
| Yield and/or gpc improved: Wheat and barley: +2–15% yield [84,168,169,170]. Rice: +4–8% yield [170]. Maize: +3–30% yield [13,109,111]. | Yield and gpc not improved: Wheat: –16% yield [167]. | ||
| Abscisic acid | Increased ABA content associated with accelerated senescence; it integrates stress signal to induce senescence onset. Negatively regulates tolerance responses through inhibition of stomatal closure to induce senescence [69]. | Acceleration of leaf senescence: It accelerates grain-filling rate and N remobilization to grains, increases activity of GS and GPT enzymes for conversion of amino acids to storage proteins [167]. Crosstalk with ethylene: senescence acceleration by increasing ethylene synthesis or sensitivity [107]. | |
| Yield and/or gpc improved: Wheat: +3% yield [107]. | Yield and gpc not improved: Wheat: –31% yield [167]. | ||
| Ethylene | Senescence-promoting hormone; increased release during leaf senescence. Cell-dismantling regulation. Activation of nutrient remobilization. | Application of ethylene inhibitor compounds to delay leaf senescence Delayed chlorophyll degradation and grain filling [113,114]. | |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hodges, J. Cheap food and feeding the world sustainably. Livest. Prod. Sci. 2005, 92, 1–16. [Google Scholar] [CrossRef]
- Teng, Z.; Chen, Y.; Meng, S.; Duan, M.; Zhang, J.; Ye, N. Environmental stimuli: A major challenge during grain filling in cereals. Int. J. Mol. Sci. 2023, 24, 2255. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Harrison, M.T.; Yan, H.; Liu, D.L.; Meinke, H.; Hoogenboom, G.; Wang, B.; Peng, B.; Guan, K.; Jaegermeyr, J.; et al. Silver lining to a climate crisis in multiple prospects for alleviating crop waterlogging under future climates. Nat. Commun. 2023, 14, 765. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Kumar, S.; Mondal, S.; Malviya, N.; Dubey, R. Physiological Basis of Cytokinin induced Drought Tolerance in Wheat (Triticum aestivum L.). JAS 2014, 1, 139–144. [Google Scholar]
- Hare, P.D.; Cress, W.A.; Van Staden, J. The involvement of cytokinins in plant responses to environmental stress. Plant Growth Regul. 1997, 23, 79–103. [Google Scholar] [CrossRef]
- Travaglia, C.; Reinoso, H.; Cohen, A.; Luna, C.; Tommasino, E.; Castillo, C.; Bottini, R. Exogenous ABA increases yield in field-grown wheat with moderate water restriction. J. Plant Growth Regul. 2010, 29, 366–374. [Google Scholar] [CrossRef]
- Davies, P.J. The plant hormone concept: Concentration, sensitivity and transport. In Plant Hormones; Springer: Dordrecht, The Netherland, 1995; pp. 13–38. [Google Scholar]
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef]
- Distelfeld, A.; Avni, R.; Fischer, A.M. Senescence, nutrient remobilization, and yield in wheat and barley. J. Exp. Bot. 2014, 65, 3783–3798. [Google Scholar] [CrossRef]
- Distelfeld, A.; Korol, A.; Dubcovsky, J.; Uauy, C.; Blake, T.; Fahima, T. Colinearity between the barley grain protein content (GPC) QTL on chromosome arm 6HS and the wheat Gpc-B1 region. Mol. Breed. 2008, 22, 25–38. [Google Scholar] [CrossRef]
- Ha, S.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S.P. Cytokinins: Metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci. 2012, 17, 172–179. [Google Scholar] [CrossRef]
- Zahir, Z.A.; Asghar, H.N.; Arshad, M. Cytokinin and its precursors for improving growth and yield of rice. Soil. Biol. Biochem. 2001, 33, 405–408. [Google Scholar] [CrossRef]
- Morris, R.O.; Blevins, D.G.; Dietrich, J.T.; Durley, R.C.; Gelvin, S.B.; Gray, J.; Reinbott, T.M. Cytokinins in plant pathogenic bacteria and developing cereal grains. Funct. Plant Biol. 1993, 20, 621–637. [Google Scholar] [CrossRef]
- Kakimoto, T. Perception and signal transduction of cytokinins. Annu. Rev. Plant Biol. 2003, 54, 605–627. [Google Scholar] [CrossRef] [PubMed]
- Criado, M.V.; Caputo, C.; Roberts, I.N.; Castro, M.A.; Barneix, A.J. Cytokinin-induced changes of nitrogen remobilization and chloroplast ultrastructure in wheat (Triticum aestivum). J. Plant Physiol. 2009, 166, 1775–1785. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Mizuno, T. Two-component phosphorelay signal transduction systems in plants: From hormone responses to circadian rhythms. Biosci. Biotechnol. Bochem. 2005, 69, 2263–2276. [Google Scholar] [CrossRef]
- Gan, S.; Amasino, R.M. Inhibition of leaf senescence by autoregulated production of cytokinin. Science 1995, 270, 1986–1988. [Google Scholar] [CrossRef]
- Jibran, R.; Hunter, D.A.; Dijkwel, P.P. Hormonal regulation of leaf senescence through integration of developmental and stress signals. Plant Mol. Biol. 2013, 82, 547–561. [Google Scholar] [CrossRef]
- Tran, L.S.P.; Urao, T.; Qin, F.; Maruyama, K.; Kakimoto, T.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 20623–20628. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Bano, A.; Saud, S.; Hassan, S.; Shan, D.; Tabassum, M.A. Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: Consequences for changing environment. Environ. Sci. Pollut. Res. 2015, 22, 4907–4921. [Google Scholar] [CrossRef]
- Santner, A.; Calderon-Villalobos, L.I.A.; Estelle, M. Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 2009, 5, 301. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.Y.; Wang, X.F.; Wu, F.Q.; Du, S.Y.; Cao, Z.; Shang, Y.; Fan, R.C. The Mg-chelatase H subunit is an abscisic acid receptor. Nature 2006, 443, 823. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.H.; Hansson, M. The barley magnesium chelatase 150-kD subunit is not an abscisic acid receptor. Plant Physiol. 2009, 150, 157–166. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, X.; Yue, Y.; Li, B.; Nie, Y.; Li, W.; Wu, W.H.; Ma, L. AG protein-coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid. Science 2007, 315, 1712–1716. [Google Scholar] [CrossRef]
- Pandey, S.; Nelson, D.C.; Assmann, S.M. Two novel GPCR-type G proteins are abscisic acid receptors in Arabidopsis. Cell 2009, 136, 136–148. [Google Scholar] [CrossRef]
- Ma, Y.; Szostkiewicz, I.; Korte, A.; Moes, D.; Yang, Y.; Christmann, A.; Grill, E. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 2009, 324, 1064–1068. [Google Scholar] [CrossRef]
- Park, S.Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Cutler, S.R. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef]
- Xing, Y.; Jia, W.; Zhang, J. AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. Plant J. 2008, 54, 440–451. [Google Scholar] [CrossRef]
- Zhang, K.; Xia, X.; Zhang, Y.; Gan, S.S. An ABA-regulated and Golgi-localized protein phosphatase controls water loss during leaf senescence in Arabidopsis. Plant J. 2012, 69, 667–678. [Google Scholar] [CrossRef]
- Bleecker, A.B.; Kende, H. Ethylene: A gaseous signal molecule in plants. Annu. Rev. Cell Dev. Biol. 2000, 16, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Wang, Y.; Liu, H.; Lei, L.; Yang, H.; Ren, D. Activation of MAPK kinase 9 induces ethylene and camalexin biosynthesis and enhances sensitivity to salt stress in Arabidopsis. J. Biol. Chem. 2008, 283, 26996–27006. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Z.; Xiao, L.T.; Lin, W.H.; Cao, Y.; Bo, X.Y. High performance liquid chromatographic determination of internal hormones in inter-subspecific hybrid rice. Chin. J. Chromatogr. 2002, 20, 148–150. [Google Scholar]
- Ju, C.; Chang, C. Mechanistic insights in ethylene perception and signal transduction. Plant Physiol. 2015, 169, 85–95. [Google Scholar] [CrossRef]
- Solano, R.; Stepanova, A.; Chao, Q.; Ecker, J.R. Nuclear events in ethylene signaling: A transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes. Dev. 1998, 12, 3703–3714. [Google Scholar] [CrossRef]
- Guo, H.; Ecker, J.R. Plant responses to ethylene gas are mediated by SCFEBF1/EBF2-dependent proteolysis of EIN3 transcription factor. Cell 2003, 115, 667–677. [Google Scholar] [CrossRef]
- Qiao, H.; Chang, K.N.; Yazaki, J.; Ecker, J.R. Interplay between ethylene, ETP1/ETP2 F-box proteins, and degradation of EIN2 triggers ethylene responses in Arabidopsis. Genes. Dev. 2009, 23, 512–521. [Google Scholar] [CrossRef]
- Kim, J.H.; Woo, H.R.; Kim, J.; Lim, P.O.; Lee, I.C.; Choi, S.H.; Nam, H.G. Trifurcate feed-forward regulation of age-dependent cell death involving miR164 in Arabidopsis. Science 2009, 323, 1053–1057. [Google Scholar] [CrossRef]
- Ciura, J.; Kruk, J. Phytohormones as targets for improving plant productivity and stress tolerance. J. Plant Physiol. 2018, 229, 32–40. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.D.C.; Glick, B.; Santoyo, G. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef]
- Najeeb, U.; Bange, M.P.; Tan, D.K.Y.; Atwell, B.J. Consequences of waterlogging in cotton and opportunities for mitigation of yield losses. AoB Plants 2015, 7, plv080. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.V.; Mendiondo, G.M.; Cantoro, R.; Auge, G.A.; Luna, V.; Masciarelli, O.; Benech-Arnold, R.L. Expression of seed dormancy in grain sorghum lines with contrasting pre-harvest sprouting behavior involves differential regulation of gibberellin metabolism genes. Plant Cell Physiol. 2011, 53, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P. The current status of research on gibberellin biosynthesis. Plant Cell Physiol. 2020, 61, 1832–1849. [Google Scholar] [CrossRef] [PubMed]
- Harberd, N.P.; Belfield, E.; Yasumura, Y. The angiosperm gibberellin-GID1-DELLA growth regulatory mechanism: How an “inhibitor of an inhibitor” enables flexible response to fluctuating environments. Plant Cell 2009, 21, 1328–1339. [Google Scholar] [CrossRef]
- Ueguchi-Tanaka, M.; Ashikari, M.; Nakajima, M.; Itoh, H.; Katoh, E.; Kobayashi, M.; Matsuoka, M. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 2005, 437, 693–698. [Google Scholar] [CrossRef] [PubMed]
- De Lucas, M.; Daviere, J.M.; Rodríguez-Falcón, M.; Pontin, M.; Iglesias-Pedraz, J.M.; Lorrain, S.; Prat, S. A molecular framework for light and gibberellin control of cell elongation. Nature 2008, 451, 480. [Google Scholar] [CrossRef]
- Achard, P.; Renou, J.P.; Berthomé, R.; Harberd, N.P.; Genschik, P. Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr. Biol. 2008, 18, 656–660. [Google Scholar] [CrossRef]
- Navarro, L.; Bari, R.; Achard, P.; Lisón, P.; Nemri, A.; Harberd, N.P.; Jones, J.D. DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr. Biol. 2008, 18, 650–655. [Google Scholar] [CrossRef]
- Saleem, M.; Fariduddin, Q.; Castroverde, C.D.M. Salicylic acid: A key regulator of redox signalling and plant immunity. Plant Physiol. Biochem. 2021, 168, 381–397. [Google Scholar] [CrossRef]
- Qi, G.; Chen, J.; Chang, M.; Chen, H.; Hall, K.; Korin, J.; Fu, Z.Q. Pandemonium breaks out: Disruption of salicylic acid-mediated defense by plant pathogens. Mol. Plant 2018, 11, 1427–1439. [Google Scholar] [CrossRef]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and Mechanism of Jasmonic Acid in Plant Responses to Abiotic and Biotic Stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.E.; Zhang, Y.; Yao, J.; Zheng, J.; Zhou, Y.; He, Q.; Moreno, J.; Lam, V.Q.; Cao, X.; Sugimoto, K.; et al. Assembly of JAZ-JAZ and JAZ-NINJA complexes in jasmonate signaling. Plant Community 2023, 4, 100639. [Google Scholar] [CrossRef] [PubMed]
- Serna-Escolano, V.; Valverde, J.M.; García-Pastor, M.E.; Valero, D.; Castillo, S.; Guillén, F.; Martínez-Romero, D.; Zapata, P.J.; Serrano, M. Pre-harvest methyl jasmonate treatments increase antioxidant systems in lemon fruit without affecting yield or other fruit quality parameters. J. Sci. Food Agric. 2019, 99, 5035–5043. [Google Scholar] [CrossRef] [PubMed]
- Abbas, K.; Li, J.; Gong, B.; Lu, Y.; Wu, X.; Lü, G.; Gao, H. Drought Stress Tolerance in Vegetables: The Functional Role of Structural Features, Key Gene Pathways, and Exogenous Hormones. Int. J. Mol. Sci. 2023, 24, 13876. [Google Scholar] [CrossRef]
- Chen, R.; Ma, J.; Luo, D.; Hou, X.; Ma, F.; Zhang, Y.; Meng, Y.; Zhang, H.; Guo, W. CaMADS, a MADS-box transcription factor from pepper, plays an important role in the response to cold, salt, and osmotic stress. Plant Sci. 2019, 280, 164–174. [Google Scholar] [CrossRef]
- Shang, C.; Liu, X.; Chen, G.; Zheng, H.; Khan, A.; Li, G.; Hu, X. SlWRKY80-mediated jasmonic acid pathway positively regulates tomato resistance to saline-alkali stress by enhancing spermidine content and stabilizing Na+/K+ homeostasis. Hortic. Res. 2024, 11, uhae028. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, C.; Qi, S.; Lin, Q.; Duan, Y. Jasmonic acid and salicylic acid induce the accumulation of sucrose and increase resistance to chilling injury in peach fruit. J. Sci. Food Agric. 2021, 101, 4250–4255. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Y.; Xu, Y.; An, Y.; Hu, Z.; Xiong, A.; Wang, G. Effects of Jasmonic Acid on Stress Response and Quality Formation in Vegetable Crops and Their Underlying Molecular Mechanisms. Plants 2024, 13, 1557. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Schumaker, K.; Zhu, J.K. Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. J. Exp. Bot. 2004, 55, 225–236. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Springer: Dordrecht, The Netherland, 2009; pp. 153–188. [Google Scholar]
- Javid, M.G.; Sorooshzadeh, A.; Moradi, F.; Modarres Sanavy, S.A.M.; Allahdadi, I. The role of phytohormones in alleviating salt stress in crop plants. Aust. J. Crop Sci. 2011, 5, 726. [Google Scholar]
- Ashraf, M. Salt tolerance of cotton: Some new advances. Crit. Rev. Plant Sci. 2002, 21, 1–30. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar, S.; Prakash, P. Exogenous application of cytokinin (6-BAP) ameliorates the adverse effect of combined drought and high temperature stress in wheat seedling. J. Pharmacogn. Phytochem. 2018, 7, 1176–1180. [Google Scholar]
- Mao, H.; Jian, C.; Cheng, X.; Chen, B.; Mei, F.; Li, F.; Kang, Z. The wheat ABA receptor gene TaPYL1-1B contributes to drought tolerance and grain yield by increasing water-use efficiency. Plant Biotechnol. J. 2022, 20, 846–861. [Google Scholar] [CrossRef]
- Dodd, I.C. Root-to-shoot signalling: Assessing the roles of ‘up’in the up and down world of long-distance signalling in planta. Plant Soil 2005, 274, 251–270. [Google Scholar] [CrossRef]
- Kang, D.J.; Seo, Y.J.; Lee, J.D.; Ishii, R.; Kim, K.U.; Shin, D.H.; Lee, I.J. Jasmonic acid differentially affects growth, ion uptake and abscisic acid concentration in salt-tolerant and salt-sensitive rice cultivars. J. Agron. Crop Sci. 2005, 191, 273–282. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, W.; Yang, J.; Ismail, A.M. Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Res. 2006, 97, 111–119. [Google Scholar] [CrossRef]
- Zhang, K.; Gan, S.S. An abscisic acid-AtNAP transcription factor-SAG113 protein phosphatase 2C regulatory chain for controlling dehydration in senescing Arabidopsis leaves. Plant Physiol. 2012, 158, 961–969. [Google Scholar] [CrossRef]
- Sauter, A.; Davies, W.J.; Hartung, W. The long-distance abscisic acid signal in the droughted plant: The fate of the hormone on its way from root to shoot. J. Exp. Bot. 2001, 52, 1991–1997. [Google Scholar] [CrossRef]
- Fricke, W.; Akhiyarova, G.; Veselov, D.; Kudoyarova, G. Rapid and tissue-specific changes in ABA and in growth rate in response to salinity in barley leaves. J. Exp. Bot. 2004, 55, 1115–1123. [Google Scholar] [CrossRef]
- HavlovA, M.; Dobrev, P.I.; Motyka, V.; Storchová, H.; Libus, J.; DobrA, J.; VankovA, R. The role of cytokinins in responses to water deficit in tobacco plants over-expressing trans-zeatin O-glucosyltransferase gene under 35S or SAG12 promoters. Plant Cell Environ. 2008, 31, 341–353. [Google Scholar] [CrossRef]
- Albacete, A.; Ghanem, M.E.; Martínez-Andújar, C.; Acosta, M.; Sánchez-Bravo, J.; Martínez, V.; Pérez-Alfocea, F. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. J. Exp. Bot. 2008, 59, 4119–4131. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, R.; Watanabe, Y.; Fujita, Y.; Le, D.T.; Kojima, M.; Werner, T.; Sakakibara, H. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 2011, 23, 2169–2183. [Google Scholar] [CrossRef] [PubMed]
- Christmann, A.; Moes, D.; Himmelbach, A.; Yang, Y.; Tang, Y.; Grill, E. Integration of abscisic acid signalling into plant responses. Plant Biol. 2006, 8, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Corot, A.; Roman, H.; Douillet, O.; Autret, H.; Perez-Garcia, M.D.; Citerne, S.; Demotes-Mainard, S. Cytokinins and abscisic acid act antagonistically in the regulation of the bud outgrowth pattern by light intensity. Front. Plant Sci. 2017, 8, 1724. [Google Scholar] [CrossRef]
- Zlobin, I.; Efimova, M.; Permykova, N.; Sokolova, I.; Kuznetsov, V.; Deineko, E. The Modification of Abscisic Acid and Cytokinin Signaling with Genome Editing to Increase Plant Drought Tolerance; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Guan, C.; Wang, X.; Feng, J.; Hong, S.; Liang, Y.; Ren, B.; Zuo, J. Cytokinin antagonizes abscisic acid-mediated inhibition of cotyledon greening by promoting the degradation of abscisic acid insensitive5 protein in Arabidopsis. Plant Physiol. 2014, 164, 1515–1526. [Google Scholar] [CrossRef]
- Shoaib, M.; Banerjee, B.P.; Hayden, M.; Kant, S. Roots’ drought adaptive traits in crop improvement. Plants 2022, 11, 2256. [Google Scholar] [CrossRef]
- Shkolnik-Inbar, D.; Bar-Zvi, D. ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. Plant Cell 2010, 22, 3560–3573. [Google Scholar] [CrossRef]
- Narayana, I.; Lalonde, S.; Saini, H.S. Water-stress-induced ethylene production in wheat: A fact or artifact? Plant Physiol. 1991, 96, 406–410. [Google Scholar] [CrossRef]
- Morgan, P.W.; Drew, M.C. Ethylene and plant responses to stress. Physiol. Plant 1997, 100, 620–630. [Google Scholar] [CrossRef]
- Sharp, R.E.; LeNoble, M.E. ABA, ethylene and the control of shoot and root growth under water stress. J. Exp. Bot. 2002, 53, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, Y.; Shi, Y.; Cui, Z.; Luo, Y.; Zheng, M.; Wang, Z. Exogenous cytokinins increase grain yield of winter wheat cultivars by improving stay-green characteristics under heat stress. PLoS ONE 2016, 11, e0155437. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, Y.; Xie, Y.; Wang, Y.; Duan, L.; Zhang, M.; Li, Z. Ethephon improved drought tolerance in maize seedlings by modulating cuticular wax biosynthesis and membrane stability. J. Plant Physiol. 2017, 214, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Hays, D.B.; Do, J.H.; Mason, R.E.; Morgan, G.; Finlayson, S.A. Heat stress induced ethylene production in developing wheat grains induces kernel abortion and increased maturation in a susceptible cultivar. Plant Sci. 2007, 172, 1113–1123. [Google Scholar] [CrossRef]
- McKeon, T.A.; Hoffman, N.E.; Yang, S.F. The effect of plant-hormone pretreatments on ethylene production and synthesis of 1-aminocyclopropane-1-carboxylic acid in water-stressed wheat leaves. Planta 1982, 155, 437–443. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene role in plant growth, development and senescence: Interaction with other phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef]
- Lin, Z.; Zhong, S.; Grierson, D. Recent advances in ethylene research. J. Exp. Bot. 2009, 60, 3311–3336. [Google Scholar] [CrossRef]
- Wang, H.; Stier, G.; Lin, J.; Liu, G.; Zhang, Z.; Chang, Y.; Jiang, C.Z. Transcriptome changes associated with delayed flower senescence on transgenic petunia by inducing expression of etr1-1, a mutant ethylene receptor. PLoS ONE 2013, 8, e65800. [Google Scholar] [CrossRef]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Fukao, T.; Bailey-Serres, J. Submergence tolerance conferred by Sub1A is mediated by SLR1 and SLRL1 restriction of gibberellin responses in rice. Proc. Natl. Acad. Sci. USA 2008, 105, 16814–16819. [Google Scholar] [CrossRef]
- Fukao, T.; Yeung, E.; Bailey-Serres, J. The submergence tolerance gene SUB1A delays leaf senescence under prolonged darkness through hormonal regulation in rice. Plant Physiol. 2012, 160, 1795–1807. [Google Scholar] [CrossRef] [PubMed]
- Sharp, R.E. Interaction with ethylene: Changing views on the role of abscisic acid in root and shoot growth responses to water stress. Plant Cell Environ. 2002, 25, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Sharipova, G.; Veselov, D.; Kudoyarova, G.; Fricke, W.; Dodd, I.C.; Katsuhara, M.; Veselov, S. Exogenous application of abscisic acid (ABA) increases root and cell hydraulic conductivity and abundance of some aquaporin isoforms in the ABA-deficient barley mutant Az34. Ann. Bot. 2016, 118, 777–785. [Google Scholar] [CrossRef]
- McAdam, S.A.; Brodribb, T.J. Linking turgor with ABA biosynthesis: Implications for stomatal responses to vapour pressure deficit across land plants. Plant Physiol. 2016, 171, 2008–2016. [Google Scholar] [CrossRef]
- Spollen, W.G.; LeNoble, M.E.; Samuels, T.D.; Bernstein, N.; Sharp, R.E. Abscisic acid accumulation maintains maize primary root elongation at low water potentials by restricting ethylene production. Plant Physiol. 2000, 122, 967–976. [Google Scholar] [CrossRef]
- Saab, I.N.; Sharp, R.E.; Pritchard, J.; Voetberg, G.S. Increased endogenous abscisic acid maintains primary root growth and inhibits shoot growth of maize seedlings at low water potentials. Plant Physiol. 1990, 93, 1329–1336. [Google Scholar] [CrossRef]
- Yemelyanov, V.V.; Shishova, M.F. The role of phytohormones in the control of plant adaptation to oxygen depletion. In Phytohormones and Abiotic Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2012; pp. 229–248. [Google Scholar]
- Iqbal, M.; Ashraf, M. Changes in hormonal balance: A possible mechanism of pre-sowing chilling-induced salt tolerance in spring wheat. J. Agron. Crop Sci. 2010, 196, 440–454. [Google Scholar] [CrossRef]
- Nayyar, H.; Walia, D.P.; Kaistha, B.L. Performance of bread wheat (Triticum aestivum) seed primed with growth-regulators and inorganic salts. Indian. J. Agric. Sci. 1995, 65, 112–116. [Google Scholar]
- Khan, S.U.; Gurmani, A.R.; Qayyum, A.; Abbasi, K.S.; Liaquat, M.; Zahoor, A. Exogenously applied gibberellic acid, indole acetic acid and kinetin as potential regulators of source-sink relationship, physiological and yield attributes in rice (Oryza sativa) genotypes under water deficit conditions. Int. J. Agric. Biol. 2016, 18, 139–145. [Google Scholar] [CrossRef]
- Horváth, E.; Szalai, G.; Janda, T. Induction of abiotic stress tolerance by salicylic acid signaling. J. Plant Growth Regul. 2007, 26, 290–300. [Google Scholar] [CrossRef]
- El-Tayeb, M.A. Response of barley grains to the interactive e. ect of salinity and salicylic acid. Plant Growth Regul. 2005, 45, 215–224. [Google Scholar] [CrossRef]
- Ananieva, E.A.; Christov, K.N.; Popova, L.P. Exogenous treatment with salicylic acid leads to increased antioxidant capacity in leaves of barley plants exposed to paraquat. J. Plant Physiol. 2004, 161, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Shah, J. The salicylic acid loop in plant defense. Curr. Opin. Plant Biol. 2003, 6, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Zhang, J.H.; Wang, Z.Q.; Zhu, Q.S.; Liu, L.J. Involvement of abscisic acid and cytokinins in the senescence and remobilization of carbon reserves in wheat subjected to water stress during grain filling. Plant Cell Environ. 2003, 26, 1621–1631. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, Y.; Zhang, X.; Li, W.; Chang, Y.; Pang, D.; Wang, Z. Interactions between cytokinin and nitrogen contribute to grain mass in wheat cultivars by regulating the flag leaf senescence process. Crop J. 2018, 6, 538–551. [Google Scholar] [CrossRef]
- Ren, B.; Zhu, Y.; Zhang, J.; Dong, S.; Liu, P.; Zhao, B. Effects of spraying exogenous hormone 6-benzyladenine (6-BA) after waterlogging on grain yield and growth of summer maize. Field Crops Res. 2016, 188, 96–104. [Google Scholar] [CrossRef]
- Younis, M.; El-Shahaby, O.; Alla, M.M.N.; El-Bastawisy, Z. Kinetin alleviates the influence of waterlogging and salinity on growth and affects the production of plant growth regulators in Vigna sinensis and Zea mays. Agronomie 2003, 23, 277–285. [Google Scholar] [CrossRef]
- Gao, Z.; Liang, X.G.; Zhang, L.; Lin, S.; Zhao, X.; Zhou, L.L.; Zhou, S.L. Spraying exogenous 6-benzyladenine and brassinolide at tasseling increases maize yield by enhancing source and sink capacity. Field Crops Res. 2017, 211, 1–9. [Google Scholar] [CrossRef]
- Gurmani, A.R.; Bano, A.; Ullah, N.; Khan, H.; Jahangir, M.; Flowers, T.J. Exogenous abscisic acid (ABA) and silicon (Si) promote salinity tolerance by reducing sodium (Na+) transport and bypass flow in rice (‘Oryza sativa’indica). Aust. J. Crop Sci. 2013, 7, 1219. [Google Scholar]
- Beltrano, J.; Carbone, A.; Montaldi, E.R.; Guiamet, J.J. Ethylene as promoter of wheat grain maturation and ear senescence. Plant Growth Regul. 1994, 15, 107–112. [Google Scholar] [CrossRef]
- Beltrano, J.; Ronco, M.G.; Montaldi, E.R. Drought stress syndrome in wheat is provoked by ethylene evolution imbalance and reversed by rewatering, aminoethoxyvinylglycine, or sodium benzoate. J. Plant Growth Regul. 1999, 18, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Singh, B. Effect of plant hormones on growth and yield of wheat irrigated with saline water. Ann. Agric. Res. 1996, 17, 209–212. [Google Scholar]
- Tuna, A.L.; Kaya, C.; Dikilitas, M.; Higgs, D. The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environ. Exp. Bot. 2008, 62, 1–9. [Google Scholar] [CrossRef]
- Kaya, C.; Tuna, A.L.; Alfredo, A.A. Gibberellic acid improves water deficit tolerance in maize plants. Acta Physiol. Plant 2006, 28, 331–337. [Google Scholar] [CrossRef]
- Fahad, S.; Bano, A. Effect of salicylic acid on physiological and biochemical characterization of maize grown in saline area. Pak. J. Bot. 2012, 44, 1433–1438. [Google Scholar]
- Gunes, A.; Inal, A.; Alpaslan, M.; Eraslan, F.; Bagci, E.G.; Cicek, N. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J. Plant Physiol. 2007, 164, 728–736. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Liu, F.; Jensen, C.R.; Andersen, M.N. A review of drought adaptation in crop plants: Changes in vegetative and reproductive physiology induced by ABA-based chemical signals. Aust. J. Agric. Res. 2005, 56, 1245–1252. [Google Scholar] [CrossRef]
- Ivanov, A.G.; Krol, M.; Maxwell, D.; Huner, N.P.A. Abscisic acid induced protection against photoinhibition of PSII correlates with enhanced activity of the xanthophyll cycle. FEBS Lett. 1995, 371, 61–64. [Google Scholar] [CrossRef]
- Parida, A.K.; Dagaonkar, V.S.; Phalak, M.S.; Umalkar, G.V.; Aurangabadkar, L.P. Alterations in photosynthetic pigments, protein and osmotic components in cotton genotypes subjected to short-term drought stress followed by recovery. Plant Biotechnol. Rep. 2007, 1, 37–48. [Google Scholar] [CrossRef]
- Ashraf, M.; Ahmad, A.; McNeilly, T. Growth and photosynthetic characteristics in pearl millet under water stress and different potassium supply. Photosynthetica 2001, 39, 389–394. [Google Scholar] [CrossRef]
- Rivero, R.M.; Gimeno, J.; Van Deynze, A.; Walia, H.; Blumwald, E. Enhanced cytokinin synthesis in tobacco plants expressing PSARK:: IPT prevents the degradation of photosynthetic protein complexes during drought. Plant Cell Physiol. 2010, 51, 1929–1941. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Agarwal, V.P.; Gupta, N.K. Efficacy of putrescine and benzyladenine on photosynthesis and productivity in relation to drought tolerance in wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2012, 18, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Bergner, C.; Teichmann, C. A role for ethylene in barley plants responding to soil water shortage. J. Plant Growth Regul. 1993, 12, 67. [Google Scholar] [CrossRef]
- Greenberg, B.M.; Huang, X.D.; Gerwing, P.; Yu, X.M.; Chang, P.; Wu, S.S.; Glick, B. Phytoremediation of salt impacted soils: Greenhouse and the field trials of plant growth promoting rhizobacteria (PGPR) to improve plant growth and salt phytoaccumulation. In Proceedings of the 33rd AMOP Technical Seminar on Environmental Contamination and Response, Halifax, NS, Canada, 7–9 June 2010; Environment Canada: Ottawa, ON, Canada, 2009; Volume 2, pp. 627–637. [Google Scholar]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd_Allah, E.F.; Hashem, A. Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef]
- Al-Hakimi, A.M.A.; Hamada, A.M. Counteraction of salinity stress on wheat plants by grain soaking in ascorbic acid, hiamine or sodium salicylate. Biol. Plant 2001, 44, 253–261. [Google Scholar] [CrossRef]
- Singh, B.; Usha, K. Salicylic acid induced physiological and biochemical changes in wheat seedlings under water stress. Plant Growth Regul. 2003, 39, 137–141. [Google Scholar] [CrossRef]
- Bandurska, H. The effect of salicylic acid on barley response to water deficit. Acta Physiol. Plant 2005, 27, 379–386. [Google Scholar] [CrossRef]
- Sedaghat, M.; Tahmasebi-Sarvestani, Z.; Emam, Y.; Mokhtassi-Bidgoli, A. Physiological and antioxidant responses of winter wheat cultivars to strigolactone and salicylic acid in drought. Plant Physiol. Biochem. 2017, 119, 59–69. [Google Scholar] [CrossRef]
- Shakirova, F.M.; Sakhabutdinova, A.R.; Bezrukova, M.V.; Fatkhutdinova, R.A.; Fatkhutdinova, D.R. Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 2003, 164, 317–322. [Google Scholar] [CrossRef]
- Bohra, J.S.; Dörffling, H.; Dörffling, K. Salinity tolerance of rice (Oryza sativa L.) with reference to endogenous and exogenous abscisic acid. J. Agron. Crop Sci. 1995, 174, 79–86. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, X.; Wang, C.; Jing, R. Overexpression of a common wheat gene TaSnRK2. 8 enhances tolerance to drought, salt and low temperature in Arabidopsis. PLoS ONE 2010, 5, e16041. [Google Scholar] [CrossRef] [PubMed]
- Gulnaz, A.; Iqbal, J.; Farooq, S.; Azam, F. Seed treatment with growth regulators and crop productivity. I. 2, 4-D as an inducer of salinity-tolerance in wheat (Triticum aestivum L.). Plant Soil 1999, 210, 209–218. [Google Scholar] [CrossRef]
- Afzal, I.; Basra, S.A.; Iqbal, A. The effects of seed soaking with plant growth regulators on seedling vigor of wheat under salinity stress. J. Stress. Physiol. Biochem. 2005, 1, 6–15. [Google Scholar]
- Iqbal, M.; Ashraf, M.; Jamil, A. Seed enhancement with cytokinins: Changes in growth and grain yield in salt stressed wheat plants. Plant Growth Regul. 2006, 50, 29–39. [Google Scholar] [CrossRef]
- Radi, A.F.; Shaddad, M.A.K.; El-Enany, A.E.; Omran, F.M. Interactive effects of plant hormones (GA3 or ABA) and salinity on growth and some metabolites of wheat seedlings. In Plant Nutrition; Springer: Dordrecht, The Netherland, 2001; pp. 436–437. [Google Scholar]
- Jamei, R.; Heidari, R.; Khara, J.; Zare, S. The interaction effects of flooding and kinetin on growth criteria, chlorophyll content, and 5-aminolevulinic acid dehydratase activity in corn seedlings. Turk. J. Biol. 2008, 32, 253–257. [Google Scholar]
- Wang, Y.; Yang, Z.M.; Zhang, Q.F.; Li, J.L. Enhanced chilling tolerance in Zoysia matrella by pre-treatment with salicylic acid, calcium chloride, hydrogen peroxide or 6-benzylaminopurine. Biol. Plant 2009, 53, 179. [Google Scholar] [CrossRef]
- Martre, P.; Jamieson, P.D.; Semenov, M.A.; Zyskowski, R.F.; Porter, J.R.; Triboi, E. Modelling protein content and composition in relation to crop nitrogen dynamics for wheat. Eur. J. Agron. 2006, 25, 138–154. [Google Scholar] [CrossRef]
- Schlüter, T.; Leide, J.; Conrad, K. Light promotes an increase of cytokinin oxidase/dehydrogenase activity during senescence of barley leaf segments. J. Plant Physiol. 2011, 168, 694–698. [Google Scholar] [CrossRef]
- Buchanan-Wollaston, V.; Page, T.; Harrison, E.; Breeze, E.; Lim, P.O.; Nam, H.G.; Leaver, C.J. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J. 2005, 42, 567–585. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H.; Schmülling, T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 2003, 15, 2532–2550. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Peng, S.; Visperas, R.M.; Sanico, A.L.; Zhu, Q.; Gu, S. Grain filling pattern and cytokinin content in the grains and roots of rice plants. Plant Growth Regul. 2000, 30, 261–270. [Google Scholar] [CrossRef]
- Wang, G.; Liu, X.; Gan, S.S. The ABA–AtNAP–SAG113 PP2C module regulates leaf senescence by dephosphorylating SAG114 SnRK3.25 in Arabidopsis. Mol. Hortic. 2023, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, K.; Jikumaru, Y.; Kamiya, Y.; Kusano, M.; Consonni, C.; Panstruga, R.; Shirasu, K. Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. Plant Cell 2009, 21, 2914–2927. [Google Scholar] [CrossRef]
- Xiao, S.; Gao, W.; Chen, Q.F.; Chan, S.W.; Zheng, S.X.; Ma, J.; Chye, M.L. Overexpression of Arabidopsis acyl-CoA binding protein ACBP3 promotes starvation-induced and age-dependent leaf senescence. Plant Cell 2010, 22, 1463–1482. [Google Scholar] [CrossRef]
- Yu, K.; Wei, J.; Ma, Q.; Yu, D.; Li, J. Senescence of aerial parts is impeded by exogenous gibberellic acid in herbaceous perennial Paris polyphylla. J. Plant Physiol. 2009, 166, 819–830. [Google Scholar] [CrossRef]
- Zhao, Y.; Christensen, S.K.; Fankhauser, C.; Cashman, J.R.; Cohen, J.D.; Weigel, D.; Chory, J. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 2001, 291, 306–309. [Google Scholar] [CrossRef]
- Kant, S.; Rothstein, S. Auxin-responsive SAUR39 gene modulates auxin level in rice. Plant Signal Behav. 2009, 4, 1174–1175. [Google Scholar] [CrossRef]
- Chen, J.; Liang, Y.; Hu, X.; Wang, X.; Tan, F.; Zhang, H.; Luo, P. Physiological characterization of ‘stay green’wheat cultivars during the grain filling stage under field growing conditions. Acta Physiol. Plant 2010, 32, 875–882. [Google Scholar] [CrossRef]
- Luo, P.; Ren, Z.; Wu, X.; Zhang, H.; Zhang, H.; Feng, J. Structural and biochemical mechanism responsible for the stay-green phenotype in common wheat. Chin. Sci. Bull. 2006, 51, 2595–2603. [Google Scholar] [CrossRef]
- Marinaccio, F.; Reyneri, A.; Blandino, M. Enhancing grain yield and quality of winter barley through agronomic strategies to prolong canopy greenness. Field Crops Res. 2015, 170, 109–118. [Google Scholar] [CrossRef]
- Kumar, U.; Joshi, A.K.; Kumari, M.; Paliwal, R.; Kumar, S.; Röder, M.S. Identification of QTLs for stay green trait in wheat (Triticum aestivum L.) in the ‘Chirya 3’בSonalika’population. Euphytica 2010, 174, 437–445. [Google Scholar] [CrossRef]
- Joshi, A.K.; Kumari, M.; Singh, V.P.; Reddy, C.M.; Kumar, S.; Rane, J.; Chand, R. Stay green trait: Variation, inheritance and its association with spot blotch resistance in spring wheat (Triticum aestivum L.). Euphytica 2007, 153, 59–71. [Google Scholar] [CrossRef]
- Tian, F.; Gong, J.; Zhang, J.; Zhang, M.; Wang, G.; Li, A.; Wang, W. Enhanced stability of thylakoid membrane proteins and antioxidant competence contribute to drought stress resistance in the tasg1 wheat stay-green mutant. J. Exp. Bot. 2013, 64, 1509–1520. [Google Scholar] [CrossRef]
- Hui, Z.; Tian, F.X.; Wang, G.K.; Wang, G.P.; Wang, W. The antioxidative defense system is involved in the delayed senescence in a wheat mutant tasg1. Plant Cell Rep. 2012, 31, 1073–1084. [Google Scholar] [CrossRef]
- Thomas, H.; Howarth, C.J. Five ways to stay green. J. Exp. Bot. 2000, 51, 329–337. [Google Scholar] [CrossRef]
- Gregersen, P.L.; Culetic, A.; Boschian, L.; Krupinska, K. Plant senescence and crop productivity. Plant Mol. Biol. 2013, 82, 603–622. [Google Scholar] [CrossRef]
- Kant, S.; Burch, D.; Badenhorst, P.; Palanisamy, R.; Mason, J.; Spangenberg, G. Regulated expression of a cytokinin biosynthesis gene IPT delays leaf senescence and improves yield under rainfed and irrigated conditions in canola (Brassica napus L.). PLoS ONE 2015, 10, e0116349. [Google Scholar] [CrossRef]
- Spano, G.; Di Fonzo, N.; Perrotta, C.; Platani, C.; Ronga, G.; Lawlor, D.W.; Shewry, P.R. Physiological characterization of ‘stay green’mutants in durum wheat. J. Exp. Bot. 2003, 54, 1415–1420. [Google Scholar] [CrossRef]
- Emebiri, L.C. QTL dissection of the loss of green colour during post-anthesis grain maturation in two-rowed barley. Theor. Appl. Genet. 2013, 126, 1873–1884. [Google Scholar] [CrossRef]
- Robson, P.R.; Donnison, I.S.; Wang, K.; Frame, B.; Pegg, S.E.; Thomas, A.; Thomas, H. Leaf senescence is delayed in maize expressing the Agrobacterium IPT gene under the control of a novel maize senescence-enhanced promoter. Plant Biotechnol. J. 2004, 2, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Jiang, D.; Dai, T.; Jing, Q.; Cao, W. Effects of exogenous ABA and cytokinin on leaf photosynthesis and grain protein accumulation in wheat ears cultured in vitro. Plant Growth Regul. 2004, 44, 25–32. [Google Scholar] [CrossRef]
- Trcková, M.; Kamínek, M.; Zmrhal, Z. Grain formation and distribution of nutrients in wheat plants after the application of synthetic cytokinin N6-(meta-hydroxybenzyl) adenosine. In Physiology and Biochemistry of Cytokinins in Plants; SPB Academic Publishers: The Hague, The Netherlands, 1992; pp. 214–247. [Google Scholar]
- Hosseini, M.S.; Salek Mearaji, H.; Tavakoli, A.; Fotovat, R. The influence of foliar application of cytokinin on wheat cultivars’ physiological traits and yield. Crop Sci. Res. Arid. Reg. 2022, 4, 1–17. [Google Scholar]
- Zhu, K.; Ren, W.; Yan, J.; Zhang, Y.; Zhang, W.; Xu, Y.; Yang, J. Grain yield and nitrogen use efficiency are increased by exogenous cytokinin application through the improvement in root physiological traits of rice. Plant Growth Regul. 2022, 97, 157–169. [Google Scholar] [CrossRef]
- Morita, S.; Yonemaru, J.I.; Takanashi, J.I. Grain growth and endosperm cell size under high night temperatures in rice (Oryza sativa L.). Ann. Bot. 2005, 95, 695–701. [Google Scholar] [CrossRef]
- Love, B.; Molero, G.; Rivera-Amado, C.; Müller, M.; Munné-Bosch, S.; Reynolds, M.P.; Foulkes, M.J. Associations between endogenous spike cytokinins and grain-number traits in spring wheat genotypes. Eur. J. Agron. 2024, 152, 127011. [Google Scholar] [CrossRef]
- Sun, W.; Lu, C.; Wen, L.; Liu, Y.; Zhou, X.; Xiao, X.; Zhang, Y. Low sucrose availability reduces basal spikelet fertility by inducing abscisic acid and jasmonic acid synthesis in wheat. J. Exp. Bot. 2024, 75, 1967–1981. [Google Scholar] [CrossRef]
- Koprna, R.; De Diego, N.; Dundálková, L.; Spíchal, L. Use of cytokinins as agrochemicals. Bioorg Med. Chem. 2016, 24, 484–492. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Wang, Z.; Zhu, Q.; Liu, L. Water deficit–induced senescence and its relationship to the remobilization of pre-stored carbon in wheat during grain filling. Agron. J. 2001, 93, 196–206. [Google Scholar] [CrossRef]
- Grossmann, K.; Kwiatkowski, J.; Caspar, G. Regulation of phytohormone levels, leaf senescence and transpiration by the strobilurin kresoxim-methyl in wheat (Triticum aestivum). J. Plant Physiol. 1999, 154, 805–808. [Google Scholar] [CrossRef]
- Sýkorová, B.; Kurešová, G.; Daskalova, S.; Trčková, M.; Hoyerová, K.; Raimanová, I.; Kamínek, M. Senescence-induced ectopic expression of the A. tumefaciens ipt gene in wheat delays leaf senescence, increases cytokinin content, nitrate influx, and nitrate reductase activity, but does not affect grain yield. J. Exp. Bot. 2008, 59, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Peng, S.; Bennett, J. Glutamine synthetase and its isoforms in rice spikelets and rachis during grain development. J. Plant Physiol. 2000, 156, 230–233. [Google Scholar] [CrossRef]
- Roitsch, T.; Ehneß, R. Regulation of source/sink relations by cytokinins. Plant Growth Regul. 2000, 32, 359–367. [Google Scholar] [CrossRef]
- Balibrea Lara, M.E.; Gonzalez Garcia, M.C.; Fatima, T.; Ehneß, R.; Lee, T.K.; Proels, R.; Roitsch, T. Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. Plant Cell 2004, 16, 1276–1287. [Google Scholar] [CrossRef]
- Gregersen, P.L. Senescence and nutrient remobilization in crop plants. In The Molecular and Physiological Basis of Nutrient Use Efficiency in Crops; Wiley-Blackwell: Oxford, UK, 2011; pp. 83–102. [Google Scholar]
- Jukanti, A.K.; Fischer, A.M. A high-grain protein content locus on barley (Hordeum vulgare) chromosome 6 is associated with increased flag leaf proteolysis and nitrogen remobilization. Physiol. Plant 2008, 132, 426–439. [Google Scholar] [CrossRef]
| Phytohormone | Treatment Type | Crop | Phytohormone Concentration (mg/L) | Application Time/Method | Target Stress/Aim | Reference |
|---|---|---|---|---|---|---|
| Cytokinin | 1 | Wheat | 10 6-BAP | Seed pre-treatment | Drought and heat | [64] |
| 3 | Wheat | 8.61 kinetin | Sprayed from 9 DPA for 5 consecutive days | Drought | [107] | |
| 3 | Wheat | 25 6-BA | Sprayed daily for 3 days after anthesis | Yield increase | [108] | |
| 3 | Wheat | 10 6-BA | Sprayed daily for 3 days after anthesis | Heat | [84] | |
| 2 | Maize | 100 6-BA | Sprayed the day after waterlogging stress | Waterlogging | [109] | |
| 2 | Maize | 50 kinetin | Sprayed on waterlogged plants | Waterlogging | [110] | |
| 3 | Maize | 25 6-BA | Sprayed at tasselling for 3 consecutive days | Yield increase | [111] | |
| Abscisic acid | 1 | Rice | 2.46 ABA | Seed pre-treatment | Salinity | [112] |
| 2–3 | Wheat | 300 ABA | 2 treatments: at shoot enlargement and anthesis | Drought | [6] | |
| Ethylene | 3 | Wheat | 8.06 AVG | Weekly from anthesis till hard dough stage | Yield increase | [113,114] |
| 2 | Wheat | 1 mg/Kg 1-MCP | Sprayed once before heat stress occurrence | Heat | [86] | |
| Gibberellins | 1 | Wheat | Not cited | Seed pre-treatment | Yield increase | [115] |
| 2 | Maize | 25–50–100 GA3 | Weekly from 10 till 45 DAG | Salinity | [116,117] | |
| 2 | Rice | 3.46 GA3 | At panicle initiation | Drought | [102] | |
| Salicylic acid | 2 | Maize | 1.38 SA | Leaf spraying at 40 DAS | Salinity | [118] |
| 1 | Barley | 138 SA | Seed pre-treatment | Salinity | [104] | |
| - | Maize | 14–69–138 SA | Incorporated into the soil (pot trial) | Salinity | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panozzo, A.; Bolla, P.K.; Barion, G.; Botton, A.; Vamerali, T. Phytohormonal Regulation of Abiotic Stress Tolerance, Leaf Senescence and Yield Response in Field Crops: A Comprehensive Review. BioTech 2025, 14, 14. https://doi.org/10.3390/biotech14010014
Panozzo A, Bolla PK, Barion G, Botton A, Vamerali T. Phytohormonal Regulation of Abiotic Stress Tolerance, Leaf Senescence and Yield Response in Field Crops: A Comprehensive Review. BioTech. 2025; 14(1):14. https://doi.org/10.3390/biotech14010014
Chicago/Turabian StylePanozzo, Anna, Pranay Kumar Bolla, Giuseppe Barion, Alessandro Botton, and Teofilo Vamerali. 2025. "Phytohormonal Regulation of Abiotic Stress Tolerance, Leaf Senescence and Yield Response in Field Crops: A Comprehensive Review" BioTech 14, no. 1: 14. https://doi.org/10.3390/biotech14010014
APA StylePanozzo, A., Bolla, P. K., Barion, G., Botton, A., & Vamerali, T. (2025). Phytohormonal Regulation of Abiotic Stress Tolerance, Leaf Senescence and Yield Response in Field Crops: A Comprehensive Review. BioTech, 14(1), 14. https://doi.org/10.3390/biotech14010014







